Introduction
Bone fractures have become a worldwide public health
concern due to their increasing incidence, the cost of treatment
and resulting absenteeism in the workplace (1–3).
Experimental models have been used in previous studies to test
novel therapies for the facilitation and acceleration of fracture
healing (4–10); however, these are not representative
of all clinical situations, such as when a hematoma is drained
and/or the periosteum is disrupted. Hematoma and periosteum
disruption are common clinical scenarios in tibial open fractures
in humans (11,12) and increase the risk of delayed union,
late union and non-union (13,14).
Therefore, it is important to have experimental models that reflect
these situations in order to study the gene and protein expression
patterns specific to these scenarios.
Fracture hematomas are a source of growth factors
and cytokines, such as platelet-derived growth factor (PDGF),
vascular endothelial growth factor (VEGF), transforming growth
factor-β2 (TGF-β2) and interleukin-1b (IL-1β) (15–17).
These growth factors promote fracture healing and induce the
expression of a number of molecules important for bone repair, such
as osteocalcin and bone morphogenetic proteins (Bmps) (15–17).
During fracture healing, periosteal cells serve a primary role in
cartilage and bone formation within the callus (18). The periosteum is a complex structure
that is a repository for pluripotent stem cells and molecular
factors that modulate cell behavior (18).
Instruments that have been designed to reproduce
long bone fractures in small species, such as rats and mice, are
composed of at least four parts: i) A frame, ii) an animal support
system, iii) a guillotine ramming system and iv) a steel weight,
similar to the one first described by Bonnarens and Einhorn
(4), which has been reproduced and
modified by other research groups (4–10).
Manufacturing these devices is an expensive and time-consuming
process (4–10). In addition, devices used for the
internal fixation of long bone diaphyseal fractures in rats and
mice typically have a very small diameter (<1 mm) and are
difficult to obtain in the majority of countries (4–10). In
the present study, a rat model of open tibial fractures with
hematoma disruption, periosteal rupture and a versatile
intramedullary fixation system was established in order to
determine gene and protein expression throughout the bone healing
process.
Materials and methods
Experimental animals
Forty 6-month-old male Wistar rats weighing 350±20 g
were obtained from the University Center for Health Sciences Animal
Research Facility (University of Guadalajara, Guadalajara, Mexico).
All animals were housed in a standard laboratory animal environment
under a 12:12-h light cycle in a controlled environment with a
temperature of 23±2°C and humidity of 50±10%. The rats had ad
libitum access to food and water.
All procedures were approved by the local Committee
of Ethics and Biosecurity at University Center for Health Sciences
(Guadalajara, Mexico) and performed in accordance with animal
