Introduction
Liver cirrhosis is a common hepatic disease, which
is characterized by the hyper-accumulation of connective tissue
components and hepatic necrosis. Chronic alcohol consumption has
been demonstrated to be a major factor that leads to the
development of hepatic cirrhosis. In patients with liver cirrhosis,
there is high coincidence of portal hypertension, esophageal
varices, and hypoalbuminaemia. Peptide growth factors and cytokines
have a pertinent role in the pathogenesis of liver cirrhosis
(1).
Fetuin-A is a glycoprotein secreted by the liver,
kidneys and choroid plexus, which has been associated with
calcification and fibrosis in rat and human studies (2,3). Fetuin
A, an extracellular inhibitor of transforming growth factor β
(4), is a profibrogenic stimulus in
liver disease (5). Circulating
fetuin-A may be a beneficial serum biomarker in the detection of
liver and vascular fibrosis progression in patients with
non-alcoholic fatty liver disease (6).
Osteoprotegerin (OPG), a secretory glycoprotein
belonging to the tumor necrosis factor (TNF) receptor superfamily,
exhibits pleiotropic effects on inflammation, endocrine function
and the immune system. Serum OPG concentrations may serve as a
noninvasive biomarker to identify patients with nonalcoholic
steatohepatitis (7). OPG inhibits
the recruitment, proliferation and activation of osteoclasts and
has a role in the regulation of bone mass, acting as a soluble
factor (8,9). α-Klotho protein is a 130 kDa,
one-transmembrane protein and its expression is confirmed in the
kidneys and the parathyroid glands; α-Klotho is a key regulator of
mineral homeostasis and bile acid/cholesterol metabolism (10,11).
Limited studies have been conducted to investigate serum Fetuin-A,
OPG and α-Klotho protein concentrations in patients with alcoholic
liver cirrhosis (12,13).
The aim of the present study was to evaluate the
concentrations of fetuin-A, OPG and α-Klotho protein in patients
with alcoholic cirrhosis in different stages of the disease in
order to determine whether these glycoproteins may be used as
markers of the severity of cirrhosis.
Materials and methods
Patients with alcoholic liver cirrhosis treated in
various hospitals of the Lublin region were randomly enrolled in
the present study. The Bioethics Committee at Medical University of
Lublin, Poland approved the protocol of the study. The study group
consisted of 40 male (74%) and 14 female (26%) patients. All
patients presented a history of heavy alcohol consumption in
absence of positivity for serological viral markers. The stages of
cirrhosis were assessed using the Child-Turcotte-Pugh criteria as
Child-Pugh scores (P-Ch) A, B and C. All patients exhibited normal
serum calcium levels and none had previously received treatment
with agents that may potentially have influenced bone metabolism.
The control group consisted of 18 healthy individuals who did not
have liver disease or drink alcohol. All patients gave their
written consent. Characteristics of the study population are
presented in Tables I and II. Cases and controls were age- and
gender-matched.
 | Table I.Characteristics of patients with
alcoholic liver cirrhosis and healthy controls. |
Table I.
Characteristics of patients with
alcoholic liver cirrhosis and healthy controls.
| Characteristic | Control (n=18) | P-Ch A (n=14) | P-Ch B (n=20) | P-Ch C (n=20) |
|---|
| Age (years) | 55.51±8.89 | 52.50±16.11 | 54.00±12.19 | 50.71±10.00 |
| Body weight
(kg) | 75.63±9.83 | 66.33±11.93 | 84.84±27.11 | 85.91±21.76 |
| Height (cm) | 173.54±10.31 | 171.33±9.86 | 177.36±11.40 | 175.45±6.69 |
| Drinking period
(years) | – | 11.16±7.40 | 13.86±7.06 | 18.17±10.73 |
| Existing
symptoms |
|
|
|
|
|
Ascites | 0 | 1 | 14 | 15 |
|
Encephalopathy | 0 | 3 | 8 | 18 |
|
Oesophageal varices | 0 | 2 | 9 | 16 |
 | Table II.Biochemical data of the study
participants. |
Table II.
Biochemical data of the study
participants.
