Introduction
Colorectal cancer (CRC) is among the most common
malignancies and the second leading cause of cancer-associated
mortality, following lung cancer (1). The 5-year survival rate for CRC is
still low because patients diagnosed with CRC have progressed to
the advanced stage (2–5). Survival rates have increased with the
introduction of irinotecan and oxaliplatin chemotherapy, as well as
the use of targeted therapies in the past decade (6–8).
Combined perioperative chemotherapy and surgery is a major
therapeutic treatment for patients with initially resectable liver
metastases from CRC (9–11). However, the development of drug
resistance in cancer cells raises a major challenge to chemotherapy
and restricts the anticancer efficacy of chemotherapeutic drugs
(12–14). Therefore, improving the sensitivity
to drug resistance remains an urgent requirement for
chemoresistance.
Human kallikrein 11 (KLK11) is a member of the human
KLK gene family and located at the chromosomal locus
19q13.3-q13.4 (15). Previous
experiments have indicated that KLK11 is ubiquitously expressed in
human brain, skin, stomach, breast, prostate, ovary and intestine
tissue (16). Recent results
demonstrated that KLKs were involved in human malignancies and that
KLK11 may be a favorable prognostic biomarker for ovarian and
prostate cancer due to the high serum levels of KLK11 in 70% of
women with ovarian cancer and in 60% of men with prostate cancer
(17). Alexopoulou et al have
shown that KLK11 mRNA expression was upregulated in colorectal
adenocarcinoma and could be considered as a new molecular
prognostic biomarker (18). However,
the value of KLK11 as a prognostic biomarker remains controversial
and more evidence is needed for further clinical application. It
has been reported that KLK11 mRNA expression could serve as a novel
and independent biomarker for diagnosis and prognosis in laryngeal
cancer (19). Unal et al have
suggested that KLK11-positive patients had higher disease-free
survival and overall survival compared to those with KLK11-negative
expression (20). However, little is
known concerning the possible involvement of KLK11 in human
CRC.
The aim of the present study was to investigate the
role of KLK11 in human CRC. Additionally, the potential use of
shRNA-mediated KLK11 gene knockdown associated with apoptosis and
drug resistance were further examined.
Materials and methods
Cell culture and reagents
Two human-derived CRC cell lines LOVO (CCL-229) and
HCT-8 (CCL-244) were obtained from the American Type Culture
Collection (Manassas, VA, USA) and cultured with RPMI-1640
(Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100
U/ml penicillin and 100 mg/ml streptomycin (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in 5% CO2 at
37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA from cells was isolated using TRIzol
reagent (Invitrogen) according to the manufacturer's directions.
Then, 1 µg total RNA was used for reverse transcription reaction
using SuperScript III reverse transcriptase (Invitrogen). qPCR was
performed using an ABI 7500 real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc., Foster City, CA, USA),
and the mRNA expression of human KLK11 and β-actin was evaluated
using a LightCycler Fast Start DNA Master SYBR Green I kit (Roche
Diagnostics GmbH, Mannheim, Germany). PCR amplification was
performed by denaturation at 95°C for 10 min, annealing and
extension at 60°C for 60 sec for 40 cycles. RT-qPCR analysis was
performed using the following primers: KLK11 forward:
5′-GTTCGAGAAGACGCGGCTAC-3′; KLK11 reverse:
5′-GGTGGGAGAGGTGAGTGAC-3′. β-actin forward: 5-CCA ACC GCG AGA AGA
TGA-3′; β-actin reverse: 5′-CCAGAGGCGTACAGGGATAG-3′. The relative
expression level of KLK11 was calculated using the ΔΔCq method
(21) and normalized against that of
β-actin. All PCR amplification was performed in triplicate and
repeated in three independent experiments.
