Introduction
Esophageal cancer is one of the most common
malignances worldwide, and is especially prevalent in China and
Japan (1,2). Patients with esophageal cancer have a
poor prognosis due to dysphagia (3).
Surgery is the only form of treatment that can provide a cure for
esophageal cancer, although it is suitable for less than a third of
patients due to late diagnosis, advanced progress and tumor
metastasis (4). In recent decades,
metallic stent insertion into the esophagus has been widely used in
the treatment of esophageal cancer as it is less invasive, prolongs
survival and improves life quality (5). However, conventional stents can only
facilitate drainage but have no antitumor effect. Furthermore, the
side-effects following stent insertion are non-negligible, and
include tumor overgrowth, tumor ingrowth and granulation tissue
hyperplasia at either end of the stent (6).
In recent years, several studies have been carried
out on the use of drug-eluting metallic stents for digestive system
carcinoma, including a 5-Fu-eluting stent for esophagal cancer and
a paclitaxel-eluting stent for biliary duct and esophagal cancers
(7,8). The majority of the results demonstrated
that self-expanding metallic stents (SEMS) combined with an
antitumor drug allowed the targeting of the drug to the wall tissue
and the maintenance of a controlled treatment dose over long
periods of time (7,8).
Paclitaxel is as a novel anti-neoplastic agent
currently used to treat several types of cancer (9). Paclitaxel has been demonstrated to be
effective at inhibiting the proliferation of human gallbladder
epithelial cells, fibroblasts, pancreatic adenocarcinoma cells and
esophageal cells (10). In addition,
Jeon et al (10) reported
that paclitaxel-eluting metallic SEMS (PEMS) inhibited tissue
hyperplasia in the esophagus, and may manage refractory benign
esophageal stricture (10).
Paclitaxel exerts its pharmacological effects by binding to
β-tubulin and by stabilizing the polymerized microtubules (11). Therefore, paclitaxel can be coated on
the SEMS in order to provide sustained release (12).
In our previous study, an esophageal squamous
carcinoma was created in rabbits using an endoscopic technique
(13). In addition, a previous study
demonstrated that the in vitro sustained release of PEMS
with 10% paclitaxel lasted for >40 days, which was sufficient
for observing the effect of the drug on the rabbit esophagus
(14). The aim of the current study
is to evaluate the safety of PEMS in the rabbit esophagus and to
investigate the effect of PEMS on esophageal tissue.
Materials and methods
Preparation of PEMSs
The SEMS used in the present study (Niti-S
polyurethane-covered stent; Garson-Flextent, Jiangsu, China) were
16 mm long, 10 mm wide in the middle and 12 mm wide at the proximal
end of the stent when fully expanded and mounted on a 7F stent
introducer set custom made by Garson-Flextent. Due to the fact that
the average diameter of the rabbit esophagus is ~5 mm, a stent with
a 12 mm diameter flare was considered sufficient to prevent stent
migration. The PEMS were loaded with 10% (wt/vol) paclitaxel
(Taxol®; Jiangsu Hongdoushan Biological Technology Co.,
Ltd., Jiangsu, China) by the State Key Laboratory of Pharmaceutical
Biotechnology, School of Life Sciences, Nanjing University
(Nanjing, China). Following the determination of the eluting stent
indices including release rates and effect on the mucosa, PEMSs
with 10% paclitaxel was shown to be the most suitable choice.
Animal study
Stent placement
All experimental procedures were performed in
accordance with the National Institutes of Health guidelines for
humane handling of animals and were approved by the Committee on
Animal Research at our institution (15). Male New Zealand white rabbits (n=48;
Jiangsu Academy of Agricultural Science, Jiangsu, China), weighing
1.5–2.0 kg and housed in an environment with a 12-h dark:light
cycle at 25°C with free access to food and water, were randomly
assigned to a PEMS group or a SEMS group (6 rabbits in each group
per time-point).
Due to the fact that the rabbit malignant stricture
model was created recently in our previous study, a normal rabbit
model (13,16) was used in the present study. A total
of 48 rabbits with malignant esophageal occlusion were fasted for
24 h prior to stent implantation. Each rabbit was anesthetized by
intraperitoneal injection with 95% pentobarbital sodium (35 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA). Each rabbit was then placed in
the left lateral position. A SEMS or PEMS was introduced into the
esophagus using the 7F stent introducer set. Prior to the placement
of the introducer at the correct site, 1–2 ml contrast medium
(Iohexol; GE Healthcare Life Sciences, Chalfont, UK) was injected
into the esophagus in order to confirm the accurate position of the
stent. The stent was then deployed in the lower esophagus. All
endoscopic procedures were performed by two well-experienced
endoscopists.
Follow-up and postmortem
examination
Following endoscopic stent placement, the animals
were fasted for a further 24 h prior to reintroduction of their
usual diet. During the follow-up, food-intake and weight were
monitored. On the 1st, 2nd, 4th and 6th week following stent
insertion, 6 rabbits in each group were sacrificed by intravascular
air embolism. The esophagus was excised and examined grossly.
Images were captured in order to examine the status of the proximal
esophageal obstruction due to inflammation hyperplasia. Each stent
was gently removed from the esophagus, and the esophagus was then
incised longitudinally. Esophageal wall hyperemia and proximal
obstruction was evaluated. Hyperemia was graded as follows: 0,
hyperemia absent; 1, hyperemia present. Proximal obstruction was
graded as follows: 0, normal; 1, stricture; 2, obstruction.
