Introduction
Human laryngeal papilloma (LP) is a benign neoplasm
mainly caused by human papillomavirus (HPV) types 6 and 11
(1). Human LP typically starts in
the commissure and anterior third of the vocal folds, and
subsequently affects the whole larynx, including the trachea,
bronchi and lung parenchyma, thereby causing a number of morbidity
and mortality cases (2). Incidence
of LP is reportedly 4.3 per 100,000 among infants and 1.8 per
100,000 among adults (3). LP is
characterized by recurrence of premalignant hyperplastic epithelial
papillomas (4) and apoptosis
resistance (5). Recurrent lesions
are often observed because surgical removal of all superficial
papillomatous lesions is difficult when the disease is widespread
(2). Thus, developing new methods to
treat LP is critical.
Epidermal growth factor receptor (EGFR) is
upregulated in LP tissues (6).
Multiple signaling molecules associated with EGFR are altered,
including mitogen-activated protein kinases (MAPKs) and
phosphatidylinositol 3-kinase (7).
Previous studies revealed that cyclooxygenase-2 (COX2) expression
is elevated in LP tissues and cells, accompanied by MAPK activation
(8,9). Phosphorylated MAPKs, including p38
MAPK, extracellular signal-regulated kinase (ERK) 1/2 and c-Jun
N-terminal kinase (JNK) are implicated in the induction of COX2
expression (10). COX2 expression is
mediated in part through the activation of p38 MAPK in LP cells,
and the inhibition of p38 MAPK activity suppresses proliferation
and enhances apoptosis of LP cells (11). Wu et al (12) demonstrated that celecoxib, a
selective COX2 inhibitor, has inhibitory effects on proliferation
and apoptosis evasion of LP cells, suggesting that COX2 serves a
crucial function in the tumorigenesis of LP. Thus, understanding
the induction of COX2 expression and activation could potentially
lead to targeted treatment of HPV-infected LP.
Isoflurane (ISO) is a widely used volatile
anesthetic, and previous studies have shown that ISO possesses
non-anesthetic effects (13,14). Notably, ISO confers
anti-proliferative and proapoptotic effects on multiple human
cancer cell lines (15). A previous
study showed that ISO reduces COX2 expression and prostaglandin
E2 (PGE2) release by inhibiting p38 MAPK
activation in murine Kupffer cells (16). However, whether ISO inhibits LP
malignancy by reducing p38 MAPK/COX2 signaling remains unclear.
The results of the present study show that
COX2/PGE2 biosynthesis was significantly upregulated in
LP tissues and cells. The enhancement in COX2 and PGE2
levels was markedly attenuated by ISO treatment in LP cells.
Molecular mechanism analysis revealed that the inhibitory effects
of ISO treatment on COX2 expression and activation were mediated by
reducing p38 MAPK activation in LP cells. Moreover, ISO
administration significantly hindered proliferation and prompted
apoptosis of the LP cells via the reduction of COX2 activity. These
results suggest that COX2 is a potential therapeutic target of LP,
and ISO may be largely beneficial for LP treatment by inhibiting
p38 MAPK/COX2 signaling.
Materials and methods
Reagents
Mouse anti-human COX2 (cat. no. 4842S), p38 MAPK
(cat. no. 9212), ERK1/2 (cat. no. 4696), JNK (cat. no. 3708S),
phosphorylated (p)-p38 MAPK (Thr180/Tyr182; cat no. 9215S), p-JNK
(Thr183/Tyr185; cat. no. 9251S) and β-actin (cat. no. 3700)
polyclonal antibodies were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Mouse anti-human p-ERK1/2 (Thr185/Tyr187;
cat. no. ab76299) polyclonal antibody was purchased from Abcam
(Cambridge, UK). Horseradish peroxidase-conjugated anti-mouse IgG
(cat. no. AP124P) was obtained from Merck Millipore (Merck KGaA,
Darmstadt, Germany). SB202190, a specific inhibitor of p38 MAPK,
was purchased from Enzo Life Sciences (Plymouth Meeting, PA, USA).
Celecoxib, a selective inhibitor of COX2, was obtained from Pfizer,
Inc. (New York, NY, USA). ISO was purchased from Baxter
International, Inc. (Deerfield, IL, USA). All other reagents were
commercially obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany) unless otherwise stated.
Tissue specimens and cell culture
LP and adjacent normal laryngeal tissues were
harvested from 5 patients who were underwent curative resection in
the Children's Hospital of Zhengzhou (Zhengzhou, China). None of
the patients had received chemotherapy or radiotherapy prior to
surgery. Demographic information of the patients is as follows:
Case 1, 4-year-old male; case 2, 4-year-old male; case 3,
6-year-old male; case 4, 8-year-old female; case 5, 1-year-old
female. Biopsies were used to establish primary cell cultures or
frozen in liquid nitrogen until use. Epithelial explant cultures of
normal laryngeal and LP cells were established in Ham's F12 with 10
µg/ml hydrocortisone and 10 ml/100 ml fetal clone II (Hyclone; GE
Healthcare, Little Chalfont, UK) as previously described (17). These cultures are >99% epithelial,
based on morphology, keratin expression, and episomal HPV DNA
(17). Normal laryngeal cells were
expanded for ≤2–3 passages, whereas LP cells were used at first
passage. Cells were trypsinized and plated at 2×104
cells/cm2 in serum-free keratinocyte growth medium (KGM;
Clonetics Corp., San Diego, CA, USA), and used for experiments
while subconfluent and proliferating. Experiments were performed at
least thrice with cells derived from different patients unless
otherwise noted. The use of human biopsies was approved by the
Institutional Review Board of Women and Infants Hospital of
Zhengzhou (Zhengzhou, China). Informed consent was signed by each
subject's guardian.