Gene silencing with the lentivirus
encoding specific shRNA
In order to silencing KLK11, the short hairpin RNA
(shRNA) were generated by ligating synthetic oligonucleotides
(Invitrogen) against the target genes into the AgeI and
EcoRI sites of pLKO.1-TRC cloning vector (provided by Dr
Xuchao Zhu; Tenth People's Hospital, Affiliated to Tongji
University, Shanghai, China). The sequences of the KLK11 shRNA
(shKLK11) and shRNA control (SCR) were as follows: KLK11-SH1 sense,
5′-CCGGCCAACAACAACCACCGCAATGCTCGCACATTGCGGTGGTCTTTGTTGGTTTTTG-3′
and antisense,
5′-AATTCAAAAACCAACAACAACCACCGCAATGCTCGCACATTGCGGTGGTCTTTGTTGG-3′;
KLK11-SH2 sense,
5′-CCGGGCAATGCTGTCACTTAATAATCTCGCAATTATTACATGACCACATCTCTTTTTG-3′
and antisense,
5′-AATTCAAAAAGCAATGCTGTCACTTAATAATCTCGCAATTATTACATGACCACATCTC-3′;
KLK11-SH3 sense,
5′-CCGGCTGGTCTGTAACCCATCTCTTCTCGCAACAAGACTGGTTACAGACCCATTTTTG-3′
and antisense,
5′-AATTCAAAAACTGGTCTGTAACCCATCTCTTCTCGCAACAAGACTGGTTACAGACCAG-3′;
control shRNA sense,
5′-CCGGAAACTACCGTTGTTATCAGTGTTCACAAGACACCTATAACAACGGTCATTTTTTTTG-3′
and antisense,
5′-AATTCAAAAAAAACTACCGTTGTTATCAGTGTCTCTTGAACACCTATAACAACGGTCATTT-3′.
Lentiviral virions were produced by co-transfection of HEK293T
cells with 5 µg pLKO.1-puro vector and 5 µg packaging and envelope
vectors using Lipofectamine 2000 (Invitrogen) according to the
manufacturer's protocol. Lentivirus was harvested 48 h after
transfection. LOVO and HCT-8 cells were infected with lentivirus
containing shKLK11 or SCR for 24 h. Two days later, the
virus-infected cells were selected by 2 µg/ml puromycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 48 h and
subjected to required assays.
Cell viability assay
Cell viability was quantified using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as previously described (22).
Briefly, 3×103 transiently transfected LOVO and HCT-8
cells (SCR or shKLK11) were seeded in 96-well plates and 20 µl MTT
solution (5 mg/ml; Sigma-Aldrich) was added to each well 72 and 96
h later. The optical density was measured using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 595 nm.
For drug sensitivity, cells were plated in 96-well
plates at 5×104 cells per well, followed by treatment
with 0, 5 or 10 µmol/lL-OHP for 24 h. The optical density was then
measured and the cell viability was calculated.
Annexin V-FITC apoptosis
detection
Apoptosis detection was performed using an Annexin V
Apoptosis Detection kit I (BD Biosciences, Franklin Lakes, NJ,
USA). In brief, cells were collected and washed with
phosphate-buffered saline (PBS). Then, 5 µl annexin V and propidium
iodide was added to the cell suspension and incubated at room
temperature in the dark for 30 min. The volume was then made up to
500 µl and the cells were analyzed using a FACSCalibur flow
cytometer (BD Biosciences).
Caspase-3 activity analysis
The activity of caspase-3 was measured using a
Caspase-3 Assay kit (Abnova Corporation, Taipei, Taiwan) according
to the manufacturer's instructions. In brief, 5×106
cells were harvested, resuspended in 50 µl chilled cell lysis
buffer and incubated on ice for 10 min. Then, 50 µl 2.0X Reaction
Buffer was added to each sample, along with 5 µl DEVD-pNA (4 mM)
substrate and incubated for 2 h at 37°C. The optical density was
measured at 405 nm using a microplate reader (Bio-Rad Laboratories,
Inc.).
Western blot analysis
Cell lysates were prepared in a buffer containing 50
mM Tris-HCl (pH 7.5), 1 mM EDTA, 1% NP-40 (v/v) and 150 mM NaCl,
supplemented with a mixture of complete protease inhibitors (Roche
Diagnostics, Basel, Switzerland). Equal quantities of protein (40
µg) were then separated on 10% SDS-PAGE and blotted onto a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.).
Blocking was performed at room temperature using Tris-buffered
saline with 0.1% Tween-20 (TBST; J&K Chemical Ltd., Shanghai,
China) containing 5% non-fat milk for 1 h. The membrane was then
incubated with primary mouse monoclonal KLK11 antibody (sc-20387;
1:500) and rabbit polyclonal β-actin (sc-47778; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), Bcl-2 (#2872; 1:1,000) and
Bax (#2772; 1:1,000) antibodies (Cell Signaling Technology, Inc.,
Danvers, MA, USA) in TBST at 4°C overnight and with the appropriate
horseradish peroxidase-conjugated secondary antibody (CW0103M;
1:3000; CWbiotech, Beijing, China) for 1 h at room temperature.