protection protocols in Mexico (NOM-062-ZOO-1999).
Fracture production and
stabilization
A tibial fracture model was designed to simulate
open fractures treated with open reduction and internal fixation.
Rats were anesthetized using Zoletil® (Virbac,
Guadalajara, Mexico; 5 mg/kg) and surgically prepared prior to
fracture and analgesia was continued for 72 h post-surgery.
Intramuscular cephalothin (40 mg/kg) was administered 30 min prior
to surgery and 24 h following surgery.
The right tibia of the rats was fractured by one of
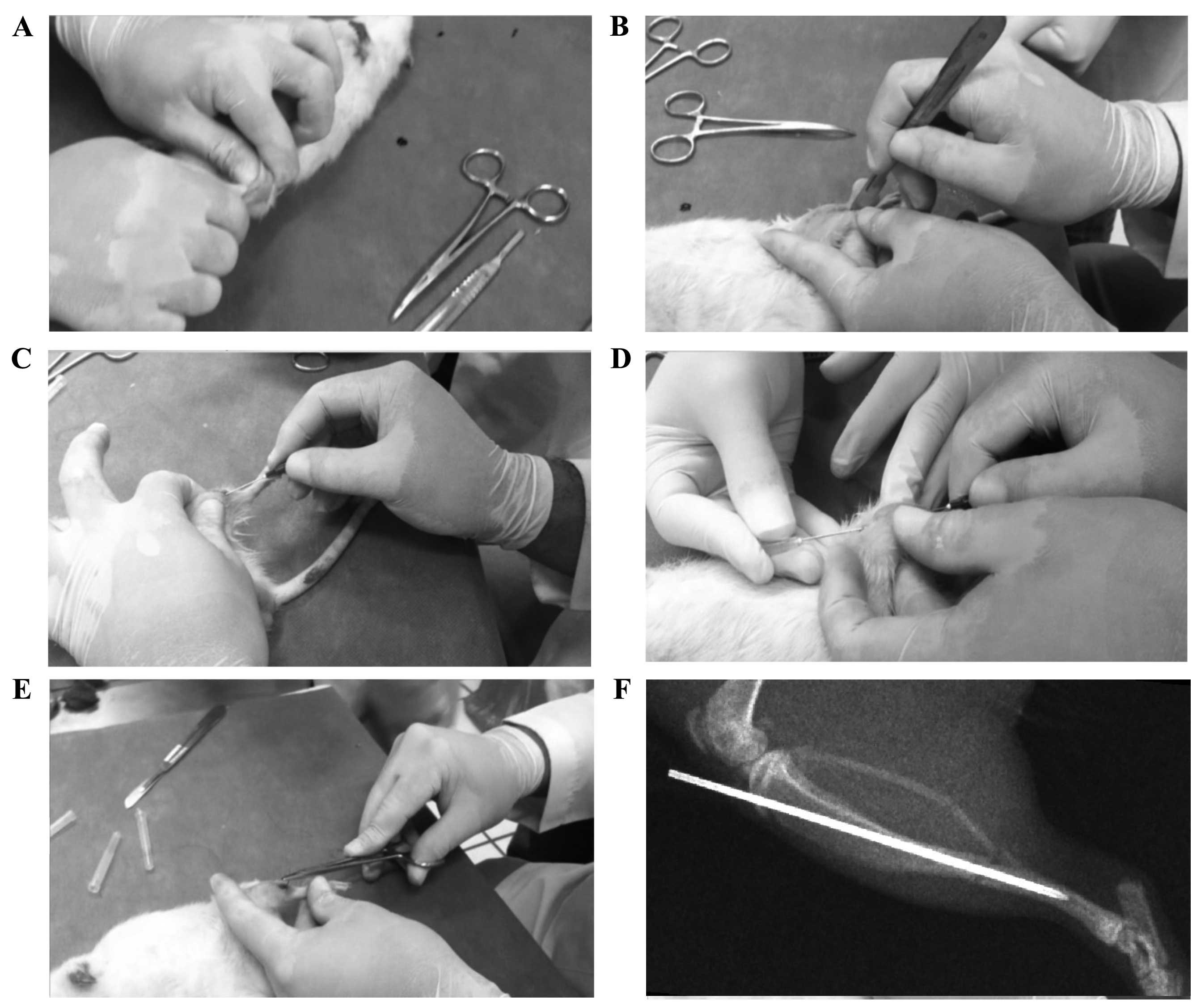
two researchers using a manual three-point bending technique
(Fig. 1A). In addition, the right
fibula was fractured. The rats were then placed in five groups
(each n=6) for sacrifice at five different time points. Following
fracture, a 4-mm incision was made over the medial side of the
tibia, the hematoma was drained and the periosteum was opened
(Fig. 1B). The fracture was
stabilized using a hypodermic 22G (0.7×38 mm) or 20G (0.9×38 mm)
needle that served as an intramedullary rod (Fig. 1C-F). For internal fixation a guide
needle was introduced in a retrograde fashion from site of fracture
towards the tibial tuberosity, then a fixation needle was passed in
an anterograde fashion through the medullary canal from the tibial
tuberosity towards the fracture site in order to stabilize the bone
fragments. The base of the needle was cut and the incisions were
closed using nylon 3–0 sutures. The length of time from fracture to
skin closing was recorded.
To verify correct needle positioning, an X-ray was
performed in 50% of the test subjects that were chosen at random.
Radiographs showed that the majority of the induced fractures were
diaphyseal with short oblique or transverse lines, regardless of
which investigator performed the fracture or the force applied
(Fig. 1F). There were two cases of
complex fracture. The reproducibility of the fracture technique was
evaluated using the inter-rater reliability Cohen Kappa statistic
(19). Before the present study,
different surgical approaches were used until reproducible hematoma
and periosteal disruption were achieved, and to find the best
fixation material.
Rats in the five groups were sacrificed using an
intraperitoneal injection of ≥200 mg/kg sodium pentobarbital
(Pisabental®; Pisa Laboratories, Tlajomulco de Zuniga,
Mexico) at 5, 14, 21, 28 and 35 days post-fracture, respectively,
in order to harvest tissue for histology, immunohistochemistry and
molecular analysis.
Histological analysis
Immediately following sacrifice the fractured tibia
were harvested and fixed in 4% paraformaldehyde in
phosphate-buffered saline at 4°C for 3 days. Specimens were then
completely decalcified using Immunocal Decalcifier (StatLab,
McKinney, TX, USA), embedded in paraffin and cut into 7-µm
sections. Sections were stained with hematoxylin and eosin for
conventional histology. Sections were examined for evidence for
bone healing using light microscopy at ×10, ×40 and ×100
magnification and representative images captured.