Experimental protocols
LP and normal laryngeal cells were cultured in KGM
for 24 h, and the cells were subsequently treated without (control)
or with 1.4% ISO for 0.5 h at 2 l/min in a metabolic chamber
(Columbus Instruments International Corporation, Columbus, OH,
USA). During ISO exposure, the ISO concentration (1.4%) was
continuously verified by sampling the exhaust gas with a Datex
Capnomac (Soma Technology, Inc., Bloomfield, CT, USA) (18). To investigate the inhibitory effects
of SB202190 or celecoxib, cells were treated with SB202190 (10 µM)
or celecoxib (5 µM) for 1 h and continuously cultured for the
indicated periods.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the frozen LP tissues
and cultured cells using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) following the manufacturer's
protocol. The RNA (50 µg) was treated with 2 µl RQ1 RNase-free
DNase (Promega Corporation, Madison, WI, USA) in 50 µl 10X DNase
buffer with 0.5 µl RNase inhibitor and diethylpyrocarbonate-ddH2O
(to a final volume of 50 µl) at 37°C for 20 min. The concentration
and quality of the RNA were measured by UV absorbance at 260 and
280 nm (260/280 nm) using a Nanodrop 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.). Reverse transcription was performed using
3 µg RNA with SuperScript™ II Reverse Transcriptase (Invitrogen),
and cDNA was generated and detected by qPCR (5 µg per reaction)
using SYBR Premix Ex Taq™ (Takara Bio, Inc., Otsu, Japan) according
to the manufacturer's protocols. GAPDH was used as endogenous
control. The relative mRNA level of COX2 was calculated using the
2−ΔΔCt method (19). The
primers used for PCR amplification were as follows: COX2, forward,
5′-TTCTCTCGGTTAGCGACCAATT-3′, and reverse,
5′-CTGAGGGCGTCTGGCTGT-3′; GAPDH, forward,
5′-GGAAATCGTGCGTGACATT-3′, and reverse, 5′-CAGGCAGCTCGTAGCTCTT-3′.
The amplification was performed for 35 cycles using a denaturing
temperature of 94°C (1 min), annealing temperature of 58°C (1.5
min), and extension temperature of 72°C (1.5 min). Results were
analyzed using ABI 7500 Real-Time PCR System software v2.0.1
(Applied Biosystems; Thermo Fisher Scientific, Inc.).
Measurement of PGE2 production
At 24 h after the LP or normal laryngeal cells were
treated with ISO or celecoxib or SB202190, PGE2
production was quantified in the culture medium from
1×105 LP or normal laryngeal cells. PGE2 was
measured using a radioimmunoassay (RIA) kit (Amersham Biosciences
Europe GmbH; GE Healthcare, Freiburg, Germany) as previously
described (20). The experiments
were performed in triplicate.
Western blot analysis
The frozen tissues and cultured cells were lysed in
RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China). For tissues, ~1 ml RIPA lysis buffer was added to 100 mg
tissues and homogenized on ice. For cells, cells in the 6-well
plates were washed with 2 ml phosphate-buffered saline (PBS;
Beyotime Institute of Biotechnology) twice and then treated with
100 µl/well RIPA lysis buffer on ice for 30 min. The homogenates or
cell lysates were sonicated on ice (five times for 5 sec each at 40
W, with 20-sec intervals between each sonication) with a Braun
Labsonic 2000 microtip sonifier (Braun, Melsungen, Germany) and
centrifuged at 12,000 × g for 10 min at 4°C. The protein
concentration of the extracts was measured by the bicinchoninic
acid method (Pierce Biotechnology; Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). Samples containing equal amounts of proteins
(25 µg) were boiled in sodium dodecyl sulfate (SDS) sample buffer
and then analyzed by 10% SDS-polyacrylamide gel electrophoresis
(Beyotime Institute of Biotechnology). The proteins were
electrotransferred to nitrocellulose membranes (Merck Millipore),
and blocked with 5% non-fat milk in Tris-buffered saline (50 mM
Tris-HCl pH 7.4, 150 mM NaCl) and 0.1% Tween 20 (TBST) with shaking
at room temperature for 1 h. Membranes were incubated with primary
antibodies targeting COX2 (1:2,000), p38 MAPK (1:1,500), ERK1/2
(1:1,000), JNK (1:1,000), p-p38 MAPK (Thr180/Tyr182; 1:1,000),
p-ERK1/2 (Thr185/Tyr187; 1:1,000), p-JNK (Thr183/Tyr185; 1:1,000)
and β-actin (1:2,000) overnight at 4°C. After washing with TBST
thrice, 5 min per time, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibody (1:2,000) in
TBST at room temperature for 1 h. Equal sample loading was
confirmed using β-actin. Protein expression was detected by
chemiluminescent film (Amersham Biosciences) using an enhanced
chemiluminescence assay kit (Pierce Biotechnology). The protein
bands were quantified using Quantity One software v4.62 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell viability assay
At 24 h after LP cells were treated with ISO or
celecoxib, the number of viable cells was determined using a Cell
Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's instructions. In
brief, cells were seeded in 96-well plates at a concentration of
1×103 per well and cultured for 1, 2, 3 and 4 days.
CCK-8 solution (10 µl) was added into each well at the indicated
time points, then the plates were stored for 2 h at 37°C. A
scanning multi-well spectrometer (Bio-Tek Instruments, Inc.,
Winooski, VT, USA) was used to measure the absorbance at 450
nm.