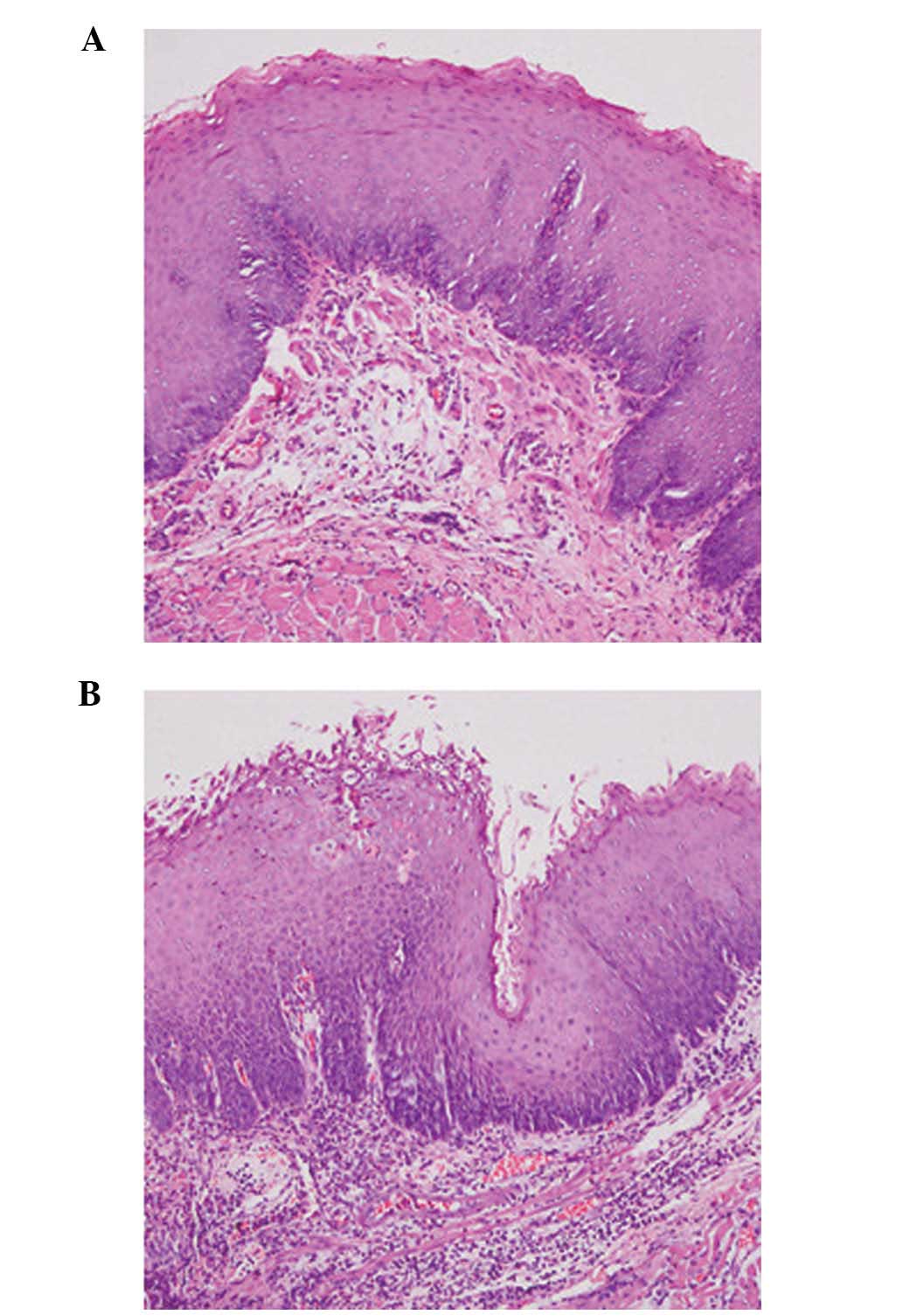
Following gross tissue evaluation, the lesion tissue samples were
fixed in 10% formalin or stored at −80°C. Tissue samples
[paclitaxel-covered segment and proximal uncovered stented segment
(the part of the stent without the membrane)] were stained with
hematoxylin and eosin (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) and examined by an experienced gastrointestinal
pathologist using a CX23 Microscope (Olympus Corporation, Tokyo,
Japan). Weight, food-intake, stent migration hyperemia and proximal
obstruction were also recorded.
A single pathologist evaluated the status of the
proximal uncovered stented segment, the thickness of the epithelial
layer and submucosal inflammatory cell infiltration. Thickening of
the epithelial layer was defined as the distance between the tissue
protruding into the lumen and the lower portion of the submucosa.
The thickened epithelial layer was defined as follows: 0, normal;
1, mild; 2, severe. The degree of submucosal inflammatory cell
infiltration was graded as follows: 0, none; 1, mild (scattered
inflammatory cells); 2, moderate (inflammatory cell infiltration in
~half of a microscopic field); 3, severe (inflammatory cells
infiltration in the majority or all of the microscopic field)
(17).
Two endoscopists performed the stent insertion and
recorded which stent (SEMS or PEMS) was inserted. Subsequently, a
pathologist blinded to the type of stent inserted examined the
tissue samples both grossly and microscopically.
Statistical analysis
The data are expressed as means ± standard error of
the mean. Continuous variables were compared by unpaired Student
t-test including food-intake following stent implantation, weight
at the time of sacrifice, proximal esophageal obstruction, tissue
hyperemia, thickness of each epithelial layer, and submucosal
inflammatory cell infiltration. One-way analysis of variance and
Fisher's exact test were used to analyze hyperemia, degree of
proximal obstruction, thickness of the epithelial layer and degree
of inflammatory cell infiltration in the SEMS and PEMS groups. SPSS
version 13.0 software (SPSS Inc., Chicago, IL, USA) was used for
all statistical analyses. P<0.05 was considered to indicate a
statistically significant result.
Results
Stent placement and follow-up
The 48 rabbits were anesthetized and the stents were
placed into their esophagus. All rabbits survived the procedure.
There were no procedure-associated complications such as abdominal
infection or pneumonia. All the stents were in situ and no
migration occurred following stent insertion in any of the rabbits.
Following insertion of the stents for 1, 2, 4 and 6 weeks, 6
rabbits were sacrificed in each group and gross and microscopic
examination of the esophageal tissue was performed. The weight and
food-intake was similar in the two groups.
Gross and microscopic findings
The middle and lower part of the esophagus was
excised from the body. Gross inspection of the excised tissue
specimens revealed no perforation or bleeding in any of the
rabbits. No adhesion was found between the esophagus and
surrounding organs. The esophagus was then incised longitudinally.
