Introduction
The pathogenesis of irritable bowel syndrome (IBS)
is complex and is believed to be multifactorial, involving the
diet, an altered neuroendocrine system, abnormal intestinal
microbiota, genetics and low-grade inflammation (1). More than 60% of patients with IBS
associate their symptom development to diet (2–4). The
most important dietary triggers are insoluble fiber and the rapidly
fermentable but poorly absorbed carbohydrates termed fermented
oligosaccharides, disaccharides, monosaccharides, and polyols
(FODMAPs) (5–9).
Enteroendocrine cells are scattered among the
various types of epithelial cells lining the gastrointestinal (GI)
lumen (10–13). Patients with IBS have abnormal
densities of enteroendocrine cells in various segments of the GI
tract (14–31). Following dietary guidance about a
low-FODMAP diet and changing the proportions of fat, protein, and
carbohydrates in their diet, patients with IBS reportedly
experience improvements in their IBS symptoms and quality of life
(32) along with normalization of
the densities of several types of enteroendocrine cells in the
stomach (33,34) and the large intestine (35,36). The
density of the total enteroendocrine cells as detected by
chromogranin A (CgA) in the ileum also changes following dietary
guidance (37). However, the types
of enteroendocrine cells that are affected in these interventions
are not clear. The present study was therefore undertaken to
determine the types of ileal enteroendocrine cells that are
affected following dietary guidance in the same cohort of patients
with IBS.
Materials and methods
Patients and controls
Male and female patients aged 18–70 years who were
referred to the Division of Gastroenterology at the Stord Hospital
(Stord, Norway) were included in the study. All of the patients
fulfilled Rome-III criteria (38)
for an IBS diagnosis. The exclusion criteria included pregnant or
lactating women, and the presence of severe psychiatric or
organic/systemic diseases, drug abuse and previous abdominal
surgery (except for appendectomy, cesarean section and
hysterectomy).
A group of 10 subjects (7 females and 3 males) with
a mean age of 51 years (age range, 26–70 years) were included in
the study as controls. Subjects in the control group did not
present symptoms associated with IBS. These control subjects
underwent colonoscopy due to health concerns not associated with
IBM, including diagnosis of a family member with cancer of the GI
tract (n=6) or a history of GI bleeding (n=4) where
the source of bleeding was identified as hemorrhoids (n=3)
or angiodysplasia (n=1).
The study was performed in accordance with the
Declaration of Helsinki (39) and
was approved by the local Committee for Medical Research Ethics in
Western Norway (no. 2010/2650-2). All of the patients provided both
oral and written consents to participate.
Study design
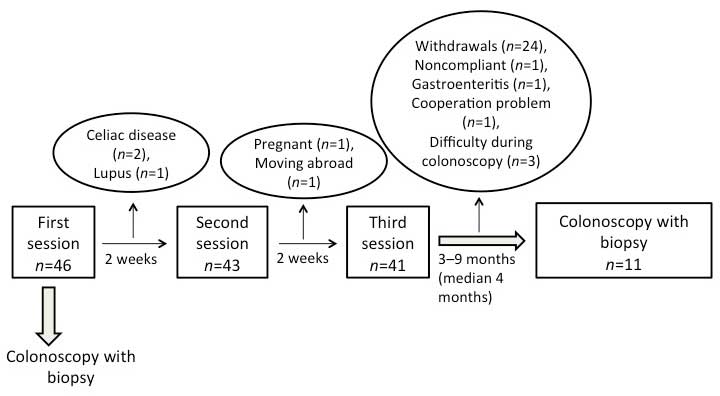
In total, 46 patients (35 females and 11 males) were
initially included in the study. Their mean age was 35 years (age
range, 18–69 years). All of the patients received physical
examinations and blood tests to exclude inflammation, infection,
and other organic diseases. The patients also received a total of
three sessions of individualized dietary guidance given by a nurse
experienced in diet and IBS. The sessions lasted for 45 min each
and were provided at intervals of at least 2 weeks (Fig. 1). The patients were examined with
colonoscopies prior to the first session and at 3–9 months (median,
4 months) following the last session of dietary guidance.
Individualized dietary guidance
Dietary guidance was delivered orally using charts,
and in written illustrations. The main focus of the first session
was to provide general information regarding IBS, and to emphasize
the importance of a regular eating pattern and the foodstuffs that
worsen IBS symptoms such as poorly absorbable FODMAPs and insoluble
dietary fiber. The patients were allowed to consume lactose-free
milk and other lactose-free dairy products during the study. For a
period of 2 weeks, the patients were instructed to test alternating
diets that were rich and then poor in protein, fat and
carbohydrates, each for 3–4 days. During this period the patients
had to register in a diary their daily consumption of food and
fluids (times and types) in addition to any associated symptoms,
including the frequency and degree of abdominal pain and abdominal
distension along with the stool frequency and consistency. No food
supplements containing probiotics, antibiotics or other medications
were allowed during the study, with the exception of where
specified otherwise.
During the second session, the information given
during the first session was briefly repeated. The nurse focused on
using the information from the diary of the patient to identify the
foodstuffs that triggered the IBS symptoms. Based on this
information the patients were instructed to alter the proportions
of protein, fat, and carbohydrates, to avoid FODMAP-rich foodstuffs
as well as insoluble fiber, and to consume vegetables and fruits
containing lower amounts of FODMAPs and insoluble fiber.
In the third session, each patient gave feedback
regarding the dietary guidance to the nurse. With the help of the
nurse, a suitable diet was designed for the patient to follow until
the end of the study.
Dietary assessment
The dietary intake was assessed using the Norwegian
Mother and Child Study food frequency questionnaire (MoBa FFQ;
www.fhi.no/dokumenter/011fbd699d.pdf) (40), which reports the frequency and the
sizes of food meal portions and beverages consumed during a certain
period of time. The nutrient content of the diet was calculated
using FoodCalc (41). The MoBa FFQ
inquires about the consumption of 225 foodstuffs and also
identifies the dietary habits of the subject, including the
consumption of any oral supplements, according to typical Norwegian
meal patterns. The questionnaire was developed and validated by the
Norwegian Institute of Public Health in Oslo, Norway (42,43). The
patients completed the MoBa FFQ form prior to the first session and
again ≥3 months following the third session of individualized
dietary guidance. The forms were delivered on the same day on which
a colonoscopy was scheduled (32).