Specific antibody binding was detected using an ECL system (GE
Healthcare, Piscataway, NJ, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS software, version
16.0 (SPSS, Inc., Chicago, IL, USA). Statistical significance was
considered to be indicated by P<0.05.
Results
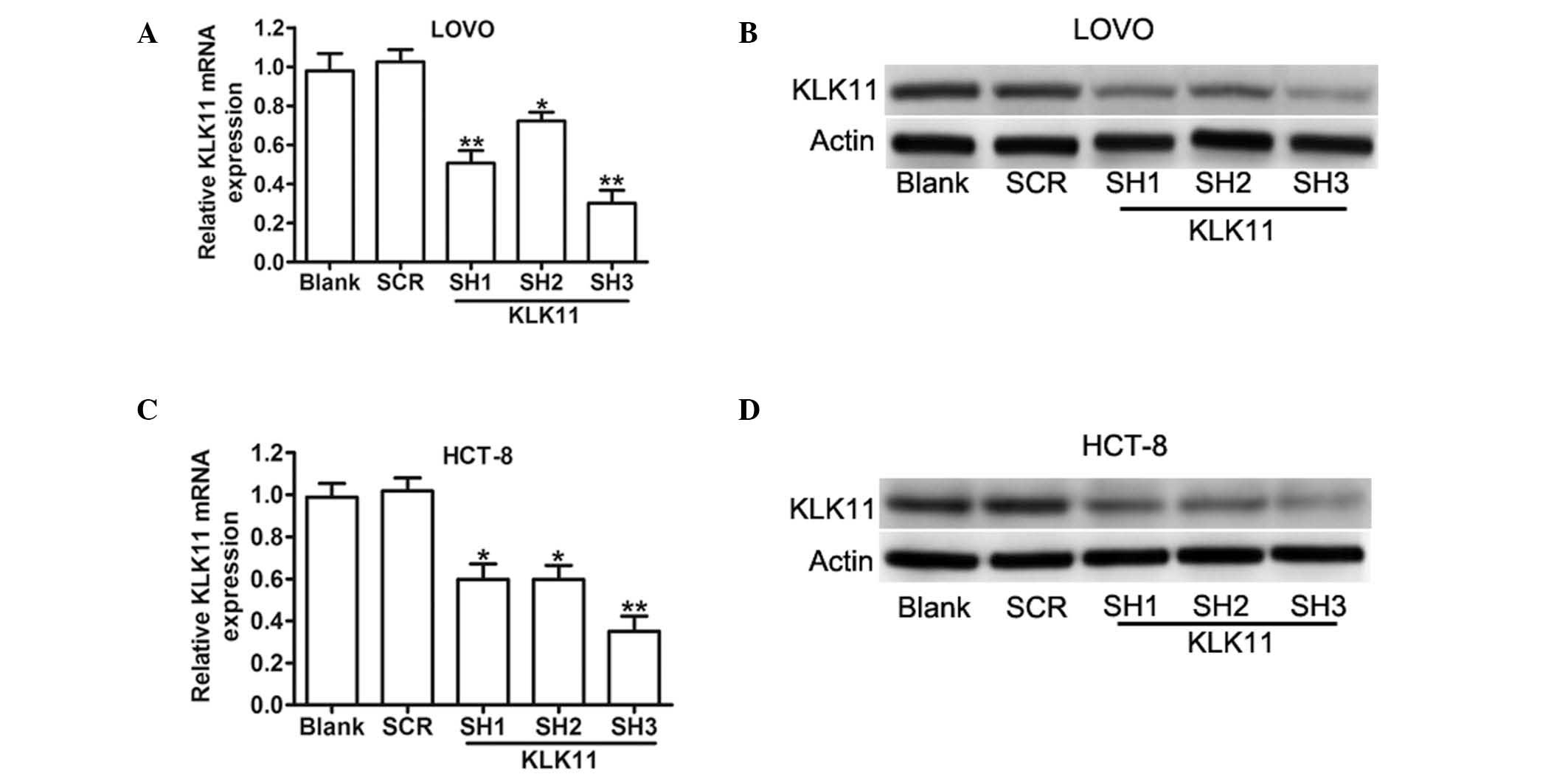
Stable knockdown of KLK11 specifically
inhibits the expression of KLK11 mRNA and protein in CRC cells
A previous study has shown that the expression of
KLK11 was upregulated in colorectal tumors (18). To determine whether KLK11 is involved
in the progression of CRC, three different lentivirus-based shRNAs
(KLK11 SH1, KLK11 SH2 and KLK11 SH3) were employed to downregulate
KLK11 expression. The mRNA and protein levels of KLK11 in stably
transfected LOVO and HCT-8 cells were confirmed using RT-qPCR and
western blot analysis (Fig. 1). The
mRNA expression levels of KLK11 were significantly decreased in
KLK11 SH3 groups in both cell lines. The subsequent assays were
performed with KLK11 SH3, which is furthermore referred to as
shKLK11. These results suggested that the lentivirus-mediated shRNA
targeting KLK11 could effectively knockdown KLK11 expression in CRC
cells.
