Introduction
Interventional therapy in acute myocardial
infarction has been widely applied in the clinic. Objective and
accurate assessment of the scope and extent of myocardial
infarction, cardiac function and ventricular remodeling prior to
and following surgery have become the focus of recent studies
(1,2). Conventional echocardiography and
speckle tracking techniques have become the preferred examination
method in clinical treatment as they are non-invasive,
reproducible, and high in sensitivity and accuracy (3). However, the measurements of this method
are not stable (4).
Cardiac magnetic resonance (CMR) imaging has high
spatial resolution, and has a high recognition rate of myocardial
infarction, degree of permeability, edema, bleeding, inflammation
and other syndromes (5). In
particular, delayed enhanced imaging (DE) was prepared to assess
the scope and extent of myocardial infarction and impaired cardiac
function, and post-processing software such as computer-aided
volume methods and visual score method (VSM) were employed as
optimal methods for quantitative detection of infracted myocardium
(6,7).
The present study used CMR to assess myocardial
infarction prior to and after AMI conducting percutaneous coronary
intervention (PCI). The correlation between cardiac prognosis and
echocardiography was compared and analyzed.
Materials and methods
Subjects
Fifty-six cases of patients with AMI (incidence time
of >24 h) were continuously selected at the FirstAffiliated
Hospital of Zhengzhou University (Henan, China) from June, 2014 to
June, 2015. There were indications of elective PCI, but no
contraindications. Exclusion criteria for the study were:
uncontrolled high blood pressure and diabetes, recent major
operations and gastrointestinal bleeding history, malignant tumor,
cerebral vascular diseases, coagulation dysfunction, severe
insufficiency of liver and kidneys, contrast agent allergy,
radiography failure, and high interventional risk of radiography
assessment. Other exclusion factors were: magnetic resonance
examination was not completed; patients exhibited cardiac shock,
malignant arrhythmia, acute left heart failure and severe disease;
less than one-year expected survival time; poor compliance;
incomplete follow-up data and loss of follow-up.
The study obtained the approval of the Ethics
Committee of the FirstAffiliated Hospital of Zhengzhou University
and informed consent of the patients and their relatives. Detection
with echocardiography and CMR was carried out in the incidence of
7–10 days to evaluate the myocardial infarction quality, VSM, wall
motion abnormality (WMA) score, left ventricular end-diastolic
diameter (LVEDD), left ventricular end-systolic diameter (LVESD),
and left ventricular ejection fraction (LVEF). For the incidence of
10–14 days, PCI was employed, and detection with echocardiography
and CMR were carried out again after 6 months, after which the
incidence of major adverse cardiac events (MACE) was compared. The
treatment of PCI was completed by the same surgical and nursing
team, in line with standard medical procedures. There were 30 men
and 26 women, aged 48–72 years, with an average age of 62.5±13.6
years. The time of incidence was from 26 to 48 h with an average of
32.4±5.7 h. Of the 56 cases, there were 21 cases of ST-segment
elevation myocardial infarction (STEMI) and 35 cases of
non-ST-elevation myocardial infarction (NSTEMI). In addition, there
were 20 cases of anterior descending artery disease, 10 cases of
circumflex artery, 20 cases of right coronary artery, and 6 cases
of ≥2 lesions. Each patient was implanted with 1–3 stents, with an
average of 1.5±0.6 mm. The length of each stent was 10–25 mm, with
and average of 15.7±4.6 mm.
Detection method of magnetic
resonance
A 3.0T superconductive magnetic resonance imager
(Avanto; Siemens, Erlangen, Germany) was utilized, with a maximum
gradient field of 45 mT/m, maximum gradient slew rate of 200
mT/m·msec. In addition, an 8 channel body surface coil and 6
channel spinal coil, wireless echocardiography vector template,
with high-pressure syringe (Ulrich Medical, Berlin, Germany) were
used. The gadopentetate dimeglumine of Schering AG (Berlin,
Germany) was employed as the contrast agent. For conventional
scanning, fast spin echo sequence was utilized to observe the
morphology of the heart and large blood vessel, cardiovascular film
was utilized for retrospective echocardiography gated to enter
procession gradient echo sequence. Left ventricular two-chamber
heart long axis, four-chamber heart long axis, left ventricular
inflow and outflow tract, left ventricular outflow tract section,
six-layer fractional movie, and cardiac function analysis were
carried out using scanning and cardiovascular film, respectively.