Colonoscopy
Colonoscopy was performed on both the patients and
controls following preparation of their bowels via consumption of
sodium picosulfate (Picoprep®; Ferring Pharmaceuticals,
Saint-Prex, Switzerland) the day before the procedure. Four
biopsies were taken from the ileum during the colonoscopy.
Histopathology and
immunohistochemistry
The biopsy samples were fixed overnight in 4%
buffered paraformaldehyde (cat. no. 329847; Den Norske Eterfabrikk,
Oslo, Norway) and embedded in paraffin (Cellwax; GCA-0305-00A;
Cellpath Ltd., Newtown, Powys, UK). The tissue samples were then
sectioned at a thickness of 5 µm using Leica SM2000 R Sliding
microtome (Leica Biosystems Nussloch GmbH, Heidelberger, Germany)
and placed on the slides, with each slide containing two tissue
samples sectioned at 50 µm apart (distance in the paraffinated
samples). The tissue sections were stained with hematoxylin
(Cellpath Ltd.) and eosin (Merck KGaA, Darmstadt, Germany), and
immunostained using the avidin-biotin complex (ABC) method with a
Vectastain ABC kit (cat. no. PK-4000; Vector Laboratories, Inc.,
Burlingame, CA, USA) and the chromogen 3,3′-diaminobenzidine
peroxidase substrate (DAB) kit (cat. no. SK-4105; Vector
Laboratories, Inc.) as described previously (40). The tissue sections were hydrated and
immersed in 0.01% hydrogen peroxide in phosphate-buffered saline
(PBS; pH=7.4) for 10 min in order to inhibit endogenous peroxidase
activity. Following washing with PBS, the tissue sections were
treated with 1% bovine serum albumin for 30 min to block
non-specific binding sites, followed by incubation with the primary
antibody at room temperature for 1 h. The following primary
antisera/antibodies were used: Monoclonal mouse anti-serotonin
(cat. no. 5HT-209; Dako, Glostrup, Denmark), polyclonal
anti-porcine peptide YY (PYY; cat. no. PYY 11A; Alpha-Diagnostic
International, Inc., San Antonio, TX, USA), polyclonal rabbit
anti-synthetic human pancreatic polypeptide (PP; cat. no. 114;
Diagnostic Biosystems, Pleasanton, CA, USA), polyclonal rabbit
anti-porcine glicentin/glucagon (also known as oxyntomodulin; cat.
no. BP508; Acris Antibodies, Herford, Germany), and polyclonal
rabbit anti-synthetic human somatostatin (cat. no. A566; Dako);
these antibodies were each diluted to 1:1,500, 1:1,000, 1:800,
1:400 and 1:200, respectively.
The sections were then washed in PBS and incubated
with biotinylated swine anti-mouse immunoglobulin G (Dako) diluted
to 1:200 for 30 min at room temperature. Following washing in PBS
buffer, the tissue sections were incubated for 30 min with
avidin-biotin-peroxidase complex (Vector Laboratories) diluted to
1:100, and then submerged in DAB and counterstained with
hematoxylin.
Computerized image analysis
The density of each type of enteroendocrine cell in
the ileum of patients with IBS and the controls was measured using
Olympus Cell D software (version 5.1; Olympus, Tokyo, Japan). The
number of enteroendocrine cells and the area of epithelial cells
were measured in 10 randomly selected fields per slide, using
Olympus BX50 DIC light microscope (Olympus, Oslo, Norway) at a
magnification of ×40. Each field represented a tissue area of 0.14
mm2. The density of the enteroendocrine cells was
expressed as the number of cells/mm2 of epithelium. The
data from the fields were tabulated, computed, and automatically
analyzed statistically. The quantification was conducted by the
same scientist (Dr Tarek Mazzawi) while he was blinded to the
identity of the tissue sections.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 6 (GraphPad Software Inc., La Jolla, CA, USA). The
data are presented as mean ± standard error of the mean values.
Comparisons of the gender and age between the controls and the
patients were conducted using Fisher's exact test and a
Mann-Whitney test, respectively. Paired t-tests were used to
compare the data from patients prior to and following dietary
guidance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients and controls
In total, 46 patients were included in the study and
received individualized dietary guidance, of which 14 (9 females
and 5 males) with a mean age of 33 years (age range, 21–44 years)
completed the study (Fig. 1). In
three of the original 46 patients (2 females and 1 male) it was
technically difficult to intubate the ileocecal valve during
colonoscopy, and so only 11 patients (7 females and 4 males) with a
mean age of 33 years (age range, 24–44 years) were included in the
final study analysis. The gender distribution did not differ
significantly between the patients and controls (P=1), whereas the
age distribution did (P=0.009). The demographic characteristics of
the subjects are summarized in Table
I.
 | Table I.Demographic characteristics of the
study subjects. |
Table I.
Demographic characteristics of the
study subjects.
| IBS subtype and
controls | Number of
patients | Age range and mean
(years) | Females/males |
|---|
| IBS-D | 5 | 31–45 (37.0) | 2/3 |
| IBS-C | 4 | 28–36 (31.0) | 3/1 |
| IBS-M | 2 | 24–30 (27.0) | 2/0 |
| Controls | 10 | 26–70 (51) | 7/3 |
Four of the 11 patients who completed the study used
one or a combination of the following: Proton-pump inhibitors
(n=2), thyroxin-substitution tablets (n=2), asthma
inhalator (n=1), angiotensin II receptor antagonist
antihypertension tablets (n=1), anti-allergy tablets
(n=3), contraceptive pills (n=2), and
antidepressant/anxiolytic tablets (n=2). These patients were
instructed not to take any kind of proton-pump inhibitors or
antacids for 1 week prior to beginning the study or during the
study.
Dietary assessment
The dietary changes in the current study are
described in detail elsewhere (32).
