Introduction
Methicillin-resistant Staphylococcus aureus
(MRSA) is a prominent human pathogen that is known for causing skin
infections, as well as hospital-acquired pneumonia, osteomyelitis
and abscesses (1); MRSA may also
result in considerable morbidity and mortality in orthopedic
patients. The mortality rate of MRSA bacteremia is twice as high as
that of patients with methicillin-sensitive Staphylococcus
aureus (MSSA) (2). In addition,
the complication rate and cost of periprosthetic joint infection
due to MRSA is considerably higher compared with that in MSSA
infection (3). Patients receiving
orthopedic implants are highly vulnerable due to the possibility of
biofilm formation and long-term morbidity (4,5).
Currently, the incidence of MRSA in orthopedic departments is
increasing (6). MRSA strains are
resistant not only to β-lactam antibiotics, but also to
fluoroquinolones and other families of antibiotics (7).
The Morus genus is part of the Moraceae
family, which includes 10–16 species of deciduous trees that are
found worldwide (8). For >1,900
years, different parts of the Morus plants, including the
branches, fruit, leaves, bark, shoot and root, have been used in
China as food and herbal medicine (9). The compounds resveratrol and
oxyresveratrol (ORV) are present in the Morus plants and
have been revealed to possess antioxidant activity (10). A previous study reported that ORV
inhibited the production of prostaglandin E2 and
nitrogen oxide (NO), the expression of inducible NO synthase (iNOS)
and the activation of nuclear factor-κB in macrophages, while
consistently reducing carrageenan-induced edema in a mouse model
(11).
ORV, a polyphenolic stilbene, is abundantly derived
from the heartwood of the traditional Thai plant, Artocarpus
lakoocha Roxburgh, which belongs to the Moraceae family
(12,13). This compound has been demonstrated to
have an inhibitory effect on the growth of Herpes simplex virus
(HSV)-1 and HSV-2 wild types, drug-resistant HSV-1 strains
(14), clinical isolates of HSV-1
and HSV-2 (15), in addition to
numerous varicella zoster virus (VZV) strains, including the wild
type, thymidine kinase-deficient and DNA polymerase VZV mutants
in vitro (16). Numerous
biological activities of ORV have also been reported, including
tyrosinase-inhibition (17) and
antioxidant (18) and anthelmintic
activities (19). Topical
application of 30% w/w ORV in petroleum jelly
suspension was reported to provide superior therapeutic efficacy
compared with oral treatment with ORV for cutaneous HSV-1 infection
in mice (14).
However, the antibacterial capacity and mechanism of
ORV against Staphylococcus aureus (S. aureus)
remain unknown. Therefore, the present study investigated the
antibacterial activities of ORV alone or in combination with
bacterial membrane-binding agents, including Triton X-100 (TX),
sodium azide (NaN3) and
N,N′-dicyclohexylcarbodiimide (DCCD). In addition, the
effects of adding peptidoglycan (PGN; derived from S.
aureus) into Müller-Hinton broth (MHB) containing ORV alone
were also examined. The aim of the present study was to gain an
insight into the antibacterial activity, survival characteristics
and changes in the bacterial morphology and mechanism of ORV
against MRSA.
Materials and methods
Isolation and purification of ORV
ORV (purity, >96.32%) was provided by the
Standardized Material Bank for New Botanical Drugs (no.
NNMBP000018) at Wonkwang University (Iksan, Korea). Twigs from the
Morus alba plant were supplied by the Herbal Medicine
Co-operative Association of Jeonbuk Province (Iksan, Korea) in
October 2010. EtOH (2 litres) was added to 2 kg of dried Morus
alba twigs for 20 days at room temperature. The dried residue
of the EtOH extract (101 g) was dissolved in 40% aqueous MeOH (1 l)
and separated with n-hexane (800 ml, twice),
CH2Cl2 (800 ml, twice) and EtOAc (800 ml,
twice), successively. A column (5×16 cm) filled with Sephadex LH-20
(GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) was used to
perform chromatography on the soluble fraction of
CH2Cl2 (8.53 g);
CH2Cl2-MeOH (ratio, 4:1 to 1:1, for each
volume of 300 ml) was used to obtain four fractions (denoted A-D).
Next, the soluble fraction of EtOAc (4.83 g) was separated by
chromatography on a silica gel (250 g) column using
CH2Cl2-MeOH (ratio, 8:1 to 4:1, for each
volume of 600 ml) in order to obtain three additional fractions
(denoted E-G). Subsequently, a silica gel (150 g) column (eluent,
n-hexane-acetone, at a ratio of 1:1) was used to perform
chromatography on fraction E (2.77 g), a fraction chosen due to its
abundance of ORV. The sample was further purified by Sephadex LH-20
column chromatography (2.5×20 cm; eluent,
CH2Cl2-MeOH, at a ratio of 4:1) in order to
obtain ORV (1.12 g, 0.056% w/w). The structure of ORV was then
identified by mass spectrometry [using a Q-TOF micro LC-MS/MS
instrument (Waters Corporation, Manchester, UK) located at Korea
University, Seoul, Korea] and nuclear magnetic resonance analyses
[recorded in CDCl3 or acetone-d6 using a JNM
ECX-400 spectrometer (JEOL, Ltd., Tokyo, Japan) operating at 400
MHz for 1H and at 100 MHz for 13C] in
accordance with our previous study (20).