At 1 week following stent insertion, 4 and 5 rabbits with hyperemia
were identified in the SEMS and PEMS group, respectively, although
this difference was not significant (P>0.05), and no proximal
obstruction at either end of the stent occurred in either groups.
Epithelial thickness mildly increased in 3 and 5 rabbits in the
SEMS and PEMS groups, respectively (P>0.05). However,
inflammatory cell infiltration was determined to be significantly
more severe in the SEMS group, as compared with the PEMS group
(P<0.05). At 2 weeks following stent insertion, proximal
stricture occurred (Table I) in 3
rabbits in the SEMS group and 4 rabbits in the PEMS group, although
this difference was not statistically significant (P>0.05).
Mucosal hyperemia occurred in 2 rabbits in the SEMS group, and 5
rabbits in the PEMS group (P>0.05). There was no statistically
significant difference in the thickness of the epithelia in the two
groups (P>0.05). Inflammatory cell infiltration remained severe
in the SEMS group and increased in the PEMS group (P<0.05). At 4
weeks following stent insertion, mucosal hyperemia occurred in 1
rabbit in the SEMS group and 4 rabbits in the PEMS group (Table II), although this difference was not
statistically significant (P>0.05). Proximal stricture occurred
in 4 rabbits in the SEMS group and 3 rabbits in the PEMS group
(P>0.05). Epithelial thickness in the SEMS group was
significantly higher, as compared with the PEMS group (P<0.05).
Inflammatory cell infiltration started to decrease in the SEMS
group, but increased in the PEMS group (P<0.05). At 6 weeks
following stent insertion, stricture occurred in the majority of
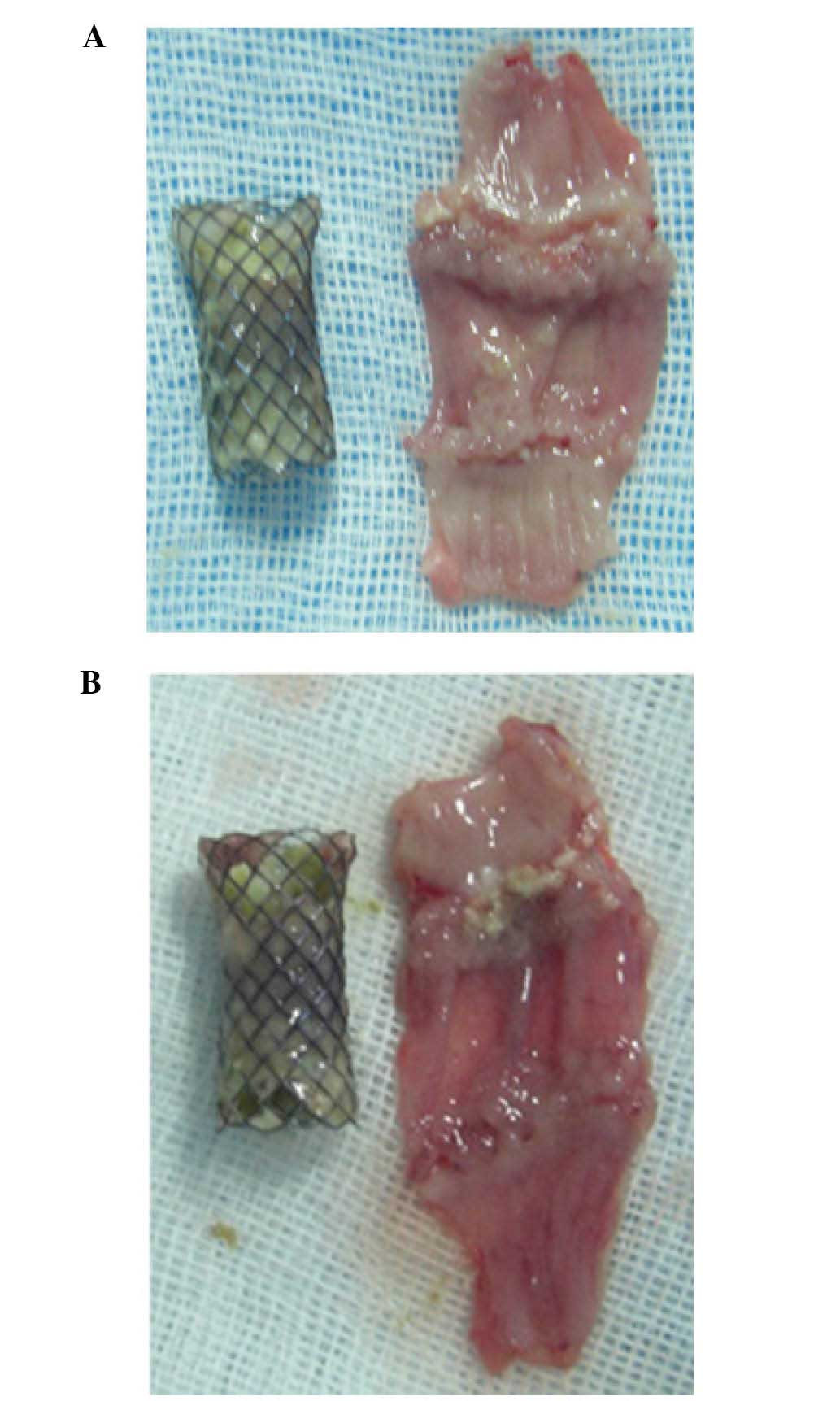
the animals, but no obstruction was observed (Table III, Fig
1A and B); the amount of stricture was not significantly
different between the SEMS and the PEMS group (P>0.05). No
hyperemia was observed in the rabbits of the SEMS group, although 3
rabbits in PEMS group exhibited hyperemia (P<0.05). Epithelial
thickness was significantly increased in the SEMS group, as
compared with the PEMS group (P<0.05). Inflammatory cell
infiltration was rarely observed in the SEMS group but remained
severe in the PEMS group (P<0.05)(Table IV; Fig.