Briefly, the total consumption of daily fruits and vegetables rich
in FODMAPs decreased significantly from 16.2±5.3 g prior to
receiving dietary guidance to 9.2±3.2 g following dietary guidance
(P=0.02). However, no significant change was observed in the total
daily consumption of fiber prior to (27.4±2.5 g) and following
(23.1±2.2 g) dietary guidance (P=0.09) (32).
Colonoscopy, histopathology, and
immunohistochemistry
The ileum was both macroscopically and
microscopically normal in both the patients and controls.
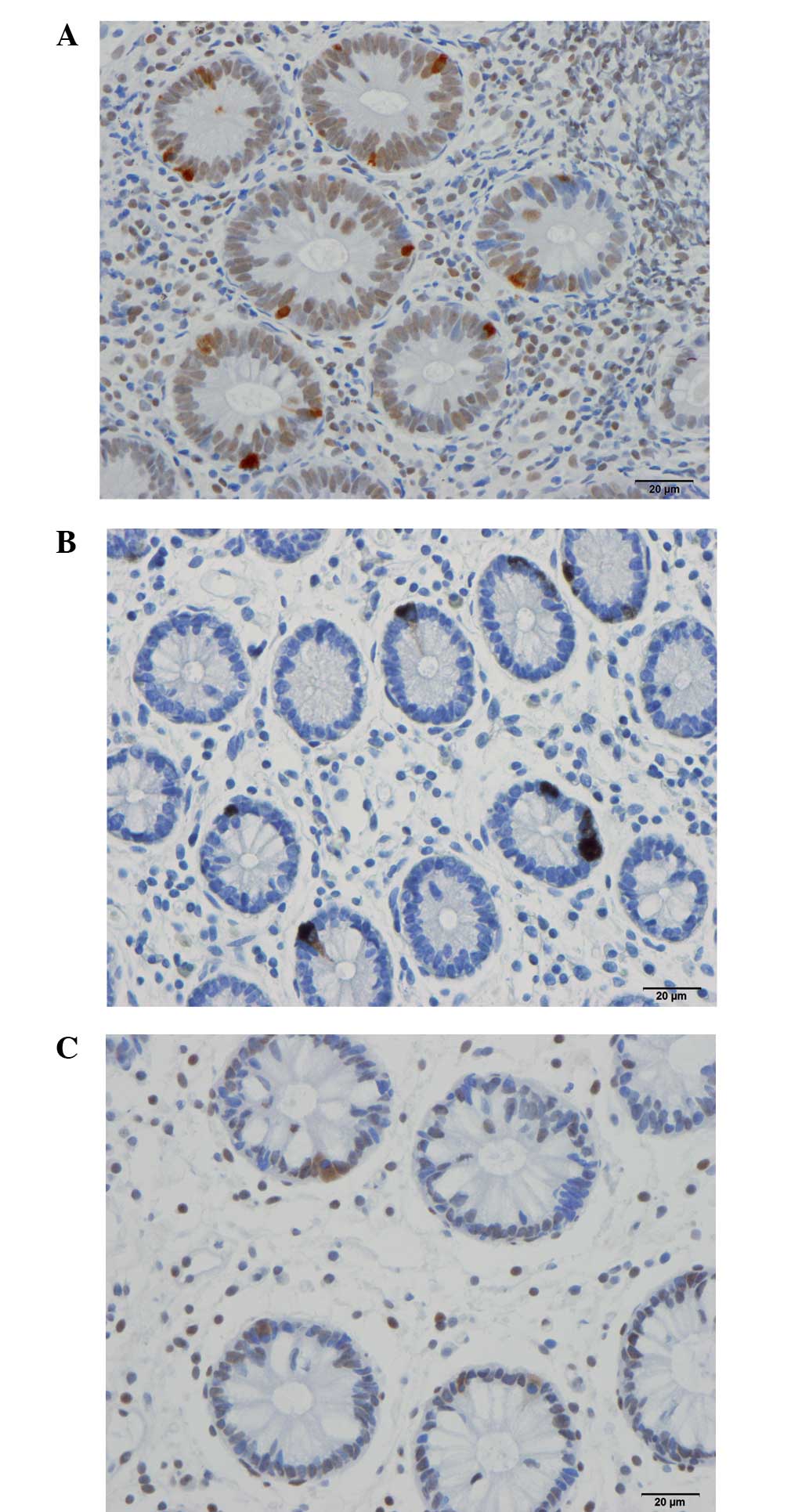
Serotonin-, PYY-, PP-, oxyntomodulin (enteroglucagon)-, and
somatostatin-immunoreactive cells were found predominantly in the
crypts of the ileum in all subjects (patients and controls). These
cells were either basket-or flask-shaped. The numbers of PP-,
oxyntomodulin (enteroglucagon)-, and somatostatin-immunoreactive
cells were too low to be reliably quantified in the examined biopsy
material.
