Introduction
The dopamine pathway in the nucleus accumbens and
ventral tegmental area serves a crucial role in the brain's reward
system, where it elicits a rewarding effect (1–3). Cocaine
is a central nervous system (CNS) stimulant and acts directly on
the dopamine transporter in the nucleus accumbens to inhibit
dopamine reuptake, thereby increasing the dopamine concentration in
the synaptic cleft, stimulating the reward system, and ultimately
leading to addiction (4).
Administration of cocaine in dopamine transporter gene knockout
mice does not increase dopamine in the nucleus accumbens, or induce
a rewarding effect (5). Therefore,
dopamine in the nucleus accumbens is of importance in the reward
mechanism of cocaine (6). The
activation of κ-opioid receptors in the CNS has been reported to
inhibit various aspects of cocaine dependence, including
cocaine-induced conditioned place preference (CPP) and
self-administration, by reducing dopamine release in the nucleus
accumbens (7,8). The amygdala is also important in the
reward effect of cocaine; destroying the amygdala has been
demonstrated to decrease cocaine induced CPP (9). The amygdala sends glutamatergic
projections to the nucleus accumbens, and activation of the
κ-opioid receptor inhibits glutamatergic transmission (10). The κ-opioid receptor is extensively
distributed throughout the ventral tegmental area, nucleus
accumbens and amygdala, all of which are markedly associated with
reward.
A previous study has confirmed that high-frequency
(100 Hz), but not low-frequency (2 Hz), electroacupuncture
suppresses cocaine-induced CPP in rats (11). The κ-opioid receptor agonist, U50488
is able to block this effect (12,13),
indicating that the κ-opioid receptor may be of importance in
cocaine addiction. Thus, the present study hypothesized that 100 Hz
electroacupuncture may suppress cocaine-induced CPP via the
κ-opioid receptor in the CNS.
The present study aimed to further elucidate cocaine
addiction, and a rat model of cocaine-induced CPP was employed to
investigate the effects of the non-specific opioid receptor
antagonist, naloxone, and the selective κ-opioid receptor
antagonist, nor-binaltorphimine (nor-BNI) on the inhibitory effect
of 100 Hz electroacupuncture in cocaine-induced CPP. The effects on
the mRNA expression levels of the κ-opioid receptor in various
regions of the brain were also assessed. Finally, the molecular
mechanisms underlying the inhibitory effects of 100 Hz
electroacupuncture on cocaine-induced CPP were investigated.
Materials and methods
Animals
A total of 36 male Sprague-Dawley rats (180–200 g)
were provided by the Institute of Zoology, Chinese Academy of
Sciences (Beijing, China) and were randomly assigned to 3 groups
(n=12 per group). They were housed at 22±1°C at a humidity of 50%
in a 12 h light/dark cycle. They were acclimatized to their
experimental surroundings for 5 days. The study was approved by the
Ethics Committee of Peking University, Health Science Center
(Beijing, China).
Reagents
Cocaine hydrochloride (Qinghai pharmaceutical
factory Co., Ltd., Xining, China), nor-binaltorphimine (nor-BNI;
Sigma-Aldrich, St. Louis, MO, USA), and the reverse
transcription-polymerase chain reaction (RT-PCR) kit (GK8030-20;
Sangon Biotech, Co., Ltd., Shanghai, China). The following reagents
were used for the reaction: 200 U/µl M-MLV reverse transcriptase
(20 µl), 5X RT reaction buffer (100 µl), 25 U/µl RNase inhibitor
(20 µl), 25 mM dNTP (20 µl), 60 µM oligo(dT)18 (20 µl),
250 µM random primer (20 µl), ddH2O (RNase and DNase
free; 1 ml) (all from Generay Biotech Co., Ltd., Shanghai, China).
β-actin primer, κ receptor primer and dynorphin primer were all
purchased from Sangon Biotech, Co., Ltd. Cocaine hydrochloride and
nor-BNI were prepared with physiological saline.
CPP
The CPP shuttle box (Med Associates, Inc., St.
Albans, VT, USA) was made of resin glass, separated into two
conditioned training boxes of equal size as follows: Box A and box
C (33×22×26 cm), and a middle box, box B (12×22×26 cm), separated
by two movable (up and down) partitions. If the partitions were
lowered, the animals would be restricted within the box. When the
partitions were elevated, the animals are able to run freely in the
shuttle box. One of the two conditioned training boxes consisted of
a white wall and a squared floor made of stainless steel. The
second consisted of a black wall and a striped floor made of
stainless steel. The middle box consisted of a gray wall and a gray
resin glass floor. A row of infrared light emission (infrared LED
monitoring system) and a receiver system were located on the
anterior and posterior walls in each box, 1.5 cm above the floor.
This system transmitted the locomotor activity of the animals to a
computer via a light-electric signal transducer. The crossing
frequencies and time spent in each box were recorded and the MED-PC
software (Med Associates Inc., St. Albans, VT, USA) was used for
the analysis.
Cocaine CPP model
Model preparation occurred in 3 stages, including
pretest, training and test, over a period of 10 days. Pretest
stage: The natural preference of rats was tested prior to training.
Partitions were removed, and the rats were placed in the middle box
with the partition in the up position, enabling them to run freely
in the shuttle box for 15 min. Their time spent in box A or C was
measured. The preference value was calculated by the following
formula: A or C/(A+C), and rats scoring between 0.4 and 0.6 were
included in the proceeding analyses. Training: CPP training
commenced 24 h after the pretest. The rats in the cocaine group
were intraperitoneally injected with 5 or 10 mg/kg cocaine or 0.5
ml physiological saline every other day. The rats administered
cocaine were placed in the non-preference box (white box), whilst
those administered physiological saline were placed in the
preference box (black box). The rats in the saline control group
were injected with physiological saline daily, and alternatively
placed in the non-preference (white) or preference (black) box
every other day. The CPP stage occurred daily over eight days, each
for 30 min (timing began immediately after the rats were placed in
the training box). Test: The CPP test was performed 24 h after the
final training. The rats were placed in the middle box and were
able to run freely in the shuttle box for 15 min. The time spent in
box A or C was recorded. The preference value was calculated
according to the aforementioned equation. The CPP model was
successfully established if the preference value in the cocaine
group was significantly increased compared with that of the control
group.
Electroacupuncture
Rats were placed in a specially made plastic
cylinder with their tails and hind limbs outside the cylinder.
Stainless steel needles were inserted at the bilateral hind limbs
at positions identical to the human Zusanli (ST36; Lateral tibial
anterior 5 mm;) and Sanyinjiao (SP6; Posterior tibial 1 mm, 3 mm
above the medial malleolus), and the other end was connected to an
Acupoint Nerve Stimulator (model HANS LH800, Beihang University,
Beijing, China). Electroacupuncture was conducted for 30 min at 100
Hz square wave, and a 0.1 msec wave width. Stimulus intensity was
increased by 1 mA every 10 min up to 3 mA. A simple restraint group
in which rats were placed in the cylinder with no acupuncture was
used as a control.