5-Ethynyl-20-deoxyuridine (EdU)
incorporation assay
At 24 h after LP cells were treated with ISO or
celecoxib, 50 µM EdU was added and the cells were incubated for 2
h, then fixed with 4% paraformaldehyde for 20 min at room
temperature. Subsequently, the cells were incubated with reaction
buffer (0.5% Triton X-100; Guangzhou RiboBio Co., Ltd., Guangzhou,
China) for 30 min. After washing twice with cold PBS, cells were
counterstained with 4′,6-diamidino-2-phenylindole (Beyotime
Institute of Biotechnology). Six random fields were selected for
observation and then photographed under an inverted fluorescent
microscope (Carl Zeiss AG, Oberkochen, Germany).
Cell cycle analysis
At 24 h after the LP cells were treated with ISO or
celecoxib, the cells were washed with PBS, fixed with ice-cold 70%
ethanol and treated with 1 mg/ml RNase for 30 min at 37°C. DNA
content staining was performed with 50 µg/ml propidium iodide (PI)
at 4°C in the dark for 30 min. A FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) was utilized for data
measurement, and the data were analyzed using CellQuest Pro
software (BD Biosciences).
Apoptosis assay by flow cytometry
For apoptosis assessment, Annexin V-fluorescein
isothiocyanate (FITC) and PI staining was performed as previously
described (21). In brief, at 24 h
after LP cells were treated with ISO or celecoxib, the cells were
harvested, centrifuged at 10,000 × g and 4°C for 5 min and
resuspended in binding buffer (BD Biosciences). Annexin V-FITC (10
µl; BD Biosciences) was added, incubated at room temperature for 15
min, and counterstained with 5 µl PI for 30 min. Annexin V-FITC and
PI fluorescence was analyzed using a FACSCalibur flow cytometer and
the results were analyzed using CellQuest software.
Nucleosomal fragmentation assay
At 24 h after LP cells were treated with ISO or
celecoxib, cell apoptosis was measured using a nucleosomal
fragmentation kit (Cell Death Detection ELISA PLUS; Roche Applied
Science, Penzberg, Germany) as previously described (16). The absorbance values were normalized
against those from control-treated cells to derive a nucleosomal
enrichment factor.
Quantitative caspase-3 activity
assay
Caspase-3 activity was detected using a
Caspase-3/CPP32 Colorimetric Assay kit (BioVision, Inc., Palo Alto,
CA, USA) as previously described (16). Briefly, at 24 h after LP cells were
treated with ISO or celecoxib, 1×106 cells were
incubated with 50 µl chilled lysis buffer on ice for 10 min. The
supernatant was then collected after 10,000 × g
centrifugation at 4°C for 10 min. Protein (150 µg) was added to 50
µl 2X reaction buffer containing 5 µl N-acetyl-Asp-Glu-Val-Asp-pNA
