Introduction
Atrial fibrillation (AF) is the most common cardiac
arrhythmia (1). AF has a prevalence
of >2.3 million in the United States (2). Thromboembolism is the most serious
complication of AF, with a 5-fold increased risk of stroke
(3). In addition, AF carries a
3-fold increased risk of heart failure (4,5), and a
2-fold increased risk of both dementia (6) and mortality (3,7).
Clinical AF can be categorized as paroxysmal,
persistent or permanent (7).
Paroxysmal AF, caused by focal drivers, particularly in the ectopic
sites in myocardial sleeves around the pulmonary veins (PVs), can
be eliminated by catheter ablation (8,9). The
proximal tunica media of the PVs, typically referred to as the
myocardial sleeve, is the primary source of supraventricular
ectopic activity. This site includes the left atrial tissue
extending to the PVs. However, the exact mechanisms by which AF
occurs in the myocardial sleeve are not well-characterized, despite
extensive studies (10–13).
Ectopic firing is driven by enhanced automaticity,
early after depolarizations and delayed after depolarizations
(DADs). The normal action potential in atrial cells remains at the
resting potential following repolarization. The resting potential
is maintained by high resting K+ permeability via inward
rectifier K+ current (IK1). Pacemaker current (If) in
atrial cells is overwhelmed by much larger IK1 with no
manifestation of automaticity (14).
Automaticity is attributed to decreased IK1 and/or enhanced If
(1). DADs are caused by abnormal
diastolic release Ca2+ from sarcoplasmic reticulum (SR)
Ca2+ stores. Ryanodine receptor 2 (RyR2) is a
specialized Ca2+ handling protein in the SR, which
releases Ca2+ in response to transmembrane
Ca2+ entry. Sarcoplasmic/endoplasmic reticulum
Ca2+-ATPase 2a (SERCA2a) is another Ca2+
handling protein, which mediates the uptake of intracellular
Ca2+ ([Ca2+]i) in cardiomyocytes to maintain
SR Ca2+ content. DADs are generated following
[Ca2+]i overload, for example in heart failure (15).
The conduction velocity of action potentials in the
atria serves an important role in AF (1). Conduction velocity is primarily
determined by inward currents causing depolarization (predominantly
Na+) and gap junction channels (connexins) providing
cell-to-cell electric continuity. In the PVs, conduction is also
affected by myocardial sleeve disconnection and pattern, which may
provide a substrate for re-entry in the veins (16–18).
In the present study, histological studies were
performed to delineate the architecture of the PVs in rats.
Furthermore, rat heart samples were immunolabeled for the following
six possible markers of ectopic beat generation: Connexin 43
(Cx43), one of the most important gap junction channels; three ion
channel proteins, the hyperpolarization-activated cyclic
nucleotide-gated channel 4 (HCN4, responsible for If current);
Nav1.5 (mediates cardiac Na+ current, INa);
Kir2.1 (responsible for cardiac IK1); and two Ca2+
handling proteins, SERCA2a and RyR2. All of the proteins being
identified were considered to serve vital roles in ectopic beat
generation, and thus may help elucidate the mechanisms of ectopic
beat generation in the myocardial sleeves of PVs.
Materials and methods
Ethical approval
The experimental procedures in the present study
were approved by the University of Manchester (Manchester, UK), and
rats were handled in accordance with the United Kingdom Animals
(Scientific Procedures) Act, 1986 (19). Rats were sacrificed in accordance
with the current UK Home Office regulations on animal
experimentation (20).
Animal and tissue preparation
In the present study 10 male adult Sprague-Dawley
rats (3 months old; 200–450 g; Charles River UK, Ltd., Margate, UK)
were used in the isolation of 7 hearts and 3 hearts + lungs. Rats
were sacrificed by cervical dislocation, followed 15 min later by
intraperitoneal injection of heparin (500 IUkg−1). The
heart + lungs were isolated by opening the thoracic cavity to
expose the trachea. The exposed trachea was raised with fine
forceps and a small V-shaped nicked on the top, followed by
injection of between 3 and 4 ml Optimal Cutting Temperature (OCT)
compound into the lungs and subsequent ligation of the trachea. The
heart + lungs were then rapidly removed en bloc and washed
in ice-cold phosphate buffered saline (PBS) solution. Hearts were
isolated by separation from the lungs, the lungs were then
dissociated from the hilum, leaving behind a fragment of lung
tissue on the hearts, in order to retain the PVs, and washed with
PBS. Samples were then embedded in OCT and frozen in isopentane
(cooled in liquid nitrogen to −50°C). Coronal, transverse and
sagittal sections of the heart were obtained. Transverse sections
of connected heart and lung tissue were also obtained. Serial 20 µm
cryosections at 100 µm intervals were collected from the samples
onto Superfrost glass slides (Fisher Scientific; Thermo Fisher
Scientific, Waltham, MA, USA) using a cryostat (Leica CM3050 S;
Leica Biosystems, Nussloch, Germany) and stored at −80°C until
required.
Histology
Bouin's fluid was used for 15 min to fix between 12
and 16 heart and heart + lung sections (at intervals of 400 µm).
Sections were then washed three times (10 min each) in 70% ethanol
and stained with Masson's trichrome as previously described
(21). Following staining, the
tissue sections were dehydrated with a gradient of ethanol (70 to
100%), cleared in Histo-Clear (National Diagnostics, Hessle, UK)
and mounted with DPX mounting medium (Merck KGaA, Darmstadt,
Germany). Using this technique, nuclei were stained dark
blue/black, cardiomyocytes were stained purple and connective
tissue blue. Stained samples were stored at room temperature prior
to analysis by light microscopy.
Antibodies
Eight primary antibodies were used in this study:
Mouse anti-Cx43 (polyclonal; 1:200; cat. no. AB1727; Chemicon,
Livingston, UK); rabbit anti-Cx43 (polyclonal; 1:200; cat. no.
C6219; Sigma-Alrich, St. Louis, MO, USA); rabbit anti-HCN4
(polyclonal; 1:50; cat. no. APC-052; Alomone Labs, Jerusalem,
Israel); mouse anti-caveolin-3 (Cav3) (monoclonal; 1:500; cat. no.
610420; BD Biosciences, Oxford, UK); mouse anti-RyR2 (clone C3-33;
monoclonal; 1:100; cat. no. MA3-916; Thermo Fisher Scientific);
mouse anti-SERCA1/2 (clone Y/1F4; monocolonal; 1:100; cat. no.
A010-21AP; Badrilla, Ltd., Leeds, UK); rabbit
anti-Nav1.5, corresponding to amino acid residues
493–511 (polyclonal; 1:50; cat. no. ASC-005; Alomone Labs); and
rabbit anti-Kir2.1, corresponding to amino acid residues 392–410
(polyclonal; 1:50; cat. no. APC-026; Alomone Labs). The secondary
antibodies used were the following: Donkey anti-mouse conjugated to
cyanine 3 (Cy3) polyclonal antibody (1:500; cat. no. AP192C;
Chemicon, Rolling Meadows, Illinois, USA); donkey anti-rabbit
conjugated to Cy3 polyclonal antibody (1:500; cat. no. AP182C;
Chemicon, Rolling Meadows, Illinois, USA); and donkey anti-rabbit
conjugated to fluorescein isothiocyanate (FITC) (polyclonal; 1:100;
cat. no. sc-2090; Santa Cruz Biotechnology, Inc., Dallas, Texas,
USA).
Immunohistochemistry
Immunohistochemistry was conducted using sections
adjacent to those used for histology. Briefly, sections were fixed
with 10% buffered formalin (Sigma-Aldrich) for 30 min and then
washed with 0.01 M PBS (Sigma-Alrich) three times at 10 min
intervals. Sections were then treated with 0.1% Triton X-100 in PBS
for 30 min, washed with PBS and blocked in 1% bovine serum albumin
(BSA; Sigma-Alrich) in PBS for 1 h at room temperature. Sections
were incubated with the appropriate primary antibody (diluted in 1%
BSA in PBS) at 4°C overnight, washed three times with PBS over 30
min and then incubated with the appropriate secondary antibodies
for between 90 and 120 min at room temperature in the dark.
Following three washes in PBS, coverslips were applied to the
slides using VECTASHIELD Mounting Medium (cat. no. H-1000; Vector
Laboratories, Inc., Burlingame, CA, USA) and sealed with a nail
varnish. Slides were stored in the dark at 4°C prior to analysis by
confocal microscopy.
Confocal microscopy
Images representing single optical sections were
acquired using a confocal laser scanning microscope (LSM 510;
Zeiss, Oberkochen, Germany) equipped with argon and helium-neon
lasers, at excitation wavelengths of 488 and 568 nm to detect FITC
and Cy3 respectively.