Administration of nor-BNI in the
lateral ventricle and nucleus
Rats were anesthetized with 50 mg/kg sodium
pentobarbital and then fixed with ear bars onto the stereotaxic
apparatus in the abdominal position. In accordance with Paxinos and
Watson Stereotaxic Coordinates (lateral ventricle: P:-1.0 mm, L:1.6
mm, H:4.0 mm, nucleus accumbens: P:1.2 mm, L:±0.8 mm, H:5.5 mm,
ventral tegmental area: P:-5.2 mm, L:±0.8 mm, H:6.8 mm, amygdala
central nuclei: P:-2.3 mm, L:±4.8 mm, H:7.0 mm, basolatera amygdala
nuclei: P:-2.3 mm, L:±3.7 mm, H:7.0 mm), the lateral ventricle or
nucleus was selected, and a 24GA plastic sleeve was inserted.
Administration of nor-BNI was performed with an inner trocar (1 mm
longer than the plastic sleeve) through the plastic sleeve.
RT-qPCR
Rats were decapitated and the nucleus accumbens and
amygdala were rapidly dissected apart and placed in liquid nitrogen
and cryopreserved at −80°C. Total RNA was extracted with TRIzol
according to the manufacturer's instructions (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). A Heraeus Biofuge Stratos
high-speed refrigerated centrifuge at 10,000xg and 4°C (Thermo
Fisher Scientific, Inc.) and ultraviolet spectrophotometer (Du 530,
Beckman Coulter, Inc., Brea, CA, USA) were used. mRNA was reverse
transcribed into cDNA. The following reagents were added to the
reaction: RNA template, 1 µl random primer (10 µM), 1 µl
oligo(dT)18 (2.4 µM) and ddH2O up to 13 µl.
The reaction was heated at 65°C for 10 mins and quickly placed on
ice for 5 mins. Then the following components were added: 5 µl 5X
RT reaction buffer, 1 µl 25 mM dNTP, 1 µl 25 U/µl RNase inhibitor,
1 µl 200 U/µ IM-MLV RTase and ddH2O up to 25 µl. The
reaction was incubated at 37°C for 1 h and then at 85°C for 5 mins
to terminate the reaction. Once the reaction was complete it was
stored at −20°C. Next, amplification of the target gene cDNA was
performed with the PCR machine (Progene, Techne, NJ, USA), and PCR
was performed. In brief, a denaturation step was carried out at
94°C for 3 mins, then the reaction was kept at 94°C for 1 min, 60°C
for 1 min and 72°C for 1 min. The cycle was repeated 20 times and
the reaction was then kept at 72°C for an extension time of 5 min.
The experiment was repeated 3 times. Product analysis was conducted
as follows: PCR products (5 µl) were mixed with X6 gel loading
buffer (1 µl); the samples were loaded into the wells of a 1%
agarose gel for electrophoresis using a PowerPAC Basic Power Supply
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 80 V for 60 min.
The samples were stained with 0.5 mg/ml ethidium bromide (Beijing
Solarbio Technology Co., Ltd., Beijing, China) for 15 min, observed
using a Gel Doc 2000 imaging system (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA), and analyzed semi-quantitatively. Results are
expressed as the intensity ratio to GAPDH products.
Statistical analysis
All data are expressed as the mean ± standard error
and were analyzed by one-way analysis of variance followed by the
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference. Data were analyzed by
GraphPad software, version 5.0 (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
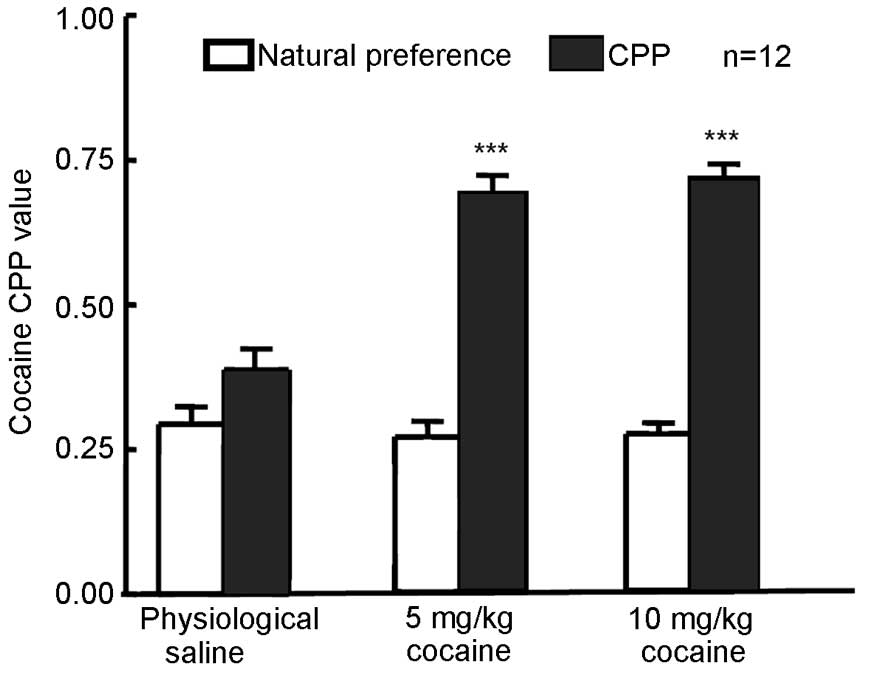
Cocaine at 5 or 10 mg/kg induces the
expression of CPP in rats
The natural preference of rats was measured in each
group prior to training and the results revealed no natural
preference following cocaine treatment compared with physiological
saline treatment (Fig. 1). Rats were
administered 5 or 10 mg/kg cocaine or physiological saline followed
by CPP training. The CPP value was measured 24 h after the final
training (Fig. 1). CPP values in
rats that received 5 or 10 mg/kg cocaine were significantly
increased (P=0.0008 for 5 mg/kg and P=0006 for 10 mg/kg) compared
with their respective natural preference values, indicating that
CPP was established in the 5 and 10 mg/kg cocaine treatment
groups.