| Variable | Control (n=18) | P-Ch A (n=14) | P-Ch B (n=20) | P-Ch C (n=20) |
|---|
| Bilirubin
(mg/dl) | 0.64±0.22 | 2.70±0.95 | 12.31±4.48 | 15.75±4.87 |
| Albumin (g/dl) | 5.23±0.54 | 4.00±0.67 | 3.80±0.84 | 2.42±0.48 |
| ALT (U/l) | 19.24±8.56 | 99.00±221.00 | 41,57±29,48 | 61.24±104.60 |
| AST (IU/l) | 17.81±5.03 |
143.00±249.0 |
96.67±101.57 | 132.00±202,06 |
| GGTP (IU/l) | 20.40±8.96 | 313.75±27.96 | 642.24±70.04 | 749.48±72.55 |
| Urea (mg/dl) | 24.40±10.07 | 38.77±6.98 | 44.81±8.54 | 51.25±5.39 |
| Blood platelets
(K/ul) | 340.2±7.96 | 166.75±11.96 | 135.46±12.28 | 105.33±7.02 |
| INR | 1.26±0.16 | 1.30±0.21 | 1.39±0.23 | 2.01±0.90 |
| MCV (fl) | 86.00±7.26 | 95.97±9.36 | 97.09±6.27 | 103.07±6.09 |
| Na (mmol/l) | 139.50±3.44 | 129.75±10.50 | 134.05±4.78 | 131.85±8.41 |
| K (mmol/l) | 4.17±0.32 | 3.59±0.42 | 4.07±0.77 | 3.86±0.60 |
| Fetuin-A
(ng/ml) | 126.92±51.20 | 79.00±13.80 | 75.77±30.06 | 57.62±31.91 |
| OPG (pmol/l) | 2.89±1.46 | 7.05±2.20 | 8.92±4.41 | 6.56±2.22 |
| α-Klotho
(pg/ml) | 645.15±131.30 | 719.00±559.00 | 1110.50±911.50 | 1091.40±774.50 |
Liver cirrhosis diagnosis was based on clinical
features, laboratory tests, abdominal ultrasound imaging and
history of heavy alcohol consumption.
The tissue sample used in the present study was
peripheral blood obtained from the ulnar vein. Blood samples (7 ml)
were collected into clot tubes between 08:00 and 10:00 following an
8–12 h overnight fast. Serum was separated by centrifugation for of
10 min at 1,300 RCF at room temperature, aliquoted and stored at
−20°C prior to analysis.
Fetuin-A
Serum fetuin-A concentration was determined using a
human fetuin-A ELISA kit (Epitope Diagnositics, Inc., San Diego,
CA, USA; cat. no. KT-800), according to the manufacturer's
protocol. The assay utilized the two-site ‘sandwich’ technique with
two selected goat anti-human polyclonal fetuin-A antibodies that
bind to different epitopes of human fetuin-A. Assay sensitivity was
5.0 ng/ml.
OPG
Serum total OPG concentration was determined using a
human OPG enzyme immunoassay kit (Quidel Corporation, San Diego,
CA, USA; cat. no. 8034), according to the manufacturer's protocol.
According to the immunocapture technique, murine monoclonal
anti-human OPG and biotin-labeled polyclonal anti-human OPG
antibodies were used. Assay sensitivity was 0.4 pmol/l.
α-Klotho protein
Serum α-Klotho concentration was determined using a
human soluble α-Klotho assay kit (Immuno-Biological Laboratories;
Tecan Group, Ltd., Männedorf, Switzerland; cat. no. JP27998),
according to the manufacturer's protocol. The kit utilizes solid
phase sandwich ELISA using two types of highly specific antibodies.
Tetramethylbenzidine was used as a coloring agent. Assay
sensitivity was 6.15 pg/ml.
Biochemical parameters
Biochemical parameters, including aspartate
transaminase (AST), alanine transaminase (ALT), bilirubin, albumin,
C-reactive protein (CRP) and platelets (PLT) were measured. Serum
AST (ASTL; cat. no. 20764949322), ALT (ALTL; cat. no. 20764957322),
bilirubin (bilirubin; cat. no. 05795397190), albumin (Albumin Gen
2; cat. no. 03183688122), CRP (CRPLX; cat. no. 2076930322) were
measured using the described kits and a COBAS 6000 Clinical
Chemistry Analyzer (all Roche Diagnostics, Basel, Switzerland). PLT
count was calculated using Fluorocell PLT (cat. no. CD994563) and
an XN-2000 hematology autoanalyzer (both Sysmex Corporation, Kobe,
Japan). Normal laboratory reference ranges were used for
comparison.
Statistical analysis
Measurable variables were characterized with
arrhythmic means (M) and standard deviation. Frequencies of
occurrence were given for qualitative variables (number and
percentage). Measurable variables were assigned qualitative
categories.
Prior to calculations, the distribution of
measurable variables was evaluated using the K-S and Lilliefors
test and the Shapiro-Wilk test, whereas homogeneity of variances
was tested via the Brown-Forsythe test. Based on P-values, the lack
of normal distribution and/or homogeneity of variances were
determined. Differences in the variables analyzed were calculated
using the Mann-Whitney or Kruskal-Wallis tests. Inter-variable
correlations were checked using the Spearman correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Inter-group differences in the concentrations of
fetuin, OPG and α-Klotho were calculated using a non-parametric
Mann-Whitney U test. The effects of biochemical parameters,
including AST, ALT, bilirubin, albumin, CRP and PLT, on the levels
of fetuin-A, OPG and α-Klotho in patients with alcoholic liver
cirrhosis were analyzed using the Spearman rank correlation test.
Fetuin-A, OPG- and α-Klotho-dependent variables and an independent
variable ‘group’, which divides patients with alcoholic liver
cirrhosis into A, B and C types, and a control group of healthy
individuals without liver cirrhosis, were compared using the
Kruskal-Wallis rank test, which is a non-parametric equivalent of
analysis of variance.