Results
Morphology of rat PVs
Heart and heart + lung samples stained with Masson's
trichrome were used for histology of the PVs. Transverse tissue
sections of heart samples were stained purple for cardiomyocytes of
the heart and PVs, blue for connective tissue and navy/black for
nuclei (Figs. 1 and 2). The transverse view of the PVs allowed
for the visualization of portions of the four PVs and left superior
vena cava. The PVs extending from the atria narrowed as they
terminated in the lungs. However, the myocardial sleeves of the PVs
varied in thickness. For example, cardiomyocytes in the right
superior PV (RSPV) formed triangular regions that extended into the
loose adventitia (Fig. 1A, indicated
by the thick red arrow). The walls of the PVs comprised three
layers as follows: A thin compact layer close to the lumen of the
intima, a middle layer of cardiomyocytes and an outer layer of
loose adventitia (Figs. 1D and
2D).
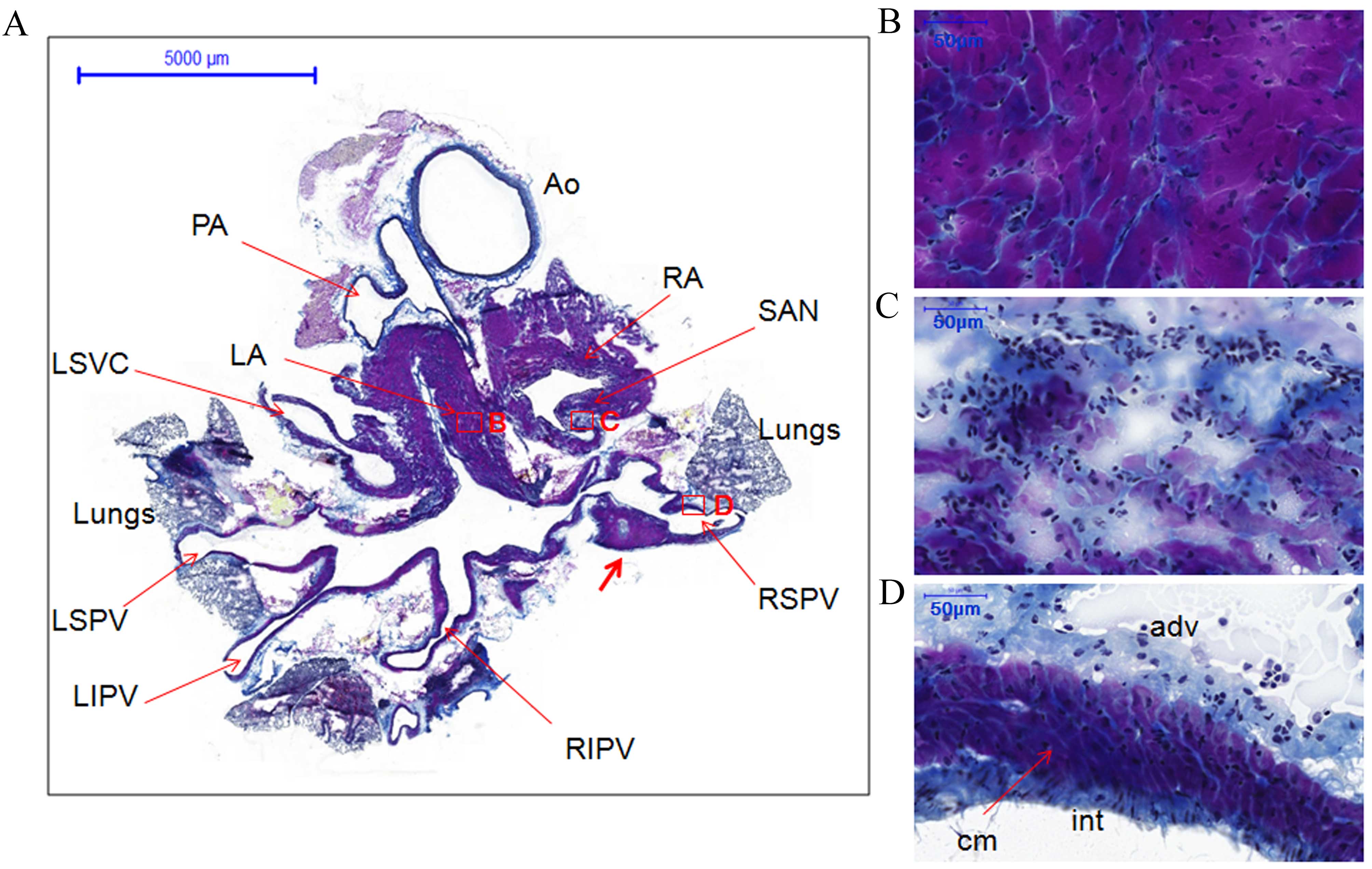 | Figure 1.Histology of the pulmonary veins from
transverse sections of the whole heart of rats without arrhythmia
stained with Masson's trichrome. (A) Whole heart section showing
the locations of different structures, boxes represent the
locations of B, C and D (scale bar, 5,000 µm). The thick red arrow
indicates cardiomyocytes in the RSPV that formed triangular regions
that extended into the loose adventitia. (B) LA (scale bar, 50 µm).
(C) SAN (scale bar, 50 µm). (D) RSPV (scale bar, 50 µm).
Cardiomyocytes of the heart and PVs are stained purple, connective
tissue blue and nuclei navy/black. AO, aorta; PA, pulmonary artery;
RA, right atrium; LA, left atrium; SAN, sinoatrial node; RSPV,
right superior pulmonary vein; RIPV, right inferior pulmonary vein;
RSPV, left superior pulmonary vein; LIPV, left inferior pulmonary
vein; LSVC, left superior vena cava; int, intima; cm,
cardiomyocytes; adv, adventitia. |
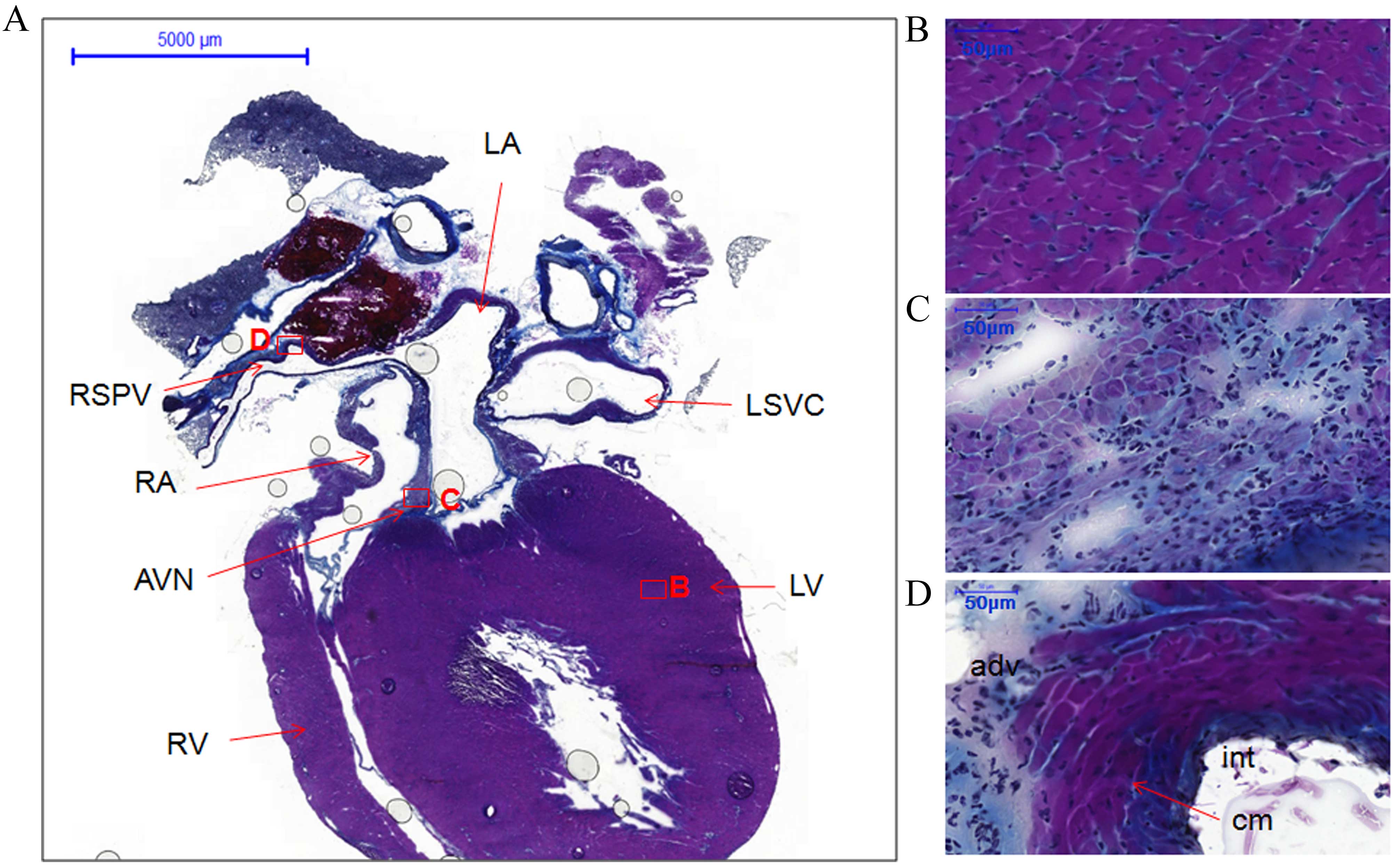 | Figure 2.Histology of the atria, ventricles,
atrioventricular node and right superior pulmonary vein from
coronal sections of the whole heart of rats without arrhythmia
stained with Masson's trichrome. (A) Coronal view of whole heart
section showing locations of different structures, boxes represent
the locations of B, C and D (scale bar, 5,000 µm). (B) LV (scale
bar, 50 µm). (C) AVN (scale bar, 50 µm). (D) RSPV (scale bar, 50
µm). LA, left atrium; RA, right atrium; LV, left ventricle; RV,
right ventricle; AVN, atrioventricular node; RSPV, right superior
pulmonary vein; LSVC, left superior vena cava; int, intima; cm,
cardiomyocytes; adv, adventitia. |
The coronal section from another heart specimen
showed the two atria, two ventricles, the atrioventricular node and
one right PV (Fig. 2A). The working
myocardium [left ventricle (LV)] and PV cardiomyocytes were heavily
stained (Fig. 2B and D,
respectively). However, the cardiomyocytes in the atrioventricular
node and the sinoatrial node were lightly stained (Figs. 2C and 1C). Furthermore, the cellular orientation
in the sinoatrial node (Fig. 1C) was
disorganized compared with the working myocardium (LA; Fig. 1B) and PVs (RSPV; Fig. 1D).