Computerized image analysis
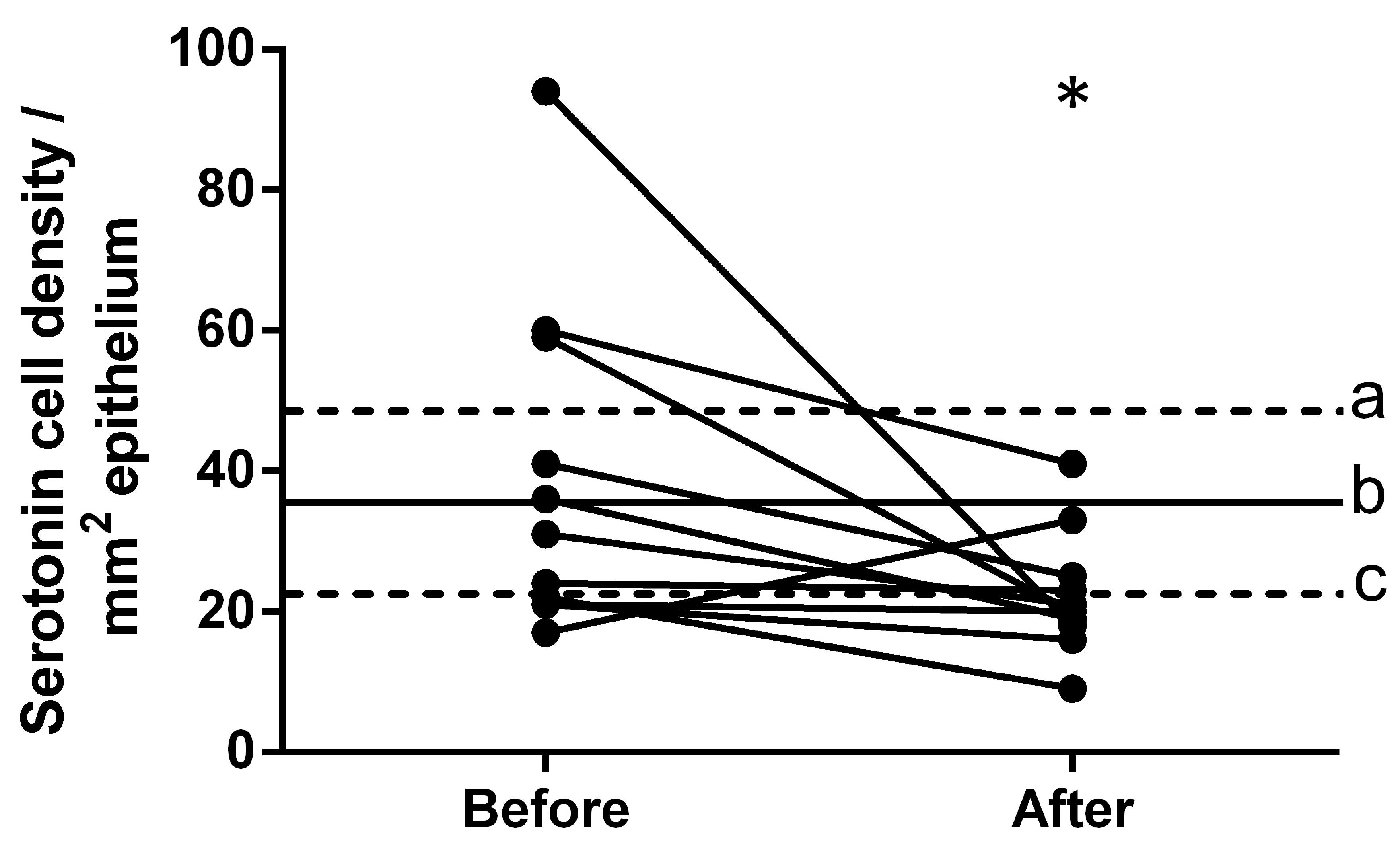
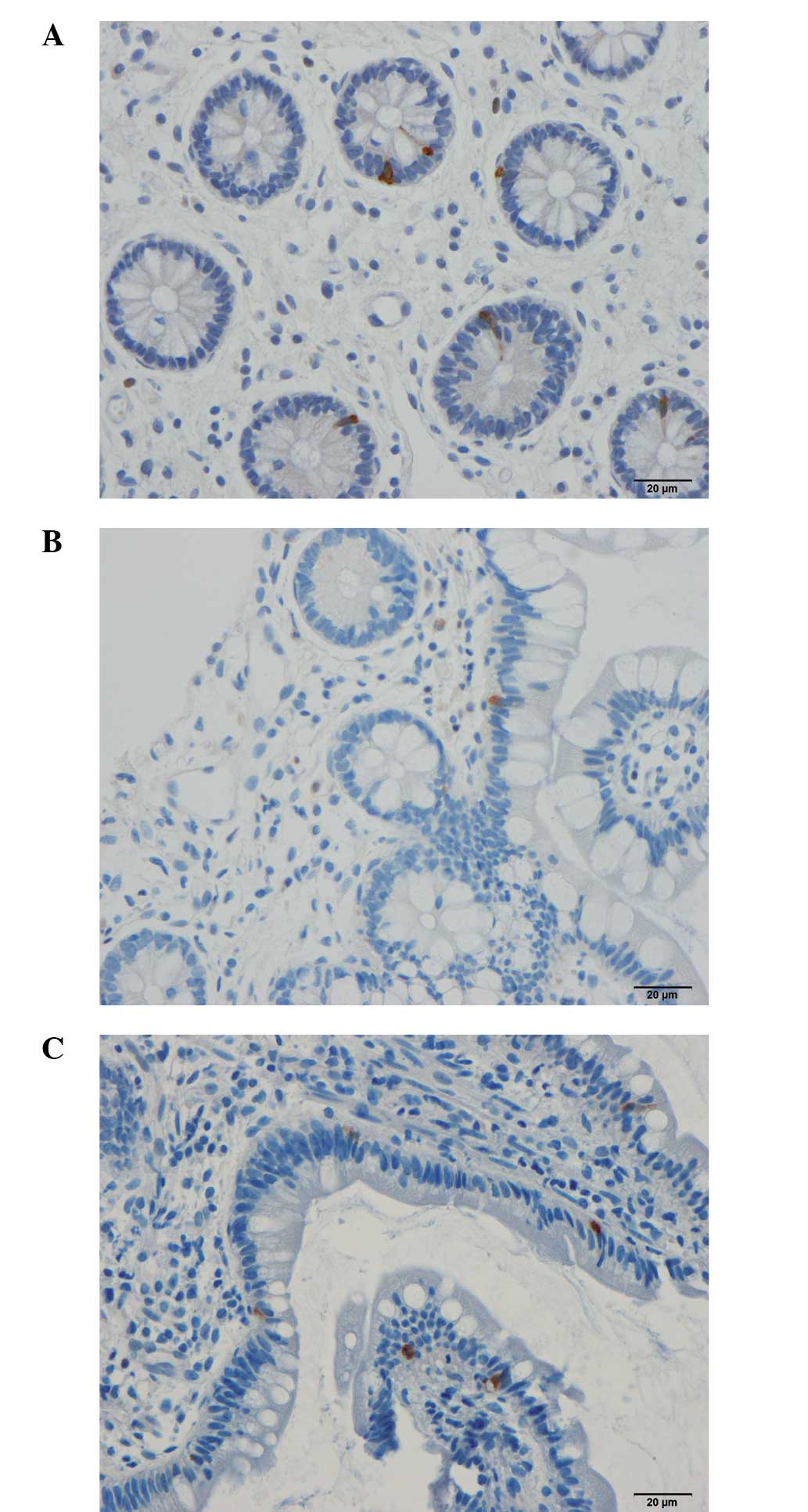
Serotonin
The density of serotonin-immunoreactive cells in the
controls was 35.5±5.7 cells/mm2. The density of these
cells in patients with IBS was 38.7±7.1 cells/mm2 prior
to dietary guidance and 22.3±2.6 cells/mm2 following
dietary guidance (Figs. 2 and
3). The densities of
serotonin-immunoreactive cells in the patients with IBS moved
closer to the mean values within the 95% confidence interval of the
controls following dietary guidance. The paired t-test
indicated a significant decrease in the density of
serotonin-immunoreactive cells in IBS patients following dietary
guidance (P=0.046).
