Introduction
The existence of skin tension has been reported for
~200 years (1). As a result of
muscle activity and respiration, the majority of the skin covering
a body is in the status of cyclic tension. Although the
pathophysiological roles of skin tension in certain diseases have
been initially investigated (2), the
biological significance of tension signal transduction in skin
cells are poorly understood, particularly the roles of tension in
the healing of skin wound (3).
Normally, cutaneous tissues and cells, particularly the dermal
tissue and fibroblasts, are the major tissues and cellular type to
receive signals and respond towards tension (1). Although the keratinocytes in the
epidermis endure tension weakly, they receive the signals from
cytokines and extra cellular matrix (ECM) secreted by human skin
fibroblasts (HFs) in dermal tissue (1,4). Once
skin tension disappears due to skin injury, multiple cellular
processes will be induced in cutaneous tissues to accelerate wound
healing (4). At the molecular level,
the sudden disappearance of skin tension may induce the expression
of various proteases in keratinocytes, which degrade particular
proteins of ECM to facilitate the migration and re-epithelization
of keratinocytes (5–8).
The maintenance of cell normal function relies
substantially on the ECM (9). During
the process of cell signal transduction, the receptors in cell
membrane bind to specific ECM components to form the ‘cross-talk’
between cells and ECM. The interaction further mediates
transmembrane signal transduction (10,11). The
interaction between cells and ECM assists in maintaining normal
cellular physiological activities, in addition to playing an
important function in multiple pathological processes, including
wound healing, scar formation and tissue fibrosis (10). Therefore, it has been widely accepted
that cell-ECM mediated transmembrane signal transduction is a major
pathway for skin to receive the outside mechanical tension signal
(10).
During the initial process of skin injury,
mechanical tension vanishes in the edge of the wound, numerous
temporary matrices are presented in the surface of wound, and
inflammatory cell infiltration is followed by neovascularization
and the aggregation of HFs (12). In
the post stage of wound healing, the surface of wound shrinks, and
the temporary matrices in the surface are substituted by mature
matrices generated from aggregated HFs, and finally scars are
formed (12). Nevertheless, it
remains unclear how the mechanical tension signal is transduced
from HFs to keratinoctyes in the process of sudden skin tension
disappearance (10).
The calcium/calmodulin-dependent serine protein
kinase (CASK), a membrane-associated guanylate kinase and a
scaffolding protein, has been demonstrated to recruit or organize
other proteins at the plasma membrane to coordinate signal
transduction pathways within the cytoplasm and nucleus (13). Sun et al (14) reported that CASK regulates the
protein and mRNA level of p21, which promotes cell proliferation in
ECV304 cells. Furthermore, CASK has been indicated to participate
in the control of the G0-G1 restriction check point of the cell
cycle in human breast cancer cells via inhibiting cyclin D1
synthesis and pRb phosphorylation (15). Thus, CASK serves a crucial function
in cell growth, while little is known about CASK effect under
cyclic stretch.
In the present study, we hypothesize that the
‘cross-talk’ among HFs, ECM (HF-secreted) and keratinocytes exists
in cutaneous tissues. In such cell-ECM-cell interaction processes,
HFs may modulate the expression levels of specific ECM proteins in
response to skin tension changes. The ECM component change would
subsequently affect the proliferation and migration of
keratinocytes. To test this hypothesis, we employed a in-house
built mechanical stretch device to apply mechanical force against
the cultured HF cells. By applying three different mechanical
stretches, including non-stretch, static stretch or cyclic stretch,
we quantified the expression levels change of ECM proteins in and
investigated the molecular signaling transduction pathways in the
process of mechanical tension-induced HF proliferation. We further
examined the effects of ECM protein expression changes on the
proliferation and migration of keratinocytes. CASK has been