Results
The findings of the present study study showed that
the concentration of fetuin-A in the control group was
significantly increased (110.00 ng/ml), as compared with patients
with alcoholic cirrhosis (3.50 ng/ml; P<0.001). In contrast,
serum OPG concentration was significantly increased in patients
with alcoholic liver cirrhosis (7.49 pmol/l), as compared with
control patients (2,46 pmol/l; P<0.001). α-Klotho protein
concentration in patients with alcoholic cirrhosis (794.85) was not
significantly different from the control group (671.1; P>0.05).
These results are shown in Table
III. Moreover, the associations between fetuin-A, OPG and
α-Klotho concentrations and the stages of cirrhosis according to
P-Ch scores were analyzed.
 | Table III.Fetuin A, OPG and α-Klotho levels in
controls and patients with alcoholic liver cirrhosis. |
Table III.
Fetuin A, OPG and α-Klotho levels in
controls and patients with alcoholic liver cirrhosis.
|
| Study group |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Marker | Control (n=18) | Patients with liver
cirrhosis (n=54) | Z test
function | P-value | Significance |
|---|
| Fetuin-A
(ng/ml) | 126.92±51.00 | 71.65±27.89 | 4.505 | 0.0001 | P<0.001 |
| OPG (pmol/l) | 2.89±1.46 | 7.74±3.48 | −5.165 | 0.0001 | P<0.001 |
| α-Klotho
(pg/ml) | 645.00±131.00 | 1004.00±789.00 | −0.904 | 0.366 | P>0.05 |
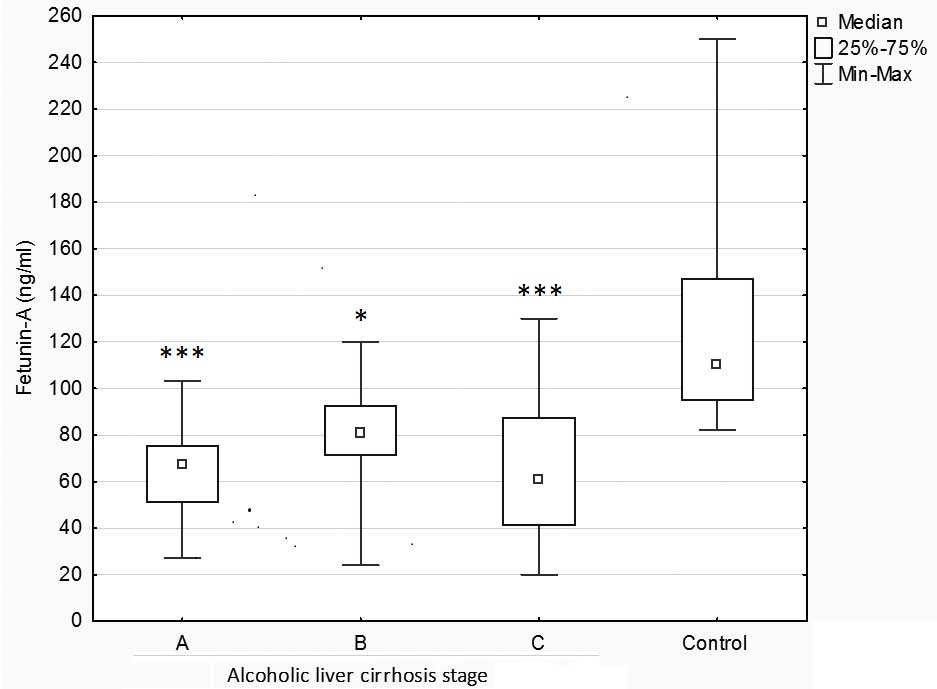
Fetuin-A concentration was significantly higher in
the control group compared to patients with alcoholic liver
cirrhosis at stages A, C (P<0.001) and B (P<0.05). No
significant differences in the concentrations of fetuin-A were
demonstrated according to the type of liver cirrhosis. However, it
should be noted that the lowest fetuin-A levels were detected in
subjects with the most severe liver cirrhosis (P-Ch C: 65.00 ng/ml
compared with P-Ch A: 77.50 ng/ml and P-Ch B: 67.50 ng/ml,
respectively; (Fig. 1).