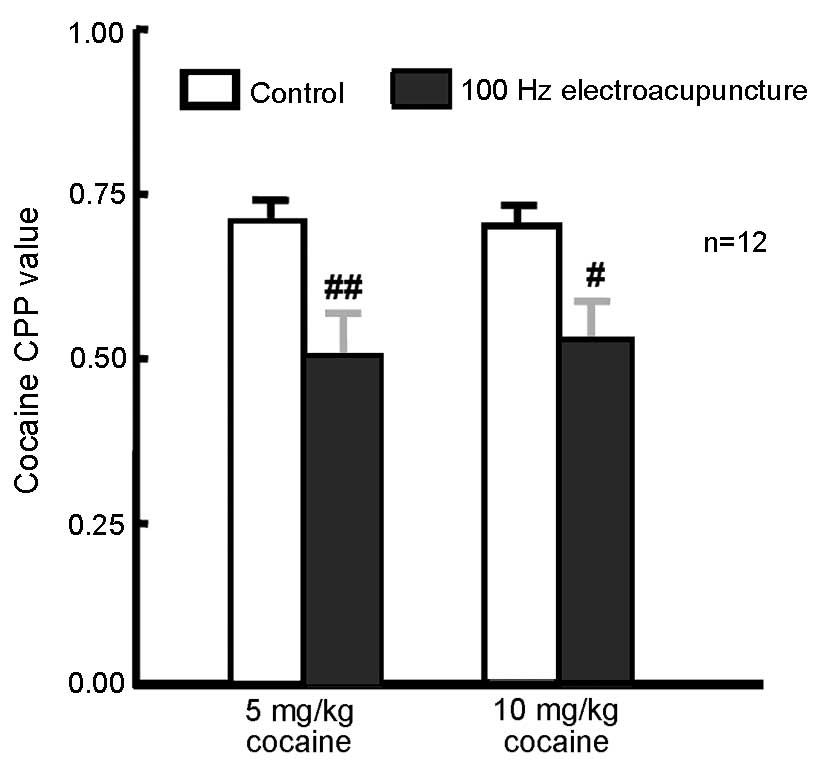
Administration of 100 Hz
electroacupuncture inhibited 5 and 10 mg/kg cocaine-induced
CPP
A total of 48 rats were randomly assigned to 2
groups of CPP training (5 and 10 mg/kg cocaine; n=24 in each
group). CPP training was conducted and CPP values were measured 24
h after the final training. Rats (n=12 from each group) received
100 Hz electroacupuncture 24 h prior to CPP measurement. The
remaining rats from each group (n=12) served as controls. CPP
values were significantly decreased in the 5 mg/kg (P=0.006) and 10
mg/kg (P=0.022) cocaine-treated rats exposed to 100 Hz
electroacupuncture compared with the respective controls (Fig. 2). These present results indicate that
100 Hz electroacupuncture inhibits 5 and 10 mg/kg cocaine-induced
CPP.
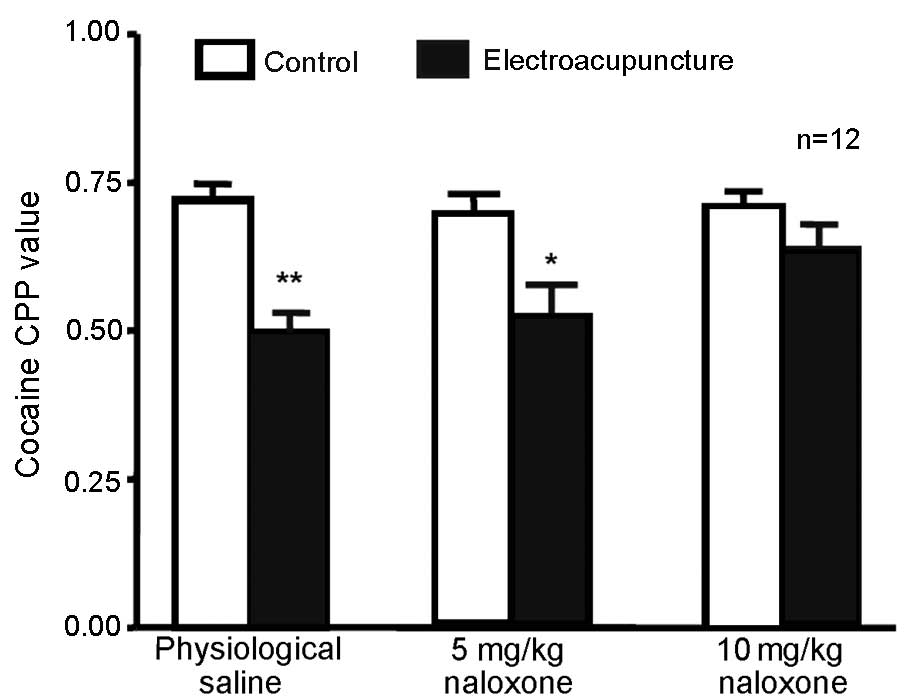
High-dose (10 mg/kg) naloxone blocks
100 Hz electroacupuncture-mediated inhibition of cocaine-induced
CPP
A total of 72 rats were randomly divided into 3
groups (n=24 in each) receiving 5 mg/kg cocaine and CPP training.
The rats were intraperitoneally injected with 5 or 10 mg/kg
naloxone or physiological saline. Next, 12 rats in each group
underwent 100 Hz single electroacupuncture 20 min after injection
of physiological saline or naloxone; CPP values were measured 24 h
after the final training. Compared with the physiological saline +
100 Hz electroacupuncture group, no significant difference in CPP
value was observed in the 5 mg/kg naloxone + 100 Hz
electroacupuncture group (Fig. 3).
This suggests that at this dose, naloxone did not inhibit the 100
Hz electroacupuncture-induced CPP. However, CPP values were
significantly higher in the 10 mg/kg naloxone + 100 Hz
electroacupuncture group compared with the saline +
electroacupuncture group (P=0.043), indicating that a higher dose
(10 mg/kg) of naloxone was able to reverse the inhibition of 100 Hz
electroacupuncture on cocaine-induced CPP.