PYY
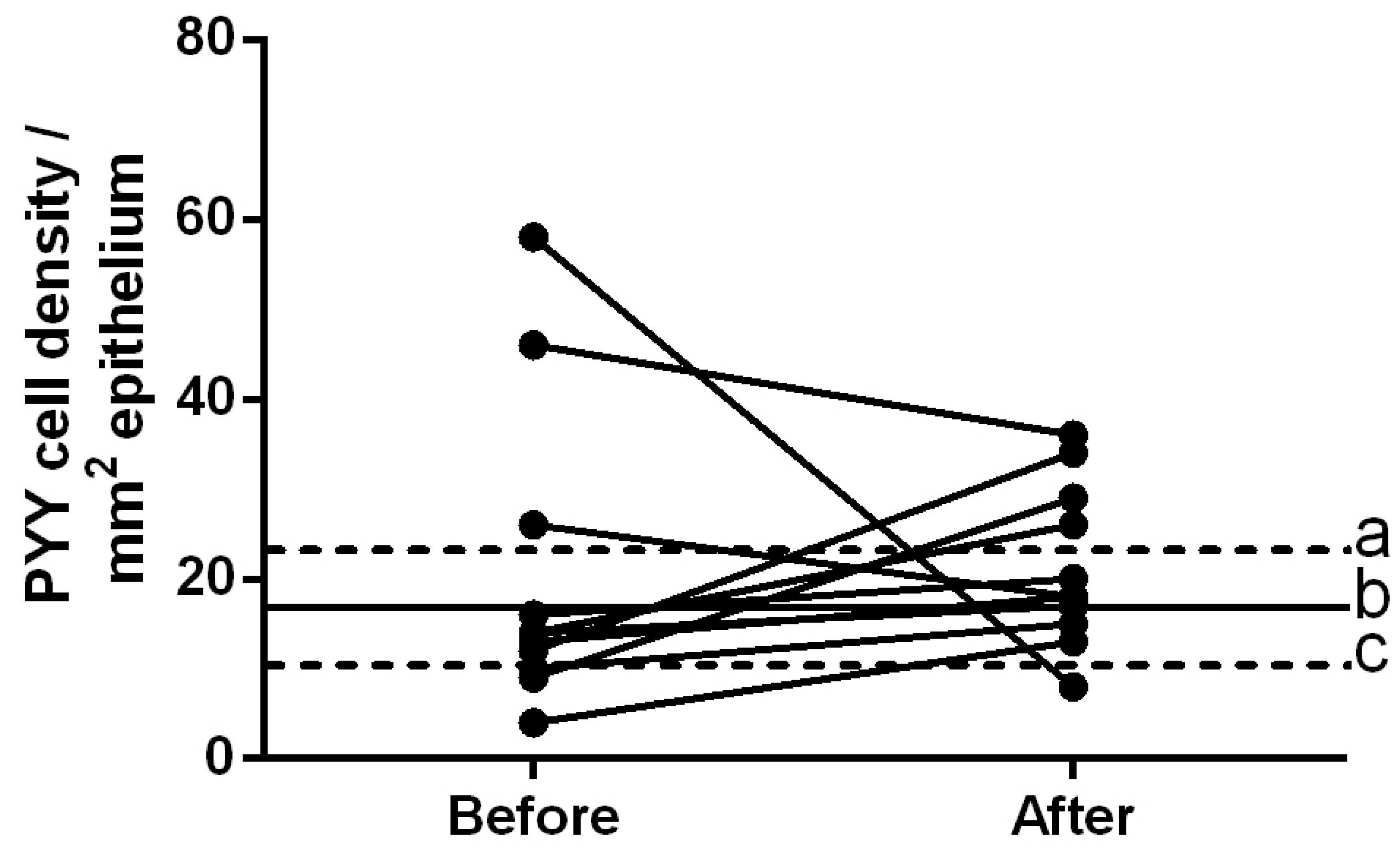
The density of PYY-immunoreactive cells in the
controls was 16.7±2.8 cells/mm2. The densities of these
cells in patients with IBS prior to and following dietary guidance
were 20.2±5.1 and 21.3±2.7 cells/mm2, respectively
(Figs. 4 and 5). The densities of PYY-immunoreactive
cells in the patients with IBS were closer to the mean values
within the 95% confidence interval of the controls following
dietary guidance. The paired t-test showed no significant
change in the density of PYY-immunoreactive cells in patients with
IBS following dietary guidance (P=0.86).
Discussion
Dropout rates of ≤48% have been reported in previous
clinical studies involving IBS (6,45–48). The
current study had an even higher dropout rate, which was likely due
to the demanding study design that included undergoing two
colonoscopies and having to follow a strict diet for a minimum of 3
months. The majority of the patients withdrew their consents when
they experienced symptom improvement following dietary guidance,
and due to their unwillingness to participate again in an invasive
examination, namely a second colonoscopy. Additional factors
further increased the dropout rate to 76%, including the exclusion
of some patients following diagnosis with organic diseases,
pregnancy, moving abroad, and technically difficult colonoscopies.