2A and B). The data was compared among different time points in
the two groups. In the SEMS group, mucosal hyperemia and
inflammatory cell infiltration decreased over time, and proximal
stricture and thickness of the epithelia increased with the time
(Fig. 3A-D). Conversely, in the PEMS
group, mucosal hyperemia decreased over time, and proximal
stricture, thickness of the epithelia and inflammatory cell
infiltration increased over time (Fig 4A
and B)
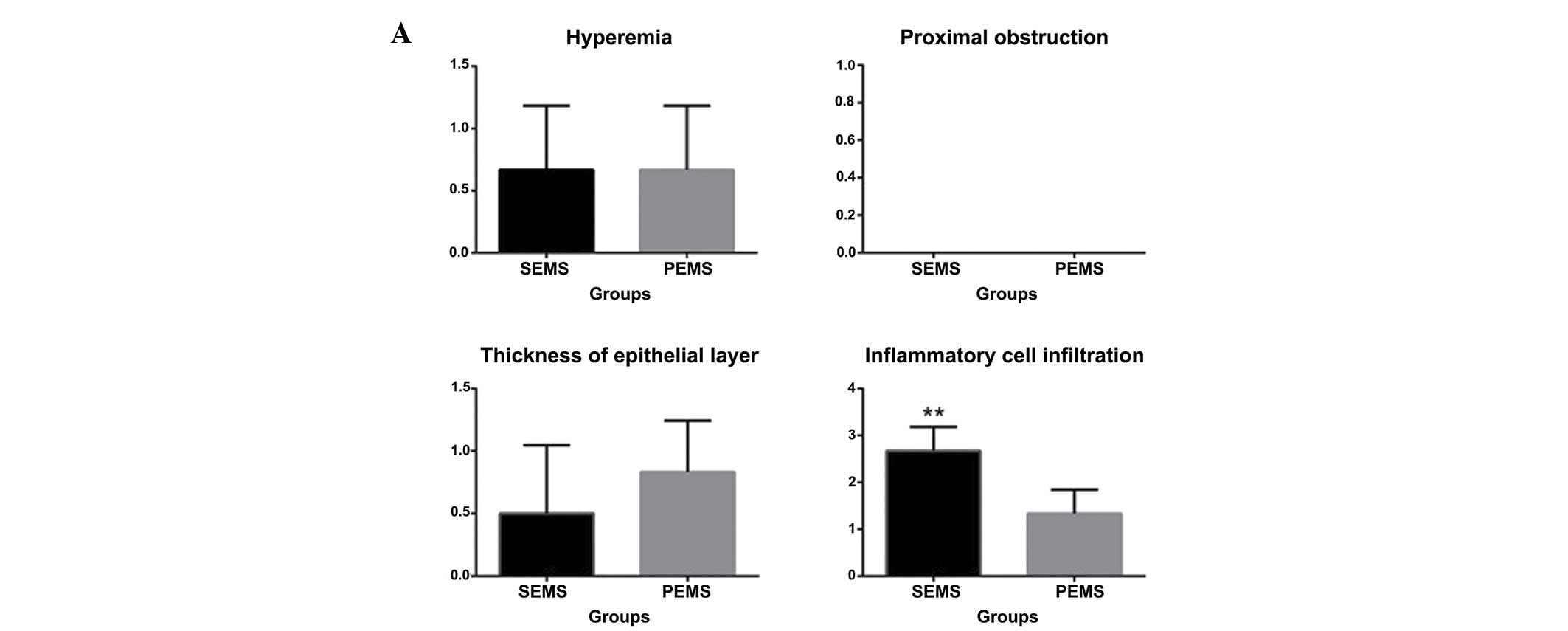 | Figure 3.Hyperemia, degree of proximal
obstruction, thickness of the epithelial layer and degree of
inflammatory cell infiltration 1, 2, 4 and 6 weeks following stent
insertion. (A) At 1 week, 4 and 5 rabbits exhibited hyperemia in
the SEMS and PEMS group, respectively, and no proximal obstruction
at either end of the stent occurred in either of the two groups.
Epithelial thickness increased in 3 rabbits in the SEMS group and 5
rabbits in the PEMS group. Inflammatory cell infiltration was
significantly higher in the SEMS group compared with the PEMS
group. (B) At 2 weeks, mucosal hyperemia occurred in 2 rabbits in
the SEMS group and 5 rabbits in the PEMS group. Proximal stricture
occurred in 3 rabbits in the SEMS group and 4 rabbits in the PEMS
group. The thickness of the epithelia was similar in both groups.