demonstrated to involve in cell growth and calcium signaling, so we
next investigated the effect of CASK on HF proliferation under
cyclic stretch.
Materials and methods
Antibody and reagents
Mouse monoclonal antibodies against human collagen
I, III, IV, fibronectin, CASK and integrin β1 were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Collagen I,
fibronectin and all other reagents were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cells and cell culture
Human skin fibroblasts [CRL2088; American Type
Culture Collection (ATCC), Manassas, VA, USA] and spontaneously
immortalized keratinocyte HaCaT (CRL2309; ATCC) were used in this
study. HaCaT cells were maintained in RPMI-1640 medium (HyClone; GE
Healthcare, Logan, UT, USA) containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Grand Island, NY, USA) and
1% penicillin/streptomycin (HyClone; GE Healthcare). HFs were
cultured in Dulbecco's modified Eagles medium (1 g/l glucose) with
10% FBS and 1% antibiotics. When reaching ~80% confluence, cells
were harvested and then transferred to the mechanical stretch
device developed in-house for an additional 6 days of culture under
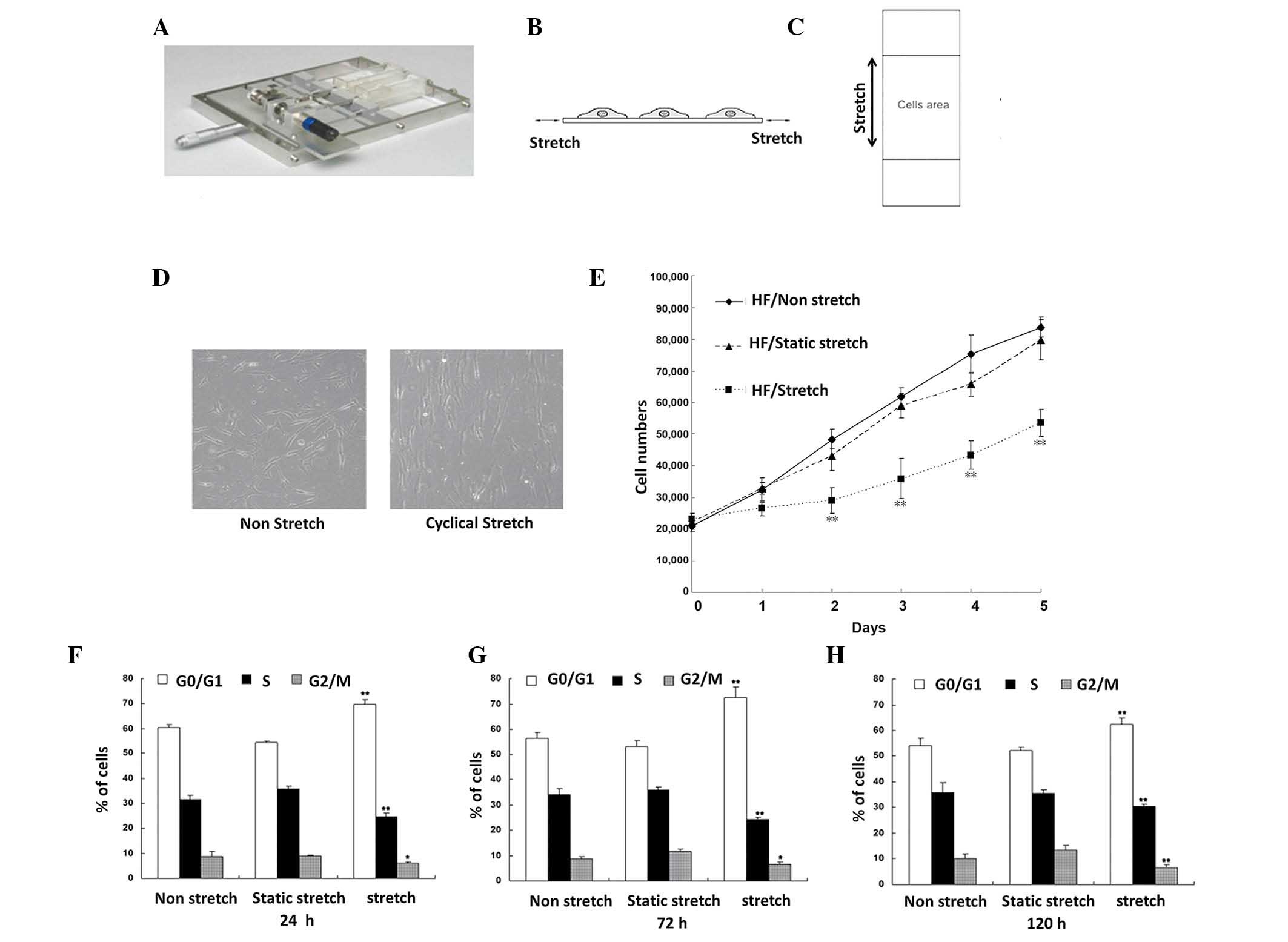
mechanical stretch (Fig. 1A). Cells
were then harvested at 0, 1, 2, 3, 4 and 5 days for various
functional investigations.
Application of cyclic stretch to
cultured cells
Mechanical stretch was applied to examine
mechano-transduction of HFs. HF cells grown in a flask were
transferred onto the silicone membrane pre-treated with oxygenized
plasma. A non-toxic stainless steel frame was used to retain the
cells inside the seeding region. The membrane was then mounted to
the mechanical stretch device and experienced a cyclic stretch with
20% strain and 1 Hz frequency for 6 days. The physiological stretch
frequency is from 0.1 (respiratory rate) to 1.25 Hz (heart rate),
all frequency in this arranges can be used for cyclic stretch. In
this study 1 Hz was used in our pre-experiments, as previous
reports have suggested that 1 Hz frequency is appropriate for
cyclic stretch investigation (16,17).
Cells under static stretch with 20% strain and non-stretch case
were used as a control.
ECM preparation and extraction
For ECM preparation, HF cells under different
stretches were maintained for up to 6 days. Cells were then washed
with sterile distilled water for 4 h followed by 1 mM NaCl for 1 h.
A total of three times of wash cycles were applied to cells.
Finally, the cells were dried overnight at 4°C. HaCaT cells were
then seeded on the prepared ECM on silicone membrane. For ECM
extraction, remaining ECM on silicone membrane was lysed with RIPA
buffer containing protease inhibitors and complete mini EDTA-free
(Roche Diagnostics, Branchburg, NJ, USA) for western blot.
ECM preparation
A certain quantity of liquid silicone was solidified
in 24-well plates to form a silicone membrane, on which 10 µg/ml
collagen I or human fibronectin (BD Biosciences, San Jose, CA, USA)
was coated for at 37°C. The silicone membrane was then washed
thoroughly with PBS and seeded with
1×104-2×104 HaCaT cells.
Western blot and
immunoprecipitation
Cells were pelleted and lysed directly using 2X
Laemmli sample buffer (1610737, Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with BME. The cell lysate was then incubated at
95°C for 5 mins and stored at −20°C until the samples were run on
an SDS-PAGE gel. Protein loading was quantified using the Pierce
BCA Protein Assay kit (23225; Thermo Fisher Scientific, Inc.).
Western blot analysis was conducted according to previous reports
(18,19). Samples of crude proteins (20 µg) were
fractionized using 4–20%SDS-PAGE Tris-glycine gels (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and transferred onto
nitrocellulose membranes. Membranes were blocked for 1 h with 5%
milk in Tris-buffered saline, then incubated with the following
primary monoclonal antibodies in 5% milk with 0.1% Tween 20 for 16
h at 4°C: Collagen I (cat. no. ab34710: Abcam, Cambridge, UK),
Collagen III (cat. no. ab7778; Abcam), Collagen IV (cat. no.
ab6586; Abcam), fibronectin (cat. no. ab3413; Abcam), integrin β1
(cat. no. ab179472; Abcam), CASK (cat. no. ab99039; Abcam),
integrin β3 (cat. no. ab119992; Abcam), β-actin (cat. no. 4970;
Cell Signaling Technology, Inc., Dancers, MA, USA), EGF (cat. no.