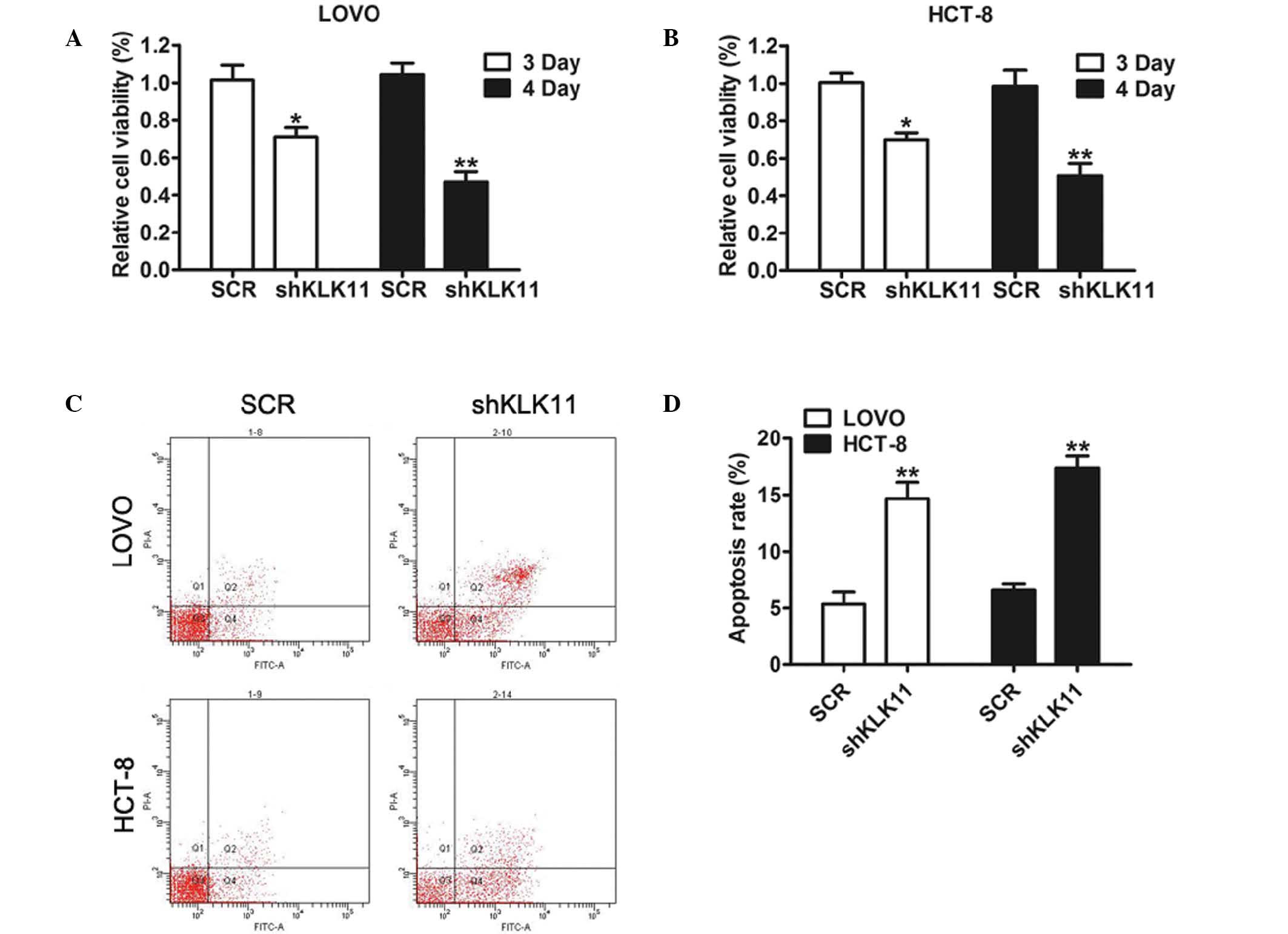
Downregulation of KLK11 inhibits
growth and enhances apoptosis of CRC cells
To determine the biological function of KLK11 in CRC
progression, MTT assays were used to examine the proliferative
ability of CRC cells. As shown in Fig.