Inflammatory cell infiltration was significantly higher in the SEMS
group compared with the PEMS group. (C) At 4 weeks, mucosal
hyperemia occurred in 1 rabbit and 4 rabbits in the SEMS and PEMS
groups, respectively. Proximal stricture occurred in 4 rabbits in
the SEMS group and 3 rabbits in the PEMS group. Epithelial
thickness in the SEMS group was significantly higher compared with
the PEMS group. Inflammatory cell infiltration was significantly
lower in the SEMS group compared with the PEMS group. (D) At 6
weeks, stricture had occurred in the majority of the rabbits
although no obstruction was observed. No hyperemia was observed in
the SEMS group, and conversely 3 rabbits exhibited hyperemia in the
PEMS group. Epithelial thickness was significantly higher in the
SEMS group compared with the PEMS group. Inflammatory cell
infiltration was significantly lower in the SEMS group compared
with the PEMS group. **P<0.05, vs. the PEMS group. SEMS,
self-expanding metallic stents; PEMS, Paclitaxel-eluting metallic
SEMS. |
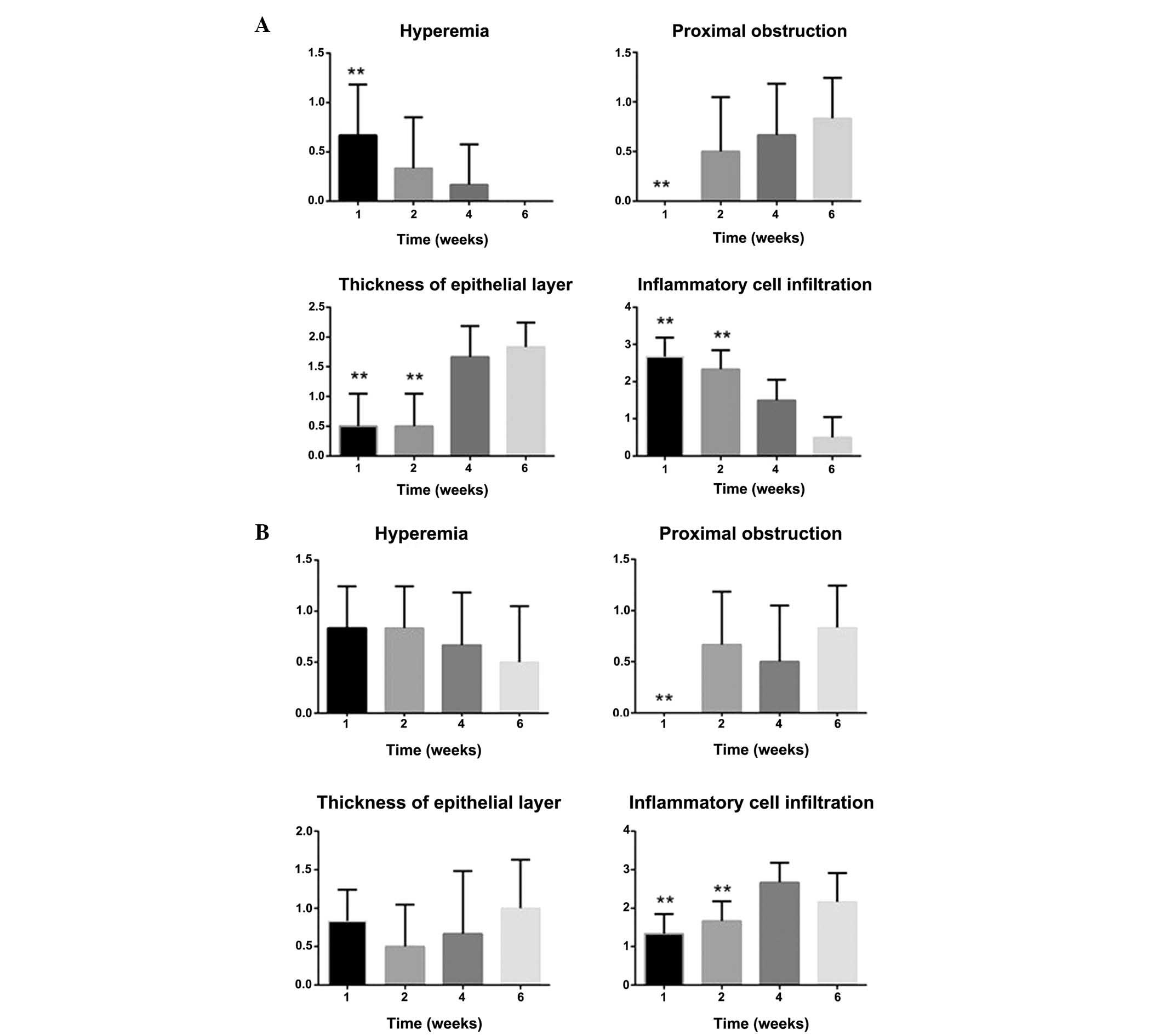 | Figure 4.Hyperemia, degree of proximal
obstruction, thickness of the epithelial layer and degree of
inflammatory cell infiltration in the SEMS and PEMS groups. (A) In
the SEMS group, mucosal hyperemia and inflammatory cell
infiltration decreased over time. Conversely, proximal stricture
and thickness of the epithelia increased with the time. (B) In the
PEMS group, mucosal hyperemia decreased over time. Conversely,
proximal stricture, thickness of the epithelia and inflammatory
cell infiltration increased over time. **P<0.05, vs. the PEMS
group. SEMS, self-expanding metallic stents; PEMS,
Paclitaxel-eluting metallic SEMS. |
 | Table I.Characteristics of the 12 rabbits
sacrificed 1 week following stent insertion. |
Table I.
Characteristics of the 12 rabbits
sacrificed 1 week following stent insertion.
|
|
|
| Gross findings | Microscopic
findings |
|---|
|
|
|
|
|
|
|---|
| Rabbit | Weight(kg) | Food-intake(g) | Migration | Hyperemia | Proximal
obstruction | Thickness of
epithelial layer | Degree of
inflammatory cell infiltration |
|---|
| SEMS 1 | 1.98 | 150 | 0 | 1 | 0 | 1 | 3 |
| SEMS 2 | 2.03 | 180 | 0 | 0 | 0 | 1 | 2 |
| SEMS 3 | 1.88 | 120 | 0 | 1 | 0 | 1 | 3 |
| SEMS 4 | 2.10 | 130 | 0 | 1 | 0 | 0 | 3 |
| SEMS 5 | 1.95 | 180 | 0 | 1 | 0 | 0 | 2 |
| SEMS 6 | 2.20 | 200 | 0 | 0 | 0 | 0 | 3 |
| PEMS 1 | 1.90 | 130 | 0 | 1 | 0 | 1 | 2 |
| PEMS 2 | 1.79 | 180 | 0 | 1 | 0 | 1 | 1 |
| PEMS 3 | 2.01 | 200 | 0 | 1 | 0 | 0 | 1 |
| PEMS 4 | 1.85 | 150 | 0 | 1 | 0 | 1 | 1 |
| PEMS 5 | 1.90 | 110 | 0 | 0 | 0 | 1 | 2 |
| PEMS 6 | 1.86 | 200 | 0 | 1 | 0 | 1 | 1 |
| P-value | 0.03 | 0.94 | 1 | 1 | 1 | 0.26 | <0.01 |
 | Table II.Characteristics of the 12 rabbits
sacrificed 2 weeks following stent insertion. |
Table II.