2646; Cell Signaling Technology, Inc.), FGF (cat. no. 9740; Cell
Signaling Technology, Inc.), PDGF (cat. no. 3169; Cell Signaling
Technology, Inc.) and IGF (cat. no. 9750; Cell Signaling
Technology, Inc.). All antibodies were diluted 1:1,000. After
washing with Tris-buffered saline Tween 20 the membranes were then
incubated for 1 h at 4°C with a goat anti-mouse secondary antibody
conjugated with horseradish peroxidase (1:5,000; 31430; Thermo
Fisher Scientific, Inc.). After washing, membranes were incubated
for 1 min with Western Lightning Chemiluminescence reagent Plus
(PerkinElmer, Inc., Waltham, MA, USA) and the signal of western
blot was exposed and developed. Band intensities were visualized
using Pierce ECL Western blotting (32106; Thermo Fischer
Scientific, Inc.) and quantified using Image J (National Institutes
of Health, Bethesda, MD, USA). Immunoprecipitation was performed as
previously reported (16). Briefly,
HFs were lysed in ice-cold RIPA buffer containing protease
inhibitors. 400 µg cell lysate proteins was then incubated with 14
µl mouse anti-human CASK (1:1,000; ab99039; Abcam) or anti-human
integrin β1 (1:1,000; ab179472; Abcam) monoclonal antibody for 2 h
at 4°C. Next, 20 µl protein G-agarose was added afterwards and
incubated at 4°C overnight on a rocker platform. Following
centrifugation at 1,000 × g for 5 min at 4°C, the agarose beads
were washed four times with RIPA buffer at 4°C and lysed in the
sample buffer for further western blot analysis as described above.
Antigen retrieval was performed using citrate buffer [10 mM citric
acid, 0.05% Tween 20, (pH 6.0)] at 95–100°C using a pre-heat
steamer.
Mammalian two-hybrid protein-protein
interaction assays
This experiment was performed as described
previously (20). Briefly, the
entire coding sequence of human CASK was cloned in-frame into a pM
vector (Clontech Laboratories, Inc., Mountain View, CA, USA)
encoding the GAL4 DNA binding domain. The cDNAs of integrin β1
extracellular or intracellular terminal were donated by Dr Xinyu
Wang of the Shanghai Institute of Biochemistry and Cell Biology
(Chinese Academy of Sciences, Shanghai, China). These were cloned
respectively in-frame into the pVP16 vector (Clontech Laboratories,
Inc.) encoding the VP16 transactivation domain. The pG5CAT reporter
vectors (0.5 µg) containing a chloramphenicol acetyltransferase
(CAT) reporter gene under the control of the GAL4 response element
and 2.5 µg of each of the above-mentioned constructed vectors were
co-transfected into 2.5×105 HFs per well in six-well
plates with the LF2000 reagent (Gibco; Thermo Fisher Scientific,
Inc.). After 48 h of transfection, cells were harvested and
extracts were assayed for CAT activity using a CAT ELISA assay kit
(Roche Diagnostics).
Analysis of cell proliferation and
migration
Cells were seeded onto silicone membranes in 24-well
plates at a density of 1–2×104 cells per well. At each
time-point, cells were harvested after trypsinization and
centrifugation at 800 × g for 5 min at 4°C. All samples were
prepared in quadruplicate and the entire experiment was repeated
twice. Cells were plated onto silicone membrane pre-coated with
collagen I or fibronectin followed by culture for ~24 h. When the
cell growth reached 80% confluence with monolayer, one 200 µl
pipette tip was used to straightly scratch cells to create wound.
Between two and four scratches were made in each well. Cells were
photographed using an inverted microscope (Olympus Corporation,
Tokyo, Japan) at day 0, 1, 2, 3, 4 and 5, and the wound area was
measured using UTHSCSA image tool software version 3.0 (University
of Texas Health Science Center, San Antonio, TX, USA). The
migration rate (MR) was calculated using the formula: MR (%) = (1 -
At / A0) × % (A0: area at 0 h; At: area at indicated times).
Cell cycle analysis
Cells were harvested at the indicated time points
and stained with propidium iodide using Cycletest Plus (BD
Biosciences, San Jose, CA, USA) according to the manufacturer's
instructions. Cells were then analyzed using FACSCalibur with
ModFit LT software, version 2.0 (BD Biosciences) to demonstrate
G0/G1, S, G2/M and hypodiploid nuclei.
Cell transfection and RNA
interference
siRNAs targeted hCASK mRNAs were designed and
synthesized by Ambion (Thermo Fisher Scientific, Inc., Austin, TX,
USA). A sequence of 19 nucleotides within the hCASK coding region
immediately downstream of AA dinucleotides was selected as the
target sequence (5,21). Two oligonucleotides were synthesized,
annealed, and inserted into the BamHI and HindIII
digestion sites of the pSilencer 3.1-H1 hygro vector (Ambion;
Thermo Fisher Scientific, Inc.), as described in our previous
report (5,21). HF cells were transfected with
expression plasmids of CASK siRNA or empty vector pBS/U6 by FuGENE6
reagent (Roche Diagnostics) according to the manufacturer's
instructions. Stable transformants were obtained under the
selection with 200 µg/ml hygromycin B (Stratagene California, La
Jolla, CA, USA). The positive stable clones were referred to as
siCASK.
Statistical analysis
Data were analyszed using GraphPad Software
(GraphPad Software, Inc., LaJolla, CA, USA) and presented as the
means ± standard error of the mean. Student's unpaired t-tests
(two-tailed) were used to determine the statistical differences
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cyclic stretch inhibited HF
proliferation
We initially employed our in-house built cell
stretch device to apply a cyclic or static stretch on HF seeded in
the central region of silicone gel membrane where the mechanical
tension is uniform (Fig. 1A-C). In
contrast to the minimal morphological change of HF cells without
mechanical stretch (Fig. 1D, left
panel), cells under cyclic stretch showed a significant
morphological change with stretch-directed orientation
perpendicular to the direction of stretch (Fig. 1D, right panel).