PVs express Cx43 but not HCN4
PV expression of Cx43 and HCN4 was investigated
(Figs. 3 and 4). HCN4 protein was detected (green signal)
in the plasma membrane of the sinoatrial node (green signal;
Fig. 3A) and the atrioventricular
node (data not shown). However, only a very weak HCN4 signal was
detected in the working myocardium [green signal; left atrium (LA);
Fig. 3B] and throughout the
myocardial sleeves (Fig. 3C and D).
Cx43 was identified in the working myocardium (red signal; LA;
Fig. 3B and green signal; LA;
Fig. 4A), the PVs (red signal;
Fig. 3C and D and green signal in
Fig. 4C and D), but had a very weak
signal in the sinoatrial node (Figs.
3B and 4B, red and green
signals, respectively). The cardiac biomarker Cav3 was detected in
cardiomyocytes of the working myocardium (red signal; LA; Fig. 4A), the cardiac conduction system,
such as the sinoatrial node (red signal; Fig. 4B), and the PVs (red signal; Fig. 4C and D). Cav3 expression in the PVs
(stained purple in Figs. 1D and
2D) confirms the presence of
cardiomyocytes in PVs.
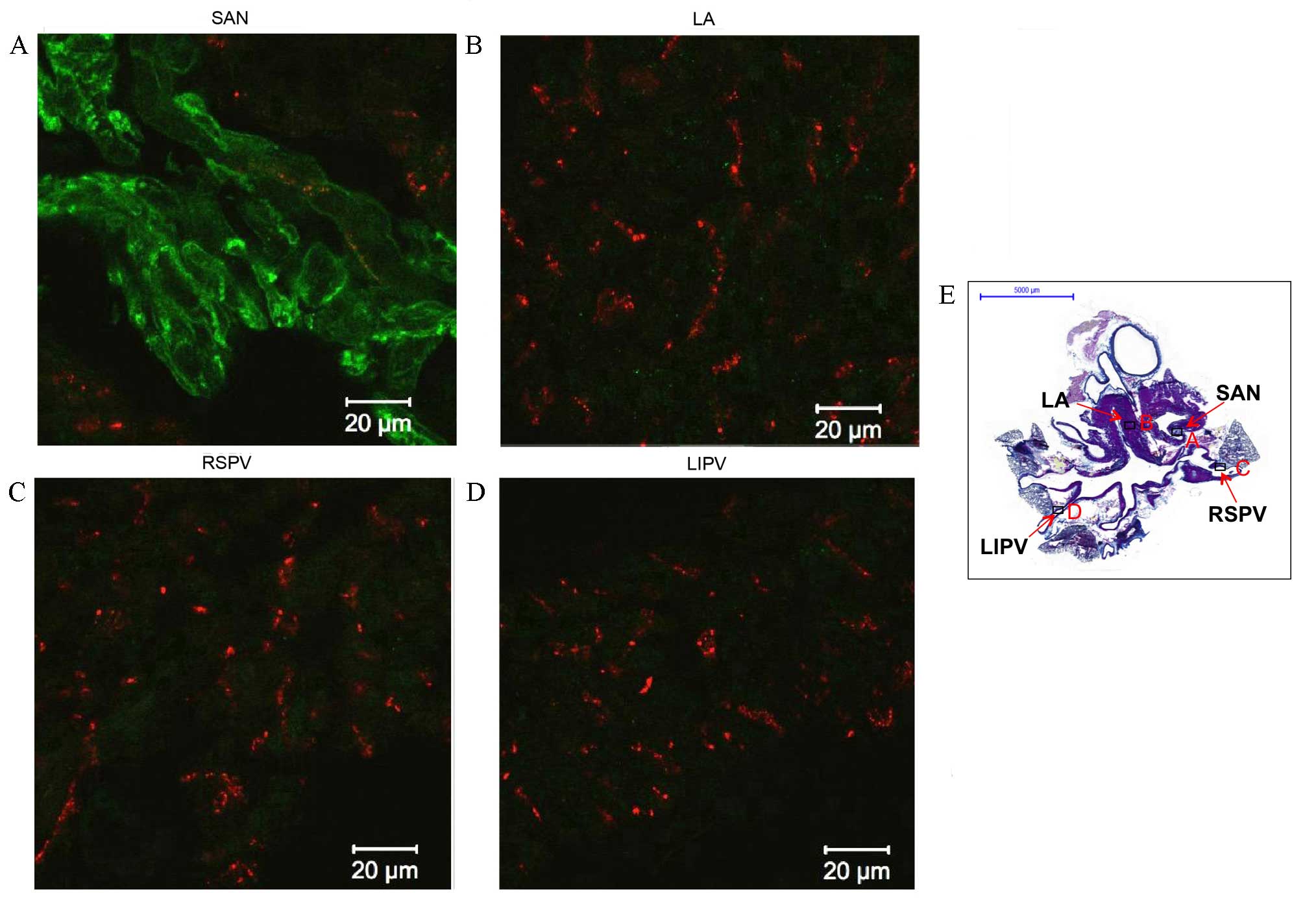 | Figure 3.Immunohistochemistry of
hyperpolarization-activated cyclic nucleotide-gated channel 4
(HCN4) and connexin 43 (Cx43) expression in transverse sections of
the whole heart of rats without arrhythmia. Expression of HCN4
(green) and Cx43 (red) in the (A) SAN, (B) LA, (C) RSPV and (D)
LIPV. Scale bar, 20 µm. (E) Masson's trichrome-stained transverse
section of the whole heart, boxes represent the locations of A, B,
C and D. Scale bar, 5,000 µm. LA, left atrium; SAN, sinoatrial
node; RSPV, right superior pulmonary vein; LIPV, left inferior
pulmonary vein. |
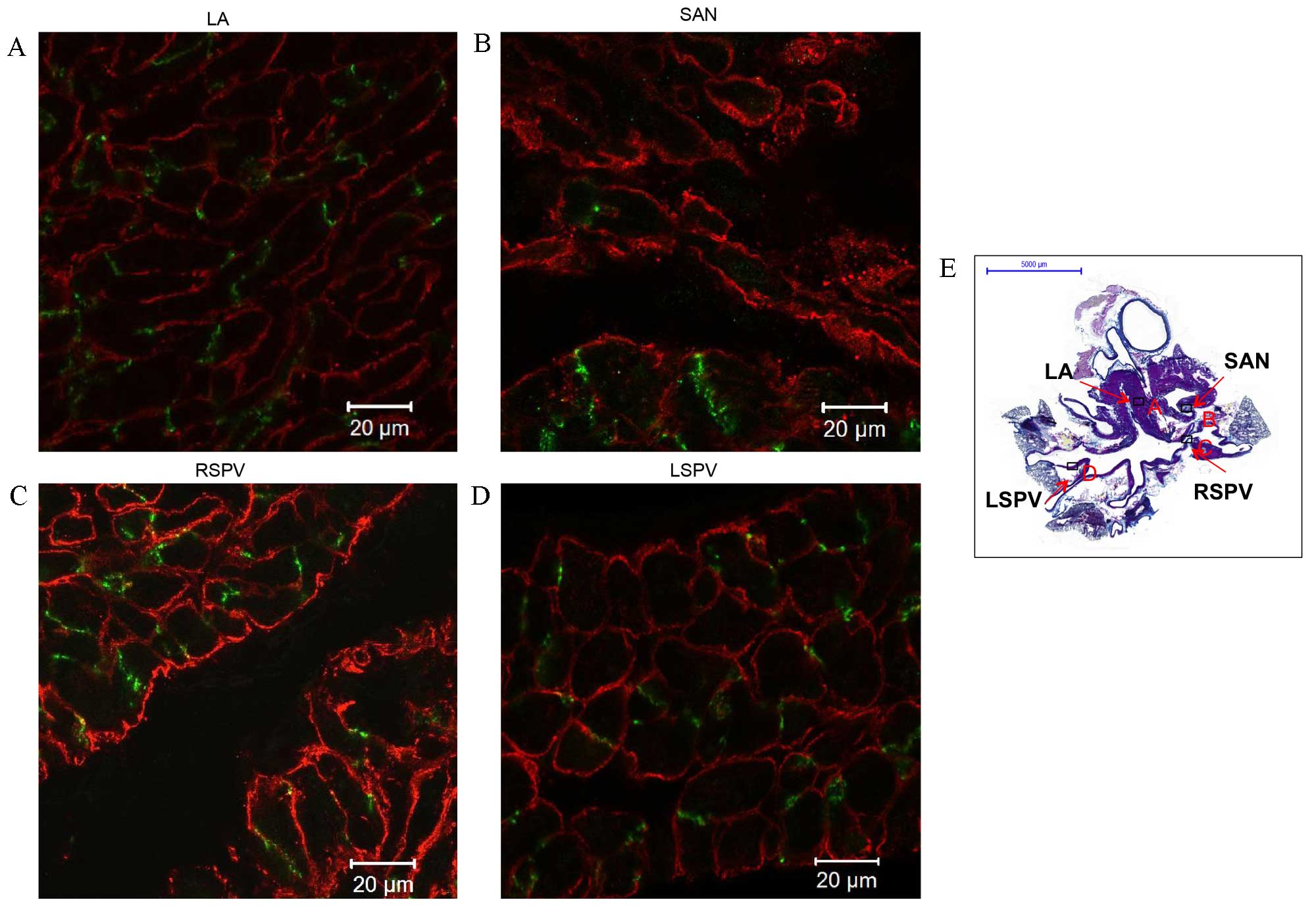 | Figure 4.Immunohistochemistry of connexin 43
(Cx43) and caveolin 3 (Cav3) expression in transverse sections of
the whole heart of rats without arrhythmia. Expression of Cx43