Nevertheless, although only a small number of patients completed
this study, the changes in diet among these patients affected the
enteroendocrine cells of the ileum in the same way as the dietary
changes affected other investigated segments of the GI tract in
previous studies (33–37). It is worth noting that neither age
nor gender affects the densities of the intestinal enteroendocrine
cells (11,35,49).
The enteroendocrine cells project specific
microvilli that interact with the GI luminal contents (particularly
with nutrients) and respond by releasing specific hormones that
regulate various functions of the GI tract (12,50–58).
Serotonin stimulates the intestinal motility, accelerates
intestinal transit (59–67), and activates the submucosal sensory
branch (termed the Meissner's plexus) of the enteric nervous system
that carries sensation from the GI tract to the central nervous
system and modulates the visceral sensitivity of the GI tract
(17,59,63–65). PYY
is a major regulator of the ‘ileal brake’ and stimulates the
absorption of water and electrolytes (17,68).
A previous study involving the same cohort of
patients with IBS investigated in the present study (37) demonstrated that the total
enteroendocrine cells of the ileum, as detected by CgA, changed
significantly following dietary guidance. These changes may be
attributed to changes in the densities of serotonin- and
PYY-immunoreactive cells. The densities of these cells became
similar to those of the control subjects after the patients
received dietary guidance. There is a dynamic interaction between
foodstuffs and enteroendocrine cells (69). These cells have a rapid turnover rate
from stem cells of ~2–6 days (70,71). It
can be speculated that a change of diet following dietary guidance
may alter the differentiation of enteroendocrine cells and explain
the observed changes in the densities of enteroendocrine cells in
the ileum as well as the other parts of the GI tract in patients
with IBS.
In conclusion, the cumulative changes in the
enteroendocrine cells throughout the GI tract, namely in the
stomach (33,34), the small intestine (37), and the large intestine (35,36), in
patients with IBS following dietary guidance may have contributed
to the improvements in the symptoms and quality of life of the
patients with IBS that were observed during the present study,
results which were concordant with those of a previous report
(32). The findings of the present
study highlight the role of the enteroendocrine cells in the
pathophysiology of IBS and the usage of dietary guidance and diet
manipulation as a first line step in the management of IBS
symptoms.
Acknowledgements
The present study was supported by a grant from
Helse-Fonna (grant no. 40415).
References
|
1
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable bowel syndrome. Nova Science Publisher. New
York: 2012.
|
|
2
|
Simrén M, Månsson A, Langkilde AM,
Svedlund J, Abrahamsson H, Bengtsson U and Björnsson ES:
Food-related gastrointestinal symptoms in the irritable bowel
syndrome. Digestion. 63:108–115. 2001. View Article : Google Scholar
|
|
3
|
Monsbakken KW, Vandvik PO and Farup PG:
Perceived food intolerance in subjects with irritable bowel
syndrome-etiology, prevalence and consequences. Eur J Clin Nutr.
60:667–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams EA, Nai X and Corfe BM: Dietary
intakes in people with irritable bowel syndrome. BMC Gastroenterol.
11:92011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Salhy M, Ostgaard H, Gundersen D,
Hatlebakk JG and Hausken T: The role of diet in the pathogenesis
and management of irritable bowel syndrome (Review). Int J Mol Med.
29:723–731. 2012.PubMed/NCBI
|
|
6
|
Ostgaard H, Hausken T, Gundersen D and
El-Salhy M: Diet and effects of diet management on quality of life
and symptoms in patients with irritable bowel syndrome. Mol Med
Rep. 5:1382–1390. 2012.PubMed/NCBI
|
|
7
|
Biesiekierski JR, Rosella O, Rose R, Liels
K, Barrett JS, Shepherd SJ, Gibson PR and Muir JG: Quantification
of fructans, galacto-oligosacharides and other short-chain
carbohydrates in processed grains and cereals. J Hum Nutr Diet.
24:154–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muir JG, Rose R, Rosella O, Liels K,
Barrett JS, Shepherd SJ and Gibson PR: Measurement of short-chain
carbohydrates in common Australian vegetables and fruits by
high-performance liquid chromatography (HPLC). J Agric Food Chem.
57:554–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muir JG, Shepherd SJ, Rosella O, Rose R,
Barrett JS and Gibson PR: Fructan and free fructose content of
common Australian vegetables and fruit. J Agric Food Chem.
55:6619–6627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Salhy M: Ghrelin in gastrointestinal
diseases and disorders: A possible role in the pathophysiology and
clinical implications (review). Int J Mol Med. 24:727–732. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sandström O and El-Salhy M: Ageing and
endocrine cells of human duodenum. Mech Ageing Dev. 108:39–48.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sternini C, Anselmi L and Rozengurt E:
Enteroendocrine cells: A site of ‘taste’ in gastrointestinal
chemosensing. Curr Opin Endocrinol Diabetes Obes. 15:73–78. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moran GW, Leslie FC, Levison SE,
Worthington J and McLaughlin JT: Enteroendocrine cells: Neglected
players in gastrointestinal disorders? Therap Adv Gastroenterol.
1:51–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coates MD, Mahoney CR, Linden DR, Sampson
JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM
and Moses PL: Molecular defects in mucosal serotonin content and
decreased serotonin reuptake transporter in ulcerative colitis and
irritable bowel syndrome. Gastroenterology. 126:1657–1664. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dizdar V, Spiller R, Singh G, Hanevik K,
Gilja OH, El-Salhy M and Hausken T: Relative importance of
abnormalities of CCK and 5-HT (serotonin) in Giardia-induced
post-infectious irritable bowel syndrome and functional dyspepsia.
Aliment Pharmacol Ther. 31:883–891. 2010.PubMed/NCBI
|
|
16
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Duodenal chromogranin a cell density as
a biomarker for the diagnosis of irritable bowel syndrome.
Gastroenterol Res Pract. 2014:4628562014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Endocrine cells in the ileum of
patients with irritable bowel syndrome. World J Gastroenterol.