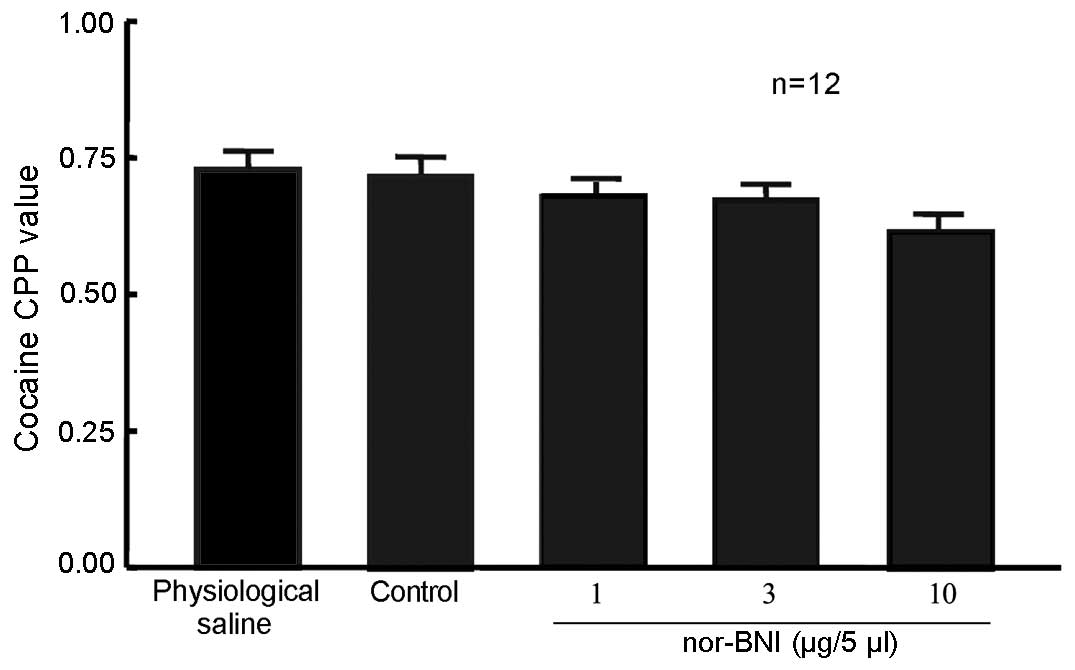
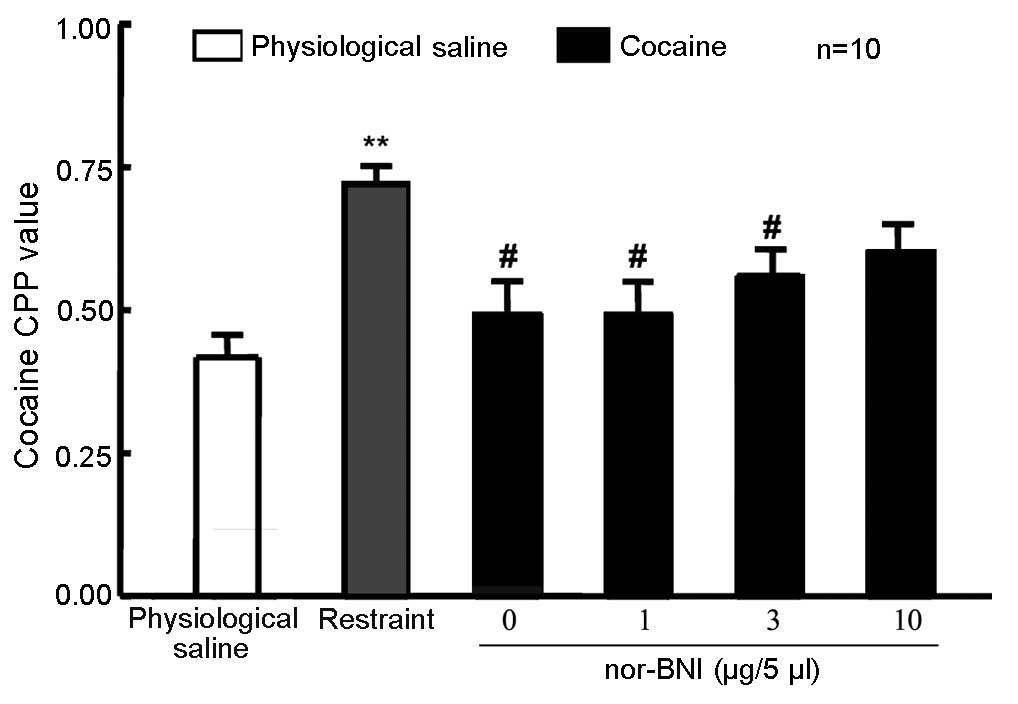
nor-BNI did not affect 10 mg/kg
cocaine-induced CPP
A total of 60 rats were randomly divided into 5
groups (n=12 in each group). In one group, rats that were left
intact served as the control. Rats in the remaining 4 groups
received lateral ventricle cannulation. After 5 days, the rats
received 10 mg/kg cocaine and CPP training, which occurred
concurrent to that of the unoperated group. Rats were then given 1,
3 or 10 µg/5 µl nor-BNI or physiological saline via the lateral
ventricle. Control rats were not administered any treatment 24 h
prior to the CPP measurement. No significant difference in the CPP
value was detected in rats exposed to the three doses of nor-BNI
compared with physiological saline and control groups (Fig. 4), suggesting that the selected dose
of nor-BNI in the present study produced no effect on 10 mg/kg
cocaine-induced CPP.
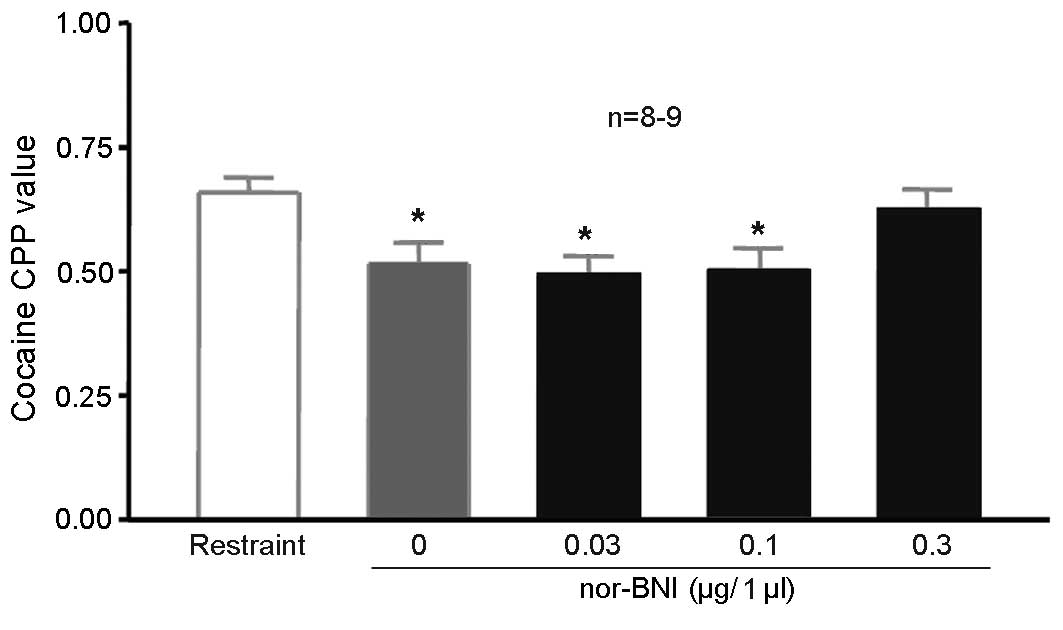
Injection of nor-BNI in the lateral
ventricle affects the inhibition of 100 Hz electroacupuncture on
cocaine CPP
A total of 60 rats were randomly divided into 6
groups (n=10 in each group). Two groups were unoperated, one
without cocaine CPP (saline-only), the other fixed in the
stereotaxic apparatus with 10 mg/kg cocaine CPP training (simple
restraint group), without electroacupuncture stimulation. The rats
in the remaining four groups received lateral ventricle
cannulation. After 5 days, the rats received 10 mg/kg cocaine CPP
training alongside the second group. The rats in the 4 groups were
given 1, 3 or 10 µg/5 µl nor-BNI or physiological saline via the
lateral ventricle. CPP values were significantly higher in the
simple restraint group compared with the physiological saline
training group, indicating the induction of cocaine CPP (P=0.043;
Fig. 5). CPP values were
significantly lower in the 0 µg/5 µl nor-BNI group (with 100 Hz
electroacupuncture) compared with those in the simple restraint
group (P=0.003; Fig. 5), indicating
that 100 Hz electroacupuncture was able to suppress cocaine-induced
CPP, as indicated by the preceding experiments. The CPP values for
rats treated with 10 µg/5 µl nor-BNI did not significantly differ
compared with the simple restraint group (Fig. 5), suggesting that 10 mg/kg reversed
the inhibitory effects of 100 Hz electroacupuncture on
cocaine-induced CPP.