Reagents
Müeller-Hinton agar (MHA) and MHB, as nutrient
media, were purchased from BD Biosciences (Franklin Lakes, NJ,
USA). NaN3 and PGN were obtained from Sigma-Aldrich
(Buchs, Switzerland) and TX, DCCD, purified lipopolysaccharide
(LPS), ampicillin (AM), oxacillin (OX), gentamicin (GT), vancomycin
(VC), norfloxacin (NR) and ciprofloxacin (CP) were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Bacterial strains and growth
conditions
Three clinical isolates of MRSA (DPS-1, −2 and −3)
were obtained from three different patients of the Department of
Plastic Surgery, Wonkwang University Hospital (Iksan, Korea), in
accordance with the methods used in a previous study (1). Two additional strains were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA); these were the S. aureus strains ATCC 33591
(methicillin-resistant) and ATCC 25923 (methicillin-susceptible)
(Table I). Prior to the experiments,
all strains were stored in 30% glycerol and frozen at −70°C. The
bacterial strains were inoculated onto MHA plates and incubated at
37°C for 24 h.
 | Table I.Determination of the mecA gene status
of the S. aureus strains used. |
Table I.
Determination of the mecA gene status
of the S. aureus strains used.
| S. aureus
strain | Class | mecA gene | β-lactamase
activity | Antibiotic
resistance |
|---|
| ATCC 33591 | MRSA | + | + | AM, OX |
| ATCC 25923 | MSSA | − | − | − |
| Clinical
isolates |
|
|
|
|
|
DPS-1 | MRSA | + | + | AM, OX |
|
DPS-2 | MRSA | + | − | AM, OX |
|
DPS-3 | MRSA | + | + | AM, OX |
Antimicrobial resistance testing
Testing for mecA gene activity was used to identify
staphylococci resistant to β-lactam antibiotics (21). Detection of the mecA gene in MRSA
strains (Table I) was performed by
polymerase chain reaction amplification, as follows: Prior to DNA
extraction, bacteria stock cultures were subcultured twice onto MHA
plates. For rapid extraction, 1–5 bacterial colonies were suspended
in 300 µl of buffer from the Easy-RED BYF total RNA extraction kit
(Intron Biotechnology, Inc., Seongnam, Korea) and heated at 100°C
for 20 min. After centrifugation at 10,000 × g for 10 min, 2 µl of
the supernatant was used for the DNA extraction. cDNA was
synthesized from RNA at 42°C for 60 min using a Power cDNA
synthesis kit (Intron Biotechnology, Inc.). PCR reactions were
performed using 2 µl of cDNA per reaction and an MRSA Primer mix
kit (Genotek Co., Daejeon, Korea) in a total reaction volume of 20
µl. The PCR amplification consisted of 30 cycles (94°C, 60 sec;
55°C, 60 sec; 72°C, 60 sec). The final PCR products were separated
on a 2% agarose gel. β-lactamase activity, indicating antibiotic
resistance, was determined using a β-lactamase activity assay kit
(Sigma-Aldrich), according to the manufacturer's protocol.
Antibacterial susceptibility
experiments
The minimum inhibitory concentration (MIC) was
determined using a broth micro-dilution method, in accordance with
the Clinical and Laboratory Standards Institute guidelines
(22). Briefly, an inoculum of the
microorganisms from the MHA plates was prepared by growing the
microorganism in a two-fold dilution of ORV in MHB for 24 h. Next,
the suspension was adjusted to a 0.5 McFarland standard turbidity
[~1.5×108 colony-forming units (CFU)/ml], with the final
inoculums adjusted to 1.5×106 CFU/ml. The MIC was
defined as the lowest concentration of ORV and the various
antibiotics that inhibited microorganism growth following
incubation at 37°C for 24 h in well plates. Subsequent to the
incubation period, the well plates were visually examined for
turbidity. Cloudiness indicated that bacterial growth had not been
inhibited by the concentration of antimicrobial agent contained in
the medium. The antibiotics AM, OX, GT, VC, NR and CP were used in
comparisons of MIC with ORV-only conditions (Table II) (21).
 | Table II.S. aureus strains used in the
experiments and MIC. |
Table II.
S. aureus strains used in the
experiments and MIC.
|
|
| MIC (µg/ml) |
|---|
|
|
|
|
|---|
| S. aureus
strain | Class | ORV | AM | OX | VC | GT | NR | CP |
|---|
| ATCC 25923 | MSSA | 125 | 31.25 | 125 | 3.9 | 62.5 | 15.6 | 31.25 |
| ATCC 33591 | MRSA | 125 | 1,000 | 500 | 1.95 | 31.25 | 250 | 500 |
| DPS-1 | MRSA | 125 | 1,000 | 1,000 | 1.95 | 250 | 31.25 | 125 |
| DPS-2 | MRSA | 125 | 1,000 | 1,000 | 3.9 | 125 | 31.25 | 125 |
| DPS-3 | MRSA | 125 | 1,000 | 1,000 | 1.95 | 125 | 31.25 | 125 |
Antibacterial activity with detergent
or ATPase inhibitors
The antibacterial activity of ORV in the presence of
a detergent, TX, was analyzed to elucidate whether the
antibacterial activity of ORV was associated with altered membrane
permeability. The activity of ORV in the presence of
ATPase-inhibiting agents, DCCD and NaN3, was also
examined to determine whether it was associated with multidrug
resistance. In order to determine the detergent-induced
permeabilization, ORV was mixed with TX (23), since the non-ionic detergent TX is
known to greatly increase antibiotic sensitivity (24). DCCD and NaN3, two
metabolic inhibitors that decrease the ATP levels by disrupting
electrochemical proton gradients in bacteria (25,26),
were used as ATPase inhibitors. The antibacterial activity of 62.5
µg/ml ORV was measured in the presence of 0.1% TX, 0.001%
NaN3 and 6.25 µg/ml DCCD compared to that of ORV alone,
determined by a reading of absorbance [or optical density measured
at a wavelength of 600 nm (OD600)] using an Epoch
microplate spectrophotometer (Bio-Tek Instruments, Inc., Winooski,
VT, USA). This measurement was indicative of cell
proliferation.