substrate (final concentration, 200 µM).
N-acetyl-Asp-Glu-Val-Asp-pNA cleavage was monitored by detecting
enzyme-catalyzed release of pNA at 405 nm after incubation at 37°C
for 2 h using a microplate reader (Bio-Tek instruments, Inc.).
Statistical analysis
All values are expressed as the mean ± standard
derivation. Intergroup differences were determined by Student's
two-tailed unpaired t-test or one-way analysis of variance,
followed by Dunnett's post hoc test as appropriate. GraphPad
v5.0 statistical software (GraphPad Software, Inc., San Diego, CA,
USA) was used to perform data analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
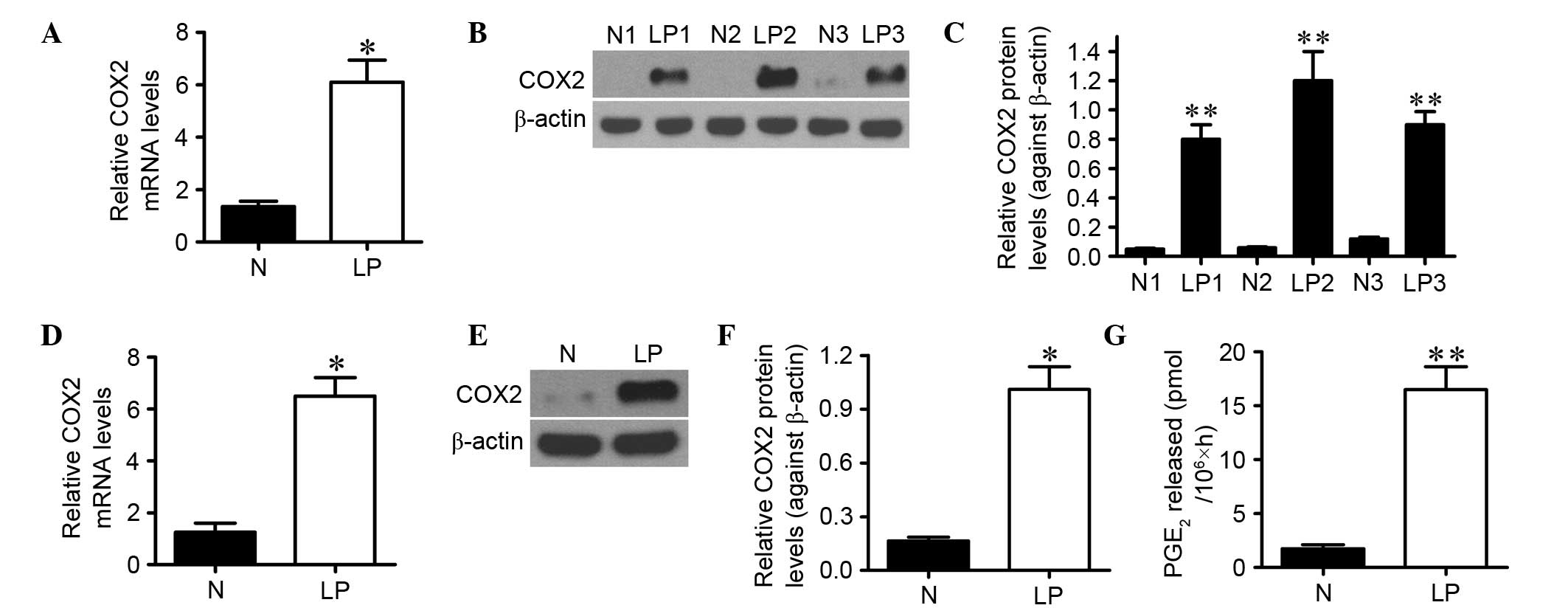
COX2 expression and PGE2
production are increased in LP tissues
The mRNA and protein levels of COX2 in LP tissues
and normal laryngeal biopsies were evaluated using RT-qPCR and
western blot analyses, respectively. As shown in Fig. 1A, the COX2 mRNA expression level was
elevated by approximately fivefold (P<0.05) in LP tissues
compared with normal laryngeal tissues. Furthermore, COX2 protein
expression was significantly higher in LP tissues than that in
normal laryngeal biopsies (P<0.01; Fig. 1B and C). Consistently, the mRNA and
protein levels of COX2 were significantly increased in cultured LP
cells derived from LP tissues compared with the normal cells
isolated from normal laryngeal tissues (Fig. 1D-F). PGE2 production, an
indicator of COX2 activity, was measured by RIA in LP and normal
laryngeal cells. As shown in Fig.
1G, PGE2 level was eightfold (P<0.01) higher in
LP cells than that in normal cells, which was in accordance with
the expression tendency of COX2. These results suggest that COX2
expression and PGE2 generation are increased in LP.
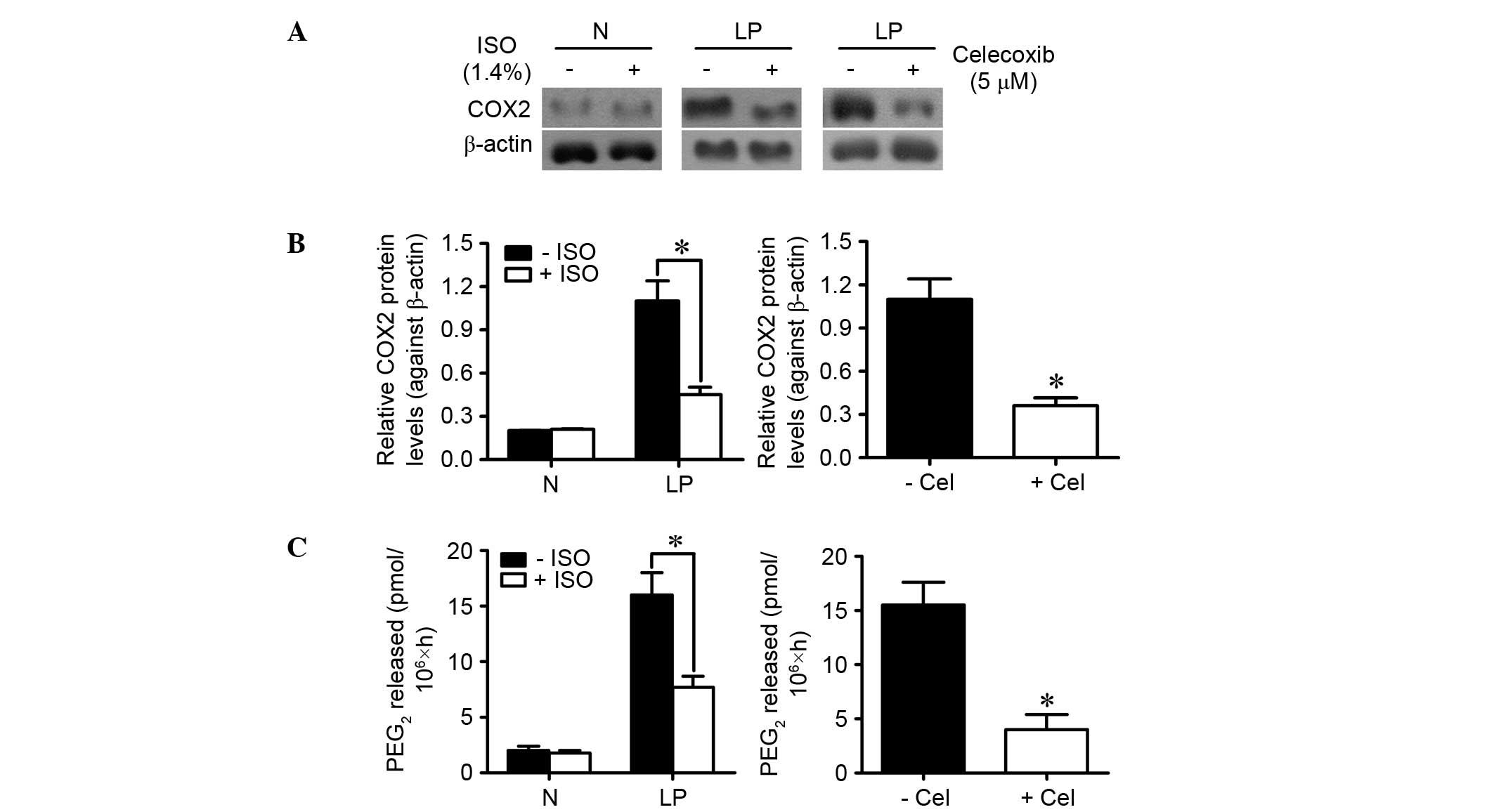
ISO reduces COX2/PGE2 biosynthesis in
LP cells
Wang et al (16) demonstrated that ISO reduces COX2
expression and PGE2 release in Kupffer cells. We
investigated whether ISO treatment inhibited COX2 expression and
PGE2 production in LP and normal laryngeal cells. As
shown in Fig. 2A and B, the
expression of COX2 was nearly threefold (P<0.05) lower in
ISO-treated LP cells than in control LP cells. Alternatively, ISO
treatment had no effect on COX2 expression in normal laryngeal
cells. PGE2 production was significantly decreased by
ISO treatment in LP cells but did not change in normal laryngeal
cells (Fig. 2C). As a positive
control, celecoxib administration also decreased
COX2/PGE2 biosynthesis in LP cells (Fig. 2A-C). These results indicate that ISO
treatment counteracts the increase in COX2 expression and
PGE2 production in LP cells.