Argus 4D software (Siemens Healthcare, Eresing, Germany) was
utilized to analyze the data. Multimodality workplace (MMWP)
workstation was to measure LVEDD, LVESD, and LVED. The contrast
agent enhanced the first-pass myocardial perfusion with a 4–5
ml/sec 0.1 mmol/kg flow rate, which was initiated at the same time
as the scanning. TSENSE EPIGER sequence was used to conduct T1WI
scanning (8), which constituted the
contrast agent phase-sensitive with inversion recovery, including 6
layers of left ventricular short axis view, 1 layer of left
ventricular two-chamber view, and four-chamber view. WMA involves
semi-quantitative scoring in the sequence of grade 0–4 (0, normal;
1, reduced movement; 2, non-movement; 3, contradictory movement;
and 4, the formation of ventricular aneurysm). Three standard short
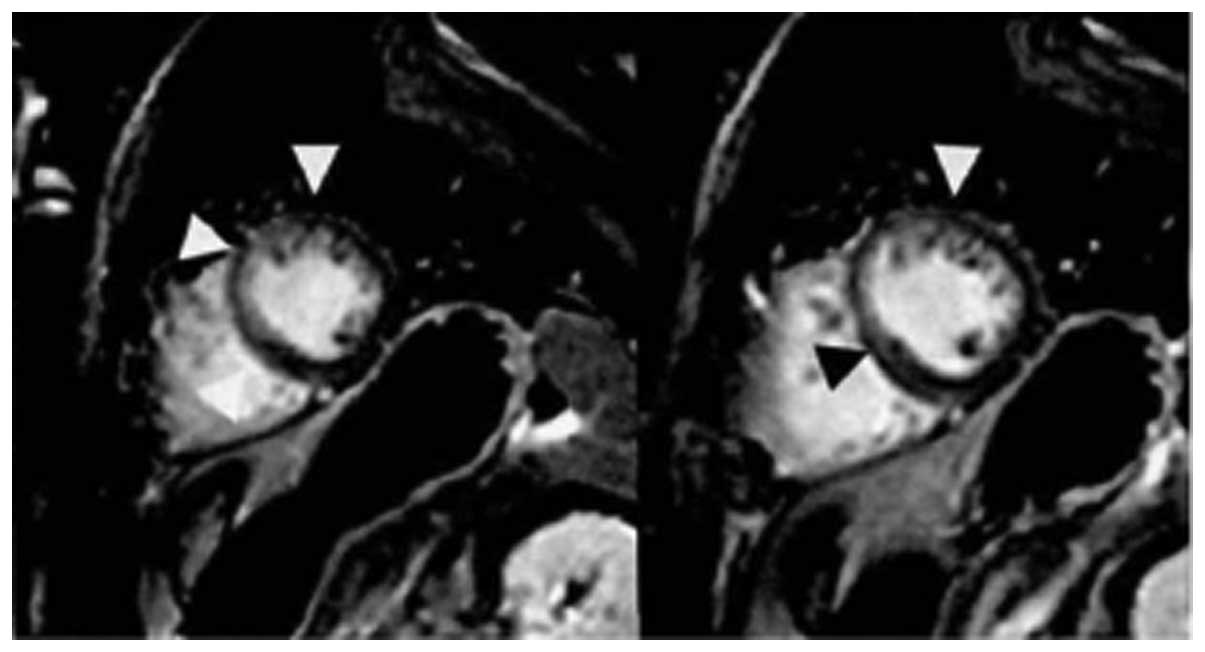
axes were selected (base, middle, apical) when the transmembrane
extent with delayed enhancement was evaluated by VSM, and each
segment was scored in accordance with the transmural extent: 0, no
enhancement; 1, 0–25% enhancement; 2, 26–50% enhancement; 3, 51–75%
enhancement; and 4, 76–100% enhancement (Fig. 1). The scores of all the stages were
added, yielding a total score of VSM. The result was independently
analyzed by two experienced physicians. If the result was
inconsistent, it would be analyzed by the third physician.
Echocardiography examination
Philips iE33 ultrasonic diagnostic apparatus and
S5-1 probe (both from Philips Medical Systems, Inc., Bothell, WA,
USA) with a frequency of 2–4 MHz were used to conduct
M-type-sampling on the standard left ventricular short axis mitral
valve under the guidance of the two-dimensional echocardiography,
to ensure the sample line be vertical to the ventricular septal
posterior wall, measuring LVEDD, LVESD, and automatically
outputting LVED according to the Teichholz correction formula
(9). The samples were measure three
times and the average was taken.