2A and B, the proliferation rates of shKLK11-infected cells
started to decrease and were reduced compared with those of the SCR
groups on days 3 and 4.
There is considerable evidence indicating that
apoptosis has a close association with cell growth (23). In the present study, apoptosis assays
were performed using CRC cells following KLK11 silencing. The
results of flow cytometric analysis indicated that the percentages
of apoptotic cells were significantly increased in shKLK11-infected
LOVO and HCT-8 cells compared with the respective SCR groups
(Fig. 2C and D). This finding
indicated that KLK11 may serve a crucial function in the
proliferation and tumorigenesis of CRC cells in vitro.
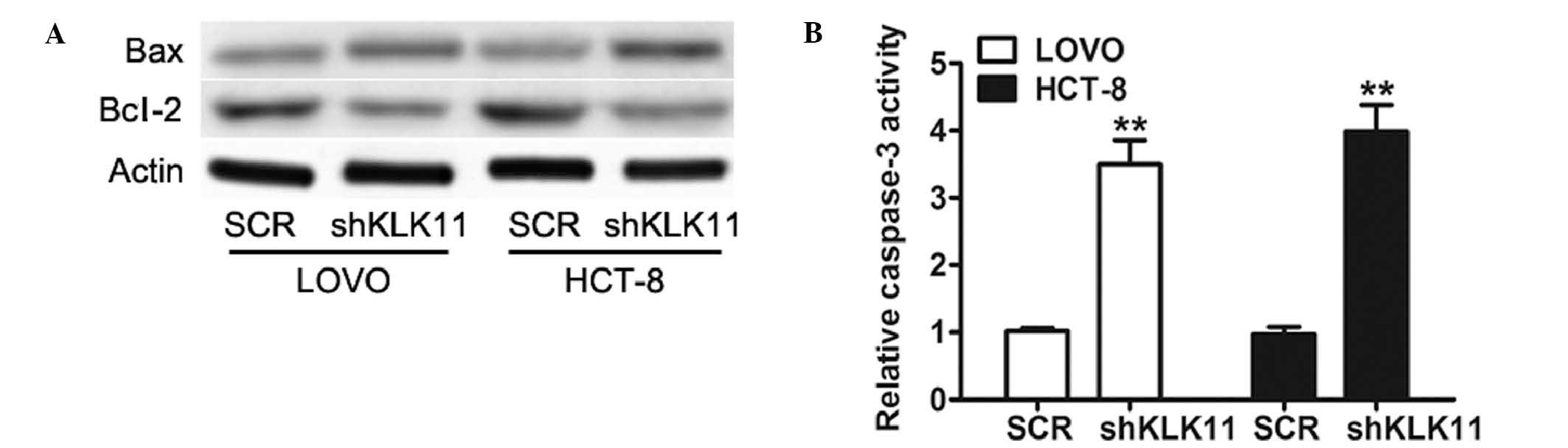
Downregulation of KLK11 expression
inactivates the apoptosis signaling pathway in CRC cells
To examine the mechanism underlying the inhibition
of cell growth, the expression of Bcl-2 and Bax, two important
proteins of the apoptosis signaling pathway (24) was investigated. Western blot analysis
showed that knockdown of KLK11 lead to a reduction of Bcl-2 and an
increase of Bax in both cell lines (Fig.
3A). Caspase-3, a crucial mediator of apoptosis, is a
frequently activated death protease, catalyzing the specific
cleavage of various key cellular proteins (25). A significant increase in caspase-3
activity was detected in shKLK11-infected LOVO and HCT-8 cells
compared with SCR groups (Fig. 3B).
Collectively, these data demonstrated that the proliferative effect
of KLK11 in CRC cells is regulated via the apoptosis pathway.