Cells undergone cyclic stretch also revealed a
decreased degree of proliferation compared with those under static
stretch or non-stretch. First, HFs cultured under cyclic stretch
had a significantly decreased number of total cells after 5 days of
culture compared with those were cultured under non-stretch or
statical stretch (both P<0.01). To further prove the decreased
cell proliferation in HFs under cyclic stretch condition, we
performed flow cytometry to demonstrate the cell cycle change in
these HFs. We found that the percentage of G0/G1 phase HF cells
after 24, 72 and 120 h of cyclic stretch was markedly higher
compared with under static stretch or non-stretch for the same
length of culture (both P<0.01; Fig.
1E). By contrast, a significantly lower percentage of cells
cultured under cyclic stretch entered S phase compared with the
other two stretch conditions (both P<0.01; Fig. 1E). Altogether, these data suggested
that cyclic stretch inhibits HF proliferation whereas static
stretch or non-stretch did not.
Cyclic stretch regulates the specific
component expression of ECM of HFs
The underlying mechanism by which cyclic stretch
inhibits HF proliferation remains elusive. Although previous
studies suggested that the autocrine or paracrine growth factors
secreted by HFs in response to stretch were involved in the process
(22), our independent
investigations with the co-culture of HF and keratinocytes showed
that the effects of HF growth factors alone was not sufficient to
explain the mechanism of proliferation inhibition (data not shown).
We hypothesized that HFs could modulate the specific component
expression of HF ECM in response to cyclic stretch, which
subsequently affected the proliferation and migration of
keratinocytes. To validate this, we extracted the ECM of HFs under
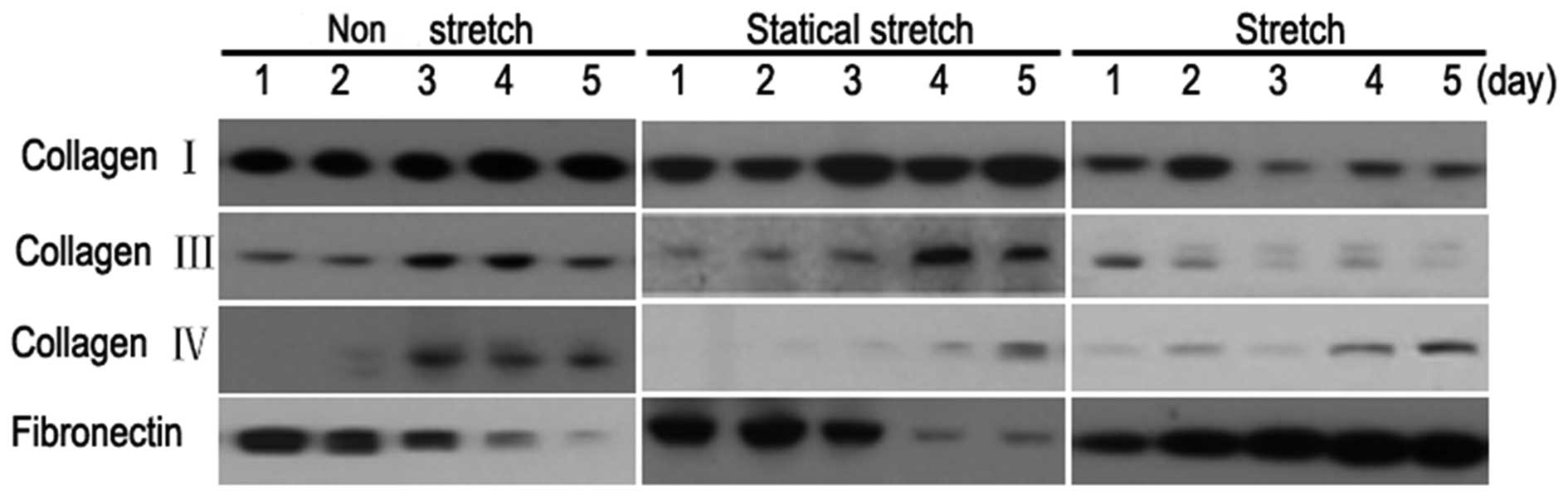
cyclic stretch, static stretch and non-stretch conditions. The
differences in the expression levels of major ECM components
including collagen I, III, IV and fibronectin were investigated
using western blot analysis. The results indicated that the
expression levels of collagen III and IV were significantly higher
in HFs cultured under cyclic stretch compared with static stretch
and non-stretch (Fig. 2). By
contrast, the expression of fibronectin was markedly reduced under
this condition compared with those cultured with static stretch and
non-stretch (Fig. 2). Furthermore,
the increase in collage III or IV and decrease in fibronectin
levels occurred in a time-dependent manner with 5 days of culture
having the most significant difference (Fig. 2).
Protein expression changes in HF ECM
in response to mechanical stretch inhibited keratinocyte
proliferation
It has previously been reported that the growth
factor in ECM modulates ECM-mediated cell proliferation (23). To verify this in the present study,
HFs seeded onto silicone membranes were cultured for six days under
these three different stretch conditions and osmotically shocked.
The remaining growth factors in ECM on silicone were eluted by high
salt elution. Subsequently, HaCaT cells were seeded on silicone
membrane containing growth factor-free ECM. The cyclic
stretch-induced HF ECM on silicone membrane was still able to
inhibit the proliferation of HaCaT cells (Fig. 3A).