Effect of exogenous PGN on ORV
activity
ORV may bind to the cell wall and disrupt its
integrity. To confirm the action of ORV upon addition of exogenous
PGN, ORV + PGN combination assays were performed using the method
described by Zhao et al (27). These assays were used to determine
whether ORV directly binds to PGN or disrupts the integrity of the
cell wall when the same concentrations of ORV and PGN were combined
(0–62.5 µg/ml, i.e. up to 50% of the MIC of ORV). LPS was used as a
control.
Transmission electron microscopy
(TEM)
Exponential-phase MRSA cultures were prepared using
overnight cultures incubated in MHB at 37°C until they reached the
mid-logarithmic phase of growth (21). Subsequently, the MHB-grown
exponential-phase MRSA cultures were treated with ORV at 50% of the
MIC, and at the MIC dose for 30 min. Subsequent to the treatment, 2
ml of the culture was collected by centrifugation at 10,000 × g for
10 min. Following removal of the supernatant, cell pellets were
fixed with modified Karnovsky's fixative (Electron Microscopy
Sciences, Hatfield, PA, USA). Energy-filtering TEM (Libra 120; Carl
Zeiss, Oberkochen, Germany) was then performed to examine the
samples at an accelerating voltage of 120 kV. Transmitted electron
signals were recorded using a 4k × 4k pixel slow-scan
charge-coupled device camera (Ultrascan 4000 SP; Gatan Inc.,
Pleasanton, CA, USA) attached to the electron microscope.
Statistical analysis
All experiments were performed three times. Data
from the experiments are presented as the mean ± SEM. Dunnett's
t-test was used for multiple comparisons. P-values <0.01 were
considered to represent a statistically significant difference.
Results and Discussion
Antibacterial agents inhibit bacterial growth
through a variety of complex mechanisms, including the disruption
of cell membranes, the inhibition of cell wall, nucleic acid and
protein synthesis and the inhibition of nucleic acid metabolism
(28). The initial and most
important step in reducing antibiotic resistance is to develop
antibiotics from natural products that do not result in any toxic
or other detrimental side effects (1,26). The
development of alternative antimicrobial drugs against infectious
diseases is therefore required.
Our previous study demonstrated the synergistic
effects of combining ORV with antibiotics in the treatment of MRSA,
revealing that combinatorial treatment effectively inhibited MRSA
growth (29). The present study
aimed to develop anti-MRSA agents using novel combinations of ORV
and antibiotics to directly address the resistance of MRSA
bacteria. Antibacterial susceptibility tests, based on
determination of cell proliferation, demonstrated the inhibitory
effect of ORV against S. aureus compared with antibiotics
AM, OX, VC, GT, NR, and CP. The results of the MIC assay performed
on five strains of S. aureus are presented in Table II. The growth of MRSA was inhibited
at 125 µg/ml ORV. The antibacterial activity of ORV had superior
potency to the antibiotics AM and OX. The MICs of VC, GT, NR and CP
were from 1.95–3.9 µg/ml, from 62.5–250 µg/ml, from 15.6–250 µg/ml
and 31.25–500 µg/ml, respectively.
To investigate the effects of enhanced membrane
permeability and ATPase inhibition, the antibacterial activity of
ORV was examined in the presence of a detergent (TX) and two
ATPase-inhibiting agents (DCCD and NaN3). TX is a
detergent known to increase the membrane permeability of various
bacterial strains, and to induce the release of lipoteichoic acid
(LTA) from the cell wall of S. aureus (30). LTA, a major constituent of the cell
wall of gram-positive bacteria, is covalently bonded to the outer
portion of PGN and is associated with the cytoplasmic membrane
(23). TX has also been revealed to
reduce methicillin resistance and increase antibiotic sensitivity
in S. aureus strains (23).