20:2383–2391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Salhy M, Gilja OH, Gundersen D and
Hausken T: Endocrine cells in the oxyntic mucosa of the stomach in
patients with irritable bowel syndrome. World J Gastrointest
Endosc. 6:176–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Salhy M, Gilja OH and Hausken T:
Chromogranin A cells in the stomachs of patients with sporadic
irritable bowel syndrome. Mol Med Rep. 10:1753–1757.
2014.PubMed/NCBI
|
|
20
|
El-Salhy M, Gundersen D, Hatlebakk JG,
Gilja OH and Hausken T: Abnormal rectal endocrine cells in patients
with irritable bowel syndrome. Regul Pept. 188:60–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Salhy M, Gundersen D, Ostgaard H,
Lomholt-Beck B, Hatlebakk JG and Hausken T: Low densities of
serotonin and peptide YY cells in the colon of patients with
irritable bowel syndrome. Dig Dis Sci. 57:873–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
El-Salhy M, Lillebø E, Reinemo A and
Salmelid L: Ghrelin in patients with irritable bowel syndrome. Int
J Mol Med. 23:703–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Salhy M, Lomholt-Beck B and Hausken T:
Chromogranin A as a possible tool in the diagnosis of irritable
bowel syndrome. Scand J Gastroenterol. 45:1435–1439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Salhy M, Mazzawi T, Gundersen D and
Hausken T: Chromogranin A cell density in the rectum of patients
with irritable bowel syndrome. Mol Med Rep. 6:1223–1225.
2012.PubMed/NCBI
|
|
25
|
El-Salhy M, Vaali K, Dizdar V and Hausken
T: Abnormal small-intestinal endocrine cells in patients with
irritable bowel syndrome. Dig Dis Sci. 55:3508–3513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Salhy M, Wendelbo IH and Gundersen D:
Reduced chromogranin A cell density in the ileum of patients with
irritable bowel syndrome. Mol Med Rep. 7:1241–1244. 2013.PubMed/NCBI
|
|
27
|
Kim HS, Lim JH, Park H and Lee SI:
Increased immunoendocrine cells in intestinal mucosa of
postinfectious irritable bowel syndrome patients 3 years after
acute Shigella infection-an observation in a small case control
study. Yonsei Med J. 51:45–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JH, Rhee PL, Kim G, Lee JH, Kim YH,
Kim JJ, Rhee JC and Song SY: Enteroendocrine cell counts correlate
with visceral hypersensitivity in patients with
diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol
Motil. 18:539–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang SH, Dong L, Luo JY, Gong J, Li L, Lu
XL and Han SP: Decreased expression of serotonin in the jejunum and
increased numbers of mast cells in the terminal ileum in patients
with irritable bowel syndrome. World J Gastroenterol. 13:6041–6047.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spiller RC, Jenkins D, Thornley JP, Hebden
JM, Wright T, Skinner M and Neal KR: Increased rectal mucosal
enteroendocrine cells, T lymphocytes, and increased gut
permeability following acute Campylobacter enteritis and in
post-dysenteric irritable bowel syndrome. Gut. 47:804–811. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK
and Cho SW: The alteration of enterochromaffin cell, mast cell, and
lamina propria T lymphocyte numbers in irritable bowel syndrome and
its relationship with psychological factors. J Gastroenterol
Hepatol. 23:1689–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mazzawi T, Hausken T, Gundersen D and
El-Salhy M: Effects of dietary guidance on the symptoms, quality of
life and habitual dietary intake of patients with irritable bowel
syndrome. Mol Med Rep. 8:845–852. 2013.PubMed/NCBI
|
|
33
|
Mazzawi T, Gundersen D, Hausken T and
El-Salhy M: Increased gastric chromogranin A cell density following
changes to diets of patients with irritable bowel syndrome. Mol Med
Rep. 10:2322–2326. 2014.PubMed/NCBI
|
|
34
|
Mazzawi T, Hausken T, Gundersen D and
El-Salhy M: Effect of dietary management on the gastric endocrine
cells in patients with irritable bowel syndrome. Eur J Clin Nutr.
69:519–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mazzawi T, Gundersen D, Hausken T and
El-Salhy M: Increased chromogranin a cell density in the large
intestine of patients with irritable bowel syndrome after receiving
dietary guidance. Gastroenterol Res Pract. 2015:8238972015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mazzawi T, Hausken T, Gundersen D and
El-Salhy M: Dietary guidance normalizes large intestinal endocrine
cells densities in patients with irritable bowel syndrome. Eur J
Clin Nutr. 70:175–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mazzawi T and El-Salhy M: Changes in small
intestinal chromogranin-A immunoreactive cells after patients with
irritable bowel syndrome receive dietary guidance Submitted.
2015.
|
|
38
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
World Medical Association: Declaration of
Helsinki. Ethical Principles for Medical Research Involving Human
Subjects. Jahrbuch für Wissenschaft Und Ethik. 14:233–238.
2009.
|
|
40
|
Meltzer HM, Brantsaeter AL, Ydersbond TA,
Alexander J and Haugen M: Methodological challenges when monitoring
the diet of pregnant women in a large study: experiences from the
Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr.
4:14–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rimestad AH, Borgejordet Å, Vesterhus KN,
Sygnestveit K, Løken EB and Trygg K: Den Store Matvaretabellen [The
Norwegian Food Composition Table]. Norwegian Food Safety Authority
(Oslo). 2001.(In Norwegian).
|
|
42
|
Masson LF, McNeill G, Tomany JO, Simpson
JA, Peace HS, Wei L, Grubb DA and Bolton-Smith C: Statistical
approaches for assessing the relative validity of a food-frequency
questionnaire: Use of correlation coefficients and the kappa
statistic. Public Health Nutr. 6:313–321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brantsaeter AL, Haugen M, Alexander J and
Meltzer HM: Validity of a new food frequency questionnaire for
pregnant women in the Norwegian Mother and Child Cohort Study
(MoBa). Matern Child Nutr. 4:28–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
El-Salhy M, Stenling R and Grimelius L:
Peptidergic innervation and endocrine cells in the human liver.