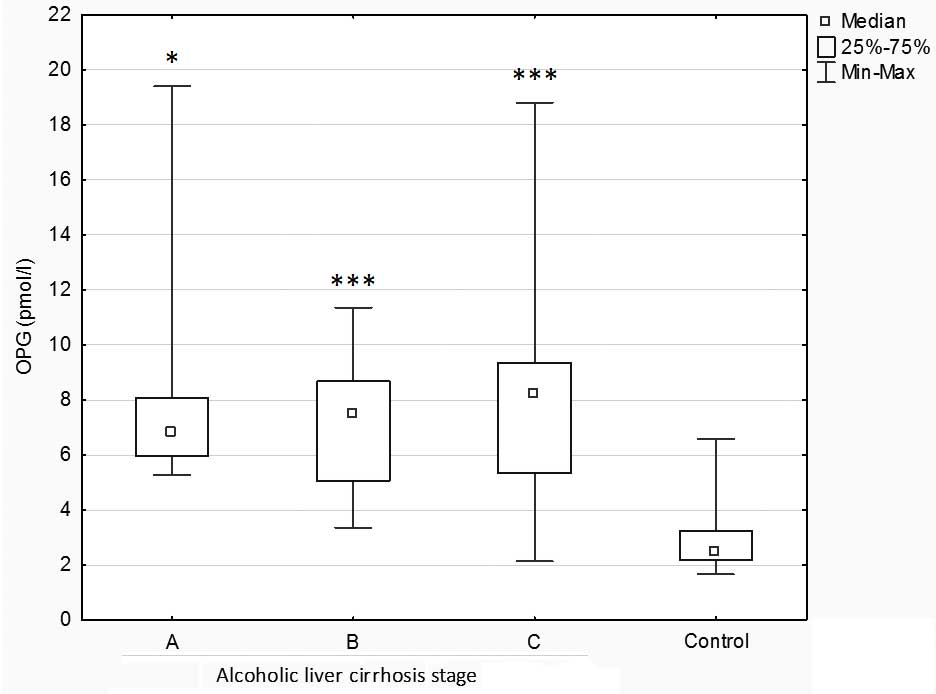
Further analysis demonstrated significantly
increased concentrations of OPG in patients with alcoholic liver
cirrhosis at stages B, C (P<0.001) and A (P<0.05) as compared
with the control group. No significant differences in OPG
concentrations were observed among the types of liver cirrhosis
(Fig. 2).
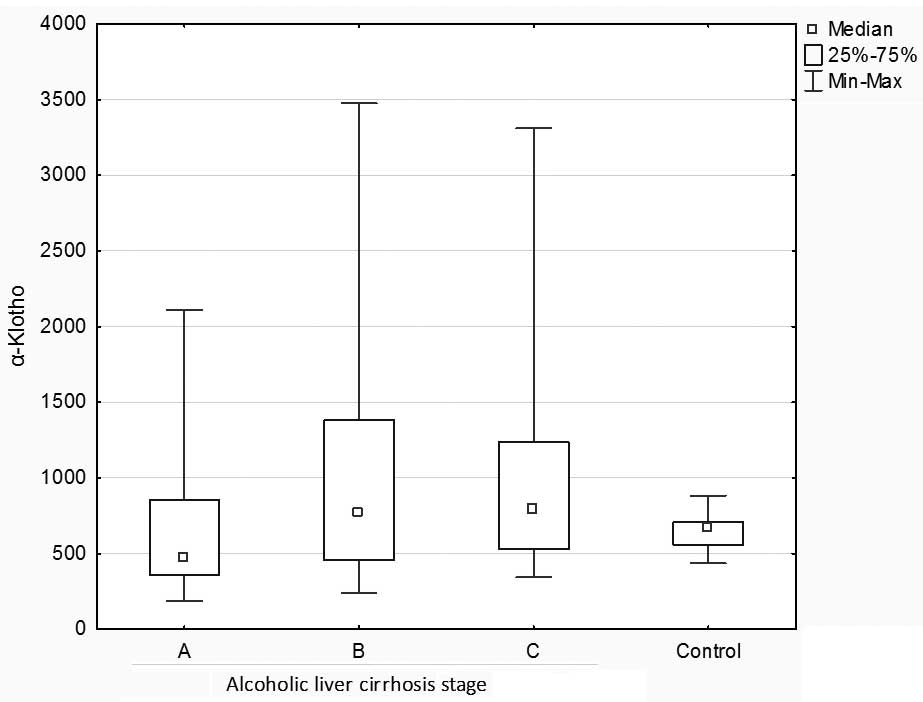
Furthermore, no statistically significant
differences in α-Klotho concentrations were demonstrated between
the control and liver cirrhosis groups, or among stages A, B and C
of alcoholic liver cirrhosis (Kruskal-Wallis: H=5.491033 P=0.1392;
Fig. 3).