Characteristics of the 12 rabbits
sacrificed 2 weeks following stent insertion.
|
|
|
| Gross findings | Microscopic
findings |
|---|
|
|
|
|
|
|
|---|
| Rabbit | Weight (kg) | Food-intake
(g) | Migration | Hyperemia | Proximal
obstruction | Thickness of
epithelial layer | Degree of
inflammatory cell infiltration |
|---|
| SEMS 1 | 2.40 | 150 | 0 | 1 | 1 | 1 | 2 |
| SEMS 2 | 2.29 | 170 | 0 | 0 | 0 | 0 | 2 |
| SEMS 3 | 2.60 | 200 | 0 | 0 | 1 | 1 | 3 |
| SEMS 4 | 2.55 | 180 | 0 | 1 | 1 | 0 | 3 |
| SEMS 5 | 2.04 | 200 | 0 | 0 | 0 | 0 | 2 |
| SEMS 6 | 2.59 | 200 | 0 | 0 | 0 | 1 | 2 |
| PEMS 1 | 2.44 | 160 | 0 | 1 | 0 | 1 | 2 |
| PEMS 2 | 2.38 | 180 | 0 | 1 | 1 | 1 | 1 |
| PEMS 3 | 2.09 | 160 | 0 | 1 | 0 | 1 | 2 |
| PEMS 4 | 2.32 | 180 | 0 | 0 | 1 | 0 | 1 |
| PEMS 5 | 2.20 | 180 | 0 | 1 | 1 | 0 | 2 |
| PEMS 6 | 2.06 | 200 | 0 | 1 | 1 | 0 | 2 |
| P-value | 0.17 | 0.54 | 1 | 0.09 | 1 | 1 | 0.04 |
 | Table III.Characteristics of the 12 rabbits
sacrificed 4 weeks following stent insertion. |
Table III.
Characteristics of the 12 rabbits
sacrificed 4 weeks following stent insertion.
|
|
|
| Gross findings | Microscopic
findings |
|---|
|
|
|
|
|
|
|---|
| Rabbit | Weight (kg) | Food-intake
(g) | Migration | Hyperemia | Proximal
obstruction | Thickness of
epithelial layer | Degree of
inflammatory cell infiltration |
|---|
| SEMS 1 | 2.57 | 200 | 0 | 0 | 1 | 2 | 1 |
| SEMS 2 | 2.39 | 190 | 0 | 1 | 1 | 2 | 2 |
| SEMS 3 | 2.80 | 200 | 0 | 0 | 1 | 1 | 1 |
| SEMS 4 | 2.75 | 180 | 0 | 0 | 0 | 2 | 2 |
| SEMS 5 | 2.34 | 180 | 0 | 0 | 0 | 2 | 1 |
| SEMS 6 | 2.70 | 200 | 0 | 0 | 1 | 1 | 2 |
| PEMS 1 | 2.87 | 200 | 0 | 1 | 0 | 1 | 3 |
| PEMS 2 | 3.02 | 180 | 0 | 0 | 0 | 1 | 2 |
| PEMS 3 | 2.31 | 170 | 0 | 1 | 0 | 0 | 3 |
| PEMS 4 | 2.29 | 150 | 0 | 1 | 1 | 0 | 3 |
| PEMS 5 | 2.40 | 180 | 0 | 0 | 1 | 2 | 2 |
| PEMS 6 | 2.23 | 200 | 0 | 1 | 1 | 0 | 3 |
| P-value | 0.66 | 0.21 | 1 | 0.09 | 1 | 0.03 | <0.01 |
 | Table IV.Characteristics of the 12 rabbits
sacrificed 6 weeks following stent insertion. |
Table IV.
Characteristics of the 12 rabbits
sacrificed 6 weeks following stent insertion.
|
|
|
| Gross findings | Microscopic
findings |
|---|
|
|
|
|
|
|
|---|
| Rabbit | Weight (kg) | Food-intake
(g) | Migration | Hyperemia | Proximal
obstruction | Thickness of
epithelial layer (µm) | Degree of
inflammatory cell infiltration |
|---|
| SEMS 1 | 2.49 | 180 | 0 | 0 | 1 | 2 | 1 |
| SEMS 2 | 2.52 | 180 | 0 | 0 | 1 | 2 | 1 |
| SEMS 3 | 2.83 | 170 | 0 | 0 | 1 | 2 | 0 |
| SEMS 4 | 2.94 | 180 | 0 | 0 | 0 | 1 | 0 |
| SEMS 5 | 2.78 | 180 | 0 | 0 | 1 | 2 | 1 |
| SEMS 6 | 2.80 | 170 | 0 | 0 | 1 | 2 | 0 |
| PEMS 1 | 2.74 | 200 | 0 | 0 | 1 | 1 | 2 |
| PEMS 2 | 2.99 | 180 | 0 | 1 | 1 | 0 | 2 |
| PEMS 3 | 2.75 | 190 | 0 | 1 | 1 | 2 | 3 |
| PEMS 4 | 2.67 | 180 | 0 | 1 | 0 | 1 | 2 |
| PEMS 5 | 2.93 | 170 | 0 | 0 | 1 | 1 | 1 |
| PEMS 6 | 2.77 | 180 | 0 | 0 | 1 | 1 | 3 |
| P-value | 0.38 | 0.19 | 1 | 0.04 | 1 | 0.02 | <0.01 |
Discussion
Esophageal carcinoma is the 6th leading cause of
cancer-associated mortality and the 8th most common cancer
worldwide (18,19). Early resection of the cancer leads to
a good prognosis (19). However,
over half of patients with esophageal cancer are not eligible for
surgical resection. Therefore, treatment of advanced esophageal
carcinoma remains challenging (20).
In recent decades, stent deployment in the esophagus has been
widely used as a palliative therapy which reduces tumor ingrowth
and facilitates drainage. The SEMS is easily inserted and provides
adequate drainage in the esophagus. Furthermore, PEMS has the
potential to inhibit tumor growth and some positive results have
been published (14,17).