MACE
MACE was defined as target vessel reconstruction,
recurrence of angina and myocardial infarction, new heart failure
and sudden cardiac death.
Statistical analysis
SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was
used to input and analyze the data. Quantitative data are presented
as mean ± standard deviation. Comparisons among groups were tested
using the independent sample t-test, and the intergroup comparison
was tested using the paired t-test. Qualitative data are expressed
as the number of case or percentage, and comparisons among groups
were tested using the χ2 test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Magnetic resonance imaging (MRI)
evaluation in myocardial infarction
Postoperative infarction quality (6.9±1.0 from
8.3±1.2), and VSM and WMA scores (7.6±1.2 and 3.7±0.5,
respectively) were significantly reduced. The difference was
statistically significant (P<0.05) (Table I).
 | Table I.MRI evaluation in myocardial
infarction. |
Table I.
MRI evaluation in myocardial
infarction.
|
| Infarction quality
(g) | VSM score | WMA score |
|---|
| Preoperative | 8.3±1.2 | 11.2±1.5 | 6.2±0.8 |
| Postoperative | 6.9±1.0 | 7.6±1.2 | 3.7±0.5 |
| t |
6.302 | 6.754 |
7.221 |
| P-value |
0.037 | 0.028 |
0.013 |
Ultrasound and magnetic resonance
evaluation in cardiac function
The comparison of ultrasound evaluation in LVEDD,
LVESD, LVEF before and after the operation indicated the difference
was of no statistical significance (P>0.05). LVEDD was increased
following evaluation by magnetic resonance prior to surgery as
compared to that by ultrasound, whereas LVESD and LVEF were
reduced. Additionally, postoperative LVEDD was reduced compared to
that prior to surgery, while LVEF was increased, and the difference
was statistically significant (P<0.05). However, no significant
change was found in LVEDD (Table
II).
 | Table II.Ultrasound and magnetic resonance
evaluation in cardiac function. |
Table II.
Ultrasound and magnetic resonance
evaluation in cardiac function.
|
| LVEDD (mm) | LVESD (mm) | LVEF (%) |
|---|
|
|
|
|
|
|---|
|
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative |
|---|
| Ultrasound | 54.6±1.2 | 54.5±1.5 | 27.5±2.2 | 27.3±2.0 | 49.6±2.4 | 51.2±3.0 |
| MRI | 56.4±1.3 | 55.7±1.2 | 25.6±2.0 | 25.4±2.1 | 45.7±2.3 | 49.7±3.3 |
| t | 5.324 | 5.124 | 5.629 | 5.748 | 6.345 | 6.528 |
| P-value | 0.039 | 0.040 | 0.035 | 0.034 | 0.032 | 0.030 |
Correlation of the two evaluation
methods and MACE
MACE occurred in 7 (12.5%) of 56 cases. The
infarction quality, and VSM and WMA scores of patients in the MACE
group were significantly higher than the group without MACE.
Post-operative LVED was lower than the group without MACE, and the
difference was statistically significant (P<0.05). There was no
difference in the evaluation of ultrasound (Table III).
 | Table III.The correlation of the two evaluation
methods and MACE. |
Table III.
The correlation of the two evaluation
methods and MACE.
| Groups | Infarction quality
(g) | VSM score | WMA score | LVEF (MRI) | LVEF
(ultrasound) |
|---|
| Group with MACE | 7.4±1.6 | 7.9±1.6 | 4.1±0.6 | 46.5±3.5 | 51.0±3.6 |
| Group without
MACE | 6.5±1.3 | 7.3±1.4 | 3.4±0.3 | 51.2±3.2 | 51.4±3.5 |
| t | 6.328 | 6.120 | 6.635 | 7.203 | 0.639 |
| P-value | 0.034 | 0.036 | 0.030 | 0.018 | 0.548 |
Discussion
Effective and accurate measurement of myocardial
infarction quality is of great value in evaluating the prognosis of
patients. Animal experiments have shown that infarction quality
measured by delayed and enhanced MRI is highly conformed with the
quality of the scar displayed by TTC staining, which is considered
to be the gold standard for the evaluation of myocardial necrosis
on histology (10,11). Cardiac MRI evaluation of myocardial
viability quality and detection of infarction quality are based on
systolic dysfunction and myocardial perfusion defects. However, the
presence of cell metabolism, survival of cell membrane integrity,
and potentially contractile reserve are useful in the enhancement
contractile response to positive inotropic agents (12). The basic principle of delayed MRI is
the use of paramagnetic contrast agents to reduce the myocardial T1
relaxation time from rapidly entering the vascular bed, and
distributing in the extravascular space (13). In addition, the aim of MRI is to
evaluate myocardial infarction size, quality, and myocardial injury
degree through the dynamic process of cardiac chamber and
myocardium (13). The strength of
myocardial tissue signal depends on the blood flow volume, tissue
perfusion, size of the extracellular space, and the distribution of
the contrast agent in the myocardium (14). A high signal area of delayed
enhancement is an irreversible necrotic myocardium. The main reason
for myocardial infarction is structural damage of myocardial cells
and microvascular damage, and the mechanism underlying the
infarction area may be the delay of non-active tissue contrast
agents clustered at the entry and exit points, and the distribution
volume of the contrast agent in the active and inactive regions
(15).