In the present study, S. aureus was
demonstrated to have an increased susceptibility to ORV in
combination with TX, compared with that of ORV alone (62.5 µg/ml),
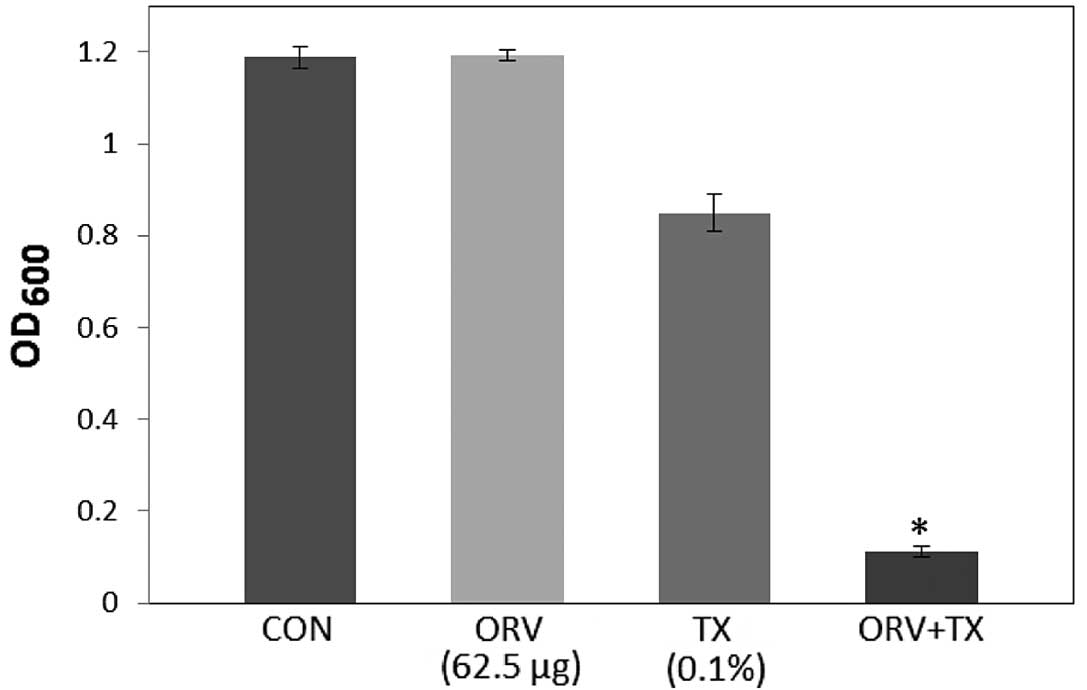
as reported in Fig. 1. In addition,
compared with the OD600 value for ORV alone (62.5
µg/ml), the OD600 value for the combination of TX with
ORV was reduced by 89.8% (Fig. 1),
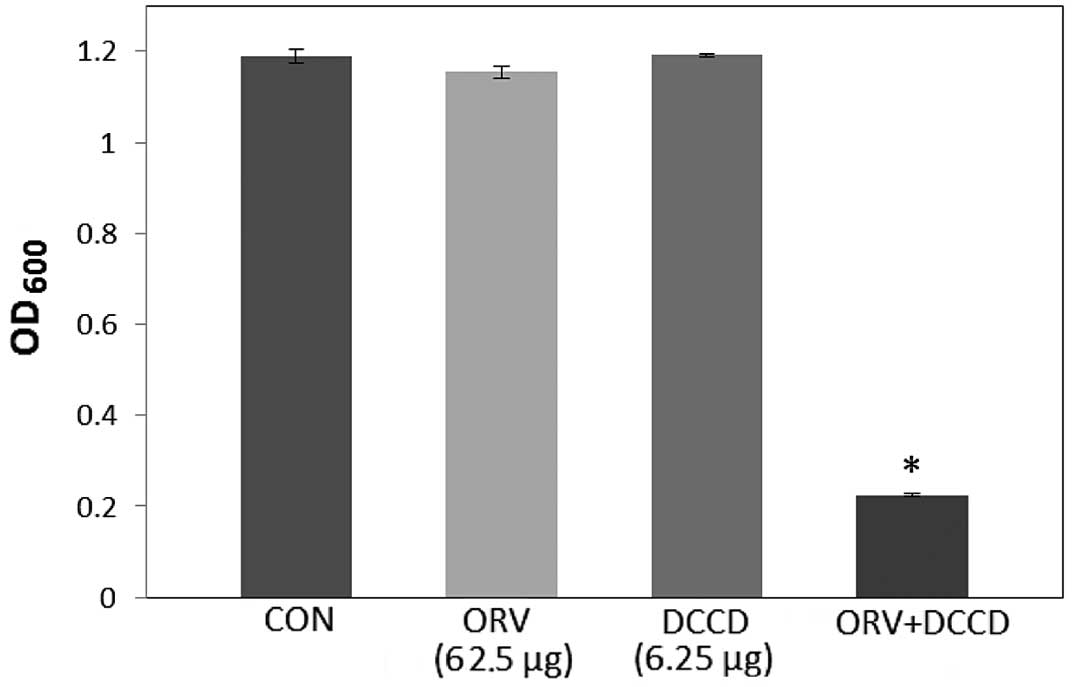
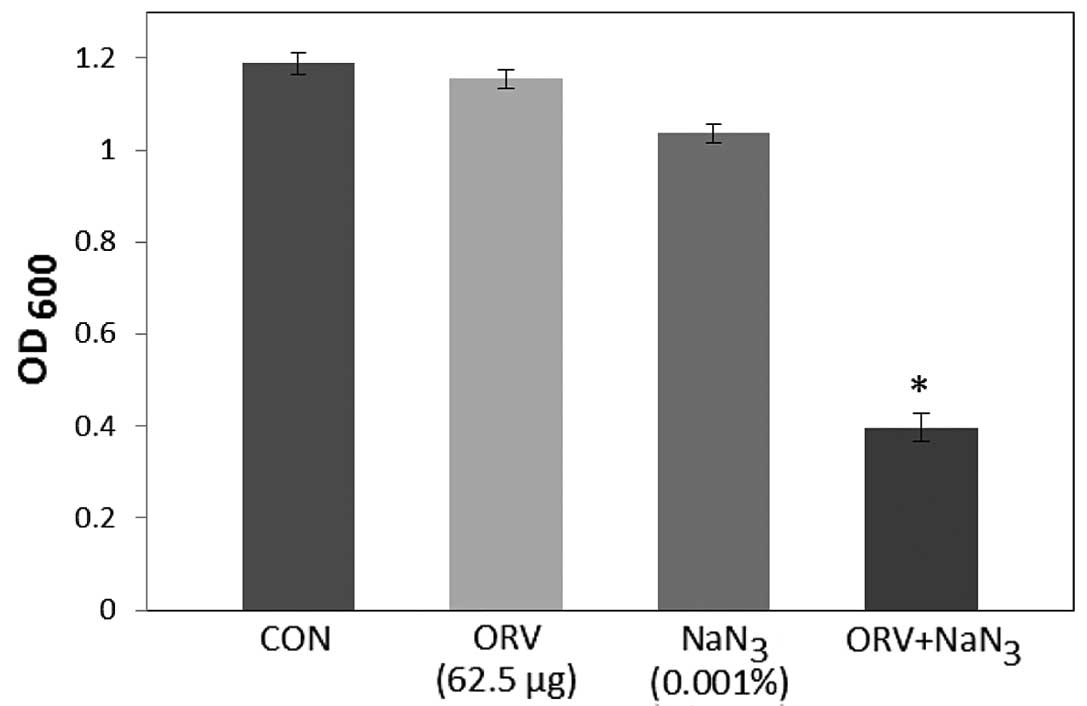
while bacterial viability in the presence of ORV with DCCD
(Fig. 2) and 0.001% NaN3
(Fig. 3) was also reduced by 80.5%
and 68.9%, respectively. DCCD impedes the ATP-binding cassette
transporter, whilst NaN3 is a metabolic inhibitor that
reduces the ATP levels by disrupting the electrochemical proton
gradients in the bacteria (21). In
the present study, S. aureus viability was markedly reduced
upon addition of ORV in combination with ATPase inhibitors (DCCD or
NaN3), compared with the use of ORV alone (Figs. 2 and 3). These results demonstrate that the