In 2005, Lee et al (21) reported on the effect of PEMS on
normal porcine bile ducts. The results demonstrated that treatment
with PEMS resulted in epithelial denudation, mucin hypersecretion
and epithelial metaplasia, which led to the hypothesis that PEMS
may have anti-tumor effects on malignant biliary stricture in
humans (21). In 2009, another study
was performed on dogs which demonstrated that the epithelial layers
were thicker in the PEMS group compared with the control group, and
revealed that the local delivery of paclitaxel resulted in marked
histological changes that may be associated with an antitumor
effect (17). Furthermore, two small
retrospective clinical studies on the use of PEMS for malignant
biliary stricture reported controversial results, indicating that
paclitaxel was unable to inhibit tumor growth and prolong
survival-time in humans (22,23).
Conversely, Guo et al (24)
revealed that Fu-eluting stents had prolonged release patterns and
retained good integrity and stability following stent deployment.
The 5-Fu concentration in stent-adjacent tissue was markedly higher
compared with that found in the serum or liver (19).
We propose that paclitaxel may also have anti-tumor
effects on squamous esophageal carcinoma. Large-sized animal models
were widely used in previous studies for stent research (14,17).
However, these models were not usually conducted under disease
conditions, and the animals were too large to be operated on and
followed up. Furthermore, studies conducted on small animals, such
as mice, used immunodeficient animals, and did not allow for stent
deployment with an endoscope (14,17,21,22).
Therefore, in present study rabbits were selected as an animal
model, as they are sufficiently large to allow for the oral
insertion of an ultra-slim endoscope and stent introducer set
(25,26).
The results presented herein revealed the safety of
PEMS and SEMS in the rabbit model. No major complications,
including massive bleeding, perforation or fatal infection were
observed. Conversely to previous studies (27,28), no
migration of the stents were observed in the present study. This
may be due to the fact that stents with larger diameters were used,
which enhanced the radical focus of the esophagus. The rabbit
weight and food-intake was normal following stent deployment, which
demonstrated that the stent did not affect the rabbits.
Following the insertion of the stent in the rabbit
esophagus, both PEMS and SEMS were demonstrated to cause tissue
hyperemia, proximal obstruction, thickening of the epithelial layer
and inflammatory cell infiltrating. In the 1st, 2nd and 4th week,
hyperemia was similar both the SEMS and PEMS groups. However, in
the 6th week, hyperemia was more marked in the PEMS group, as
compared with the SEMS group. Hyperemia was marked in the 1st and
2nd week in the SEMS group but then decreased in subsequent weeks.
Conversely, hyperemia was low in the 1st and 2nd week in the PEMS
group but then increased in the following weeks. This may be due to
the fact that paclitaxel had the effect of promoting inflammation
thereby causing persistent tissue hyperemia. In the 1st week no
proximal obstruction of the uncovered stent segments was observed
in either group, although in the following weeks stricture was
noted in both groups. However, there was no significant difference
between the two groups. The proximal obstruction of the proximal
uncovered stent is associated with mechanical stimulation between
the stent and esophageal mucosa and tissue overgrowth. Following
microscopic observation, it was apparent that the thickness of the
epithelia was similar in the SEMS and PEMS groups in the 1st and
2nd weeks, although by 4 weeks the epithelial thickness was
significantly different. In the 4th and 6th week, the epithelial
layer was markedly thicker in the SEMS group compared with the PEMS
group. Mavi et al (29)
reported that inflammation promotes the growth of esophageal
epithelia and fiber hyperplasia. However, the results of the
present study were not concordant with those of previous reports
(17,21). The mechanism underlying the
association between inflammation and the epithelia merits further
study. Inflammatory cell infiltration was markedly high in the SEMS
group in the 1st and 2nd week, and decreased over the following
weeks. Conversely, inflammatory cell infiltration was low in the
PEMS group at the 1st and 2nd week, and increased in the 4th and
6th week. The inflammatory cell infiltration was different at
different time points in the PEMS group. We think the reason for
the change of inflammatory cell infiltration was the same as that
of hyperemia. Notably, persistent inflammatory cell infiltration in
the PEMS group also revealed the sustained release of paclitaxel at
6 weeks. The PEMS may inhibit tumor growth through the sustained
released of paclitaxel, which exhibits anti-tumor effects and
activates inflammation.
The limitations of the present study included the
fact that the experiments were carried out on normal rabbit
esophagus, and results obtained from a rabbit model may not
generalize to the effect of PEMS in human patients with esophageal
carcinoma. In addition, the mechanism underlying the effects of
sustained released of paclitaxel on normal esophageal and cancerous
cells requires further study.
In conclusion, endoscopic stent insertion into
rabbit esophagus is safe and easily carried out. PEMS exhibited a
steady release pattern of paclitaxel and may provide an alternative
tool in the management of human esophageal squamous carcinoma.
Acknowledgements
The present study was partially supported by grants
from the National Natural Science Foundation of China (grant nos.
81172266, 81273464, 81202474 and 30973651), the Science and
Technology Support Program of Jiangsu Province (grant no.
BE2010719), the Natural Science Foundation of Jiangsu Province
(grant no. BK2011859) and Jiangsu Innovation of Medical Team and
Leading Talents Cultivation (grant no. LJ201127). The authors would
also like to thank Professor Yiqiao Hu at State Key Laboratory of
Pharmaceutical Biotechnology, Nanjing University for generously
providing the key techniques of producing the paclitaxel-eluting
covered metal stents.
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xing D, Tan W and Lin D: Genetic
polymorphisms and susceptibility to esophageal cancer among Chinese
population (review). Oncol Rep. 10:1615–1623. 2003.PubMed/NCBI
|
|
3
|
Layke JC and Lopez PP: Esophageal cancer:
A review and update. Am Fam Physician. 73:2187–2194.
2006.PubMed/NCBI
|
|
4
|
Griffin SM and Lamb P: Oesophageal cancer.
Surgery. 24:97–100. 2006.
|
|
5
|
Johnson E, Enden T, Noreng HJ, Holck-Steen
A, Gjerlaug BE, Morken T, Johannessen HO and Drolsum A: Survival
and complications after insertion of self-expandablemetal stents
for malignant oesophageal stenosis. Scand J Gastroenterol.