(green) and Cav3 (red) in the (A) LA, (B) SAN, (C) RSPV and (D)
LSPV. Scale bar, 20 µm. (E) Masson's trichrome-stained transverse
section of the whole heart, boxes represent the locations of A, B,
C and D. Scale bar, 5,000 µm. LA, left atrium; SAN, sinoatrial
node; RSPV, right superior pulmonary vein; LSPV, left superior
pulmonary vein. |
Shared protein expression patterns
between the myocardial sleeves of PVs and the working
myocardium
Protein expression patterns of the myocardial
sleeves of PVs and the working myocardium were investigated to
identify any similarities (Figs.
5–8). The present study detected
that Nav1.5 expression (green signal) in the working
myocardium, including the right ventricle (RV) and left atrial
appendage (LAA) (Fig. 5A and B,
respectively). Nav1.5 was also expressed in PV tissue,
such as the left PV (LPV; Fig. 5C and
D). Expression of Nav1.5 was higher in the RV
(Fig. 5A) compared with the other
tissues investigated. Kir2.1 expression (red signal) was similar to
that of Nav1.5, and was detected in the working
myocardium (such as the RV and LAA in Fig. 6A and B respectively) and PVs (such as
LPV shown in Fig. 6C and D). Kir2.1
was more highly expressed in the RV (Fig. 6A) compared with the other tissues
investigated. The striated pattern of Kir2.1 expression observed in
Fig. 6A likely corresponds to
t-tubules. The Kir2.1 signal intensity in the PVs (Fig. 6C and D) was comparable with that in
the LAA (Fig. 6B), but was weaker
compared with the RV (Fig. 6A).
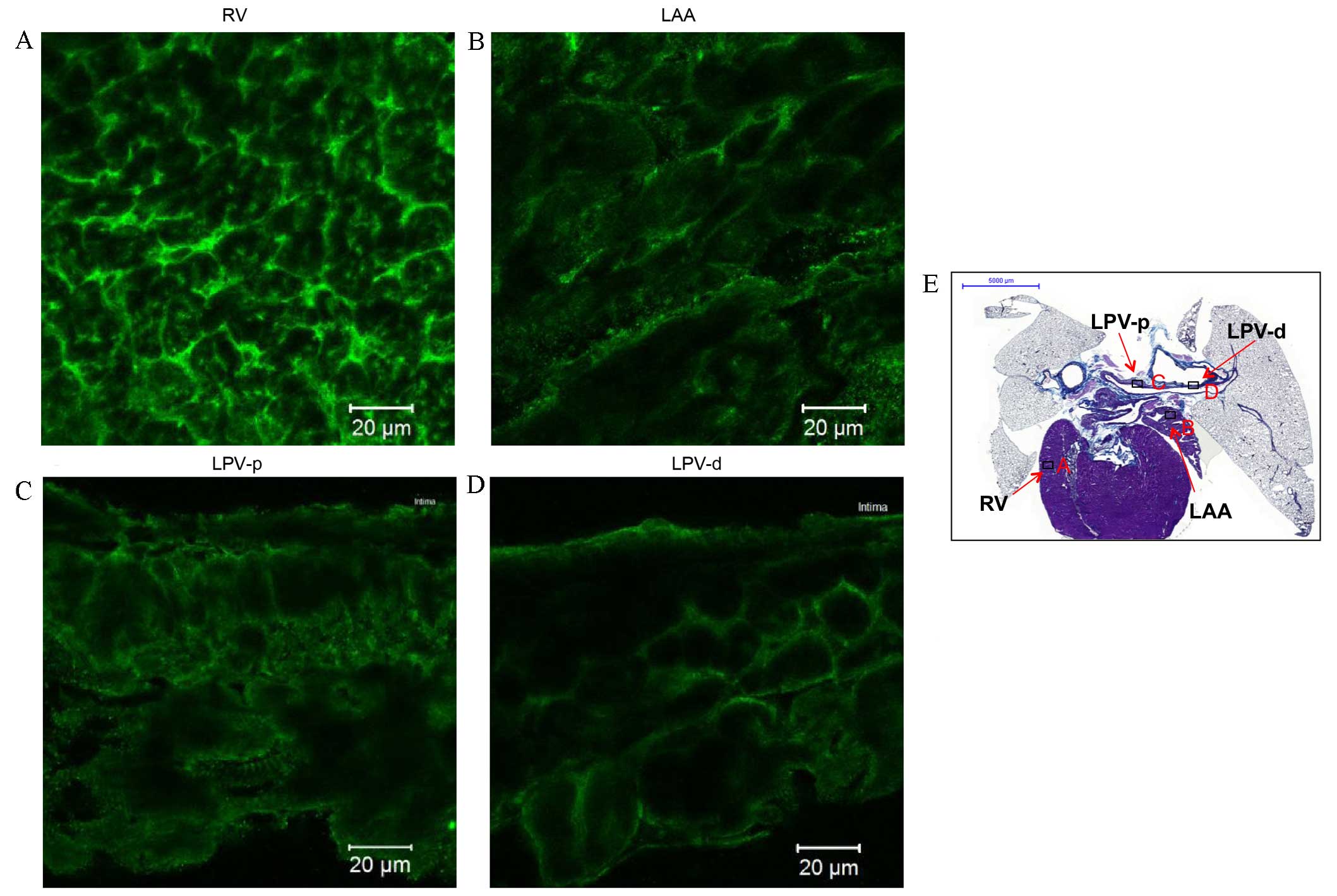 | Figure 5.Immunohistochemistry of
Nav1.5 expression in transverse heart + lung sections of
rats without arrhythmia. Expression of Nav1.5 in the (A)
RV, (B) LAA, (C) LPV-p and (D) LPV-d. Scale bar, 20 µm. (E)
Masson's trichrome-stained transverse section of the whole heart,
boxes represent the locations of A, B, C and D. Scale bar, 5,000