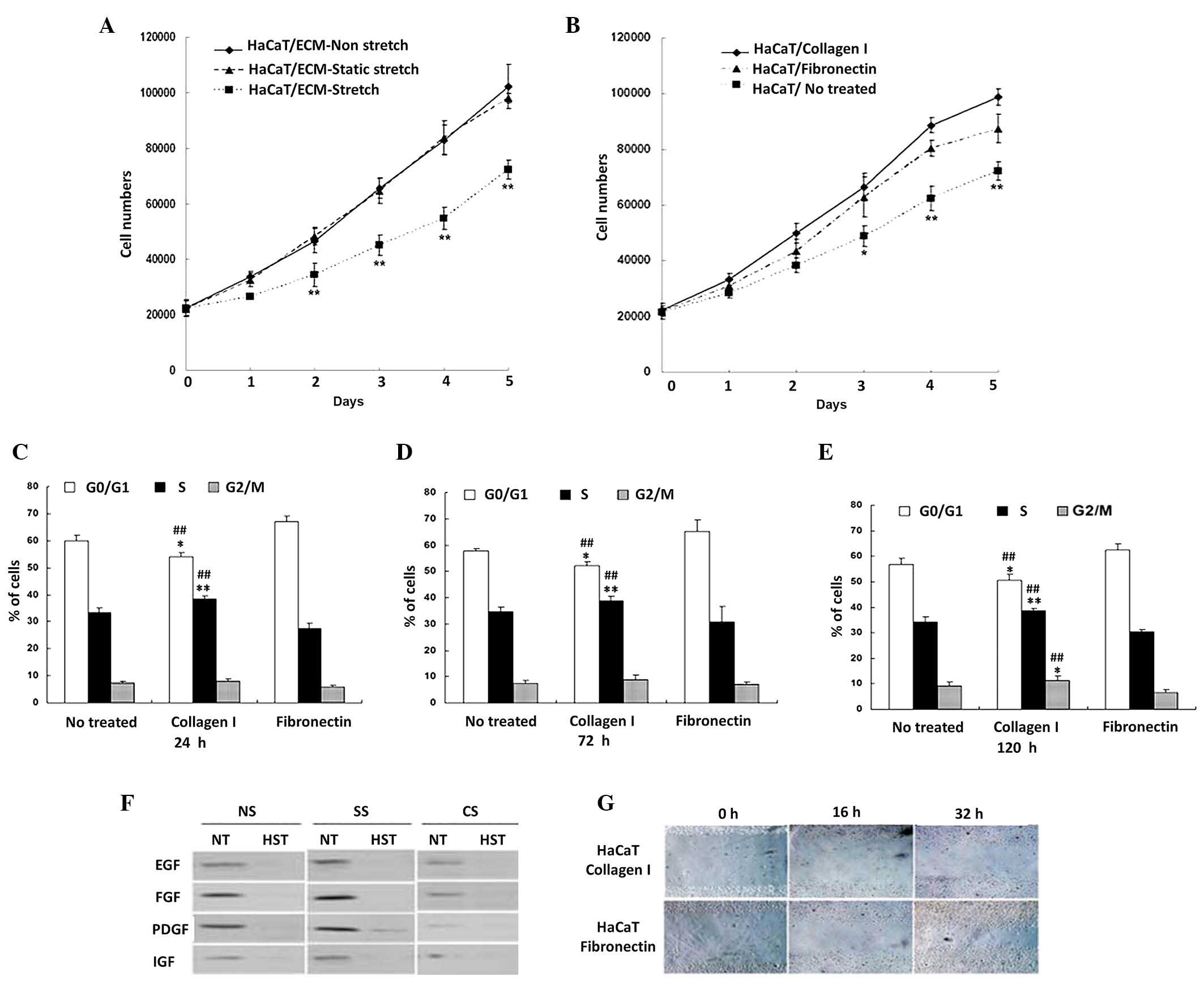 | Figure 3.Cyclic stretch induced HF ECM
inhibited the proliferation of keratinocytes. (A) Total HaCaT cell
numbers after 5 days of culture on silicone gel membranes with the
prepared ECM from HFs under cyclic stretch, static stretch or
non-stretch (n=3). **P<0.01 vs. non-stretch or static stretch
group. (B) Total HaCaT cell numbers after 5 days of culture on
silicone gel membranes coated with collagen I, fibronectin or blank
matrix for indicated period of times. **P<0.01 vs. fibrinogen
coated or no treated membrane. Cell cycle profiles of HaCaT cells
cultured on silicone gel membranes with collagen I, fibronectin or
blank matrix for (C) 24, (D) 72 and (E) 120 h. **P<0.01,
##P<0.01 vs. no treated membrane. (F) Western blot of
growth factors including EGF, FGF, PDGF and IGF in extracted HF ECM
upon S, SS or NS treated HF. ECM on silicone gel membranes were
eluted with HST or NT. (G) Scratch migration assay of HaCaT cells
on the silicone gel membrane with collagen I or fibronectin matrix.
The influence of matrix proteins on cellular migration by
wound-healing assay at the time of 16 and 32 h of post scratched
respectively. *P<0.05 vs static stretch or without stretch
group. HF, human skin fibroblast; ECM, extracellular matrix; S,
cyclic stretch; SS, static stretch; NS, non-stretch; HST, high salt
solution; NT, without high salt solution. |
Differential roles of collagen I and
fibronectin in cell proliferation and migration
To further investigate the roles of different ECM
components in cell proliferation, we prepared specific silicone gel
membranes with single collagen I, fibronectin matrix or blank
matrix. HaCaT proliferation analysis showed that membranes coated
with collagen I or fibronection matrix promoted cell proliferation
when compared with membrane without any ECM component coating
(Fig. 3B). Furthermore, the collagen
I-coated membrane had an even greater capability to promote cell
proliferation compared with fibronectin-coated membrane (Fig. 3B). Data of cell cycle analysis was
also consistent with this finding. We found that the percentage of
G0/G1 cells in collagen I matrix group was significantly lower than
that in fibronectin matrix and blank matrix groups (both
P<0.01). In contrast, of the percentage of cells in S phase were
markedly higher in collagen I matrix group compared with the other
two groups (both P<0.01; Fig.