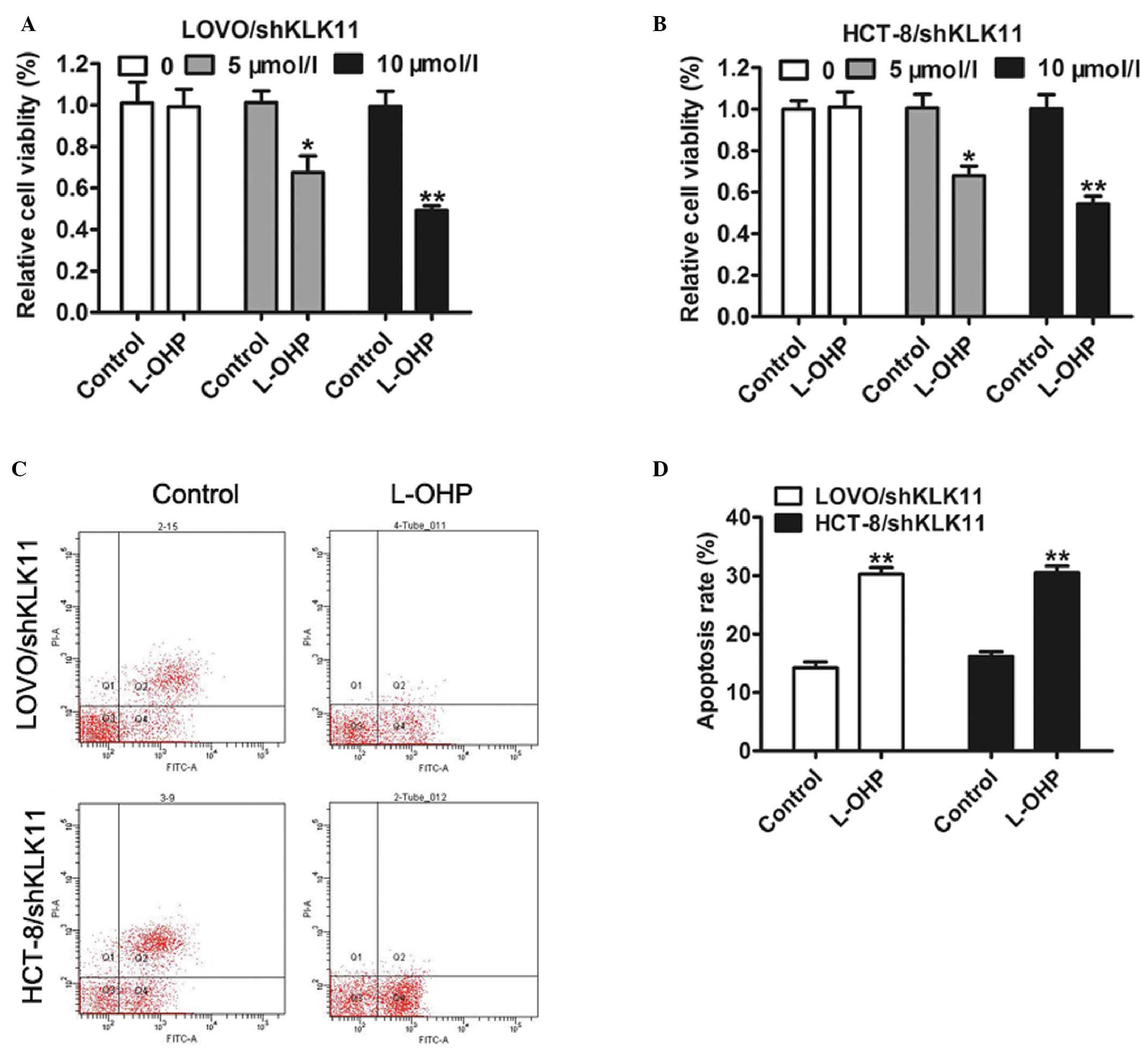
KLK11 silencing enhances sensitivity
of CRC cells to L-OHP and L-OHP-induced apoptosis in vitro
Our previous study has shown that dysregulation of
KLK11 expression had an association with FOLFOX4 chemotherapy in
human CRC cells (26). However,
whether KLK11 played a key role in affecting the sensitivity of CRC
cells was not fully understood. To elaborate on this, LOVO and
HCT-8 cells with stable KLK11 silencing were treated with 0, 5 or
10 µmol/l L-OHP for 24 h. The results of MTT assay suggested that
knockdown of KLK11 led to a significant reduction in the viability
of CRC cells in response to L-OHP in a dose-dependent manner
compared with control (Fig. 4A and
B). Furthermore, flow cytometric analysis showed that the
apoptotic rate of cells with stable KLK11 silencing treated with
L-OHP was significantly higher than that of cells treated with
control (Fig. 4C and D).