µm. RV; right ventricle; LAA, left arterial appendage; LPV-p,
proximal left pulmonary vein; LPV-d, distal pulmonary vein. |
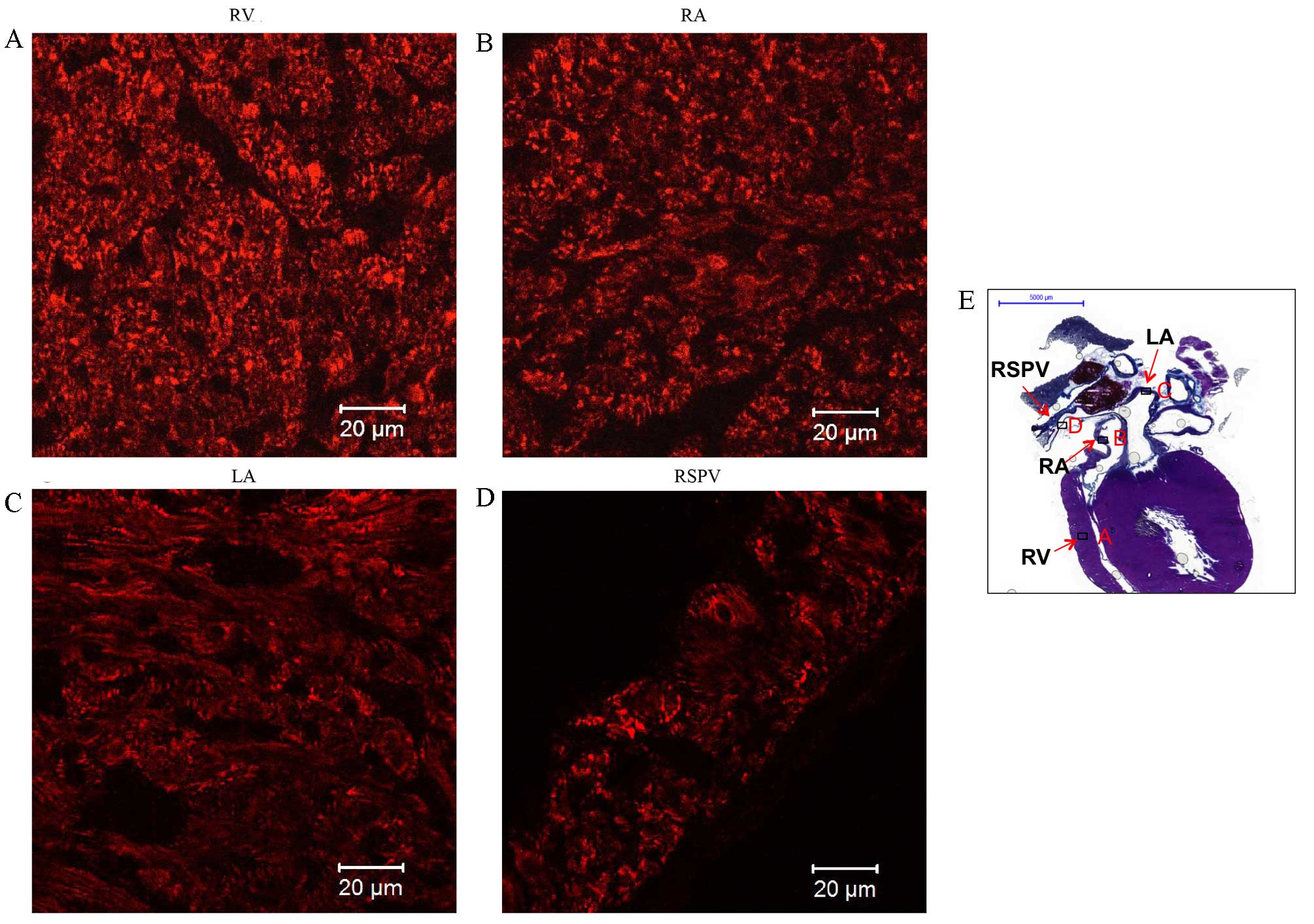 | Figure 8.Immunolabeling of
sarcoplasmic/endoplasmic reticulum calcium-ATPase 2a (SERCA2a)
expression in coronal sections of the whole heart of rats without
arrhythmia. Expression of SERCA2a in the (A) RV, (B) RA, (C) LA and
(D) RSPV. Scale bar, 20 µm. (E) Masson's trichrome-stained coronal
section of the whole heart, boxes represent the locations of A, B,
C and D, scale bar, 5,000 µm RV, right ventricle; RA, right atrium;
LA, left atrium; RSPV, right superior pulmonary vein. |
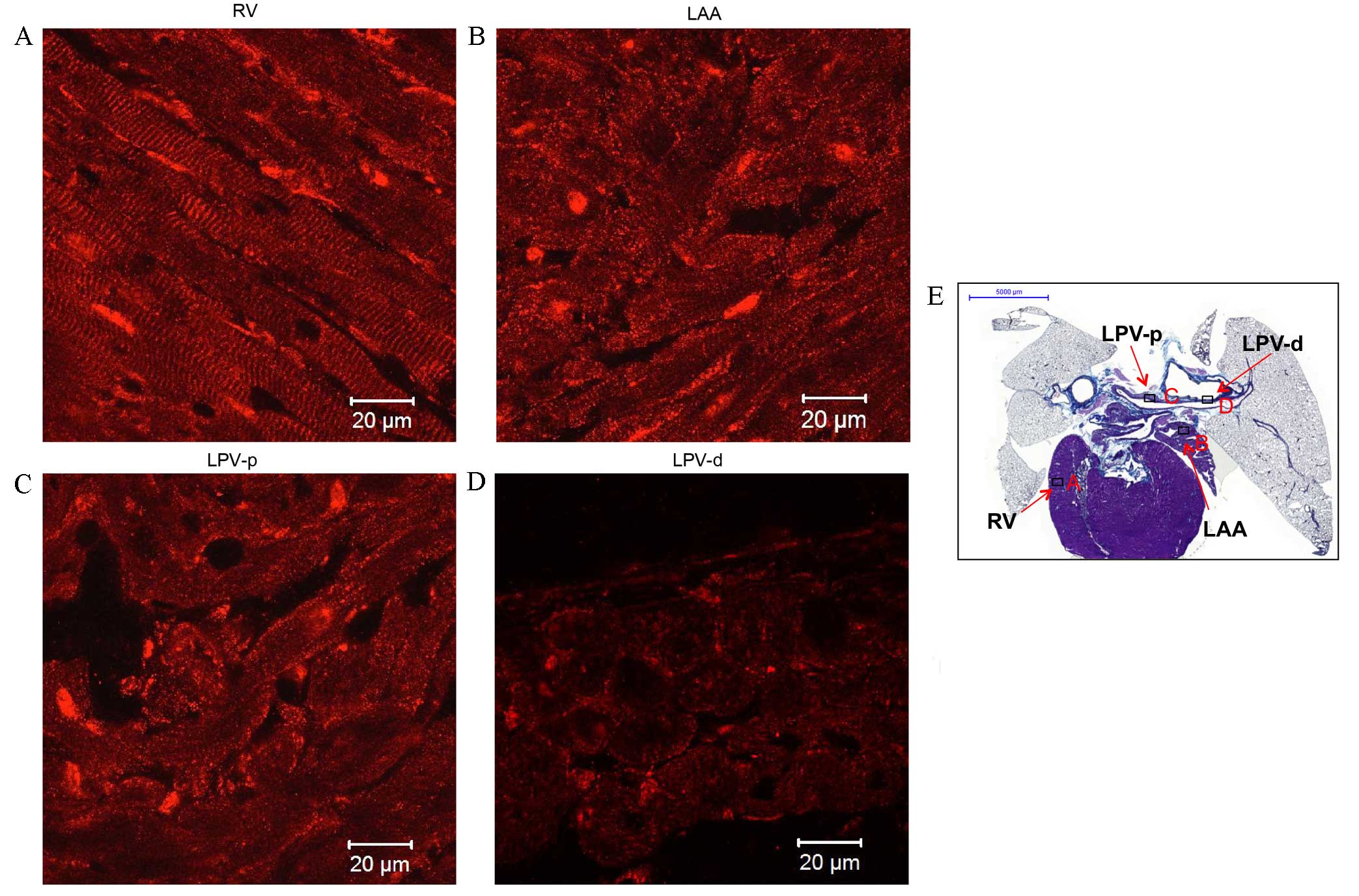 | Figure 6.Immunohistochemistry of Kir2.1
expression in transverse heart + lung sections of rats without
arrhythmia. Expression of Kir2.1 in the (A) RV, (B) LAA, (C) LPV-p
and (D) LPV-d. Scale bar, 20 µm. (E) Masson's trichrome-stained
transverse section of the whole heart, boxes represent the
locations of A, B, C and D. Scale bar, 5,000 µm. RV; right
ventricle; LAA, left arterial appendage; LPV-p, proximal left
pulmonary vein; LPV-d, distal pulmonary vein. |
RyR2 (red signal) was abundantly expressed in a
striated pattern intracellularly in the working myocardium,
particularly in the area close to the cell surface (Fig. 7A and C), and in the LPV (Fig. 7D). Two layers of myofibers were
oriented differently in the LPV: One layer circumferentially around
the lumen and the other longitudinal to the LPV (Fig. 7D). In addition, an intense and
uniform reticular pattern of SERCA2a expression was identified in
the working myocardium, including the RV, right atrium (RA) and LA
(Fig. 8A-C, respectively). SERCA2a
expression in the RSPV (Fig. 8D) was
similar to that of the working myocardium. A summary of the
proteins expressed in the working myocardium, sinoatrial node and
PVs is shown in Table I.