ISO reduces COX2 expression and
activity by inactivating p38 MAPK in LP cells
MAPK activation has been implicated to be involved
in the upregulation of COX2 (10).
The present study investigated whether ISO treatment hindered COX2
expression and PGE2 production by reducing p38 MAPK
activation in LP cells. Western blot results showed that ISO
treatment significantly reduced the phosphorylation of p38 MAPK
(Thr180/Tyr182) but not those of ERK1/2 (Thr185/Tyr187) and JNK
(Thr183/Tyr185) in LP cells (Fig. 3A and
B). However, ISO treatment had no effect on phosphorylation of
MAPKs in normal laryngeal cells (Fig. 3A
and B). In addition, it was found that treatment with 10 µM
SB202190 for 1 h, which is a p38 MAPK activation inhibitor, led to
a notable reduction of COX2 expression in LP cells (Fig. 3C and D). Furthermore, PGE2
production was significantly reduced in SB202190-treated LP cells
(Fig. 3E). These findings indicate
that ISO treatment reduces COX2 expression and activity in LP cells
by inhibiting p38 MAPK activation.
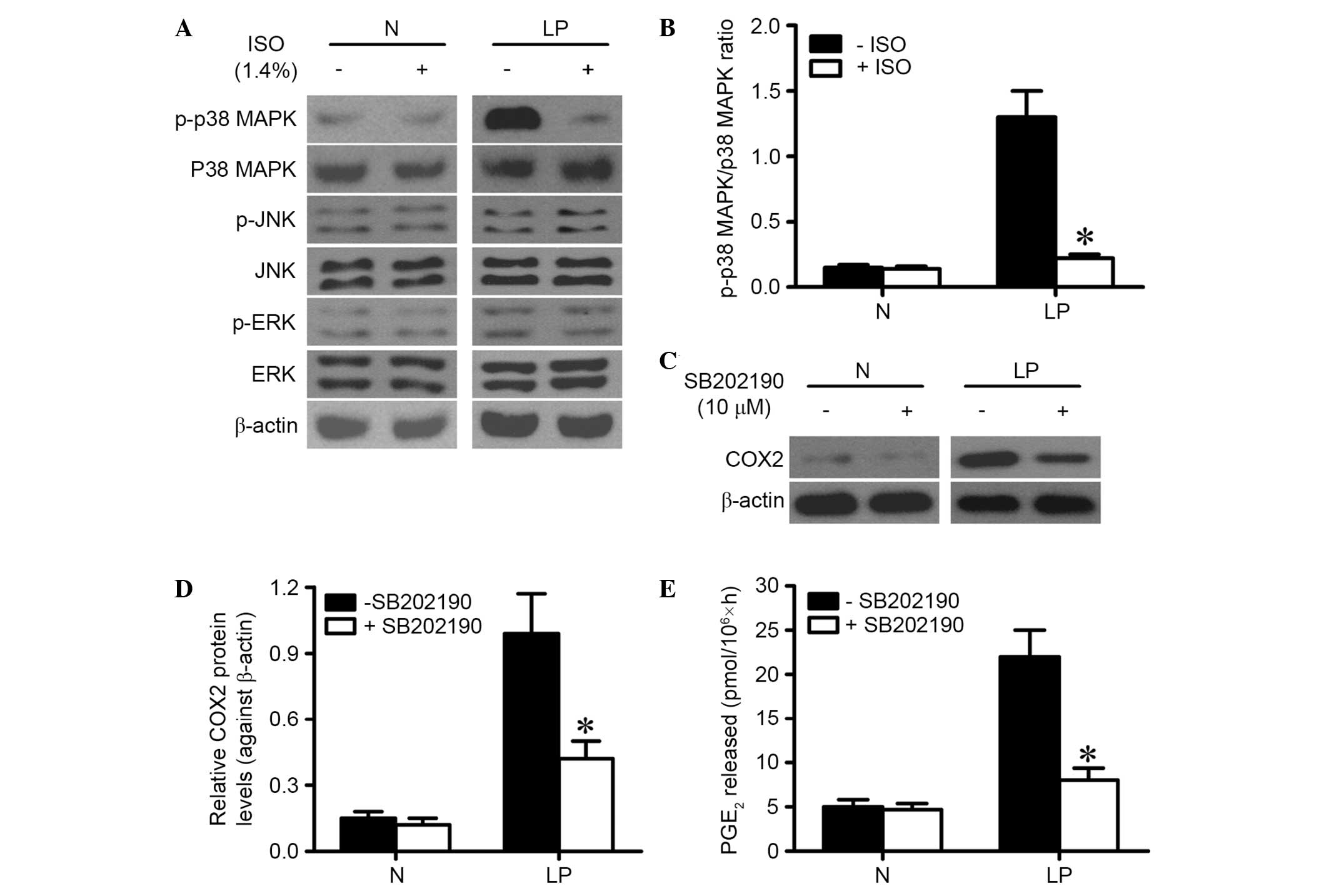 | Figure 3.ISO treatment inhibited COX2
expression and PGE2 production via inactivation of p38
MAPK in LP cells. (A) LP and N cells were treated with or without
1.4% ISO for 0.5 h. The cells were continuously cultured for 6 h.