Immunohistochemical analysis
Immunohistochemical analysis was performed on tissue
from the fracture site at the time of maximum expression of the
respective genes. The sections described above were dewaxed and
heat-mediated antigen retrieval was performed using the pressure
cooker method with citrate buffer at pH 6.0 for 25 min. Tissues
were washed in 0.1 M phosphate-buffered saline (PBS) three times
for 5 min each time. After this, slides were placed in PolyDetector
AP Blocker (cat. no. BSB 0055; Bio-SB, Inc., Santa Barbara, CA,
USA) for 5 min and washed in 0.1 M PBS buffer three times for 5 min
each time. Following this, immunohistochemical staining was carried
out using antibodies obtained from Abcam (Cambridge, MA, USA). The
tissues were incubated with the primary antibodies overnight al
4°C: Anti-Bmp-7 (cat. no. ab15640; 1:50 dilution), anti-Bmp-6 (cat.
no. ab56023; 1:50 dilution), anti-Tgf-β2 (cat. no. ab53778; 1:50
dilution), anti-Il-1β (cat. no. ab9722; 1:50 dilution) and
anti-osteocalcin (cat. no. ab13420; 1:50 dilution). Subsequently,
tissues were washed in 0.1 M PBS buffer three times for 5 min each
time. Immunohistochemical staining was continued using a Dako LSAB
System-HRP system (K0675; Agilent Technologies, USA); the
biotinylated secondary antibody and streptavidin-conjugated
horseradish peroxidase were used according to the manufacturer's
protocol. Immunodetection was performed using diaminobenzidine
tetrahydrochloride (DAB; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany). Slides were counterstained with hematoxylin for 2 min
(cat. no. BSB 0024; Bio-SB, Inc.) and mounted with
Entellan® (cat. no. 107960; Merck-Millipore). Antibodies
Bmp-6, Bmp-7, Tgf-β2, Il-1β, and Bglap (osteocalcin) were chosen
because of their distinctive expression profiles in different
stages of bone healing (inflammation, cartilage formation,
cartilage resorption, primary bone formation, bone resorption and
secondary bone formation) (20).
Tissues were visualized by light microscopy using an optical
microscope with 40X objective (Motic BA210; Carlsbad, CA, USA).
Molecular analysis
Gene expression of Bmp-6, Bmp-7,
Tgf-β2, Il-1β, and Bglap was determined using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). Total RNA was extracted using TRIzol reagent with the
PureLink Micro-to-Midi Total RNA Purification System (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Extracted RNA
was quantified using spectrophotometry (NanoDrop 2000C; Thermo
Scientific, Inc.). RT-qPCR was performed in two phases:
Complementary DNA (cDNA) synthesis and mRNA expression
measurements. Firstly, cDNA synthesis for each gene was carried out
using a High Capacity cDNA Reverse Transcription kit (cat. no.
4368814; Thermo Scientific, Inc.). The final reaction contained 2
µg total RNA, 240 ng random primers, 2 units RNase inhibitor, 10 mM
DTT, 0.5 mM dNTPs and 200 units reverse transcriptase. The
following conditions were used: 65°C for 5 min, 4°C for 5 min, 25°C
for 10 min, 37°C for 50 min, 70°C for 15 min and 4°C for 5 min.
Secondly, mRNA expression measurements was performed
by qPCR using a Rotor Gene 3000 Thermocycler (Corbett Research;
Qiagen GmbH, Hilden, Germany) under the following conditions: 1
cycle at 50°C for 2 min; 1 cycle at 94°C for 5 min; 45 cycles at
94°C for 30 sec; and 45 cycles at 60°C for 40 sec. For the
reaction, 2 µg cDNA (9 µl final volume) was used with 10 µl
TaqMan® Universal Master Mix II (cat. no. 4440049;
Applied Biosystems; Thermo Fisher Scientific, Inc.) and 1 µl TaqMan
probe and primer sets (TaqMan® Gene Expression Assay;
Applied Biosystems) for Il-1β (cat. no. Rn00676330_m1),
Tgf-β2 (cat. no. Rn00579674_m1), Bmp-6 (cat.
no. Rn00432095_m1), Bmp-7 (cat. no. Rn01528889_m1),
Bglap (cat. no. Rn00566386_g1) and Gapdh (RHK-1; Real
Time Primers LLC, Elkins Park, PA, USA). The TaqMan probe and
primer set used is a fluorophore-based detection system containing
FAM-MGB dye and quencher, allowing quantitative measurements of the
accumulated product during the exponential stages of PCR. Finally,
levels of gene expression were calculated using the
2−ΔΔCq method described by Livak and Schmittgen
(21).
Statistical analysis
Descriptive and inferential statistical analysis was
performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA).
One-way analysis of variance was used to compare means between
groups. To identify the reproducibility of the fracture model
between operators the Kappa Cohen coefficient was used. Chi-squared
test was used to compare qualitative variables. P<0.05 was
considered to indicate a statistically significant result.