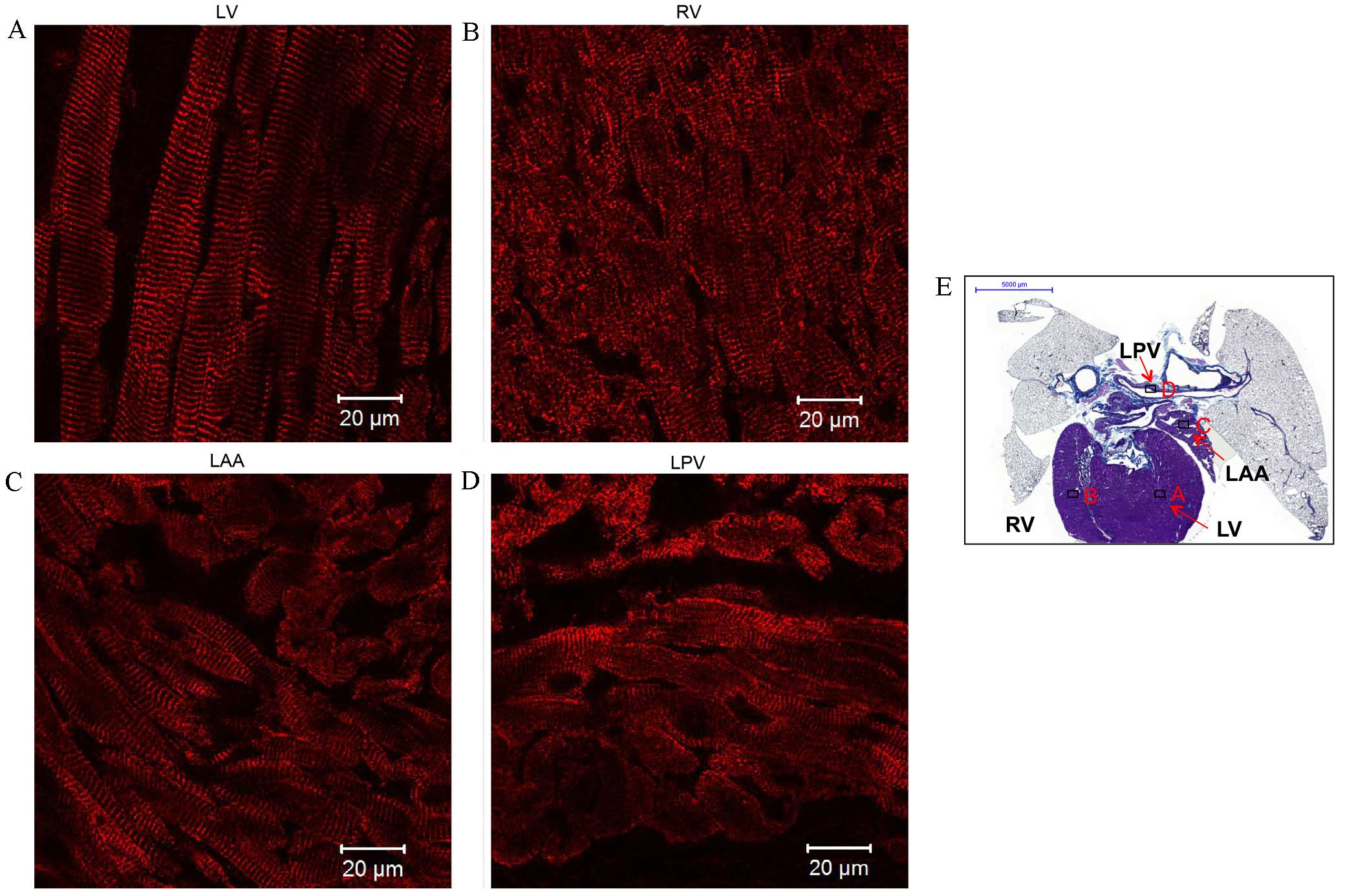 | Figure 7.Immunohistochemistry of ryanodine
receptor 2 (RyR2) expression in transverse sections of the heart +
lungs of rats without arrhythmia. Expression of RyR2 in the (A) LV,
(B) RV, (C) LAA and (D) LPV. Scale bar, 20 µm. (E) Masson's
trichrome-stained transverse section of the whole heart, boxes
represent the locations of A, B, C and D. Scale bar, 5,000 µm. LV,
left ventricle; RV, right ventricle; LAA, left atrial appendage;
LPV, left pulmonary vein. |
 | Table I.Proteins expressed in the working
myocardium, sinoatrial node and pulmonary veins. |
Table I.
Proteins expressed in the working
myocardium, sinoatrial node and pulmonary veins.
| Protein | PVs | LA | LV | RV | RA | SAN |
|---|
| Cav3 | + | + | + | + | + | + |
| Cx43 | + | + | + | + | + | − |
| HCN4 | − | − | − | − | − | + |
|
Nav1.5 | ± | ± | + | + | ± | N/A |
| Kir2.1 | ± | ± | + | + | ± | N/A |
| RyR2 | + | + | + | + | + | N/A |
| SERCA2a | + | + | + | + | + | N/A |
Discussion
In the present study the histology and
immunohistochemistry of the PVs in adult rats without arrhythmia
was investigated. It was observed that the myocardial sleeves of
the PVs extended from the atria into the lungs. PV cardiomyocytes
were determined to express Cx43, Cav3, Nav1.5, Kir2.1,
RyR2 and SERCA2a, but not HCN4, at levels similar to the LA.
In 2008, a study reported the differences in the
histology of PVs between rodents and humans (22). Mice and rat cardiomyocytes were found
to typically extend along the PVs from the hilus into the lung.
However human extrapericardiac cardiomyocytes were found in <30%
of PVs and exhibited an incomplete sleeve at the hilus.
Furthermore, in humans, cardiomyocytes were determined to occur
significantly more often in the RPVs than the LPVs and never in PVs
within the lungs. The present study identified the presence of rat
cardiomyocytes in PVs within the lungs, through staining of the
cardiomyocytes and identifying expression Cav3, which is
concentrated in the caveolae of cardiomyocytes.
Consistent with the present study's identification
of the presence of rat cardiomyocytes in PVs within the lung, a
previous study demonstrated the occurrence of electrically-induced
action potentials in rat cardiomyocytes of the distal PV in all
lobes of the lung (23). The action
potentials were triangular and atrial (23). In addition, prior research showed
that the shape of PV action potentials was similar to that recorded
in the left atrial cells of dogs and humans (17,24).
However, another study reported that, compared with the left atrial
free-wall, canine PV cardiomyocytes were characterized by lower
negative resting membrane potentials, a lower maximum phase 0
upstroke velocity and shorter action potential duration (25).
Gap junction channels consisting of connexins
mediate the electric coupling between cardiomyocytes. Electric
coupling in the working myocardium is strong, allowing rapid
conduction of action potentials. However, poor electric coupling
within the sinoatrial node results in slow conduction (26,27),
which protects the pacemaking tissue from the hyperpolarizing
effect of the surrounding atrial muscle (28). Gap junctions are clustered channels
consisting of two hemichannels, each formed by 6 Cx proteins, which
connect the cytoplasm of adjacent cardiomyocytes (29). Three principal connexins are
expressed in cardiomyocytes, Cx43, Cx40, and Cx45 (30). Cx43 is expressed in all chambers of
the heart. In the present study, Cx43 expression in the PVs was
similar to that in the working myocardium, but in contrast to that
in the cardiac conduction system (such as the sinoatrial node).