anti-MRSA activity of ORV is enhanced by changes in the membrane
permeability and a reduced ATP level.
The cell wall of gram-negative bacteria typically
contains <10% of PGN, whereas the PGN content in gram-positive
bacteria ranges from 5–95% (31).
The gram-positive bacterial cell wall consists of 30–50 PGN sheets
outside the cell membrane, which is important in cell division and
osmotic protection (32). ORV
directly binds the cell wall and affects its integrity. In the
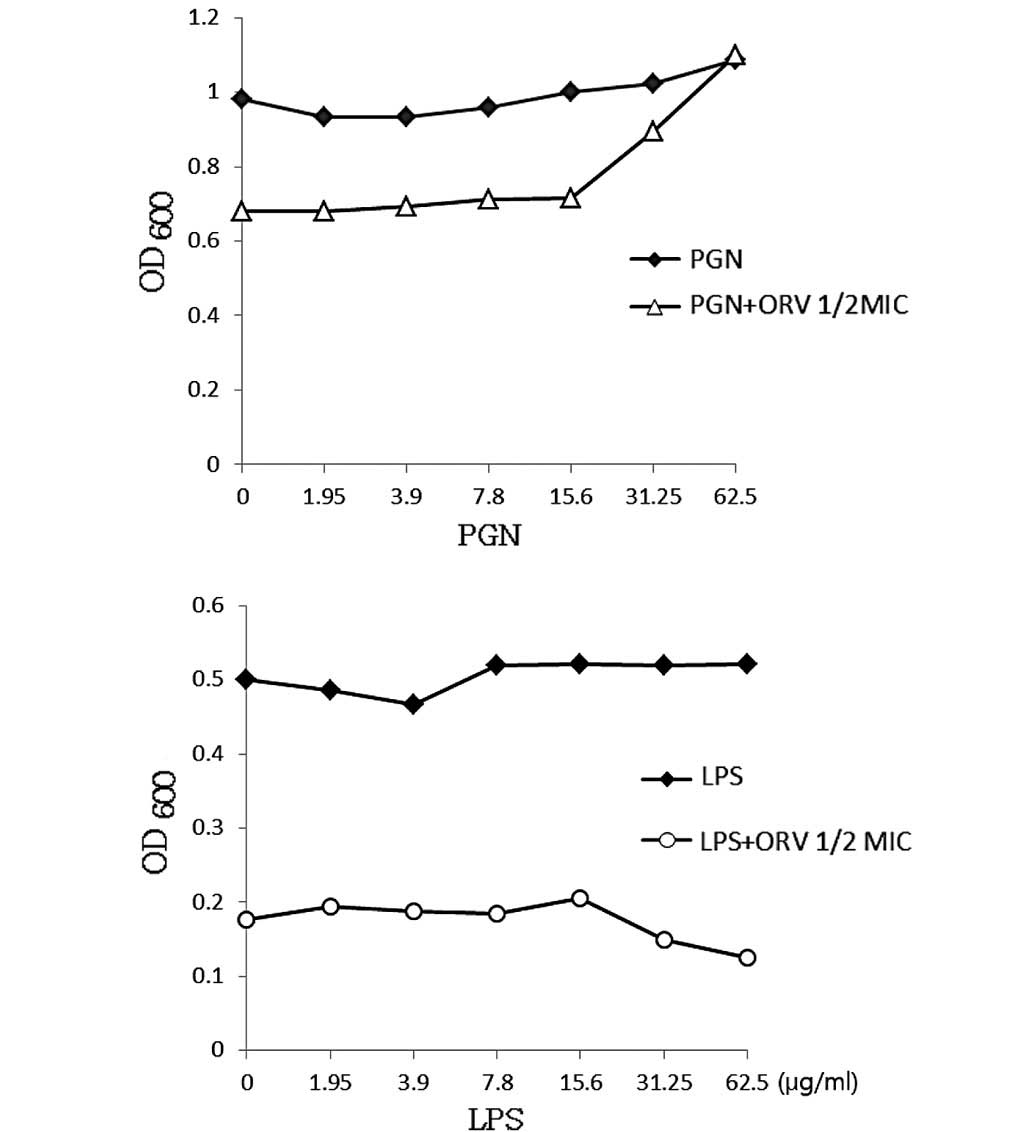
present study, ORV-induced inhibition of bacterial growth (Fig. 4) indicated that ORV interfered with
bacterial biosynthesis. PGN at a dose of 62.5 µg/ml completely
blocked the antibacterial activity of ORV, indicating the direct
binding of ORV with PGN on the cell wall. The cell wall ORV-binding
effect of PGN was confirmed by the addition of PGN derived from
S. aureus into MHB containing ORV alone (62.5 µg/ml). Under
the same conditions, LPS, which was used as the control, did not