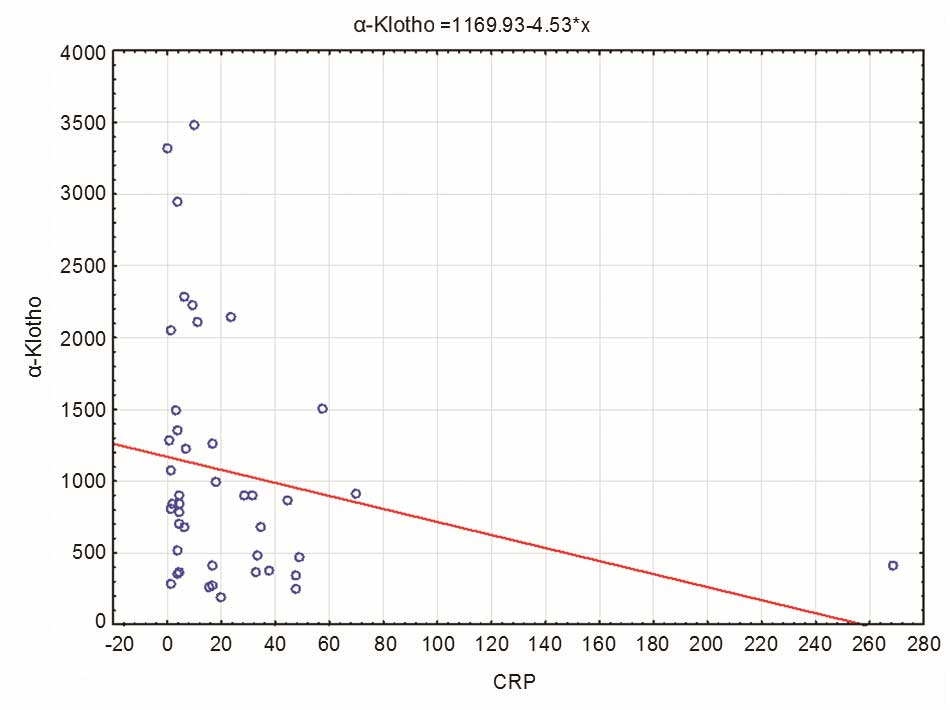
The results revealed a significant negative
correlation between the levels of α-Klotho and CRP in patients with
liver cirrhosis (Fig. 4), indicating
that an increase in CRP concentration may lead to a decrease in
α-Klotho concentration [R Spearman=−0.304; t(N-2)=−2.039; P=0.047].
No significant correlations were detected for the remaining
biochemical variables.
Discussion
Fetuin-A, which is also referred to as alpha
2-Heremans Schmid glycoprotein, is a multifunctional plasma agent
with a molecular weight of ~60 kDa and a half-life of several days
that is predominantly secreted by the liver in adults (>95%)
(14). Fetuin-A induces inflammatory
cytokine expression and inhibits adiponectin expression. An
increase of fetuin A serum levels has been observed in patients
with metabolic syndrome, type 2 diabetes and non-alcoholic fatty
liver disease (15). Furthermore,
fetuin-A has been demonstrated to act as an antagonist of
hepatocyte growth factor/scatter factor and as a regulator in
tissue regeneration (16), and it
may be a relevant biomarker of aortic valve disease (17).
Ohnishi et al (18) indicated that serum fetuin-A
concentrations were reduced in alcoholic subjects with liver
cirrhosis, which may be a consequence of reduced fetuin-A synthesis
by hepatocytes. The present study showed that patients with
alcoholic cirrhosis had significantly lower fetuin-A levels than
healthy subjects. These results are consistent with previous
findings reported by Kalabay et al (19), who demonstrated the same relationship
between fetuin-A levels and cirrhosis and hepatocellular carcinoma
(HCC). In addition, according to Kalabay et al (13), the tendency to decrease serum
fetuin-A concentration is a reliable and sensitive indicator of
mortality in patients with alcoholic liver cirrhosis.
Moreover, it has been suggested that decreased serum
fetuin-A levels indicate hepatocyte dysfunction rather than an
acute phase response (20). This
hypothesis may be supported by the lack of correlation between
fetuin-A concentration and CRP levels in patients with alcoholic
liver cirrhosis in the present study. An association between the
severity of liver cirrhosis, assessed by P-Ch scores, and fetuin-A
levels has not been determined at present. However, the findings of
the present study did not reveal a correlation between fetuin-A
concentration and the stage of alcoholic liver cirrhosis, and
selected laboratory parameters (ALT, ASP, CRP, bilirubin, albumins
and PLT). Notably, the lowest fetuin-A levels were detected in
subjects with the most severe liver cirrhosis.
Another protein investigated in the present study
was OPG. OPG is a glycoprotein member of the TNF ligand family that
is coded by a gene located on chromosome 8, and consists of 380
amino acids. The primary biological action of OPG is the inhibition
of osteooclast activity and differentiation from osteooclast
precursors (19). In the present
study, it was demonstrated that serum OPG levels were significantly
increased in patients with alcoholic liver cirrhosis than in
healthy subjects. No correlations were detected between the
severity of alcoholic liver cirrhosis, according to P-Ch scores (A,
B, C) and OPG levels. Previous studies have indicated increased OPG
levels in patients with primary biliary and viral cirrhosis
(21,22). Furthermore, a rise in glycoprotein
concentrations has been reported in subjects with alcoholic liver
cirrhosis, in accordance with the results of the present study. In
one such report, Fábrega et al (23) reported an increase of OPG levels in
30 patients with cirrhosis compared with 20 controls. Additionally,
OPG levels were found to be higher in patients with P-Ch C,
compared with those with P-Ch A. OPG is synthesized in the liver,
and expression of OPG mRNA has been detected in hepatocytes, bile
duct epithelium, Kupffer cells and lymphocytes (23). Moreover, the inflammatory cells that
infiltrate the liver in chronic alcoholic liver disease may have a
role in serum elevation of OPG in these subjects. Osteoblasts are
another primary source of OPG (24).