41:252–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rozanes I, Poyanli A and Acunaș B:
Palliative treatment of inoperable malignant esophageal strictures
with metal stents: One center's experience with four different
stents. Eur J Radiol. 43:196–203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Q, Guo S and Wang Z: A type of
esophageal stent coating composed of one 5-fluorouracil-containing
EVA layer and one drug-free protective layer: In vitro release,
permeation and mechanical properties. J Control Release.
118:318–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kipshidze N: Current status of drug
eluting stents. Curr Pharm Des. 16:39772010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
López de Cicco R, Watson JC, Bassi DE,
Litwin S and Klein-Szanto AJ: Simultaneous expression of furin and
vascular endothelial growth factor in human oral tongue squamous
cell carcinoma progression. Clin Cancer Res. 10:4480–4488. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeon SR, Eun SH, Shim CS, Ryu CB, Kim JO,
Cho JY, Lee JS, Lee MS and Jin SY: Effect of drug-eluting metal
stents in benign esophageal stricture: An in vivo animal study.
Endoscopy. 41:449–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Wang F, Sun D and Wang R: A review
of the ligands and related targeting strategies for active
targeting of paclitaxel to tumours. J Drug Target. 15:1–13. 2016.
View Article : Google Scholar
|
|
12
|
Rowinsky EK and Donehower RC: Paclitaxel
(taxol). N Engl J Med. 332:1004–1014. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Zhang Y, Zhong H, Fan Z, Jiang G,
Shen Y, Song H, Tao Z and Wang K: Comparison of endoscopic
submuscosal implantation vs. surgical intramuscular implantation of
VX2 fragments for establishing a rabbit esophageal tumor model
formimicking human esophageal squamous carcinoma. PLoS One.
9:e853262014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee DK, Kim HS, Kim KS, Lee WJ, Kim HK,
Won YH, Byun YR, Kim MY, Baik SK and Kwon SO: The effect on porcine
bile duct of a metallic stent covered with a
Paclitaxel-incorporated membrane. Gastrointest Endosc. 61:296–301.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stokes WS: Best practices for the use of
animals in toxicological research and testing. Ann N Y Acad Sci.
1245:17–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang J, Shuang J, Xiong G, Wang X, Zhang
Y, Tang X, Fan Z, Shen Y, Song H and Liu Z: Establishing a rabbit
model of malignant esophagostenosis using the endoscopic
implantation technique for studies on stent innovation. J Transl
Med. 12:402014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SS, Shin JH, Han JM, Cho CH, Kim MH,
Lee SK, Kim JH, Kim KR, Shin KM, Won YH and Song HY: Histologic
influence of Paclitaxel-eluting covered metallic stents in a canine
biliary model. Gastrointest Endosc. 69:1140–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang HZ, Jin GF and Shen HB:
Epidemiologic differences in esophageal cancer between Asian and
Western populations. Chin J Canc. 31:281–286. 2012. View Article : Google Scholar
|
|
19
|
Müller JM, Erasmi H, Stelzner M, Zieren U
and Pichlmaier H: Surgical therapy of oesophageal carcinoma. Br J
Surg. 77:845–857. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Talukdar FR, Ghosh SK, Laskar RS and
Mondal R: Epigenetic, genetic and environmental interactions in
esophageal squamous cell carcinoma from northeast India. PLoS One.
8:e609962013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee DK, Kim HS, Kim KS, Lee WJ, Kim HK,
Won YH, Byun YR, Kim MY, Baik SK and Kwon SO: The effect on porcine
bile duct of a metallic stent covered with a
paclitaxel-incorporated membrane. Gastrointest Endosc. 61:296–301.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suk KT, Kim JW, Kim HS, Baik SK, Oh SJ,
Lee SJ, Kim HG, Lee DH, Won YH and Lee DK: Human application of a
metallic stent covered with a Paclitaxel-incorporated membrane for
malignant biliary obstruction: Multicenter pilot study.
Gastrointest Endosc. 66:798–803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song TJ, Lee SS, Yun SC, do H Park, Seo
DW, Lee SK and Kim MH: Paclitaxel-eluting covered metal stents
versus covered metal stents for distal malignant biliary
obstruction: A prospective comparative pilot study. Gastrointest
Endosc. 73:727–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo SR, Wang ZM, Zhang YQ, Lei L, Shi JM,
Chen KM and Yu Z: In vivo evaluation of 5-fluorouracil-containing
self-expandable nitinol stent in rabbits: Efficiency in long-term
local drug delivery. J Pharm Sci. 99:3009–3018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim EY, Park YS, Shin JH, Cho YJ, Shin DH,
Yoon HK and Song HY: The effectiveness of erythromycin in reducing
stent-related tissue hyperplasia: An experimental study with a rat
esophageal model. Acta Radiol. 53:868–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kapisiz A, Karabulut R, Sonmez K,
Turkyilmaz Z, Poyraz A, Gulbahar O, Onal B, Ozbayoglu A and
Basaklar AC: Effect of stent placement, balloon or cutting balloon
dilatation on stricture formation after caustic esophageal burn in
rats. Eur J Pediatr Surg. 21:258–262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Speer E, Dunst CM, Shada A, Reavis KM and
Swanström LL: Covered stents in cervical anastomoses following
esophagectomy. Surg Endosc. Nov 11–2015.(Epub ahead of print).
|
|
28
|
Fuccio L, Hassan C, Frazzoni L, Miglio R
and Repici A: Clinical outcomes following stent placement in
refractory benign esophageal stricture: A systematic review and
meta-analysis. Endoscopy. 48:141–8. 2016.PubMed/NCBI
|
|
29
|
Mavi P, Niranjan R, Dutt P, Zaidi A,
Shukla JS, Korfhagen T and Mishra A: Allergen-induced resistin-like
molecule (Relm)-α promotes esophageal epithelial cell hyperplasia
in eosinophilic esophagitis. Am J Physiol Gastrointest Liver
Physiol. 307:G499–G507. 2014. View Article : Google Scholar : PubMed/NCBI
|