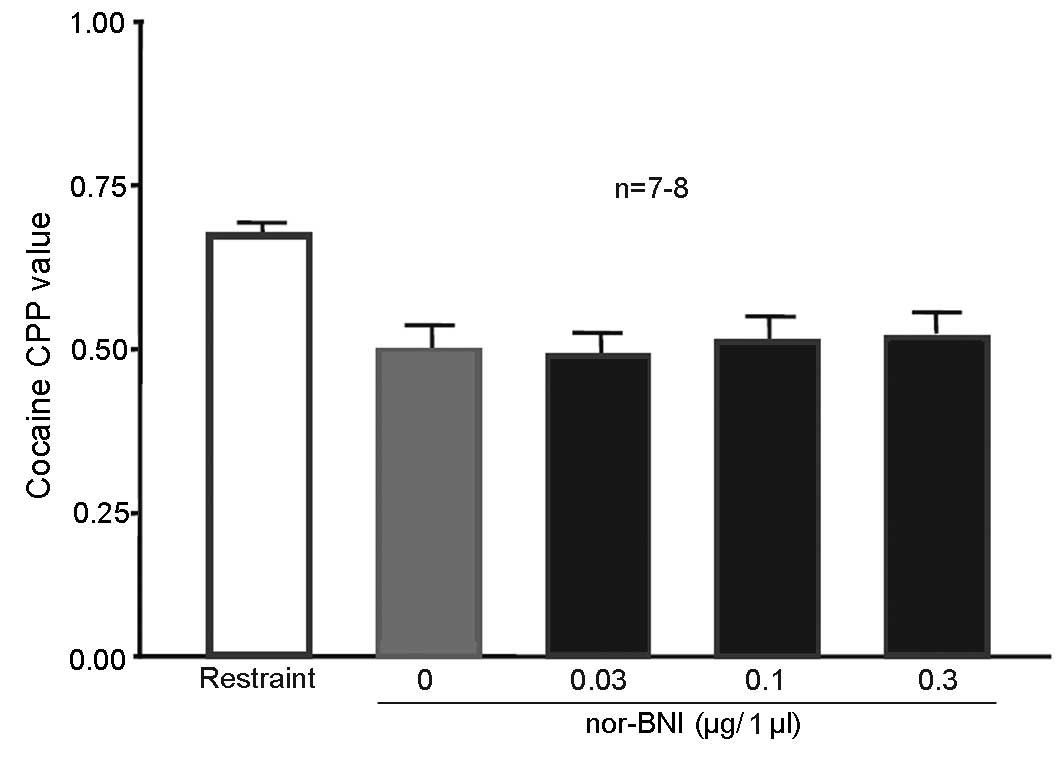
Injection of 0.3 µg/1 µl nor-BNI in
the nucleus accumbens blocked 100 Hz electroacupuncture-mediated
inhibition of cocaine-induced CPP
A total of 50 rats were randomly assigned to 5
groups (n=10 in each group). One of the groups served as the simple
restraint group, and remained intact. The nucleus accumbens of rats
in the remaining 4 groups received cannulation prior to training.
After 5 days, all rats received 10 mg/kg cocaine CPP training. The
restraint group was subjected to simple restraint 24 h prior to CPP
measurement. Rats in the remaining 4 groups were given 0.03, 0.1 or
0.3 µg/1 µl nor-BNI or physiological saline to the bilateral
nucleus accumbens 15 min prior to the administration of 100 Hz
electroacupuncture. Rats were decapitated following CPP value
measurement. The entire brain was then obtained and sliced into
sections for observation. Rats with their cannula at the designated
place (P:1.2 mm, L:±0.8 mm, H:5.5 mm) (n=9 for the 0 and 0.1 µg/l
nor-BNI group; n=8 for the 0.03 and the 0.3 µg/l nor-BNI group)
were selected for statistical analysis. CPP values were
significantly lower in rats injected with physiological saline (0
µg/1 µl nor-BNI group) in the nucleus accumbens compared with the
simple restraint group [P=0.045 (NS), 0.037 (0.03 µg/1 µl nor-BNI)
and 0.043 (0.1 µg/1 µl nor-BNI); Fig.
6], indicating that 100 Hz electroacupuncture inhibited
cocaine-induced CPP. CPP values of rats injected with 0.3 µg/1 µl
in the nucleus accumbens did not significantly differ compared with
the simple restraint group, suggesting that the addition of nor-BNI
at this dose in the nucleus accumbens reversed the inhibitory
effects of 100 Hz electroacupuncture on cocaine-induced CPP.
Injection of nor-BNI in the ventral
tegmental area of the midbrain did not affect 100 Hz
electroacupuncture-mediated inhibition of cocaine-induced CPP
A total of 50 rats were randomly assigned to 5
groups (n=10 in each group). The first group was the simple
restraint group and was used as a control. The ventral tegmental
area of rats in the remaining 4 groups received cannulation prior
to training. After 5 days, all rats received 10 mg/kg cocaine CPP
training. Rats in the restraint group were subjected to simple
restraint 24 h prior to CPP measurement, whilst rats in the
remaining four groups were administered 0.03, 0.1 or 0.3 µg/1 µl
nor-BNI or physiological saline in the ventral tegmental area 15
min prior to 100 Hz electroacupuncture. Rats were decapitated after
measurement, and the entire brain was dissected and sliced into
sections for observation. Rats with their cannula at the designated
place (n=7 for the 0 and 0.03 µg/l nor-BNI group; n=8 for the 0.1
and 0.3 µg/l nor-BNI group) were selected for statistical analysis.
CPP values were significantly lower in rats administered
physiological saline (0 µg/1 µl group) in the ventral tegmental
area compared with the simple restraint group (P=0.024 (NS), 0.023
(0.03 µg/1 µl nor-BNI),0.034 (0.1 µg/1 µl nor-BNI) and 0.039 (0.3
µg/1 µl nor-BNI); Fig. 7),
indicating that 100 Hz electroacupuncture inhibited cocaine-induced
CPP. CPP values were significantly lower in rats injected with
different doses of nor-BNI in the ventral tegmental area than those
in the simple restraint group (P<0.05), indicating that the
doses administered did not affect the inhibitory effects of 100 Hz
electroacupuncture on cocaine CPP expression.