Western blot analysis was performed to assess the phosphorylation
of p38 MAPK, ERK1/2 and JNK. β-actin was used as internal control.
(B) Ratio of p-p38 MAPK to p38 MAPK is indicated above the bands.
(C) LP and N cells were treated with or without 10 µM SB202190 for
1 h and then washed by PBS. At 24 h after SB202190 treatment,
western blot analysis was used to analyze the protein expression of
COX2. β-actin was used as internal control. (D) COX2 expression in
was quantified and normalized against β-actin. (E) LP and N cells
were treated with or without 10 µM SB202190 for 1 h. At 24 h after
SB202190 treatment, the quantity of PGE2 in the culture
medium was determined by radioimmunoassay. Representative data are
from three independent experiments and expressed as the mean ±
standard deviation. *P<0.05. ISO, isoflurane; N, normal
laryngeal tissues or cells; LP, laryngeal papilloma tissues or
cells; p38 MAPK, p38 mitogen-activated protein kinase; JNK, c-Jun
N-terminal kinase; ERK, extracellular signal-regulated kinase;
COX2, cyclooxygenase 2; PGE2, prostaglandin
E2. |
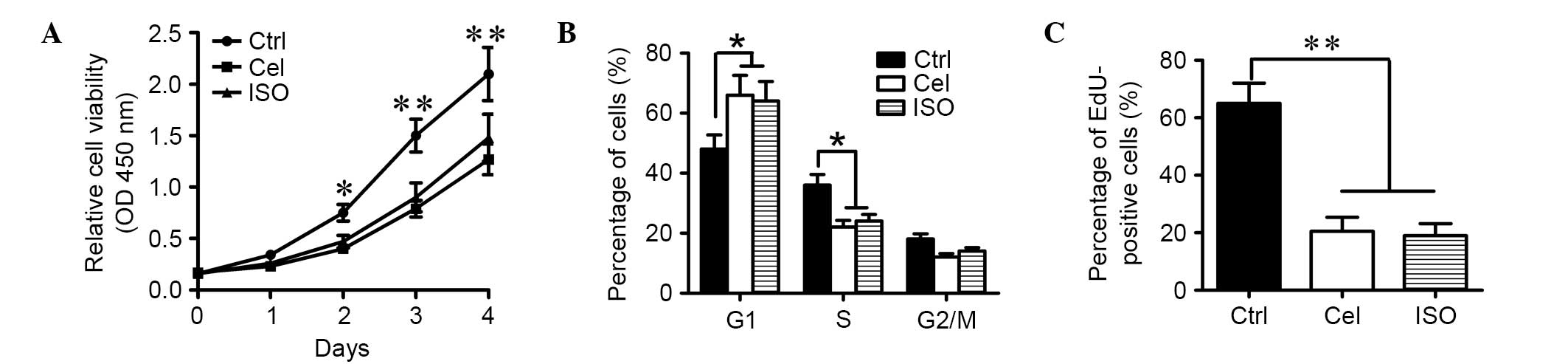
ISO inhibits LP cell proliferation by
decreasing COX2 activity
COX2 activity is involved in increased proliferation
of LP cells (11). To investigate
whether the inhibitory effects of ISO on LP cell viability and
proliferation depend on the reduction of COX2 activity, CCK-8, flow
cytometry and EdU incorporation assays were performed. As shown in
Fig. 4A, ISO administration
significantly reduced the viability of LP cells. Cell cycle was
arrested at the G1 phase, with 66% of ISO-treated LP cells in G0/G1
compared with 48% of control cells (P<0.05; Fig. 4B). The percentage of EdU
incorporation was also decreased, with 22% of ISO-treated LP cells
compared with 64% of control cells (P<0.01; Fig. 4C). All these results were consistent
with the inhibitory effects of celecoxib on LP cell viability and
proliferation (Fig. 4A-C),
suggesting that ISO treatment inhibits the viability and
proliferation of LP cells, probably by reducing COX2 activity.