Results
Evaluation of fracture production
method
The mean elapsed time from tibial fracture to
closure of the incision was 3.8±0.44 min and was equivalent in all
groups (P=0.711). The Cohen Kappa inter-rater coefficient for the
type of fracture produced by the two researchers was 0.82,
indicating high reproducibility. All fractures (performed as shown
in Fig. 1-E) exhibited minimal
displacement of the bone ends (<0.5 mm), as determined by random
radiographic examination (Fig. 1F),
and no surgical complications (infection, surgical wound dehiscence
or fracture rotation-separation) were identified.
Histological analysis of fracture
healing
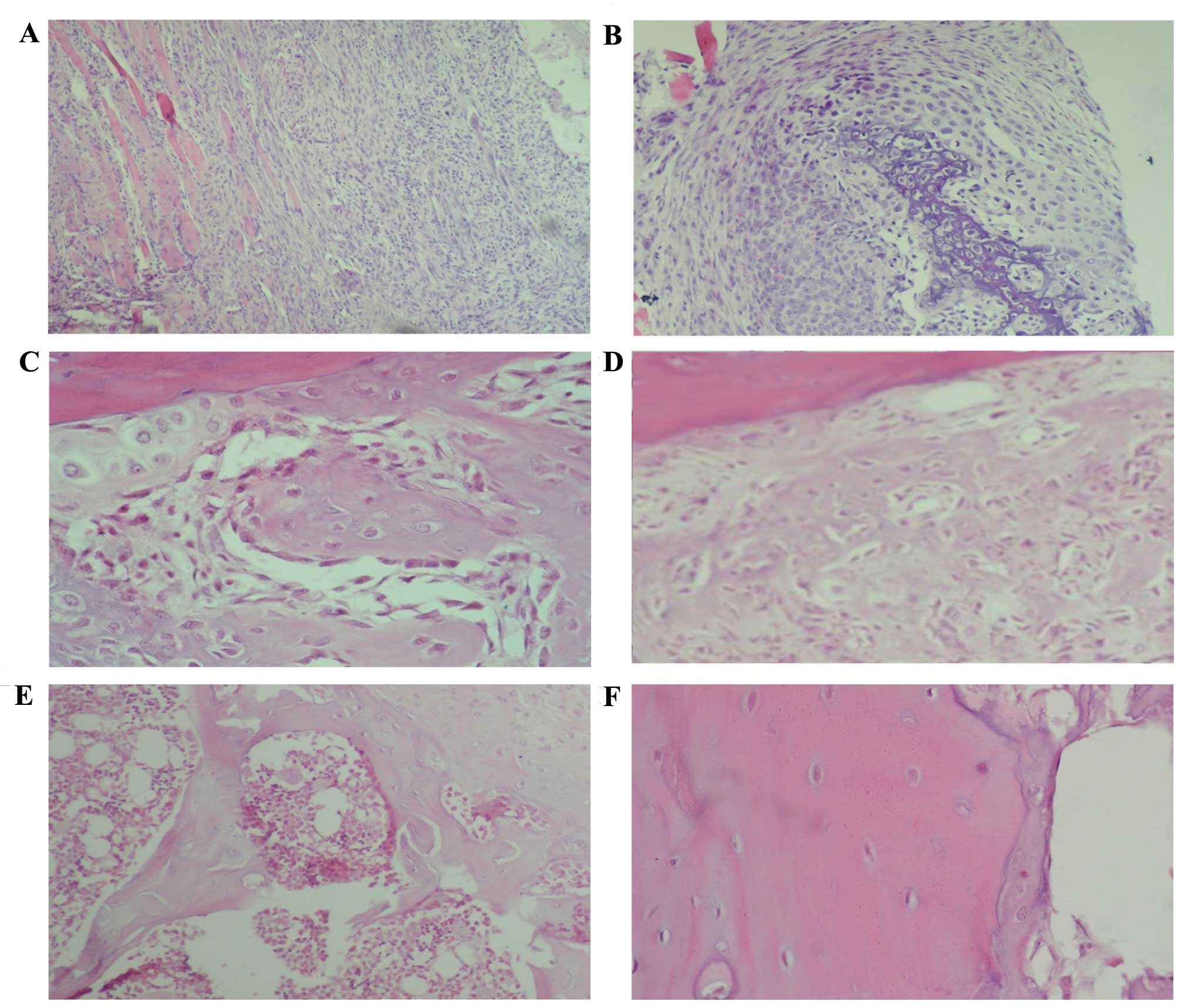
On day 5 post-fracture, inflammation was predominant
(Fig. 2A and B), while 14 days
following fracture there were numerous zones of cartilage and
primary bone formation (Fig. 2C). On
day 21 post-fracture, the formation of a large amount of primary
bone was observed (Fig. 2D). On day
28 post-fracture, a histological pattern compatible with primary
bone resorption and secondary bone formation was identified, while
35 days following fracture a completely calcified bone and
histologically normal bone area was observed (Fig. 2E and F).