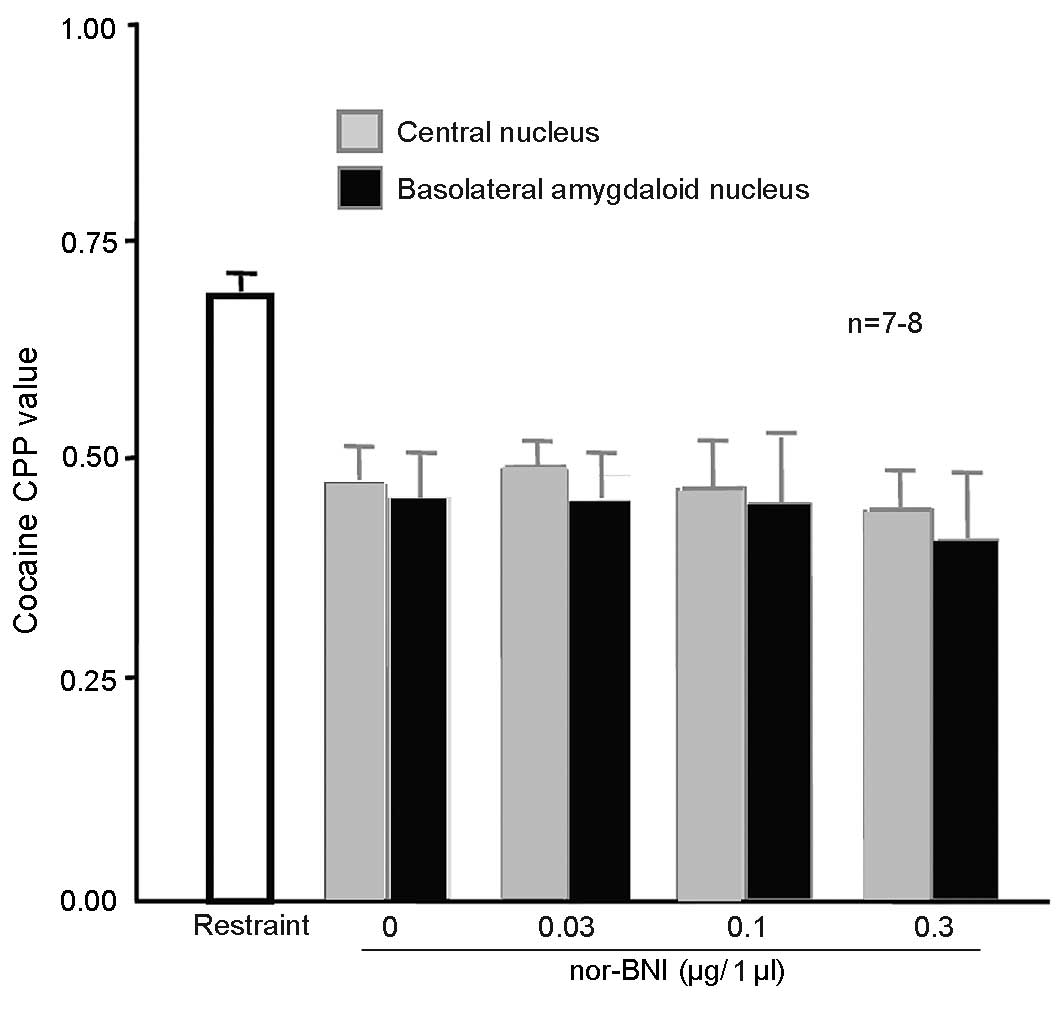
Injection of nor-BNI in the amygdala
did not affect 100 Hz electroacupuncture-mediated inhibition of
cocaine-induced CPP
A total of 108 rats were randomly assigned into 9
groups (n=12 in each group). The first group was the simple
restraint group. In the other groups, the amygdala central nuclei
received cannulation prior to training. The basolateral amygdaloid
nuclei in the remaining 4 groups received cannulation prior to
training. After 5 days, the 8 groups received 10 mg/kg cocaine CPP
training, which was administered simultaneous to that of rats in
the first group. The first group was subjected to simple restraint
24 h prior to CPP measurement. The remaining rats were administered
0.1, 0.3 or 1 µg/1 µl nor-BNI or physiological saline in the
bilateral amygdala central nuclei or bilateral basolateral
amygdaloid nuclei 15 min prior to the administration of 100 Hz
electroacupuncture. After measurement, the rats were decapitated
and their brains were dissected and sliced into sections for
observation. Rats with their cannula at the designated place (n=8
for the 0, 0.1 and 0.3 µg/l nor-BNI and n=7 for the 0.03 µg/l
nor-BNI group) were selected for statistical analysis. CPP values
were significantly lower in the two regions of the brains of the
rats injected with physiological saline in the amygdala, compared
with the simple restraint group [P=0.037 (NS in amygdala central
nuclei); Fig. 8], indicating that
100 Hz electroacupuncture inhibited cocaine-induced CPP. CPP values
were significantly lower in the brains of the rats injected with
different doses of nor-BNI in the two regions of the amygdala,
compared with the simple restraint group [0.043 (0.03 µg/1 µl
nor-BNI in amygdala central nuclei), 0.032 (0.1 µg/1 µl nor-BNI in
amygdala central nuclei) and 0.027 (0.3 µg/1 µl nor-BNI in amygdala
central nuclei); P=0.031 (NS in basolatera amygdala nuclei), 0.029
(0.03 µg/1 µl nor-BNI in basolatera amygdala nuclei), 0.024 (0.1
µg/1 µl nor-BNI in basolatera amygdala nuclei) and 0.018 (0.3 µg/1
µl nor-BNI in basolatera amygdala nuclei), indicating that the
doses did not alter the inhibitory effects of 100 Hz
electroacupuncture on cocaine-induced CPP.