Recent findings show that revascularization is not
affected by MRI on the first day following stent implantation, and
no stent artifacts are produced, which can accurately determine the
quality of myocardial infarction, the degree of permeability, the
degree of ventricular wall motion, and left ventricular function
(16). Thus, the infarction quality,
and VSM and WMA socres were significantly reduced, with the
difference being statistically significant. In the present study,
the difference was of no statistical significance in the comparison
of ultrasound evaluation in LVEDD, LVESD, and LVEF prior to and
following surgery. LVEDD was increased by the evaluation of
magnetic resonance imaging prior to surgery compared to that by
ultrasound, whereas LVESD and LVEF were reduced. Additionally,
postoperative LVEDD was reduced compared to that prior to surgery,
whereas LVEF was increased, with the difference being statistically
significant, albeit no significant change was found in LVEDD.
Magnetic resonance measurement is generally lower than that for
ultrasound, considering that the shape of the ventricular cavity
may be irregular following myocardial infarction, which makes left
ventricular volume or inner measurement prone to bias under
echocardiography (17). In addition,
over-reliance on ultrasound techniques and experience of the
operator may cause endocardial inappropriate depiction and is
subjected to the limitation of the acoustic window, adding
difficulty in distinguishing the myocardial, endocardial, and
epicardial adipose layer, resulting in the measured value being
extremely large (17). However, CMR
is an objective, quantitative indication that does not assume the
geometry of the left ventricle, especially for existing ventricular
remodeling and expansion of the heart chamber to obtain better
ejection fraction, and has strong repeatability and good
consistency (18). The infarction
quality, and VSM and WMA scores of the patients in the MACE group
were significantly higher than the group without MACE.
Postoperative LVEF was lower than the group without MACE, and the
difference was statistically significant, whereas there was no
difference found in the ultrasound evaluation of LVEF. The results
suggest that CMR measurement of myocardial infarction and the
changes in cardiac function are more sensitive, which is associated
with prognosis.
In conclusion, CMR evaluation of AMI with elective
PCI treatment in myocardial infarction remodeling and cardiac
function is more sensitive and accurate compared to
echocardiography. However, further investigations are required to
confirm the above results.
References
|
1
|
Elmariah S, Smith SJ and Fuster V: Late
medical versus interventional therapy for stable ST-segment
elevation myocardial infarction. Nat Clin Pract Cardiovasc Med.
5:42–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Degeare VS, Dangas G, Stone GW and Grines
CL: Interventional procedures in acute myocardial infarction. Am
Heart J. 141:15–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woo JS, Yu TK, Kim WS, Kim KS and Kim W:
Early prediction of myocardial viability after acute myocardial
infarction by two-dimensional speckle tracking imaging. J Geriatr
Cardiol. 12:474–481. 2015.PubMed/NCBI
|
|
4
|
Jankauskienė E, Orda P, Rumbinaitė E,
Žaliaduonytė-Pekšienė D, Steponavičiutė R, Krasauskienė A,
Vaškelytė JJ and Bunevičius R: Left ventricular function by
speckle-tracking echocardiography in patients with low-T3 syndrome
and acute myocardial infarction. Medicina (Kaunas). 51:209–216.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stuckey DJ, Ishii H, Chen QZ, Boccaccini
AR, Hansen U, Carr CA, Roether JA, Jawad H, Tyler DJ, Ali NN,
Clarke K and Harding SE: Magnetic resonance imaging evaluation of
remodeling by cardiac elastomeric tissue scaffold biomaterials in a
rat model of myocardial infarction. Tissue Eng Part A.