Gene expression patterns during
fracture healing
Gapdh gene was used as a control for gene
expression (Fig. 3A). Molecular
analysis revealed a biphasic peak of Il-1β expression in the
fractured tibia, the first on day 5 and the second on day 28
following fracture (Fig. 3B). During
the first peak, Il-1β expression increased 36-fold by day 5
post-fracture and then decreased until day 21 (Fig. 3B). The second peak was a 37-fold
increase (from basal levels) on day 28 (Fig. 3B). Tgf-β2 was expressed
from day 5 to day 21 when it reached its highest levels equivalent
to 8 times its basal level, prior to dropping on days 28 and 35 to
reach basal levels (Fig. 3B).
Bglap expression increased from day 5 until day 28 as
follows: A 2-fold increase on day 5, a 32-fold increase on day 14
and a 51-fold increase on day 21 when it reached its peak
expression level (Fig. 3B). On day
28, the relative expression level of Bglap dropped to
3.5-fold and normalized at day 35 (Fig.
3B). Bmp-6 was consistently expressed throughout the
fracture healing processes, whereas Bmp-7 expression
increased 14-fold 14 days post-fracture, reaching a peak of 26
times its basal level on day 21 (Fig.
3C).
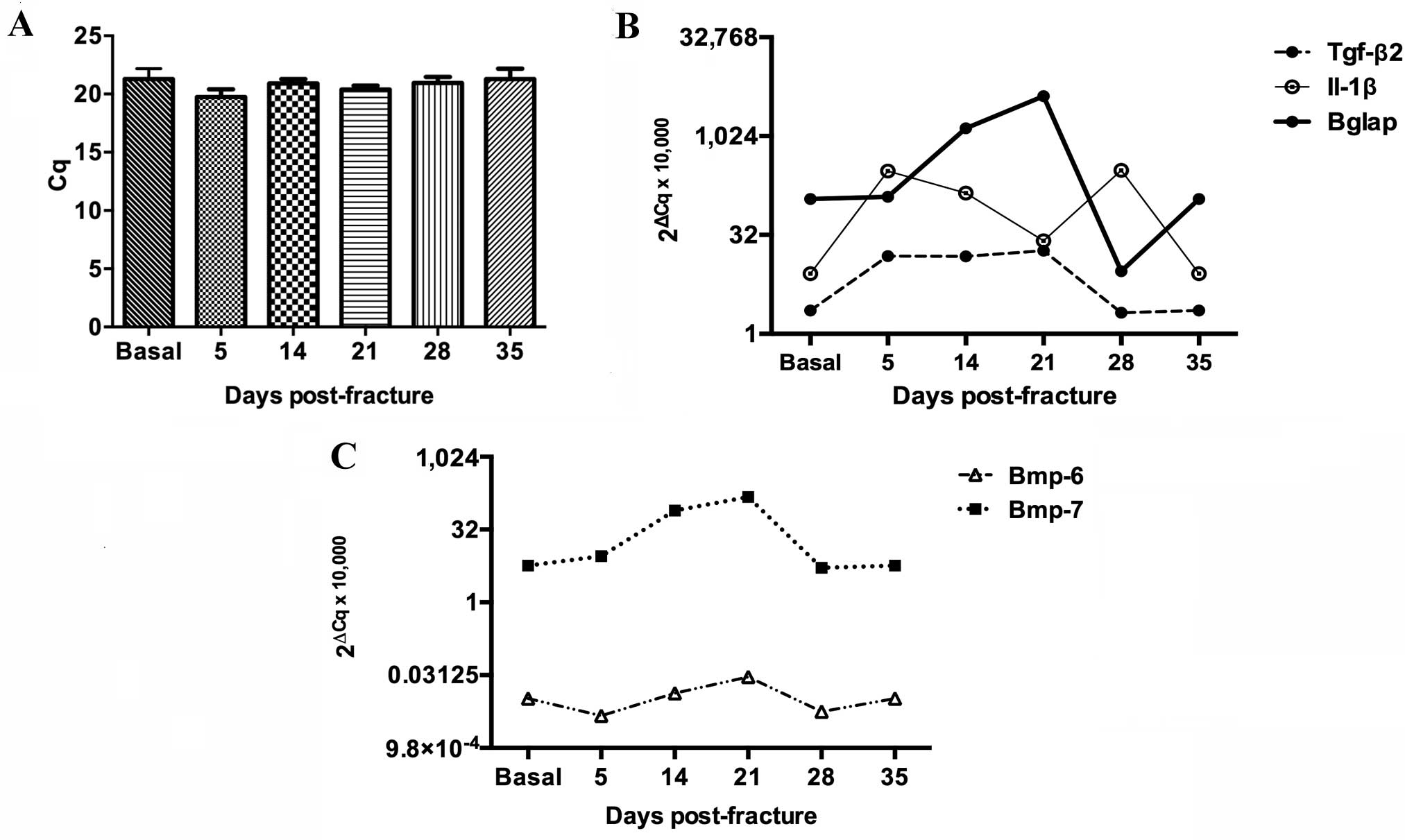 | Figure 3.Molecular profiling of Il-1β,
Tgf-β2, Bglap, Bmp-7 and Bmp-6 gene expression during the bone
healing process. Messenger RNA levels of the studied genes were
analyzed by reverse transcription-quantitative polymerase chain
reaction. (A) Expression of the control, Gadph, showing
stable expression levels. (B) Expression levels of Il-1β,
Tgf-β2 and Bglap. (C) Expression levels of Bmp-6
and Bmp-7. Tgf-β2, transforming growth factor β2;
IL-1β, interleukin-1β; Bglap, bone γ-carboxyglutamic
acid-containing protein; Bmp-6, bone morphogenetic
protein-6; Bmp-7, bone morphogenetic protein-7. |
Protein expression patterns during
fracture healing
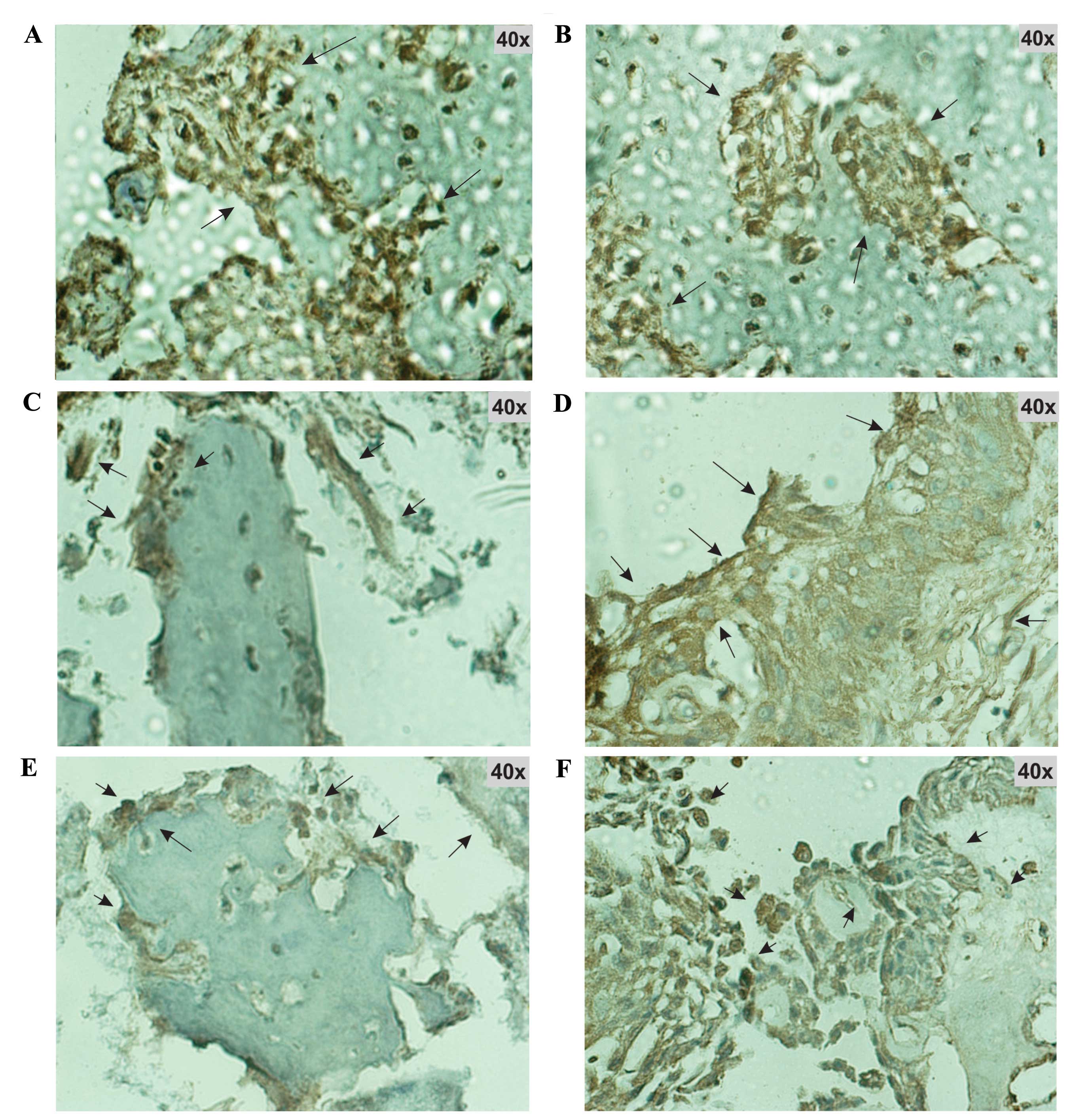
Expression of the protein products of Il-1β,
Tgf-β2, Bmp-6, Bmp-7 and Bglap was confirmed by
immunohistochemistry. Immunohistochemical analysis was performed on
tissue from the fracture site at the time of maximum expression of
their respective genes. At this time all of the studied proteins
were found to be markedly expressed (Fig. 4).