demonstrate any such effect.
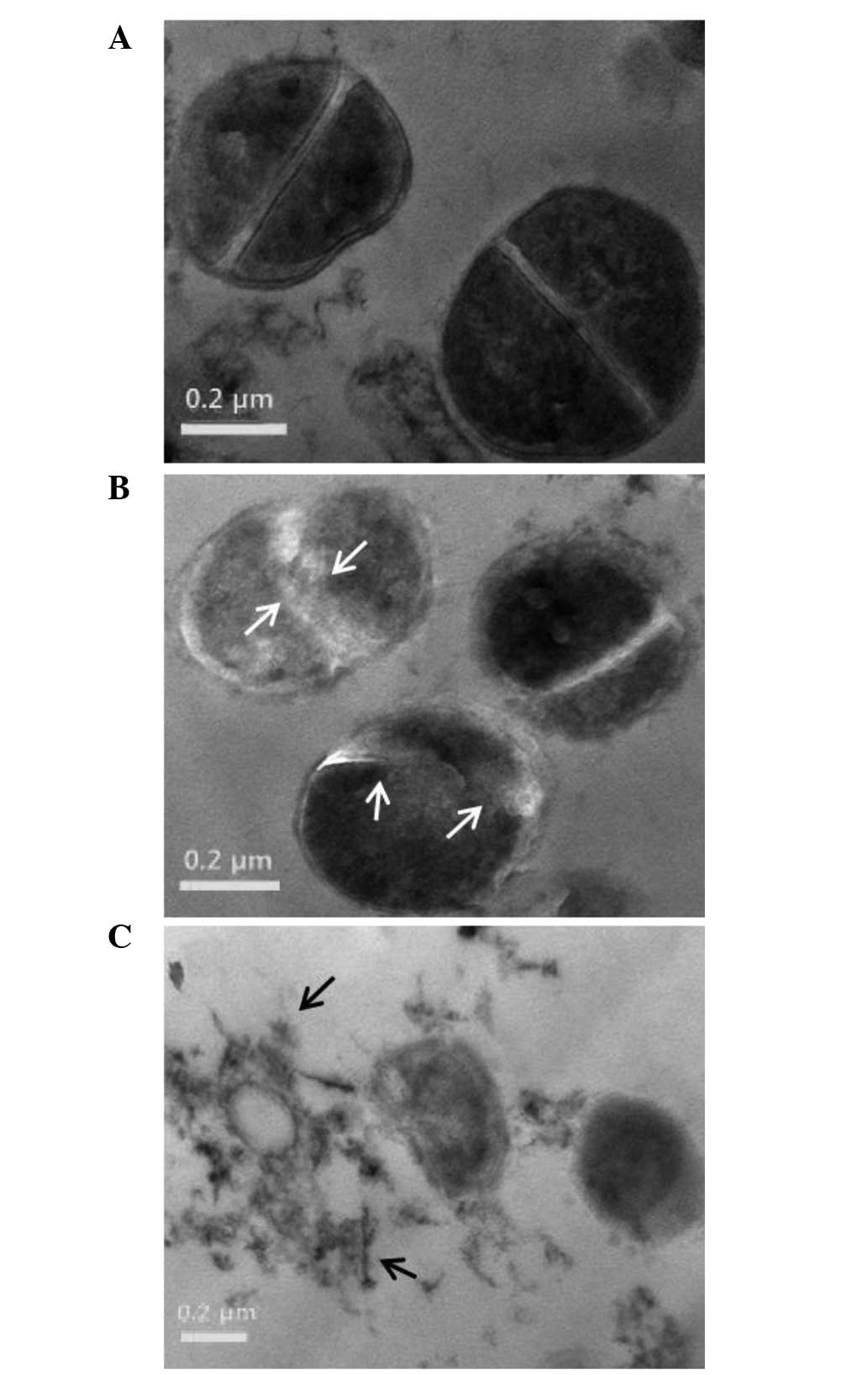
The cell morphology of ORV-treated cells was
observed using TEM, which confirmed weakening of the cell wall and
lytic effects of ORV on the S. aureus strain, ATCC 33591
(33). The micrographs reported in
Fig. 5 illustrate the cell wall and
membrane damage following ORV treatment in S. aureus. The
control cells demonstrated normal S. aureus morphology with
distinct septa (Fig. 5A), whereas
bacterial cells treated with ORV at a dose of 62.5 µg/ml (i.e. 50%
of the MIC) had a deformed septum and midline disruption (Fig. 5B). In addition, upon exposure of
S. aureus to a higher dose of ORV (125 µg/ml, i.e. the MIC),
cell division and ghost cells were observed (Fig. 5C). Distinct septa were rarely
discerned in the treated cells. TEM observation of ORV-treated MRSA
cells substantiates the results indicating that ORV treatment
induced altered expression of cell wall- and cell
division-associated genes in MRSA.