Collectively, these results suggest that knockdown of KLK11 could
increase the chemosensitivity of CRC cells to L-OHP by inducing
apoptosis enhancement in vitro.
Discussion
Overexpression of KLK11 is a general feature in
numerous human malignancies including CRC, and the overexpression
is often correlated with malignant behavior (27). In the present study, it was found
that KLK11 silencing inhibited the growth and increased the
apoptosis of CRC cells. Downregulation of KLK11 also increased
caspase-3 activity by activating the apoptosis signaling pathway,
which induced a reduction of the Bcl-2/Bax ratio.
KLK11 is a member of the KLK family, which are
dysregulated in multiple tumors (17). Previous experiments have shown that
KLK11 was upregulated in malignant CRC tissues in comparison with
noncancerous tissues, and was associated with highly invasive and
positive nodal status (18).
Therefore, we hypothesized that KLK11 might be an oncogene in
colorectal tumors. As expected, lentivirus-mediated KLK11 silencing
was able to effectively suppress the proliferation of colon cancer
cells in vitro. Furthermore, knocking down KLK11 resulted in
a significant upregulation of apoptosis in CRC cells. From these
data, we suggest that KLK11 has a positive impact on the
progression of CRC cells in vitro.
In order to determine the underlying mechanisms by
which KLK11 is involved in cell growth, the present study analyzed
apoptosis signaling in CRC cells. Bcl-2 and Bax, two crucial
regulatory proteins that play important roles in the induction of
apoptosis have been reported to regulate cancer growth (28,29). The
results of the present study indicated that KLK11 silencing may
activate the apoptosis signaling pathway by increasing the
expression level of Bax and decreasing the expression level of
Bcl-2, which induced a reduction of the Bcl-2/Bax ratio.
Furthermore, the downregulation of KLK11 promotes caspase-3
activity, which results in the death of tumor cells.
There is considerable evidence supporting the
hypothesis that mechanisms involved in resistance to chemotherapy
correlate with apoptosis (30). The
present study next investigated whether the KLK11 silencing was
associated with sensitivity to L-OHP. Consistent with the above
data, KLK11 silencing resulted in a higher inhibition of cell
growth and apoptosis following exposure to L-OHP.
Based on these data, we conclude that knockdown of
KLK11 could inhibit cell proliferation, induce apoptosis and
increase the sensitivity of CRC cells to L-OHP in vitro,
which may offer a novel therapeutic approach for L-OHP-resistant
CRC treatment. Further studies are required to determine whether
these findings are present in vivo.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Talbot R and Kirkham S: Colorectal cancer.
Lancet. 376:330author reply 331–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SY, Choi EJ, Yun JA, Jung ES, Oh ST,
Kim JG, Kang WK and Lee SH: Syndecan-1 expression is associated
with tumor size and EGFR expression in colorectal carcinoma: A
clinicopathological study of 230 cases. Int J Med Sci. 12:92–99.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuo IM, Huang SF, Chiang JM, Yeh CY, Chan
KM, Chen JS and Yu MC: Clinical features and prognosis in
hepatectomy for colorectal cancer with centrally located liver
metastasis. World J Surg Oncol. 13:922015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Nordlinger B, Adam R, Köhne
CH, Pozzo C, Poston G, Ychou M and Rougier P: European Colorectal
Metastases Treatment Group: Towards a pan-European consensus on the
treatment of patients with colorectal liver metastases. Eur J
Cancer. 42:2212–2221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Díaz-Rubio E, Tabernero J, Gómez-España A,
Massutí B, Sastre J, Chaves M, Abad A, Carrato A, Queralt B, Reina
JJ, et al: Phase III study of capecitabine plus oxaliplatin
compared with continuous-infusion fluorouracil plus oxaliplatin as
first-line therapy in metastatic colorectal cancer: Final report of
the Spanish Cooperative Group for the Treatment of Digestive Tumors
Trial. J Clin Oncol. 25:4224–4230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nordlinger B, Sorbye H, Glimelius B,
Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole
ET, Finch-Jones M, et al: Perioperative chemotherapy with FOLFOX4
and surgery versus surgery alone for resectable liver metastases