Discussion
The present study introduces a simple and highly
reproducible fracture model, which can be used for future research
on therapies aimed at improving the bone healing process. This
method will be more accessible for developing countries, because
the fracture is manually produced and the hypodermic needle
(0.7–0.9 mm diameter) required for internal fixation of the
fracture is readily available. By contrast, other experimental
models of long bone fracture in small species require materials
that are difficult to obtain and/or specialized equipment for
fracture reproduction and stabilization. For example, the apparatus
used by Bonnarens and Einhorn (4) to
fracture rat femurs was made of four parts and was followed by the
use of 0.45 mm Steinmann pins to stabilize these fractures.
Hiltunen et al (5) modified
this apparatus to achieve a reproducible tibia fracture in mice and
used 0.2 mm stainless steel rods to stabilize these fractures
(5). Kon et al (6) used the fracture technique of Bonnarens
and Einhorn (4) and 23–25 G spinal
needles as internal fixators, whereas Nakajima et al
(7) used a modified version of the
fracture technique used by Bonnarens and Einhorn (4) and 1.1 mm Kirschner wires as internal
tutors to stabilize fractures. Techniques used by a number of other
studies, to reproduce and stabilize diaphyseal fractures of mouse
and rat tibias, are modifications of the previously described
models (8–10).
The fracture model used in the current study
reproduces two scenarios frequently encountered in human open
tibial fractures, namely, hematoma rupture and periosteal
disruption. These situations increase the risk of delayed
consolidation and non-union, due to their importance in the bone
healing process, particularly the loss of growth factors that
occurs if the hematoma is disrupted (13–18). The
reproduction of these conditions is one of the benefits that the
model used in the present study offers. The characterization of the
expression profiles of gene and proteins throughout the bone
healing process in the current study is important since it more
accurately reflects what occurs in human open tibial fractures.
A clear expression pattern for each studied gene and
its respective protein was noted at every stage of bone healing
process. In agreement with previous findings (20,22),
when inflammatory cells predominated (day 5 post-fracture), gene
and protein expression of Il-1β increased. This cytokine is
highly expressed during the early stages of bone healing, where it
induces osteoblast proliferation and a slight acceleration in
endochondral ossification, which facilitates bone formation
(20,22). Expression of Il-1β gene and
protein was also markedly increased 28 days following fracture,
when bone remodeling and secondary bone formation were observed via
histological analysis. During this phase, Il-1β contributes to bone
remodeling and mineralization (6,20).
Tgf-β2 expression between days 5 and
21 reflects its role in cartilage formation, the periosteal
response and endochondral ossification, as described in previous
reports (6,20). In addition, Tgf-β2 induces
chondrocyte proliferation in the early stages of bone healing
(20,23).
Bmps are essential to bone biology and fracture
healing, due to their role in inflammatory response modulation,
cartilage formation, the periosteal response, cartilage resorption
and primary bone formation (20).
The constitutive expression of Bmp-6 throughout the fracture
healing processes in the current study has been previously reported
(8). Bmp-7 participates in
osteoclast recruitment and cartilage resorption (20). Bmp-7 was identified to be
strongly expressed from day 14 to 21 post-fracture, reaching its
peak level on day 21, when cartilage resorption and primary bone
formation are highly active (8,20).
Bglap is expressed abundantly in the bone,
particularly during the mineralization stage of osteogenesis
(20,24,25). In
the present study, Bglap in the majority of the cases, had
the highest expression levels of all the studied genes, basally and
in every time point measured of the bone healing process. The
protein product of Bglap, osteocalcin, is the most abundant
non-collagenous protein in the bone (24,25).
Osteocalcin has a high affinity for calcium and hydroxyapatite, and
serves as a chemoattractant and activator for cells with bone
resorption properties (24,25). These functions make osteocalcin
essential for bone health, bone remodeling and bone healing.
In conclusion, in the present study a simple rat
model of tibial open fractures with hematoma and periosteal
disruption was established, which produced clear and well-defined
expression patterns for Il-1β, Tgf-β2, Bmp-6,
Bmp-7 and Bglap and their protein products throughout
the bone healing process. This model will stimulate further
research in the area of bone healing, particularly in the testing
of therapeutic interventions aimed at enhancing the bone-formation
process.