In conclusion, the results of the present study
suggests that ORV has antibacterial activity against MRSA. However,
additional in vivo experiments are required to confirm that
ORV may be effective against MRSA infections, which will be
addressed in subsequent studies.
Acknowledgements
The present study was supported by grants from the
Ministry of Food and Drug Safety (grant.no. 12172MFDS990; 2014),
and the National Research Foundation of Korea (grant.no.
2008-0062484; funded by the Korean government).
References
|
1
|
Mun SH, Kang OH, Joung DK, Kim SB, Choi
JG, Shin DW and Kwon DY: In vitro anti-MRSA activity of
carvone with gentamicin. Exp Ther Med. 7:891–896. 2014.PubMed/NCBI
|
|
2
|
Whitby M, McLaws ML and Berry G: Risk of
death from methicillin-resistant Staphylococcus aureus
bacteraemia: A meta-analysis. Med J Aust. 175:264–267.
2001.PubMed/NCBI
|
|
3
|
Bozic KJ and Ries MD: The impact of
infection after total hiparthroplasty on hospital and surgeon
resource utilization. J Bone Joint Surg Am. 8:1746–1751. 2005.
View Article : Google Scholar
|
|
4
|
Gracia E, Fernández A, Conchello P,
Laclériga A, Paniagua L, Seral F and Amorena B: Adherence of
Staphylococcus aureus slime-producing strain variants to
biomaterials used in orthopaedic surgery. Int Orthop. 21:46–51.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seghrouchni K, van Delden C, Dominguez D,
Benkabouche M, Bernard L, Assal M, Hoffmeyer P and Uçkay I:
Remission after treatment of osteoarticular infections due to
Pseudomonas aeruginosa versus Staphylococcus aureus:
A case-controlled study. Int Orthop. 36:1065–1071. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Lucas-Villarrubia JC, Lopez-Franco M,
Granizo JJ, De Lucas-Garcia JC and Gomez-Barrena E: Strategy to
control methicillin resistant Staphylococcus aureus
post-operative infection in orthopaedic surgery. Int Orthop.
28:16–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aqil F, Ahmad I and Owais M: Evaluation of
anti-methicillin resistant Staphylococcus aureus (MRSA)
activity and synergy of some bioactive plant extracts. Biotechnol
J. 1:1093–1102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iqbal S, Younas U, Sirajuddin Chan KW,
Sarfraz RA and Uddin K: Proximate composition and antioxidant
potential of leaves from three varieties of Mulberry (Morus sp.): A
comparative study. Int J Mol Sci. 13:6651–6664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singab AN, El-Beshbishy HA, Yonekawa M,
Nomura T and Fukai T: Hypoglycemic effect of Egyptian Morus
alba root bark extract: Effect on diabetes and lipid
peroxidation of streptozotocin-induced diabetic rats. J
Ethnopharmacol. 100:333–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin WY, Na MK, An RB, Lee HY, Bae KH and
Kang SS: Antioxidant compounds from twig of Morus alba. Nat Prod
Sci. 13:129–132. 2002.
|
|
11
|
Chung KO, Kim BY, Lee MH, Kim YR, Chung
HY, Park JH and Moon JO: In-vitro and in-vivo
anti-inflammatory effect of oxyresveratrol from Morus alba
L. J Pharm Pharmacol. 55:1695–1700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sritularak B, De-Eknamkul W and
Likhitwitayawuid K: Tyrosinase inhibitors form Artocarpus lakoocha.
Thai J Pharm Sci. 22:149–155. 1998.
|
|
13
|
Likhitwitayawuid K, Sritularak B and
Benchanak K: Phenolics with antiviral activity from Millettia
erythrocalyx and Artocarpus lakoocha. Nat Prod Res. 19:177–182.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuanasa T, Phromjai J, Lipipun V,
Likhitwitayawuid K, Suzuki M, Pramyothin P, Hattori M and Shiraki
K: Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol
derived from Thai medicinal plant: Mechanism of action and
therapeutic efficacy on cutaneous HSV-1 infection in mice.