This finding indicates that the electric coupling between PV
cardiomyocytes is similar to that in the working myocardium.
Previous studies have found that presence of AF was accompanied
with a reduction in atrial Cx43, and that Cx43 gene therapy
prevents persistent AF in animal models (31,32),
indicating that Cx43 serves a critical role in AF. Therefore, the
abundant PV expression of Cx43 found in the present study strongly
suggests that PV cardiomyocytes are electrically well coupled, in
contrast with the sinoatrial node.
The present study investigated three key cardiac ion
channels; HCN4, Nav1.5 and Kir2.1. Firstly, HCN4 protein
was identified to be present in the sinoatrial node, but only in
very small quantities in the atria and PVs. HCN4 is the primary HCN
isoform, responsible for the pacemaker current (If). HCN4 is highly
expressed in the sinoatrial nodes, but not PVs, of small mammals
and humans (33). However, one study
reported that sinoatrial node-like tissue is present in human PVs
containing the principal cells of the sinus node (P cells),
transitional cells and Purkinje cells (34). In addition, HCN4 expression in
interstitial Cajal cells is capable of generating a repetitive
electrical rhythm within human PVs, particularly in patients with a
history of AF (35). The present
study determined that there were no ectopic focus cells that
expressed HCN4 in the PVs of adult rats without arrhythmia.
Interestingly, a recent study found that expression levels of HCN2
and HCN4 channel mRNA and protein were lower in the sinoatrial
node, and higher in the atrium and PVs of aged dogs, suggesting
that atrial electrophysiology and regional HCN2 and HCN4 channel
expression are associated with the onset and maintenance of
age-related AF (36).
Secondly, the present study identified that
Nav1.5, responsible for cardiac INa, is present in the
PVs and the working myocardium of rats without AF. Expression of
Nav1.5 in the PVs, although weaker than that in the
ventricles, was similar to that in the atria. In contrast, a
previous study found that Nav1.5 was absent in the
majority of sinoatrial node cells (28). This suggests that there is
differential expression of Nav1.5 in PVs and the
sinoatrial node.
Thirdly, the results of the present study determined
that the Kir2.1 expression profile in the PVs is similar to that of
Nav1.5. Kir2.1 expression is associated with IK1. In the
working myocardium, IK1 serves an important role in the final phase
repolarization of the action potential and in the generation of
resting membrane potential (37).
Weak Kir2.1 expression in the sinoatrial node compared with the
atrial muscle leads to a lack of stable resting potential in the
sinoatrial node (21,28). Chen et al (38) reported that canine PV cardiomyocytes
with spontaneous activity have a significantly lower IK1 density.
However, the present study found that the Kir2.1 expression in the
PVs was similar to that in the atrium. This result suggests that
unlike the sinoatrial node cells, cardiomyocytes of the PVs have a
more stable resting potential. Although, a previous study suggested
that lower levels and the subcellular distribution of Kir2.3 in
canine PVs may contribute to their smaller IK1 density (39).
Finally, the present study investigated two
Ca2+-handling proteins, RyR2 and SERCA2a. In the heart,
[Ca2+]i movements regulate subsequent mechanical
contractions. In cardiac excitation-contraction coupling, RyR2
represents the SER Ca2+ release channel. SERCA2a is a
Ca2+-ATPase that transfers Ca2+ from the
cytosol of cardiomyocytes to the lumen of the SR, at the expense of
ATP hydrolysis. During systole, Ca2+ is released from
the SR through the RyR2. Subsequently, Ca2+ binds to
troponin C and initiates the cross-bridge movement of the
myofibrils, resulting in force development and contraction. During
diastole, SERCA2a rapidly sequesters Ca2+ into the SR
for cardiac relaxation (40,41). The present study compared the
expression of RyR2 and SERCA2a between PVs and working myocytes.
RyR2 and SERCA2a were identified in the PVs and the working
myocytes, as previously reported (42,43).
Their pattern of expression in PV cardiomyocytes resembled the
pattern found in the atria, which explains the molecular basis of
their contractile response to electrical excitation (44,45). In
addition, intracellular Ca2+ serves an important role in
pacemaking in small mammals (46).
RyR2 dysregulation and enhanced SERCA2a activity increase SR
Ca2+ leakage and release events, causing DADs in
paroxysmal AF. Although the expression of the Na+-
Ca2+ exchanger does not vary between tissues, RyR2 and
SERCA2a were identified to be less abundant in the sinoatrial node
than in atrial cells in humans (21). In the present study, similar
expression patterns of RyR2 and SERCA2a were found between PV
cardiomyocytes and atrial cells in adult rats without arrhythmia.
This indicates different expression patterns of RyR2 and SERCA2a
between PV cardiomyocytes and the sinoatrial node. PV
arrhythmogenesis, caused by abnormal Ca2+ regulation
likely occurs only under pathological conditions, such as heart
failure and dilated atria, resulting in remodeling of
Ca2+-handling proteins and altered intracellular
Ca2+ dynamics (47).
The present study had a number of limitations.
Firstly, data was derived from adult rats without arrhythmia, which
does not address the clinical realities of AF. Electrical
remodeling in the myocardial sleeves of the PVs under pathological
conditions may contribute to ectopic beats. Secondly, only
histological and immunohistochemical studies were conducted. Minor
differences between cardiomyocytes of rat PVs vs. left atrium were
undetectable due to methodological constraints. Therefore, further
investigations are needed to elucidate the molecular mechanisms
contributing to the generation of ectopic beats.
In conclusion, the expression of Cx43, the three key
cardiac ion channels (HCN4, Nav1.5 and Kir2.1) and the two
Ca2+-handling proteins (RyR2 and SERCA2a) in
the PVs of adult rats without arrhythmia is similar to that in the
working myocardium, but unlike the sinoatrial node. The results of
the present study suggested that PV cardiomyocytes of adult rats
without arrhythmia are electrically well coupled and have a stable
resting potential. This indicates that ectopic beats originating in
the myocardial sleeves surrounding the PVs are likely associated
with pathological conditions, such as heart failure.
Acknowledgements
The authors would like to thank Mr Joseph Yanni
Gerges from the Institute of Cardiovascular Sciences, Faculty of
Medical and Human Sciences, University of Manchester, UK for
assistance with immunohistochemical staining.
References
|
1
|
Iwasaki YK, Nishida K, Kato T and Nattel
S: Atrial fibrillation pathophysiology: Implications for
management. Circulation. 124:2264–2274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen LY and Shen WK: Epidemiology of
atrial fibrillation: A current perspective. Heart Rhythm. 4(3
Suppl): S1–S6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kannel WB, Wolf PA, Benjamin EJ and Levy
D: Prevalence, incidence, prognosis, and predisposing conditions
for atrial fibrillation: Population-based estimates. Am J Cardiol.
82:2N–9N. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang TJ, Larson MG, Levy D, Vasan RS, Leip
EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB and Benjamin EJ:
Temporal relations of atrial fibrillation and congestive heart
failure and their joint influence on mortality: The framingham
heart study. Circulation. 107:2920–2925. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stewart S, Hart CL, Hole DJ and McMurray
JJ: A population-based study of the long-term risks associated with
atrial fibrillation: 20-year follow-up of the Renfrew/Paisley
study. Am J Med. 113:359–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ott A, Breteler MM, de Bruyne MC, van
Harskamp F, Grobbee DE and Hofman A: Atrial fibrillation and
dementia in a population-based study. The rotterdam study. Stroke.
28:316–321. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
January CT, Wann LS, Alpert JS, Calkins H,
Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD,
Field ME, et al: 2014 AHA/ACC/HRS guideline for the management of
patients with atrial fibrillation: A report of the American College
of Cardiology/American Heart Association Task Force on Practice
Guidelines and the Heart Rhythm Society. J Am Coll Cardiol.
64:e1–e76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haissaguerre M, Jaïs P, Shah DC, Takahashi
A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P and
Clémenty J: Spontaneous initiation of atrial fibrillation by
ectopic beats originating in the pulmonary veins. N Engl J Med.
339:659–666. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cappato R, Calkins H, Chen SA, Davies W,
Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, et al:
Updated worldwide survey on the methods, efficacy, and safety of
catheter ablation for human atrial fibrillation. Circ Arrhythm
Electrophysiol. 3:32–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Po SS, Li Y, Tang D, Liu H, Geng N,
Jackman WM, Scherlag B, Lazzara R and Patterson E: Rapid and stable
re-entry within the pulmonary vein as a mechanism initiating
paroxysmal atrial fibrillation. J Am Coll Cardiol. 45:1871–1877.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Corradi D, Callegari S, Gelsomino S,
Lorusso R and Macchi E: Morphology and pathophysiology of target
anatomical sites for ablation procedures in patients with atrial
fibrillation: Part II: Pulmonary veins, caval veins, ganglionated
plexi, and ligament of Marshall. Int J Cardiol. 168:1769–1778.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan AY, Li H, Wachsmann-Hogiu S, Chen LS,
Chen PS and Fishbein MC: Autonomic innervation and segmental
muscular disconnections at the human pulmonary vein-atrial
junction: Implications for catheter ablation of atrial-pulmonary
vein junction. J Am Coll Cardiol. 48:132–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nathan H and Gloobe H: Myocardial
atrio-venous junctions and extensions (sleeves) over the pulmonary
and caval veins. Anatomical observations in various mammals.