3C). Next, we applied cell scratch experiment to analyze HaCaT
migration in silicone gel membrane with different matrices. To
exclude the effects of cell proliferation to cell migration, cells
were treated for 48 h before scratch experiment with 10 µg/ml
mitomycin for 1 h. In the fibronectin matrix group, the healing
area was 36.7 and 65.5% at 16 and 32 h after scratching (Fig. 3E). However, the healing area was
quite lower in collagen I matrix group which was 17.8 and 37.2% at
16 and 32 h after scratching with representative images shown in
Fig. 3E. In addition, since the high
salt elution almost totally removed the growth factors in ECM, such
an observation suggests that ECM-induced inhibition of cell
proliferation under cyclic stretch was not a growth factors-related
effect which was further proved by the western blot (Fig. 3D). Collectively, these results
demonstrated that collagen I has a greater capability to promote
cell proliferation while fibronectin has a higher capability to
promote cell migration.
CASK suppression attenuates the
effects of cyclic stretch-induced inhibition of HF
proliferation
To investigate the underlying mechanism by which
mechanical stretch inhibits cell proliferation, we performed an
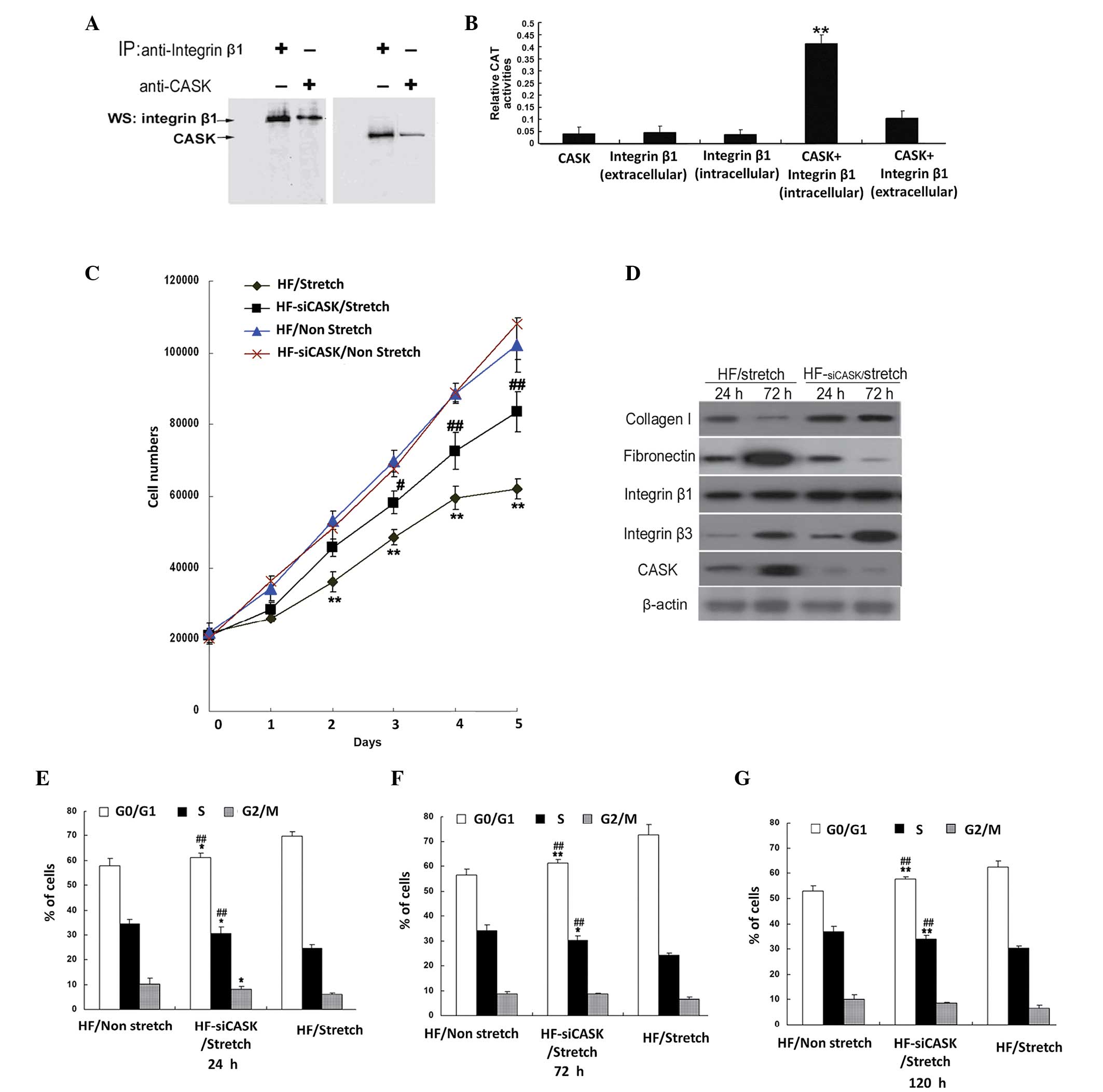
immunoprecipitation assay for integrin β1, one of the most
important integrin protein in ECM, and found that it bound with
CASK (Fig. 4A). With mammalian
two-hybrid analysis, we confirmed that CASK directly interacted
with integrin β1 (Fig. 4B). Using
siRNA interference to suppress the expression of CASK in HFs under
cyclic stretch, the results showed that CASK knockdown attenuated
the inhibition of cell proliferation under cyclic stretch (Fig. 4C), indicating the important role of
CASK in cyclic stretch related cell proliferation inhibition. Cell
cycle analysis also indicated that the portion of G0/G1 cells in
CASK knockdown HF cells was significantly lower compared with HF
cells with normal CASK expression under cyclic stretch, while the
percentage of HF cells in S phase was significantly higher (all
P<0.01; Fig. 4D). Western blot
further demonstrated that the expression of collagen I increased
and fibronectin decreased in CASK knockdown group compared with
normal CASK expression HF cells (Fig.