Scand J Gastroenterol. 28:809–815. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Enck P, Klosterhalfen S and Kruis W:
Determination of placebo effect in irritable bowel syndrome. Dtsch
Med Wochenschr. 130:1934–1937. 2005.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Abdul-Baki H, El Hajj II, Elzahabi L, Azar
C, Aoun E, Skoury A, Chaar H and Sharara AI: A randomized
controlled trial of imipramine in patients with irritable bowel
syndrome. World J Gastroenterol. 15:3636–3642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zernicke KA, Campbell TS, Blustein PK,
Fung TS, Johnson JA, Bacon SL and Carlson LE: Mindfulness-based
stress reduction for the treatment of irritable bowel syndrome
symptoms: A randomized wait-list controlled trial. Int J Behav Med.
20:385–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Halmos EP, Power VA, Shepherd SJ, Gibson
PR and Muir JG: A diet low in FODMAPs reduces symptoms of irritable
bowel syndrome. Gastroenterology. 146:67–75.e5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sandström O and el-Salhy M: Human rectal
endocrine cells and aging. Mech Ageing Dev. 108:219–226. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee J, Cummings BP, Martin E, Sharp JW,
Graham JL, Stanhope KL, Havel PJ and Raybould HE: Glucose sensing
by gut endocrine cells and activation of the vagal afferent pathway
is impaired in a rodent model of type 2 diabetes mellitus. Am J
Physiol Regul Integr Comp Physiol. 302:R657–R666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Parker HE, Reimann F and Gribble FM:
Molecular mechanisms underlying nutrient-stimulated incretin
secretion. Expert Rev Mol Med. 12:e12010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Raybould HE: Nutrient sensing in the
gastrointestinal tract: Possible role for nutrient transporters. J
Physiol Biochem. 64:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
San Gabriel A, Nakamura E, Uneyama H and
Torii K: Taste, visceral information and exocrine reflexes with
glutamate through umami receptors. J Med Invest. 56(Suppl):
S209–S217. 2009.
|
|
54
|
Rudholm T, Wallin B, Theodorsson E,
Näslund E and Hellström PM: Release of regulatory gut peptides
somatostatin, neurotensin and vasoactive intestinal peptide by acid
and hyperosmolal solutions in the intestine in conscious rats.
Regul Pept. 152:8–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sternini C: Taste receptors in the
gastrointestinal tract. IV. Functional implications of bitter taste
receptors in gastrointestinal chemosensing. Am J Physiol
Gastrointest Liver Physiol. 292:G457–G461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Buchan AM: Nutrient tasting and signaling
mechanisms in the Gut III. Endocrine cell recognition of luminal
nutrients. Am J Physiol. 277:G1103–G1107. 1999.PubMed/NCBI
|
|
57
|
Montero-Hadjadje M, Elias S, Chevalier L,
Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM,
Driouich A, et al: Chromogranin A promotes peptide hormone sorting
to mobile granules in constitutively and regulated secreting cells:
Role of conserved N- and C-terminal peptides. J Biol Chem.
284:12420–12431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shooshtarizadeh P, Zhang D, Chich JF,
Gasnier C, Schneider F, Haïkel Y, Aunis D and Metz-Boutigue MH: The
antimicrobial peptides derived from chromogranin/secretogranin
family, new actors of innate immunity. Regul Pept. 165:102–110.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gershon MD and Tack J: The serotonin
signaling system: From basic understanding to drug development for
functional GI disorders. Gastroenterology. 132:397–414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tack JF, Janssens J, Vantrappen G and Wood
JD: Actions of 5-hydroxytryptamine on myenteric neurons in guinea
pig gastric antrum. Am J Physiol. 263:G838–G846. 1992.PubMed/NCBI
|
|
61
|
Michel K, Sann H, Schaaf C and Schemann M:
Subpopulations of gastric myenteric neurons are differentially
activated via distinct serotonin receptors: Projection,
neurochemical coding, and functional implications. J Neurosci.
17:8009–8017. 1997.PubMed/NCBI
|
|
62
|
Tack J, Coulie B, Wilmer A, Andrioli A and
Janssens J: Influence of sumatriptan on gastric fundus tone and on
the perception of gastric distension in man. Gut. 46:468–473. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gershon MD: Plasticity in serotonin
control mechanisms in the gut. Curr Opin Pharmacol. 3:600–607.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gershon MD: 5-Hydroxytryptamine
(serotonin) in the gastrointestinal tract. Curr Opin Endocrinol
Diabetes Obes. 20:14–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gershon MD: Serotonin is a sword and a
shield of the bowel: Serotonin plays offense and defense. Trans Am
Clin Climatol Assoc. 123:268–280; discussion 280. 2012.PubMed/NCBI
|
|
66
|
Gershon MD: Review article: Roles played
by 5-hydroxytryptamine in the physiology of the bowel. Aliment
Pharmacol Ther. 13(Suppl 2): S15–S30. 1999.
|
|
67
|
Gershon MD, Wade PR, Kirchgessner AL and
Tamir H: 5-HT receptor subtypes outside the central nervous system.
Roles in the physiology of the gut. Neuropsychopharmacology.
3:385–395. 1990.PubMed/NCBI
|
|
68
|
El-Salhy M, Mazzawi T, Gundersen D,
Hatlebakk JG and Hausken T: The role of peptide YY in
gastrointestinal diseases and disorders (review). Int J Mol Med.
31:275–282. 2013.PubMed/NCBI
|
|
69
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Interaction between ingested nutrients
and gut endocrine cells in patients with irritable bowel syndrome
(review). Int J Mol Med. 34:363–371. 2014.PubMed/NCBI
|
|
70
|
Höcker M and Wiedenmann B: Molecular
mechanisms of enteroendocrine differentiation. Ann N Y Acad Sci.
859:160–174. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Inokuchi H, Fujimoto S and Kawai K:
Cellular kinetics of gastrointestinal mucosa, with special
reference to gut endocrine cells. Arch Histol Jpn. 46:137–157.
1983. View Article : Google Scholar : PubMed/NCBI
|