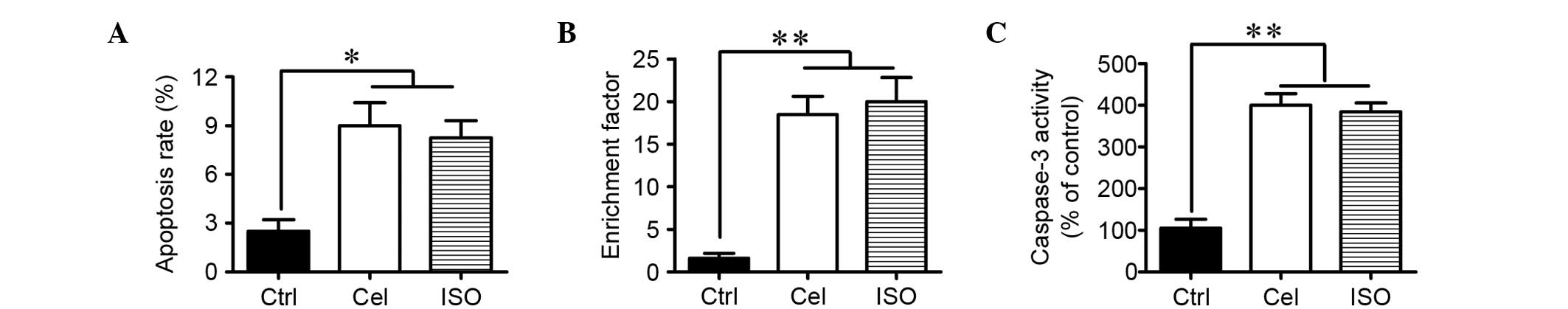
ISO promotes LP cell apoptosis by
inhibiting COX2 activity
Increase in COX2 activity has been shown to inhibit
LP cell apoptosis (12). Flow
cytometry, nucleosomal fragmentation and caspase-3 activity assays
were employed to investigate whether ISO induced LP cell apoptosis
by inhibiting COX2 activity. Flow cytometry analysis showed that
ISO treatment significantly increased the percentage of apoptotic
LP cells compared with the control cells (Fig. 5A). The significant increases in
nucleosomal fragmentation and caspase-3 activity were observed in
ISO-treated LP cells compared with the control cells (Fig. 5B and C). The above results were
consistent with the proapoptotic effects of celecoxib on LP cells
(Fig. 5). Thus, ISO administration
promotes the apoptosis of LP cells probably by inhibiting COX2
activity.
Discussion
Cultured LP cells are a model system for the study
of HPV-infected LP. In contrast to immortalized cell lines, these
primary cells closely reflect the biology of in vivo
papilloma. In the present report, it was found that ISO treatment
significantly inhibited proliferation and prompted apoptosis in LP
cells by reducing p38 MAPK/COX2 signaling. The key findings are as
follows: First, COX2 was highly expressed in LP tissues and cells
compared to normal laryngeal tissues and cells. Second, ISO
treatment significantly inhibited COX2 expression and
PGE2 production in LP cells. Third, the inhibitory
effects of ISO on COX2 expression and activity depend on the
inactivation of p38 MAPK in LP cells. Finally, ISO markedly
decreased the proliferation and apoptosis resistance of LP cells by
inhibiting COX2 activity.
COX2, an inducible enzyme, serves a crucial function
in the production of prostaglandins under physiological and
pathophysiological conditions, which can be rapidly induced by
various stimulants, such as growth factors, carcinogens and
proinflammatory cytokines (22).
COX2 expression is enhanced in a variety of inflammatory and
neoplastic diseases (23). Notably,
COX2 expression is upregulated in numerous types of HPV-infected
cells, including respiratory papillomas (24), head and neck tumors (25–27),
cervical cancers and penile cancers (28,29). Wu
et al (11) reported that
COX2 upregulation is mediated in part by Rac1-dependent activation
of p38 MAPK in papilloma cells. Wang et al (16) demonstrated that ISO administration
markedly decreased COX2 expression via the inhibition of p38 MAPK
activation in zymosan-stimulated murine Kupffer cells. The present
results showed that ISO treatment significantly suppressed COX2
expression and activity by inhibiting p38 MAPK activation.
Activation of p38 MAPK can exert either proapoptotic
or antiapoptotic effects in different cell types and cell
microenvironments (30). p38 MAPK
activation has been shown to increase cell viability in LP cells
(11). This effect may be due to the
increased levels of COX2 induced by p38 MAPK activation (12). Previous studies showed that COX2
upregulation enhances proliferation and apoptosis resistance, and
inactivation of COX2 induces cell apoptosis in cancer cells
(31–33). Treatment with celecoxib, an inhibitor
of COX2, could effectively suppress cell proliferation and induce
cell apoptosis in LP cells, and these effects are mediated in part
by PGE2 (12). Notably,
excessive production of PGE2 has been reported to
promote cell proliferation and inhibit cell apoptosis (34). ISO inhibits proliferation of several
human cancer cell lines (15). The
present results showed that ISO treatment markedly decreased LP
cell viability and proliferation by inhibiting COX2 activity.
Additionally, ISO has been demonstrated to induce apoptosis in
different cell types (35,36), and prolonged ISO treatment can induce
apoptosis in cancer cells (15). A
previous study suggested that the effect of ISO on apoptosis
depends on the mitochondrial pathway (37), which is regulated by Bcl-2 family
proteins; this pathway also involves the release of cytochrome c
from the mitochondria to the cytosol (38). The released cytochrome c activates
caspase-9, and consequently induces caspase-3 activation,
ultimately leading to cell apoptosis (39). Thus, the present findings showed that
ISO treatment significantly increased LP cell apoptosis by
inhibiting COX2 activity.