from colorectal cancer (EORTC Intergroup trial 40983): A randomised
controlled trial. Lancet. 371:1007–1016. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niitsu H, Hinoi T, Shimomura M, Egi H,
Hattori M, Ishizaki Y, Adachi T, Saito Y, Miguchi M, Sawada H, et
al: Up-front systemic chemotherapy is a feasible option compared to
primary tumor resection followed by chemotherapy for colorectal
cancer with unresectable synchronous metastases. World J Surg
Oncol. 13:1622015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tu J, Yu Y, Liu W and Chen S: Significance
of human epidermal growth factor receptor 2 expression in
colorectal cancer. Exp Ther Med. 9:17–24. 2015.PubMed/NCBI
|
|
12
|
Tol J, Koopman M, Cats A, Rodenburg CJ,
Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ,
Sinnige HA, et al: Chemotherapy, bevacizumab and cetuximab in
metastatic colorectal cancer. N Engl J Med. 360:563–572. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seki K, Tsuduki Y, Ioroi T, Yamane M,
Yamauchi H, Shiraishi Y, Ogawa T, Nakata I, Nishiguchi K,
Matsubayashi T, et al: Serum lactate dehydrogenase levels as a
predictive marker of oxaliplatin-induced hypersensitivity reactions
in Japanese patients with advanced colorectal cancer. Int J Med
Sci. 11:641–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Zhang C, Guo J, Huang J, Xi X,
Zhang L and Cui X: Super-compact treatment with a high dose of
moxifloxacin in patients with drug-resistant tuberculosis and its
resistance mechanisms. Exp Ther Med. 9:1314–1318. 2015.PubMed/NCBI
|
|
15
|
Gan L, Lee I, Smith R, Argonza-Barrett R,
Lei H, McCuaig J, Moss P, Paeper B and Wang K: Sequencing and
expression analysis of the serine protease gene cluster located in
chromosome 19q13 region. Gene. 257:119–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura T, Mitsui S, Okui A, Miki T and
Yamaguchi N: Molecular cloning and expression of a variant form of
hippostasin/KLK11 in prostate. Prostate. 54:299–305. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mavridis K and Scorilas A: Prognostic
value and biological role of the kallikrein-related peptidases in
human malignancies. Future Oncol. 6:269–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alexopoulou DK, Kontos CK, Christodoulou
S, Papadopoulos IN and Scorilas A: KLK11 mRNA expression predicts
poor disease-free and overall survival in colorectal adenocarcinoma
patients. Biomark Med. 8:671–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patsis C, Yiotakis I and Scorilas A:
Diagnostic and prognostic significance of human kallikrein 11
(KLK11) mRNA expression levels in patients with laryngeal cancer.
Clin Biochem. 45:623–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Unal D, Tasdemir A, Oguz A, Eroglu C,
Cihan YB, Turak EE, Karaman H and Soyuer S: Is human kallikrein-11
in gastric cancer treated with surgery and adjuvant
chemoradiotherapy associated with survival? Pathol Res Pract.
209:779–783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL,
Ye QH, Qin LX and Wu XZ: microRNA-29a suppresses cell proliferation
by targeting SPARC in hepatocellular carcinoma. Int J Mol Med.
30:1321–1326. 2012.PubMed/NCBI
|
|
23
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou F, Yang Y and Xing D: Bcl-2 and
Bcl-xL play important roles in the crosstalk between autophagy and
apoptosis. FEBS J. 278:403–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Lu X, Chi P and Pan J:
Identification of HOXB8 and KLK11 expression levels as potential
biomarkers to predict the effects of FOLFOX4 chemotherapy. Future
Oncol. 9:727–736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen YG, Wang Q, Zhou CZ, Yan DW, Qiu GQ,
Yang C, Tang HM and Peng ZH: Identification and validation of
Kallikrein-ralated peptidase 11 as a novel prognostic marker of
gastric cancer based on immunohistochemistry. J Surg Oncol.
104:516–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pettersson F, Dalgleish AG, Bissonnette RP
and Colston KW: Retinoids cause apoptosis in pancreatic cancer
cells via activation of RAR-gamma and altered expression of
Bcl-2/Bax. Br J Cancer. 87:555–561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan W, Xiaoyun H, Haifeng Q, Jing L,
Weixu H, Ruofan D, Jinjin Y and Zongji S: MicroRNA-218 enhances the
radiosensitivity of human cervical cancer via promoting radiation
induced apoptosis. Int J Med Sci. 11:691–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|