In patients with liver cirrhosis assessed in previous studies,
plasma levels of TNF-α, interleukin (IL)-1 and IL-6 were elevated
(25,26). OPG is likely to be stimulated by
proinflammatory cytokines. Elevated TNF-α and IL-6 levels are
strongly associated with OPG levels (27). TNF-α and IL-6 enhance bone
resorption; therefore, their association with OPG suggests a
protective effect of raised OPG on bone loss. Gaudio et al
(28) have previously shown an
increase in OPG levels, possibly in response to enhanced bone loss
in patients with liver cirrhosis. The present study indicates that
OPG is not a good indicator of the severity of cirrhosis.
The final protein tested in patients with liver
cirrhosis was α-Klotho, which is essential for mineral metabolism.
α-Klotho participates in the regulation of parathyroid hormone
secretion and vitamin D biosynthesis, in the transepithelial
transport of calcium ions in the choroid plexus and kidney, and in
renal phosphate re-absorption (28).
The association between α-Klotho serum concentration and alcoholic
liver cirrhosis has not yet been studied.
The results of the present study did not indicate
statistically significant differences in α-Klotho levels among
patients with alcoholic liver cirrhosis and controls. The stage of
disease had no effect on α-Klotho concentration. Previous studies
have characterized α-Klotho as an anti-aging hormone that modulates
antioxidant enzyme expression levels (29) and has an anti-inflammatory function
(30). Therefore, an adequate tissue
level of α-Klotho may provide protection against oxidative stress
and inflammation (31,32). Systemic and local inflammation is
associated with decreased renal α-Klotho expression levels
(33). In the present study,
α-Klotho concentration was negatively correlated with serum CRP
levels in patients with alcoholic liver cirrhosis, as increased
levels of CRP resulted in reduced concentrations of α-Klotho.
Therefore, it can be speculated that alcoholic liver cirrhosis,
which is a chronic inflammatory disorder, may be associated with
impaired α-Klotho expression and reduced soluble α-Klotho
concentrations. A similar negative correlation between α-Klotho
concentration and CRP level has been detected in a study conducted
by Navarro-González et al (34). This study showed that α-Klotho
concentration was reduced in patients with significant coronary
artery disease. Furthermore, α-Klotho has been revealed to have
tumor suppressive properties during various malignant
transformations by factor pathway (35), which is associated with cancer risk
and tumor progression. Xie et al (36) measured mRNA and protein expression
levels of α-Klotho in 64 HCC tumor tissues using real-time
polymerase chain reaction and immunohistochemistry, respectively,
demonstrating that a loss of α-Klotho expression in HCC cells was a
predictive factor for the poor prognosis of the disease. Similarly,
Shu et al (37) reported that
exogenous expression of the Klotho gene significantly
inhibited the proliferation of HCC cells, induced HCC cell
apoptosis and decreased HCC cell migration, using a Matrigel
invasion chamber assay. Conversely, following the analysis of 52
hepatoma patients, Chen et al (38) reported that immunohistochemical
α-Klotho staining was significantly associated with liver
cirrhosis, tumor multiplicity and venous invasion, and the survival
rate of subjects with high α-Klotho expression was significantly
decreased compared to patients with low α-Klotho expression.
In conclusion, fetuin-A concentration levels are
significantly reduced in patients with alcoholic liver cirrhosis
compared with controls, whereas α-Klotho levels are consistent
between the groups. No statistically significant differences in the
concentrations of fetuin-A, OPG and α-Klotho were demonstrated
according to the type of cirrhosis. Furthermore, a significant
negative correlation was demonstrated between the levels of
α-Klotho and CRP in patients with alcoholic liver cirrhosis. These
results indicate that fetuin-A, OPG and α-Klotho may not be useful
markers of the severity of liver cirrhosis, but fetuin-A and
ostoprotegerin concentration levels may be used as biomarkers of
alcoholic liver cirrhosis.
Acknowledgements
This study was supported by the Medical University
of Lublin, Poland (grant no. DS507).