Thorax. 25:317–324. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stillitano F, Lonardo G, Zicha S, Varro A,
Cerbai E, Mugelli A and Nattel S: Molecular basis of funny current
(If) in normal and failing human heart. J Mol Cell Cardiol.
45:289–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeh YH, Wakili R, Qi XY, Chartier D,
Boknik P, Kääb S, Ravens U, Coutu P, Dobrev D and Nattel S:
Calcium-handling abnormalities underlying atrial arrhythmogenesis
and contractile dysfunction in dogs with congestive heart failure.
Circ Arrhythm Electrophysiol. 1:93–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hocini M, Ho SY, Kawara T, Linnenbank AC,
Potse M, Shah D, Jaïs P, Janse MJ, Haïssaguerre M and De Bakker JM:
Electrical conduction in canine pulmonary veins:
Electrophysiological and anatomic correlation. Circulation.
105:2442–2448. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verheule S, Wilson EE, Arora R, Engle SK,
Scott LR and Olgin JE: Tissue structure and connexin expression of
canine pulmonary veins. Cardiovasc Res. 55:727–738. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arora R, Verheule S, Scott L, Navarrete A,
Katari V, Wilson E, Vaz D and Olgin JE: Arrhythmogenic substrate of
the pulmonary veins assessed by high-resolution optical mapping.
Circulation. 107:1816–1821. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hollands C: The Animals (scientific
procedures) Act 1986. Lancet. 2:32–33. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Combes RD and Balls M: The Three
Rs-opportunities for improving animal welfare and the quality of
scientific research. Altern Lab Anim. 42:245–259. 2014.PubMed/NCBI
|
|
21
|
Chandler NJ, Greener ID, Tellez JO, Inada
S, Musa H, Molenaar P, Difrancesco D, Baruscotti M, Longhi R,
Anderson RH, et al: Molecular architecture of the human sinus node:
Insights into the function of the cardiac pacemaker. Circulation.
119:1562–1575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mueller-Hoecker J, Beitinger F, Fernandez
B, Bahlmann O, Assmann G, Troidl C, Dimomeletis I, Kääb S and
Deindl E: Of rodents and humans: A light microscopic and
ultrastructural study on cardiomyocytes in pulmonary veins. Int J
Med Sci. 5:152–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Logantha SJ, Cruickshank SF, Rowan EG and
Drummond RM: Spontaneous and electrically evoked Ca2+
transients in cardiomyocytes of the rat pulmonary vein. Cell
Calcium. 48:150–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spach MS, Barr RC and Jewett PH: Spread of
excitation from the atrium into thoracic veins in human beings and
dogs. Am J Cardiol. 30:844–854. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ehrlich JR, Cha TJ, Zhang L, Chartier D,
Melnyk P, Hohnloser SH and Nattel S: Cellular electrophysiology of
canine pulmonary vein cardiomyocytes: Action potential and ionic
current properties. J Physiol. 551:801–813. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boyett MR, Honjo H and Kodama I: The
sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc
Res. 47:658–687. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boyett MR, Inada S, Yoo S, Li J, Liu J,
Tellez J, Greener ID, Honjo H, Billeter R, Lei M, et al: Connexins
in the sinoatrial and atrioventricular nodes. Adv Cardiol.
42:175–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dobrzynski H, Boyett MR and Anderson RH:
New insights into pacemaker activity: Promoting understanding of
sick sinus syndrome. Circulation. 115:1921–1932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saez JC, Berthoud VM, Branes MC, Martinez
AD and Beyer EC: Plasma membrane channels formed by connexins:
Their regulation and functions. Physiol Rev. 83:1359–1400. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Severs NJ, Bruce AF, Dupont E and Rothery
S: Remodelling of gap junctions and connexin expression in diseased
myocardium. Cardiovasc Res. 80:9–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bikou O, Thomas D, Trappe K, Lugenbiel P,
Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA and Bauer
A: Connexin 43 gene therapy prevents persistent atrial fibrillation
in a porcine model. Cardiovasc Res. 92:218–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Igarashi T, Finet JE, Takeuchi A, Fujino
Y, Strom M, Greener ID, Rosenbaum DS and Donahue JK: Connexin gene
transfer preserves conduction velocity and prevents atrial
fibrillation. Circulation. 125:216–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto M, Dobrzynski H, Tellez J, Niwa
R, Billeter R, Honjo H, Kodama I and Boyett MR: Extended atrial
conduction system characterised by the expression of the HCN4
channel and connexin45. Cardiovasc Res. 72:271–281. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perez-Lugones A, McMahon JT, Ratliff NB,
Saliba WI, Schweikert RA, Marrouche NF, Saad EB, Navia JL, McCarthy
PM, Tchou P, et al: Evidence of specialized conduction cells in
human pulmonary veins of patients with atrial fibrillation. J
Cardiovasc Electrophysiol. 14:803–809. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morel E, Meyronet D, Thivolet-Bejuy F and
Chevalier P: Identification and distribution of interstitial Cajal
cells in human pulmonary veins. Heart Rhythm. 5:1063–1067. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li YD, Hong YF, Zhang Y, Zhou XH, Ji YT,
Li HL, Hu GJ, Li JX, Sun L, Zhang JH, et al: Association between
reversal in the expression of hyperpolarization-activated cyclic
nucleotide-gated (HCN) channel and age-related atrial fibrillation.
Med Sci Monit. 20:2292–2297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Priori SG, Pandit SV, Rivolta I, Berenfeld
O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta
MR, Gudapakkam S, et al: A novel form of short QT syndrome (SQT3)
is caused by a mutation in the KCNJ2 gene. Circ Res. 96:800–807.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P,
Chang MS and Lin CI: Effects of rapid atrial pacing on the
arrhythmogenic activity of single cardiomyocytes from pulmonary
veins: Implication in initiation of atrial fibrillation.
Circulation. 104:2849–2854. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Melnyk P, Ehrlich JR, Pourrier M,
Villeneuve L, Cha TJ and Nattel S: Comparison of ion channel
distribution and expression in cardiomyocytes of canine pulmonary
veins versus left atrium. Cardiovasc Res. 65:104–116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marks AR: Calcium cycling proteins and
heart failure: Mechanisms and therapeutics. J Clin Invest.
123:46–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Frank KF, Bölck B, Erdmann E and Schwinger
RH: Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac
contraction and relaxation. Cardiovasc Res. 57:20–27. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Musa H, Lei M, Honjo H, Jones SA,
Dobrzynski H, Lancaster MK, Takagishi Y, Henderson Z, Kodama I and
Boyett MR: Heterogeneous expression of Ca(2+) handling proteins in
rabbit sinoatrial node. J Histochem Cytochem. 50:311–324. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lyashkov AE, Juhaszova M, Dobrzynski H,
Vinogradova TM, Maltsev VA, Juhasz O, Spurgeon HA, Sollott SJ and
Lakatta EG: Calcium cycling protein density and functional
importance to automaticity of isolated sinoatrial nodal cells are
independent of cell size. Circ Res. 100:1723–1731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thiagalingam A, Reddy VY, Cury RC, Abbara
S, Holmvang G, Thangaroopan M, Ruskin JN and d'Avila A: Pulmonary
vein contraction: Characterization of dynamic changes in pulmonary
vein morphology using multiphase multislice computed tomography
scanning. Heart Rhythm. 5:1645–1650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rietdorf K, Masoud S, McDonald F,
Sanderson MJ and Bootman MD: Pulmonary vein sleeve cell
excitation-contraction-coupling becomes dysynchronized by
spontaneous calcium transients. Biochem Soc Trans. 43:410–416.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bogdanov KY, Vinogradova TM and Lakatta
EG: Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+)
exchanger: Molecular partners in pacemaker regulation. Circ Res.
88:1254–1258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Honjo H, Boyett MR, Niwa R, Inada S,
Yamamoto M, Mitsui K, Horiuchi T, Shibata N, Kamiya K and Kodama I:
Pacing-induced spontaneous activity in myocardial sleeves of
pulmonary veins after treatment with ryanodine. Circulation.
107:1937–1943. 2003. View Article : Google Scholar : PubMed/NCBI
|






