4E). Collectively, these data implied that cyclic
stretch-induced inhibition of HF proliferation could possibly links
with the integrin β1-CASK signal pathway, which might regulate the
expression of specific protein expression in HF ECM.
Discussion
The capacity for tension endurance by skin tissues
is crucial for skin development, maintenance of skin normal
physiological functions and skin injure repair (2,3). Using
an in-house built cell mechanical stretch device, we found that the
cyclic tension changed in skin tissues could be mimicked in
vitro and the impact of cyclic tension change on proliferation
and migration of HF and keratinocytes were further investigated in
the current study. The results indicated that cyclic stretch
significantly inhibited the proliferation of HF. Such inhibition of
HF proliferation was associated with altered ECM protein levels in
HF cells. We further showed that cultured keratinocytes on the
altered of HF ECM inhibited the proliferation of keratinocytes,
suggesting that the secreted ECM by HF under cyclic stretch might
transmit the signal of cell proliferation inhibition. Data obtained
from this study indicated a possible ‘cross-talk’ between HFs, ECM
and keratinocytes in cutaneous tissues. Such interaction maybe
involved in the processes underlying skin development,
physiological skin function maintenance, skin injure repair and the
formation of scars.
Although it is well known that multiple growth
factors in ECM are able to regulate cell proliferation (24,25), we
demonstrated that growth factor-free ECM from cyclic stretched HF
cells after removing the growth factors in ECM by high salt elution
was able to induce inhibit the proliferation of keratinocytes. By
analyzing the expression levels of major ECM components, we showed
that the expression levels of collagen I and III, and fibronectin
changed in response to cyclic stretch. We therefore concluded that
not only the changes of growth factors in ECM, but also the changes
of protein expression levels of ECM induced by skin tension
alteration modulated the cell proliferation and migration in skin
tissues.
Integrins are receptors coupling ECM components
outside a cell to the cytoskeleton inside the cell, through the
interaction with their ligands including collagen I and fibronectin
(25). Integrins transmit the
extracellular signals into the cells to in regulate multiple
cellular processes in cell proliferation (26–29).
Peripheral plasma membrane protein CASK could bind cell-surface
proteins to coordinate signal transduction pathways (29). We showed in the present study that
CASK was a partner of integrin β1, and the integrin β1-CASK pathway
may be involved in the cyclic stretch-induced inhibition of HFs
proliferation. By knocking down the CASK expression with siRNA
interference, we observed that the inhibition of HF proliferation
was attenuated and the expression of collagen I increased while
fibronectin decreased under cyclic stretch condition, suggesting
that CASK was one of the upstream molecules in regulating the
expression of ECM proteins in response to skin tension.
Comprehensively, the present study indicated a possible molecular
mechanism that skin tissues adapt cyclic stretch by regulation of
the proliferation and migration of HFs and keratinocytes through
integrin β1-CASK signal pathway.
Since skin injury is associated with a sudden
disappearance of local tension and would healing requires skin cell
proliferation and migration, we further hypothesized that the local
tension disappearance in injured skin might discharge the effects
of proliferation inhibition, which subsequently initiate the
process of wound healing. Previous studies have indicated that the
disappearance of skin tension upregulated the expression of
multiple proteinases in keratinocytes, which contributed to the
cell proliferation and migration and promotes re-epithelization of
skin (7–10). The present study further showed that
the disappearance of skin tension could regulate the expression
levels of major ECM components, which is consistent with a previous
report indicating changes of mRNA levels of major ECM components in
response to mechanical stretch (30). These results indicate the existence
of other mechanisms by which the changes of skin tension could
affect the biological functions of HF and keratinocytes by
regulating the expression level of ECM components.
Although all biological functions of collagen I and
III, and fibronectin in pathological and physiological states are
not elaborately classified (31,32), our
results showed that different ECM components have differential
biological functions, where collagen I and III might preferably
regulate keratinocyte proliferation while fibronectin had a more
dominant role in modulating keratinocyte migration. Since collagen
and fibronectin are integrin ligands, the subtle functional
differences between collagen and fibronectin in ECM implied the
elaborate regulation of integrin β1-CASK signal pathway in response
to the changes of skin tension.
In conclusion, our results demonstrated the
existence of HF-ECM-keratinocyte interaction in cutaneous tissues.
The integrin β1-CASK signal pathway in HFs may be involved in the
outside-in signal transduction of extracellular stretch and altered
the expression and secretion of ECM components in this process.
Acknowledgements
This study was supported by grants from the National
High Technology Research and Development Program of China (grant
no. 2011CB710904) and the National Natural Science Foundation of
China (grant no. 81372813).
References
|
1
|
Silver FH, Siperko LM and Seehra GP:
Mechanobiology of force transduction in dermal tissue. Skin Res
Technol. 9:3–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ingber DE: Mechanobiology and diseases of
mechanotransduction. Ann Med. 35:564–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ingber DE: Cellular mechanotransduction:
Putting all the pieces together again. FASEB J. 20:811–27. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhadal N, Wall IB, Porter SR, Broad S,
Lindahl GE, Whawell S and Lewis MP: The effect of mechanical strain
on protease production by keratinocytes. Br J Dermatol.
158:396–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jensen PJ and Lavker RM: Urokinase is a
positive regulator of epidermal proliferation in vivo. J Invest
Dermatol. 112:240–224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McNeill H and Jensen PJ: A high-affinity
receptor for urokinase plasminogen activator on human
keratinocytes: Characterization and potential modulation during
migration. Cell Regul. 1:843–852. 1990.PubMed/NCBI
|
|
7
|
Wang XQ, Sun P and Paller AS: Gangliosides
inhibit urokinase-type plasminogen activator (uPA)-dependent
squamous carcinoma cell migration by preventing uPA
receptor/alphabeta integrin/epidermal growth factor receptor
interactions. J Invest Dermatol. 124:839–848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bissell MJ, Hall HG and Parry G: How does
the extracellular matrix direct gene expression? J Theor Biol.