Glossary
Abbreviations
Abbreviations:
|
OPG
|
osteoprotegerin
|
|
TNFR
|
tumor necrosis factor receptor
|
|
P-Ch
|
Child-Pugh score
|
|
HGF
|
hepatocyte growth factor
|
|
SF
|
scatter factor
|
|
TNF
|
tumor necrosis factor
|
|
IL
|
interleukin
|
|
HCC
|
hepatocellular carcinoma
|
References
|
1
|
Prystupa A, Szpetnar M,
Boguszewska-Czubara A, Grzybowski A, Sak J and Załuska W: Activity
of MMP1 and MMP13 and amino acid metabolism in patients with
alcoholic liver cirrhosis. Med Sci Monit. 21:1008–1014. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schinke T, Amendt C, Trindl A, Pöschke O,
Müller-Esterl W and Jahnen-Dechent W: The serum protein alpha2-HS
glycoprotein/fetuin inhibits apatite formation in vitro and in
mineralizing calvaria cells. A possible role in mineralization and
calcium homeostasis. J Biol Chem. 271:20789–20796. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karabakan M, Bozkurt A, Gunay M, Aktas BK,
Hirik E, Aydın M and Nuhoglu B: Association between serum fetuin-A
level and erectile function. Andrologia. 48:787–792. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swallow CJ, Partridge EA, Macmillan JC,
Tajirian T, DiGuglielmo GM, Hay K, Szweras M, Jahnen-Dechent W,
Wrana JL, Redston M, et al: Alpha2HS-glycoprotein, an antagonist of
transforming growth factor beta in vivo, inhibits intestinal tumor
progression. Cancer Res. 64:6402–6409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sato M, Kamada Y, Takeda Y, Kida S, Ohara
Y, Fujii H, Akita M, Mizutani K, Yoshida Y, Yamada M, et al:
Fetuin-A negatively correlates with liver and vascular fibrosis in
nonalcoholic fatty liver disease subjects. Liver Int. 35:925–935.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yilmaz Y, Yonal O, Kurt R, Oral AY, Eren
F, Ozdogan O, Ari F, Celikel CA, Korkmaz S, Ulukaya E, et al: Serum
levels of osteoprotegerin in the spectrum of nonalcoholic fatty
liver disease. Scand J Clin Lab Invest. 70:541–546. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lipton A, Ali SM, Leitzel K, Chinchilli V,
Witters L, Engle L, Holloway D, Bekker P and Dunstan CR: Serum
osteoprotegerin levels in healthy controls and cancer patients.
Clin Cancer Res. 8:2306–2310. 2002.PubMed/NCBI
|
|
9
|
Kainuma S, Otsuka T, Kuroyanagi G,
Yamamoto N, Matsushima-Nishiwaki R, Kozawa O and Tokuda H: Possible
involvement of AMP-activated protein kinase in PGE1-induced
synthesis of osteoprotegerin in osteoblasts. Exp Ther Med.
11:2042–2048. 2016.PubMed/NCBI
|
|
10
|
Tomiyama K, Maeda R, Urakawa I, Yamazaki
Y, Tanaka T, Ito S, Nabeshima Y, Tomita T, Odori S, Hosoda K, et
al: Relevant use of Klotho in FGF19 subfamily signaling system in
vivo. Proc Natl Acad Sci USA. 107:1666–1671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamazaki Y, Imura A, Urakawa I, Shimada T,
Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y,
et al: Establishment of sandwich ELISA for soluble alpha-Klotho
measurement: Age-dependent change of souble alpha-Klotho levels in
healthy subjects. Biochem Biophys Res Commun. 398:513–518. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Valdecasas-Campelo E,
González-Reimers E, Santolaria-Fernández F, De la Vega-Prieto MJ,
Milena-Abril A, Sánchez-Pérez MJ, Martínez-Riera A and
Gómez-Rodríguez Mde L: Serum osteoprotegerin and RANKL levels in
chronic alcoholic liver disease. Alcohol Alcohol. 41:261–266. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalabay L, Gráf L, Vörös K, Jakab L, Benko
Z, Telegdy L, Fekete B, Prohászka Z and Füst G: Human serum fetuin
A/alpha2HS-glycoprotein level is associated with long-term survival
in patients with alcoholic liver cirrhosis, comparison with the
Child-Pugh and MELD scores. BMC Gastroenterol. 7:152007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dabrowska AM, Tarach JS, Wojtysiak-Duma B
and Duma D: Fetuin-A (AHSG) and its usefulness in clinical
practice. Review of the literature. Biomed Pap Med Fac Univ Palacky
Olomouc Czech Repub. 159:352–359. 2015.PubMed/NCBI
|
|
15
|
Denecke B, Gräber S, Schäfer C, Heiss A,
Wöltje M and Jahnen-Dechent W: Tissue distribution and activity
testing suggest a similar but not identical function of fetuin-B
and fetuin-A. Biochem J. 376:135–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ix JH, Shlipak MG, Brandenburg VM, Ali S,
Ketteler M and Whooley MA: Association between human fetuin-A and
the metabolic syndrome: Data from the heart and soul study.
Circulation. 113:1760–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng YI, Sun R, Li X, Liu M, Chen S and
Zhang P: Pathophysiology of valvular heart disease. Exp Ther Med.
11:1184–1188. 2016.PubMed/NCBI
|
|
18
|
Ohnishi T, Nakamura O, Arakaki N and
Daikuhara Y: Effect of phosphorylated rat fetuin on the growth of
hepatocytes in primary cultures in the presence of human
hepatocyte-growth factor. Evidence that phosphorylated fetuin is a
natural modulator of hepatocyte-growth factor. Eur J Biochem.
243:753–761. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalabay L, Jakab L, Prohászka Z, Füst G,
Benkö Z, Telegdy L, Lörincz Z, Závodszky P, Arnaud P and Fekete B:
Human fetuin/alpha2HS-glycoprotein level as a novel indicator of
liver cell function and short-term mortality in patients with liver
cirrhosis and liver cancer. Eur J Gastroenterol Hepatol.