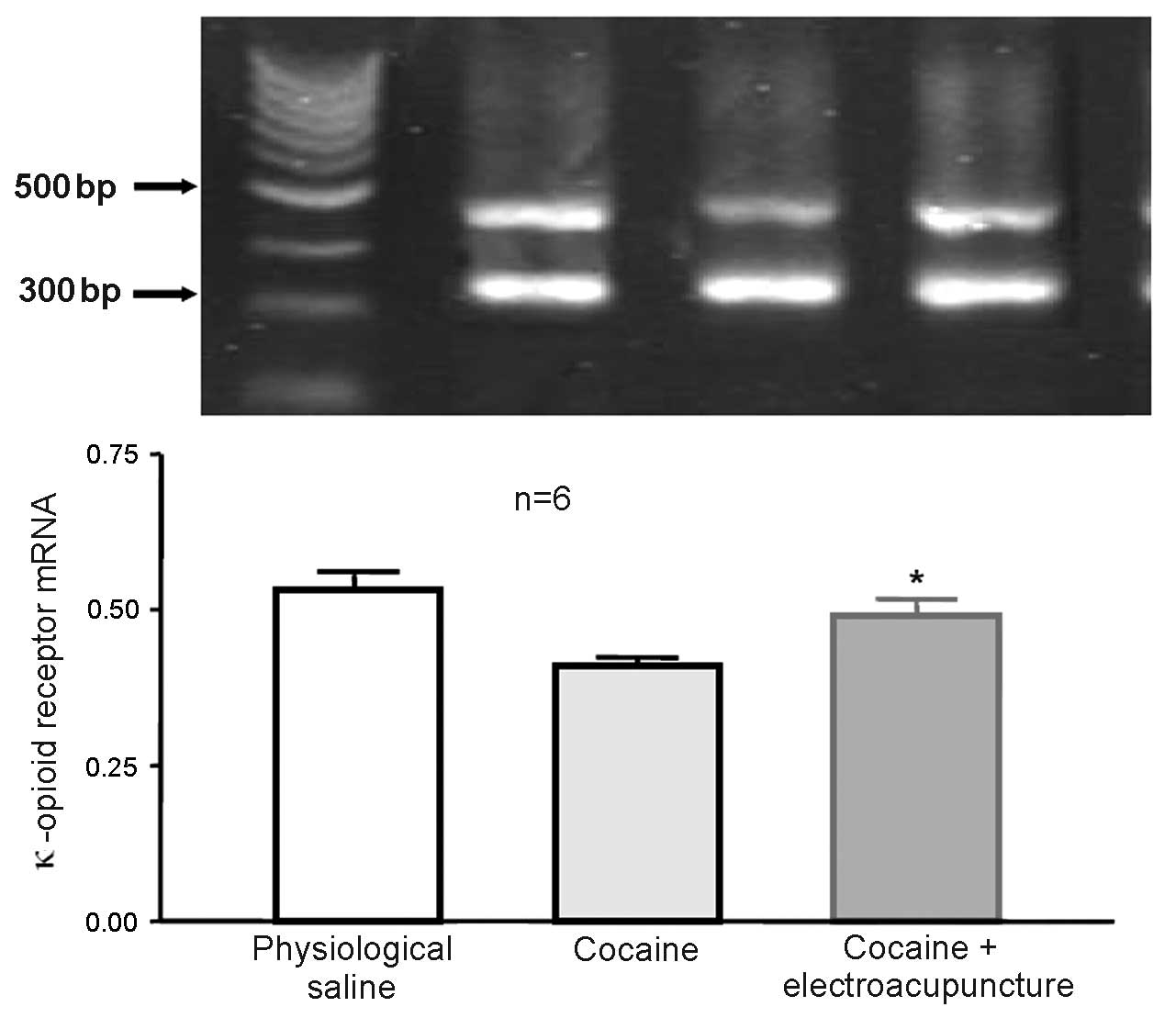
Administration of 100 Hz
electroacupuncture in cocaine-induced CPP rats led to increased
mRNA expression levels of κ-opioid receptor in the nucleus
accumbens
A total of 24 rats were randomly assigned to 3
groups (n=8 in each group). Some mice died and were excluded from
the study. The control group was administered physiological saline
during CPP training, whilst the remaining 2 groups (cocaine-induced
CPP and 100 Hz electroacupuncture + cocaine-induced CPP) received
10 mg/kg cocaine during CPP training. Subsequent to final training,
the electroacupuncture treatment group received 100 Hz
electroacupuncture stimulation. After 2 days, rats from each group
were then decapitated and the nucleus accumbens was collected for
RT-qPCR in order to measure the expression of κ-opioid receptor
mRNA expression levels. mRNA expression levels of the κ-opioid
receptor were significantly increased in the nucleus accumbens of
the cocaine-induced CPP rats previously exposed to 100 Hz
electroacupuncture compared with cocaine-induced CPP rats (P=0.032;
Fig. 9).
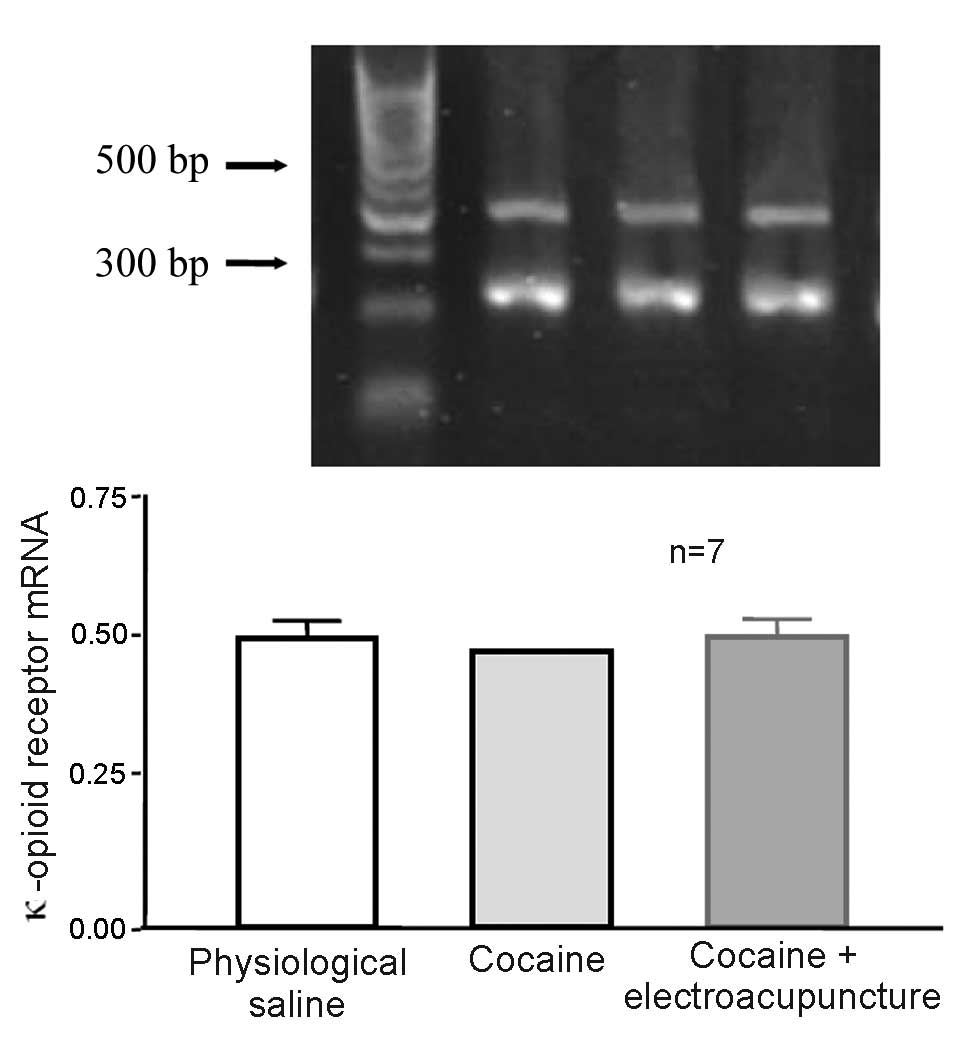
Effect of 100 Hz electroacupuncture in
cocaine-induced CPP rats did not affect mRNA expression levels of
κ-opioid receptor in the amygdala
A total of 24 rats were randomly assigned to 3
groups (n=8 per group). Some mice died and were excluded from the
study. The control group was administered physiological saline,
whilst the remaining 2 groups (cocaine-induced CPP and 100 Hz
electroacupuncture on cocaine-induced CPP) received 10 mg/kg
cocaine CPP training. After final training, the electroacupuncture
treatment group received 100 Hz electroacupuncture stimulation
whilst the remaining rats were left intact. Two days later, rats
from each group were decapitated and the amygdala was collected for
RT-qPCR to measure the mRNA expression levels of κ-opioid receptor.
mRNA expression levels of the κ-opioid receptor were not
significantly different in the amygdala of cocaine-induced CPP rats
exposed to 100 Hz electroacupuncture and cocaine-induced CPP rats
(P>0.05; Fig. 10).