99:31–68. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aszódi A, Legate KR, Nakchbandi I and
Fässler R: What mouse mutants teach us about extracellular matrix
function. Annu Rev Cell Dev Biol. 22:591–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Myllyharju J and Kivirikko KI: Collagens,
modifying enzymes and their mutations in humans, flies and worms.
Trends Genet. 20:33–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carrion K, Dyo J, Patel V, Sasik R,
Mohamed SA, Hardiman G and Nigam V: The long non-coding HOTAIR is
modulated by cyclic stretch and WNT/β-CATENIN in human aortic valve
cells and is a novel repressor of calcification genes. PLoS One.
9:e965772014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu ZQ, Wang D, Xiang D, Yuan YX and Wang
Y: Calcium/calmodulin-dependent serine protein kinase is involved
in exendin-4-induced insulin secretion in INS-1 cells. Metabolism.
63:120–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun R, Su Y, Zhao X, Qi J, Luo X, Yang Z,
Yao Y, Luo X and Xia Z: Human calcium/calmodulin-dependent serine
protein kinase regulates the expression of p21 via the E2A
transcription factor. Biochem J. 419:457–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodriguez-Mora OG, LaHair MM, McCubrey JA
and Franklin RA: Calcium/calmodulin-dependent kinase I and
calcium/calmodulin-dependent kinase kinase participate in the
control of cell cycle progression in MCF-7 human breast cancer
cells. Cancer Res. 65:5408–5416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soltow QA, Lira VA, Betters JL, Long JH,
Sellman JE, Zeanah EH and Criswell DS: Nitric oxide regulates
stretch-induced proliferation in C2C12
myoblasts. J Muscle Res Cell Motil. 31:215–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eckes B, Nischt R and Krieg T: Cell-matrix
interactions in dermal repair and scarring. Fibrogenesis Tissue
Repair. 3:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin J, Ren W, Duan J, Wu L, Chen S, Li T,
Yin Y and Wu G: Dietary arginine supplementation enhances
intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth
depression in mycotoxin-challenged pigs. Amino Acids. 46:883–892.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin J, Wu MM, Xiao H, Ren WK, Duan JL,
Yang G, Li TJ and Yin YL: Development of an antioxidant system
after early weaning in piglets. J Anim Sci. 92:612–619. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Qiu J, Yang C, Yang X, Chen X,
Jiang J and Luo X: Identification of a novel estrogen receptor
beta1 binding partner, inhibitor of differentiation-1, and role of
ERbeta1 in human breast cancer cells. Cancer Lett. 278:210–219.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi J, Su Y, Sun R, Zhang F and Luo X, Yang
Z and Luo X: CASK inhibits ECV304 cell growth and interacts with
Id1. Biochem Biophys Res Commun. 328:517–521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurita M, Okazaki M, Fujino T, Takushima A
and Harii K: Cyclic stretch induces upregulation of endothelin-1
with keratinocytes in vitro: Possible role in mechanical
stress-induced hyperpigmentation. Biochem Biophys Res Commun.
409:103–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuentes-Calvo I, Blázquez-Medela AM, Eleno
N, Santos E, López-Novoa JM and Martínez-Salgado C: H-Ras isoform
modulates extracellular matrix synthesis, proliferation and
migration in fibroblasts. Am J Physiol Cell Physiol. 302:C686–C697.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh P, Chen C, Pal-Ghosh S, Stepp MA,
Sheppard D and Van De Water L: Loss of integrin alpha9beta1 results
in defects in proliferation, causing poor re-epithelialization
during cutaneous wound healing. J Invest Dermatol. 129:217–228.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bush KA and Pins GD: Carbodiimide
conjugation of fibronectin on collagen basal lamina analogs
enhances cellular binding domains and epithelialization. Tissue Eng
Part A. 16:829–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Araki E, Momota Y, Togo T, Tanioka M,
Hozumi K, Nomizu M, Miyachi Y and Utani A: Clustering of syndecan-4
and integrin beta1 by laminin alpha 3 chain-derived peptide
promotes keratinocyte migration. Mol Biol Cell. 20:3012–3024. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukoyama Y, Utani A, Matsui S, Zhou S,
Miyachi Y and Matsuyoshi N: T-cadherin enhances cell-matrix
adhesiveness by regulating beta1 integrin trafficking in cutaneous
squamous carcinoma cells. Genes Cells. 12:787–796. 2007.PubMed/NCBI
|
|
28
|
Rodius S, Indra G, Thibault C, Pfister V
and Georges-Labouesse E: Loss of alpha6 integrins in keratinocytes
leads to an increase in TGFbeta and AP1 signaling and in expression
of differentiation genes. J Cell Physiol. 212:439–449. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsueh YP, Wang TF, Yang FC and Sheng M:
Nuclear translocation and transcription regulation by the
membrane-associated guanylate kinase CASK/LIN-2. Nature.
404:298–302. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawaguchi N, Majima T, Funakoshi T,
Shimode K, Harada K, Minami A and Nishimura S: Effect of cyclic
three-dimensional strain on cell proliferation and collagen
synthesis of fibroblast-seeded chitosan-hyaluronan hybrid polymer
fiber. J Orthop Sci. 15:569–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Teti A: Regulation of cellular functions
by extracellular matrix. J Am Soc Nephrol. 2(Suppl): S83–S87.
1992.PubMed/NCBI
|
|
32
|
Wang H, Yan X, Shen L, Li S, Wang S, Hou
XL, Shi C, Yang Y, Dai J and Tan Q: Acceleration of wound healing
in acute full-thickness skin wounds using a collagen-binding
peptide with an affinity for MSCs. Burn Trauma. 2:181–186. 2014.
View Article : Google Scholar
|