14:389–394. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jezequel M, Seta NS, Corbic MM, Feger JM
and Durand GM: Modifications of concanavalin A patterns of alpha
1-acid glycoprotein and alpha 2-HS glycoprotein in alcoholic liver
disease. Clin Chim Acta. 176:49–57. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hofbauer LC, Khosla S, Dunstan CR, Lacey
DL, Boyle WJ and Riggs BL: The roles of osteoprotegerin and
osteoprotegerin ligand in the paracrine regulation of bone
resorption. J Bone Miner Res. 15:2–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szalay F, Hegedus D, Lakatos PL, Tornai I,
Bajnok E, Dunkel K and Lakatos P: High serum osteoprotegerin and
low RANKL in primary biliary cirrhosis. J Hepatol. 38:395–400.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fábrega E, Orive A, García-Suarez C,
García-Unzueta M, Amado J Antonio and Pons-Romero F:
Osteoprotegerin and RANKL in alcoholic liver cirrhosis. Liver Int.
25:305–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moschen AR, Kaser A, Stadlmann S, Millonig
G, Kaser S, Mühllechner P, Habior A, Graziadei I, Vogel W and Tilg
H: The RANKL/OPG system and bone mineral density in patients with
chronic liver disease. J Hepatol. 43:973–983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon BS, Wang S, Udagawa N, Haridas V, Lee
ZH, Kim KK, Oh KO, Greene J, Li Y, Su J, et al: TR1, a new member
of the tumor necrosis factor receptor superfamily, induces
fibroblast proliferation and inhibits osteoclastogenesis and bone
resorption. FASEB J. 12:845–854. 1998.PubMed/NCBI
|
|
26
|
Daniluk J, Szuster-Ciesielska A, Drabko J
and Kandefer-Szerszeń M: Serum cytokine levels in alcohol-related
liver cirrhosis. Alcohol. 23:29–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasso GR, Florencio-Silva R, Simões RS,
Baracat MC, Júnior JM Soares and Baracat EC: Elevated serum
osteoprotegerin levels in women: Friend or foe? Rev Assoc Med Bras
(1992). 61:524–529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gaudio A, Lasco A, Morabito N, Atteritano
M, Vergara C, Catalano A, Fries W, Trifiletti A and Frisina N:
Hepatic osteodystrophy: Does the osteoprotegerin/receptor activator
of nuclear factor-kB ligand system play a role? J Endocrinol
Invest. 28:677–682. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsujikawa H, Kurotaki Y, Fujimori T,
Fukuda K and Nabeshima Y: Klotho, a gene related to a syndrome
resembling human premature aging, functions in a negative
regulatory circuit of vitamin D endocrine system. Mol Endocrinol.
17:2393–2403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuro-o M: Klotho as a regulator of
oxidative stress and senescence. Biol Chem. 389:233–241. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Banerjee S, Dey N, LeJeune WS,
Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG and Choudhary S:
Klotho depletion contributes to increased inflammation in kidney of
the db/db mouse model of diabetes via RelA (serine)536
phosphorylation. Diabetes. 60:1907–1916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martín-Núñez E, Donate-Correa J,
Muros-de-Fuentes M, Mora-Fernández C and Navarro-González JF:
Implications of Klotho in vascular health and disease. World J
Cardiol. 6:1262–1269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moreno JA, Izquierdo MC, Sanchez-Niño MD,
Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez
R, Selgas R, Ruiz-Ortega M, et al: The inflammatory cytokines TWEAK
and TNFα reduce renal klotho expression through NFκB. J Am Soc
Nephrol. 22:1315–1325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Navarro-González JF, Donate-Correa J, de
Fuentes M Muros, Pérez-Hernández H, Martínez-Sanz R and
Mora-Fernández C: Reduced Klotho is associated with the presence
and severity of coronary artery disease. Heart. 100:34–40. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie B, Zhou J, Shu G, Liu DC, Zhou J, Chen
J and Yuan L: Restoration of klotho gene expression induces
apoptosis and autophagy in gastric cancer cells: Tumor suppressive
role of klotho in gastric cancer. Cancer Cell Int. 13:182013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q
and Shu G: Epigenetic silencing of Klotho expression correlates
with poor prognosis of human hepatocellular carcinoma. Hum Pathol.
44:795–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shu G, Xie B, Ren F, Liu DC and Zhou J, Li
Q, Chen J, Yuan L and Zhou J: Restoration of klotho expression
induces apoptosis and autophagy in hepatocellular carcinoma cells.
Cell Onco (Dordr). 36:121–129. 2013. View Article : Google Scholar
|
|
38
|
Chen L, Liu H, Liu J, Zhu Y, Xu L, He H,
Zhang H, Wang S, Wu Q, Liu W, et al: Klotho endows hepatoma cells
with resistance to anoikis via VEGFR2/PAK1 activation in
hepatocellular carcinoma. PLoS One. 8:e584132013. View Article : Google Scholar : PubMed/NCBI
|