Antiviral Res. 80:62–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lipipun V, Sasivimolphan P, Yoshida Y,
Daikoku T, Sritularak B, Ritthidej G, Likhitwitayawuid K,
Pramyothin P, Hattori M and Shiraki K: Topical cream-based
oxyresveratrol in the treatment of cutaneous HSV-1 infection in
mice. Antiviral Res. 91:154–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasivimolphan P, Lipipun V,
Likhitwitayawuid K, Takemoto M, Pramyothin P, Hattori M and Shiraki
K: Inhibitory activity of oxyresveratrol on wild-type and
drug-resistant varicella-zoster virus replication in vitro.
Antiviral Res. 84:95–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YM, Yun J, Lee CK, Lee H, Min KR and
Kim Y: Oxyresveratrol and hydroxystilbene compounds. Inhibitory
effect on tyrosinase and mechanism of action. J Biol Chem.
227:16340–16344. 2002. View Article : Google Scholar
|
|
18
|
Lorenz P, Roychowdhury S, Engelmann M,
Wolf G and Horn TF: Oxyresveratrol and resveratrol are potent
antioxidants and free radical scavengers: Effect on nitrosative and
oxidative stress derived from microglial cells. Nitric Oxide.
9:64–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saowakon N, Tansatit T, Wanichanon C,
Chanakul W, Reutrakul V and Sobhon P: Fasciola gigantica:
Anthelmintic effect of the aqueous extract of Artocarpus lakoocha.
Exp Parasitol. 122:289–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu F, Komatsu K, Kawasaki K, Saito K, Yao
X and Kano Y: A novel stilbene glucoside, oxyresveratrol
3′-O-beta-glucopyranoside, from the root bark of Morus alba.
Planta Med. 62:559–561. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mun SH, Joung DK, Kim SB, Park SJ, Seo YS,
Gong R, Choi JG, Shin DW, Rho JR, Kang OH and Kwon DY: The
mechanism of antimicrobial activity of sophoraflavanone B against
methicillin-resistant Staphylococcus aureus. Foodborne
Pathog Dis. 11:234–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clinical and Laboratory Standards
Institute: Performance Standards for Antimicrobial Susceptibility
Testing; Twenty-Fourth Informational Supplement. CLSI document
M100-S24. Clinical and Laboratory Standards Institute. (Wayne, PA).
2014.
|
|
23
|
Cordwell SJ, Larsen MR, Cole RT and Walsh
BJ: Comparative proteomics of Staphylococcus aureus and the
response of methicillin-resistant and methicillin-sensitive strains
to Triton X-100. Microbiology. 148:2765–2781. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibata H, Saito H, Yomota C, Kawanishi T
and Okuda H: Alterations in the detergent-induced membrane
permeability and solubilization of saturated
phosphatidylcholine/cholesterol liposomes: Effects of poly(ethylene
glycol)-conjugated lipid. Chem Pharm Bull (Tokyo). 60:1105–1111.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Linnett PE and Beechey RB: Inhibitors of
the ATP synthetase system. Methods Enzymol. 55:472–518. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung HJ and Lee DG: Synergistic
antibacterial effect between silybin and
N,N'-dicyclohexylcarbodiimide in clinical Pseudomonas
aeruginosa isolates. J Microbiol. 46:462–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao WH, Hu ZQ, Okubo S, Hara Y and
Shimamura T: Mechanism of synergy between epigallocatechin gallate
and beta-lactams against methicillin-resistant Staphylococcus
aureus. Antimicrob Agents Chemother. 45:1737–1742. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al-Habib A, Al-Saleh E, Safer AM and Afzal
M: Bactericidal effect of grape seed extract on
methicillin-resistant Staphylococcus aureus (MRSA). J
Toxicol Sci. 35:357–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Joung DK, Choi SH, Kang OH, Kim SB, Mun
SH, Seo YS, Kang DH, Gong R, Shin DW, Kim YC and Kwon DY:
Synergistic effects of oxyresveratrol in conjunction with
antibiotics against methicillin-resistant Staphylococcus
aureus. Mol Med Rep. 12:663–667. 2015.PubMed/NCBI
|
|
30
|
Komatsuzawa H, Ohta K, Sugai M, Fujiwara
T, Glanzmann P, Berger-Bächi B and Suginaka H: Tn551-mediated
insertional inactivation of the fmtB gene encoding a cell
wall-associated protein abolishes methicillin resistance in
Staphylococcus aureus. J Antimicrob Chemother. 45:421–431.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farca AM, Nebbia P and Re G: Potentiation
of antibiotic activity by EDTA-tromethamine against three
clinically isolated gram-positive resistant bacteria. An in vitro
investigation. Vet Res Commun. 18:1–6. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lorian V and Atkinson B: Effect of serum
on gram-positive cocci grown in the presence of penicillin. J
Infect Dis. 138:865–871. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muthaiyan A, Martin EM, Natesan S,
Crandall PG, Wilkinson BJ and Ricke SC: Antimicrobial effect and
mode of action of terpeneless cold-pressed Valencia orange
essential oil on methicillin-resistant Staphylococcus
aureus. J Appl Microbiol. 112:1020–1033. 2012. View Article : Google Scholar : PubMed/NCBI
|