Discussion
The results of the present study indicated that 100
Hz single electroacupuncture inhibited 5 and 10 mg/kg
cocaine-induced CPP. The present study also revealed that the
high-dose (10 mg/kg) naloxone significantly blocked 100 Hz
electroacupuncture-mediated inhibition of cocaine-induced CPP. The
several types of opioid receptor each display different affinities
for naloxone. A low dose (1–5 mg/kg) predominately antagonizes µ
and δ receptors, whilst a high dose (10–20 mg/kg) blocks the
κ-opioid receptor (14). The present
study hypothesized that 100 Hz electroacupuncture exerted its
inhibitory effects through the κ-opioid receptor. The present study
revealed that 10 µg/5 µl nor-BNI blocked 100 Hz
electroacupuncture-mediated inhibition of cocaine-induced CPP, thus
confirming the hypothesis.
The κ-opioid receptor is extensively distributed
throughout the brain, including the nucleus accumbens, ventral
tegmental area and amygdala, which are brain regions strongly
associated with reward. Previous studies in numerous animal models
have confirmed that the activation of the κ-opioid receptor
antagonizes the drug-seeking behavior mediated by cocaine (15–16).
κ-Opioid receptor activation in the nucleus accumbens suppresses
dopamine release by presynaptic inhibition, thus reducing the
dopamine concentration in the synaptic cleft of the nucleus
accumbens and resulting in aversion to cocaine (10,17).
Results from the present study demonstrated that the injection of
0.3 µg/1 µl nor-BNI in the nucleus accumbens blocked 100 Hz
electroacupuncture-mediated inhibition of cocaine-induced CPP.
However, the injection of nor-BNI in the ventral tegmental area and
amygdala was not observed to produce a significant effect. The
results indicate that the κ-opioid receptor in the nucleus
accumbens serves an important role in 100 Hz
electroacupuncture-mediated inhibition of cocaine-induced CPP.
Previous studies have demonstrated that 100 Hz
electroacupuncture-mediated inhibition of cocaine-induced CPP lasts
for a duration of 24 h, and have postulated that electroacupuncture
may regulate κ-opioid receptor synthesis at the gene level
(9,18). The results of the present study
revealed that 100 Hz electroacupuncture significantly increased
mRNA expression levels of the κ-opioid receptor in the nucleus
accumbens but not in the amygdala of rats with cocaine-induced CPP.
The present results demonstrated that 100 Hz electroacupuncture was
able to restore the function of the κ-opioid receptor and inhibit
cocaine-induced CPP, possibly by regulating the mRNA expression
levels of the κ-opioid receptor in the nucleus accumbens.
In conclusion, the results of the present study
demonstrated that 100 Hz electroacupuncture inhibits
cocaine-induced CPP via the κ-opioid receptor in the nucleus
accumbens of rats.
References
|
1
|
Nestler EJ: Molecular basis of long-term
plasticity underlying addiction. Nat Rev Neurosci. 2:119–128. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Girault JA and Greengard P: The
neurobiology of dopamine signaling. Arch Neurol. 61:641–644. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montague PR and Berns GS: Neural economics
and the biological substrates of valuation. Neuron. 36:265–284.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramamoorthy S, Samuvel DJ, Balasubramaniam
A, See RE and Jayanthi LD: Altered dopamine transporter function
and phosphorylation following chronic cocaine self-administration
and extinction in rats. Biochem Biophys Res Commun. 391:1517–1521.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giros B, Jaber M, Jones SR, Wightman RM
and Caron MG: Hyperlocomotion and indifference to cocaine and
amphetamine in mice lacking the dopamine transporter. Nature.
379:606–612. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen R, Tilley MR, Wei H, Zhou F, Zhou FM,
Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, et al: Abolished
cocaine reward in mice with a cocaine- insensitive dopamine
transporter. Proc Natl Acad Sci USA. 103:9333–9338. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kallupi M, Wee S, Edwards S, Whitfield TW
Jr, Oleata CS, Luu G, Schmeichel BE, Koob GF and Roberto M: Kappa
opioid receptor-mediated dysregulation of gamma-aminobutyric
acidergic transmission in the central amygdala in cocaine
addiction. Biol Psychiatry. 74:520–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spanagel R, Herz A and Shippenberg TS:
Opposing tonically active endogenous opioid systems modulate the
mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA.
89:2046–2050. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown EE and Fibiger HC: Differential
effects of excitotoxic lesions of the amygdala on cocaine-induced
conditioned locomotion and conditioned place preference.
Psychopharmacology (Berl). 11:123–130. 1993. View Article : Google Scholar
|
|
10
|
Thompson AC, Zapata A, Justice JM Jr,
Vaughan RA, Sharpe1 LG and Shippenberg TS: κ-opioid receptor
activation by U69593 modifies dopamine uptake in the nucleus
accumbens of the rat and opposes the effects of repeated cocaine
exposure on dopamine uptake. J Neurosci. 20:9333–9340.
2000.PubMed/NCBI
|
|
11
|
Ren YH, Wang B, Luo F, Cui CL, Zheng JW
and Han JS: Peripheral electric stimulation attenuates the
expression of cocaine-induced place preference in rats. Brain Res.
95:129–135. 2002. View Article : Google Scholar
|
|
12
|
Schindler AG, Li S and Chavkin C:
Behavioral stress may increase the rewarding valence of
cocaine-associated cues through a dynorphin/kappa opioid receptor
mediated mechanism without affecting associative learning or memory
retrieval mechanisms. Neuropsychopharmacology. 35:1932–1942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morani AS, Kivell B, Prisinzano TE and
Schenk S: Effect of kappa-opioid receptor agonists U69593, U50488H,
spiradoline and salvinorin A on cocaine-induced drug-seeking in
rats. Pharmacol Biochem Behav. 94:244–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tiseo PJ and Yaksh TL: Dose-dependent
antagonism of spinal opioid receptor agonists by naloxone and
naltrindole: Additional evidence for delta-opioid receptor subtypes
in the rat. Eur J Pharmacol. 236:89–96. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shippenberg TS, Chefer VI, Zapata A and
Heidbreder CA: Modulation of the behavioral and neurochemical
effects of psychostimulants by kappa-opioid receptor systems. Ann
NY Acad Sci. 93:50–73. 2001.
|
|
16
|
Wee S and Koob GF: The role of the
dynorphin-kappa opioid system in the reinforcing effects of drugs
of abuse. Psychopharmacology (Berl). 210:121–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mu P, Neumann PA, Panksepp J, Schlüter OM
and Dong Y: Exposure to cocaine alters dynorphin-mediated
regulation of excitatory synaptic transmission in nucleus accumbens
neurons. Biol Psychiatry. 69:228–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo HF, Tian J, Wang X, Fang Y, Hou Y and
Han J: Brain substrates activated by electroacupuncture (EA) of
different frequencies (II): Role of Fos/Jun proteins in EA-induced
transcription of preproenkephalin and preprodynorphin genes. Brain
Res Mol Brain Res. 43:167–173. 1996. View Article : Google Scholar : PubMed/NCBI
|