16:3395–3402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsadok Y, Friedman Z, Haluska BA, Hoffmann
R and Adam D: Myocardial strain assessment by cine cardiac magnetic
resonance imaging using non-rigid registration. Magn Reson Imaging.
34:381–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romero J, Lupercio F, Díaz JC,
Goodman-Meza D, Haramati LB, Levsky JM, Shaban N, Piña I and Garcia
MJ: Microvascular obstruction detected by cardiac MRI after AMI for
the prediction of LV remodeling and MACE: a meta-analysis of
prospective trials. Int J Cardiol. 202:344–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamuro M, Tadamura E, Kanao S, Okayama
S, Okamoto J, Urayama S, Kimura T, Komeda M, Kita T and Togashi K:
Cardiac functional analysis by free-breath real-time cine CMR with
a spatiotemporal filtering method, TSENSE: Comparison with
breath-hold cine CMR. J Cardiovasc Magn Reson. 8:801–807. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scopinaro F, Banci M, Vania A, Tavolaro R,
Schillaci O, Tisei A, Werner B, Digilio G, Ventriglia F and
Colloridi V: Radioisotope assessment of heart damage in
hypertransfused thalassaemic patients. Eur J Nucl Med. 20:603–608.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suhail MS, Wilson MW, Hetts SW and Saeed
M: Magnetic resonance imaging characterization of circumferential
and longitudinal strain under various coronary interventions in
swine. World J Radiol. 5:472–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haase J, Bayar R, Hackenbroch M, Storger
H, Hofmann M, Schwarz CE, Reinemer H, Schwarz F, Ruef J and Sommer
T: Relationship between size of myocardial infarctions assessed by
delayed contrast-enhanced MRI after primary PCI, biochemical
markers, and time to intervention. J Interv Cardiol. 17:367–373.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saeed M, Van TA, Krug R, Hetts SW and
Wilson MW: Cardiac MR imaging: current status and future direction.
Cardiovasc Diagn Ther. 5:290–310. 2015.PubMed/NCBI
|
|
13
|
Park CH, Choi EY, Yoon YW, Kwon HM, Hong
BK, Lee BK, Min PK, Greiser A, Paek MY, Hwang SH, et al:
Quantitative T2 mapping after reperfusion therapy in patients with
acute myocardial infarction: a comparison with late gadolinium
enhancement and cine MR imaging. Magn Reson Imaging. 33:1246–1252.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma N, Cheng H, Lu M, Liu Q, Chen X, Yin G,
Zhu H, Zhang L, Meng X, Tang Y, et al: Magnetic resonance imaging
with superparamagnetic iron oxide fails to track the long-term fate
of mesenchymal stem cells transplanted into heart. Sci Rep.
5:90582015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bönner F, Merx MW, Klingel K, Begovatz P,
Flögel U, Sager M, Temme S, Jacoby C, Salehi Ravesh M, Grapentin C,
et al: Monocyte imaging after myocardial infarction with 19F MRI at
3 T: a pilot study in explanted porcine hearts. Eur Heart J
Cardiovasc Imaging. 16:612–620. 2015.PubMed/NCBI
|
|
16
|
Rischpler C, Langwieser N, Souvatzoglou M,
Batrice A, van Marwick S, Snajberk J, Ibrahim T, Laugwitz KL,
Nekolla SG and Schwaiger M: PET/MRI early after myocardial
infarction: evaluation of viability with late gadolinium
enhancement transmurality vs. 18F-FDG uptake. Eur Heart J
Cardiovasc Imaging. 16:661–669. 2015.PubMed/NCBI
|
|
17
|
No authors listed: Recommendations for
cardiac chamber quantification by echocardiography in adults: an
update from the American Society of Echocardiography and the
European Association of, Cardiovascular Imaging. Eur Heart J
Cardiovasc Imaging. 17:4122016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kidambi A, Biglands JD, Higgins DM, Ripley
DP, Zaman A, Broadbent DA, McDiarmid AK, Swoboda PP, Al Musa T,
Erhayiem B, et al: Susceptibility-weighted cardiovascular magnetic
resonance in comparison to T2 and T2 star imaging for detection of
intramyocardial hemorrhage following acute myocardial infarction at
3 Tesla. J Cardiovasc Magn Reson. 16:862014. View Article : Google Scholar : PubMed/NCBI
|