References
|
1
|
Brooks PM: The burden of musculoskeletal
disease-a global perspective. Clin Rheumatol. 25:778–781. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnell O and Kanis JA: An estimate of the
worldwide prevalence and disability associated with osteoporotic
fractures. Osteoporos Int. 17:1726–1733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riera-Espinoza G: Epidemiology of
osteoporosis in Latin America 2008. Salud Publica Mex. 51(Suppl 1):
S52–S55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonnarens F and Einhorn TA: Production of
a standard closed fracture in laboratory animal bone. J Orthop Res.
2:97–101. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiltunen A, Vuorio E and Aro HT: A
standardized experimental fracture in the mouse tibia. J Orthop
Res. 11:305–312. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh
N, Graves D, Gerstenfeld LC and Einhorn TA: Expression of
osteoprotegerin, receptor activator of NF-kappaB ligand
(osteoprotegerin ligand) and related proinflammatory cytokines
during fracture healing. J Bone Miner Res. 16:1004–1014. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakajima F, Ogasawara A, Goto K, Moriya H,
Ninomiya Y, Einhorn TA and Yamazaki M: Spatial and temporal gene
expression in chondrogenesis during fracture healing and the
effects of basic fibroblast growth factor. J Orthop Res.
19:935–944. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho TJ, Gerstenfeld LC and Einhorn TA:
Differential temporal expression of members of the transforming
growth factor beta superfamily during murine fracture healing. J
Bone Miner Res. 17:513–520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thompson Z, Miclau T, Hu D and Helms JA: A
model for intramembranous ossification during fracture healing. J
Orthop Res. 20:1091–1098. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wildemann B, Schmidmaier G, Brenner N,
Hüning M, Stange R, Haas NP and Raschke M: Quantification,
localization, and expression of IGF-I and TGF-beta1 during growth
factor-stimulated fracture healing. Calcif Tissue Int. 74:388–397.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Court-Brown CM, Rimmer S, Prakash U and
McQueen MM: The epidemiology of open long bone fractures. Injury.
29:529–534. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Court-Brown CM and McBirnie J: The
epidemiology of tibial fractures. J Bone Joint Surg Br. 77:417–421.
1995.PubMed/NCBI
|
|
13
|
Papakostidis C, Kanakaris NK, Pretel J,
Faour O, Morell DJ and Giannoudis PV: Prevalence of complications
of open tibial shaft fractures stratified as per the
Gustilo-Anderson classification. Injury. 42:1408–1415. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gaebler C, Berger U, Schandelmaier P,
Greitbauer M, Schauwecker HH, Applegate B, Zych G and Vécsei V:
Rates and odds ratios for complications in closed and open tibial
fractures treated with unreamed, small diameter tibial nails: A
multicenter analysis of 467 cases. J Orthop Trauma. 15:415–423.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kolar P, Schmidt-Bleek K, Schell H, Gaber
T, Toben D, Schmidmaier G, Perka C, Buttgereit F and Duda GN: The
early fracture hematoma and its potential role in fracture healing.
Tissue Eng Part B Rev. 16:427–434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marsell R and Einhorn TA: The role of
endogenous bone morphogenetic proteins in normal skeletal repair.
Injury. 40(Suppl 3): S4–S7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grundnes O and Reikerås O: The importance
of the hematoma for fracture healing in rats. Acta Orthop Scand.
64:340–342. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colnot C, Zhang X and Tate ML Knothe:
Current insights on the regenerative potential of the periosteum:
Molecular, cellular, and endogenous engineering approaches. J
Orthop Res. 30:1869–1878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landis JR and Koch GG: The measurement of
observer agreement for categorical data. Biometrics. 33:159–174.
1977. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ai-Aql ZS, Alagl AS, Graves DT,
Gerstenfeld LC and Einhorn TA: Molecular mechanisms controlling
bone formation during fracture healing and distraction
osteogenesis. J Dent Res. 87:107–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lange J, Sapozhnikova A, Lu C, Hu D, Li X,
Miclau T III and Marcucio RS: Action of IL-1beta during fracture
healing. J Orthop Res. 28:778–784. 2010.PubMed/NCBI
|
|
23
|
Wildemann B, Schmidmaier G, Ordel S,
Stange R, Haas NP and Raschke M: Cell proliferation and
differentiation during fracture healing are influenced by locally
applied IGF-I and TGF-beta1: Comparison of two proliferation
markers, PCNA and BrdU. J Biomed Mater Res B Appl Biomater.
65:150–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hauschka PV, Lian JB, Cole DE and Gundberg
CM: Osteocalcin and matrix Gla protein: Vitamin K-dependent
proteins in bone. Physiol Rev. 69:990–1047. 1989.PubMed/NCBI
|
|
25
|
Villafán-Bernal JR, Sánchez-Enríquez S and
Muñoz-Valle JF: Molecular modulation of osteocalcin and its
relevance in diabetes (Review). Int J Mol Med. 28:283–293.
2011.PubMed/NCBI
|