In summary, the present study showed that COX2 is
highly expressed in HPV-infected LP tissues and cells compared with
normal laryngeal tissues and cells, and PGE2 production
is increased in LP cells. Notably, it was found that ISO treatment
significantly reduces COX2 enhancement and PGE2 release
in LP cells by inhibiting the activation of p38 MAPK. In addition,
ISO significantly inhibits cell proliferation and apoptosis
resistance by inhibiting COX2 activity. Collectively, ISO may be a
potential agent for LP treatment by inhibiting p38 MAPK/COX2
activation.
References
|
1
|
Watanabe A, Tsujie H, Taniguchi M,
Hosokawa M, Fujita M and Sasaki S: Laryngoscopic detection of
pharyngeal carcinoma in situ with narrowband imaging. Laryngoscope.
116:650–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katsenos S and Becker HD: Respiratory
papillomatosis: A rare chronic disease, difficult to treat, with
potential to lung cancer transformation: Apropos of two cases and a
brief literature review. Case Rep Oncol. 4:162–171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katada C, Nakayama M, Tanabe S, Koizumi W,
Masaki T, Takeda M, Okamoto M and Saigenji K: Narrow band imaging
for detecting metachronous superficial oropharyngeal and
hypopharyngeal squamous cell carcinomas after chemoradiotherapy for
head and neck cancers. Laryngoscope. 118:1787–1790. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steinberg BM, Meade R, Kalinowski S and
Abramson AL: Abnormal differentiation of human
papillomavirus-induced laryngeal papillomas. Arch Otolaryngol Head
Neck Surg. 116:1167–1171. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Poetker DM, Sandler AD, Scott DL, Smith RJ
and Bauman NM: Survivin expression in juvenile-onset recurrent
respiratory papillomatosis. Ann Otol Rhinol Laryngol. 111:957–961.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnston D, Hall H, DiLorenzo TP and
Steinberg BM: Elevation of the epidermal growth factor receptor and
dependent signaling in human papillomavirus-infected laryngeal
papillomas. Cancer Res. 59:968–974. 1999.PubMed/NCBI
|
|
7
|
Zhang P and Steinberg BM: Overexpression
of PTEN/MMAC1 and decreased activation of Akt in human
papillomavirus-infected laryngeal papillomas. Cancer Res.
60:1457–1462. 2000.PubMed/NCBI
|
|
8
|
Vambutas A, Di Lorenzo TP and Steinberg
BM: Laryngeal papilloma cells have high levels of epidermal growth
factor receptor and respond to epidermal growth factor by a
decrease in epithelial differentiation. Cancer Res. 53:910–914.
1993.PubMed/NCBI
|
|
9
|
Johnston D, Hall H, DiLorenzo TP and
Steinberg BM: Elevation of the epidermal growth factor receptor and
dependent signaling in human papillomavirus-infected laryngeal
papillomas. Cancer Res. 59:968–974. 1999.PubMed/NCBI
|
|
10
|
Tanabe T and Tohnai N: Cyclooxygenase
isozymes and their gene structures and expression. Prostaglandins
Other Lipid Mediat 68–69. 95–114. 2002. View Article : Google Scholar
|
|
11
|
Wu R, Coniglio SJ, Chan A, Symons MH and
Steinberg BM: Up-regulation of Rac1 by epidermal growth factor
mediates COX-2 expression in recurrent respiratory papillomas. Mol
Med. 13:143–150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu R, Abramson AL, Shikowitz MJ,
Dannenberg AJ and Steinberg BM: Epidermal growth factor induced
cyclooxygenase-2 expression is mediated through
phosphatidylinositol-3 kinase, not mitogen-activated
protein/extracellular signal-regulated kinase kinase, in recurrent
respiratory papillomas. Clin Cancer Res. 11:6155–6161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li JT, Wang H, Li W, Wang LF, Hou LC, Mu
JL, Liu X, Chen HJ, Xie KL, Li NL and Gao CF: Anesthetic isoflurane
posttreatment attenuates experimental lung injury by inhibiting
inflammation and apoptosis. Mediat Inflamm. 2013:1089282013.
View Article : Google Scholar
|
|
14
|
Wang H, Fan J, Li NL, Li JT, Yuan SF, Yi
J, Wang L, Chen JH, Lv YG, Yao Q, et al: A subanesthetic dose of
isoflurane during postconditioning ameliorates zymosan-induced
neutrophil inflammation lung injury and mortality in mice. Mediat
Inflamm. 2013:4796282013. View Article : Google Scholar
|
|
15
|
Kvolik S, Glavas-Obrovac L, Bares V and
Karner I: Effects of inhalation anesthetics halothane, sevoflurane
and isoflurane on human cell lines. Life Sci. 77:2369–2383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Wang L, Li NL, Li JT, Yu F, Zhao
YL, Wang L, Yi J, Wang L, Bian JF, et al: Subanesthetic isoflurane
reduces zymosan-induced inflammation in murine kupffer cells by
inhibiting ROS-activated p38 MAPK/NF-kB signaling. Oxid Med Cell
Longev. 2014:8516922014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steinberg BM, Abramson AL and Meade RP:
Culture of human laryngeal papilloma cells in vitro. Otolaryngol
Head Neck Surg. 90:728–735. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawaraguchi Y, Horikawa YT, Murphy AN,
Murray F, Miyanohara A, Ali SS, Head BP, Patel PM, Roth DM and
Patel HH: Volatile anesthetics protect cancer cells against tumor
necrosis factor-related apoptosis-inducing ligand-induced apoptosis
via caveolins. Anesthesiology. 115:499–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olson PN, Bowen RA, Behrendt MD, Olson JD
and Nett TM: Validation of radioimmunoassays to measure
prostaglandins F2 alpha and E2 in canine endometrium and plasma. Am
J Vet Res. 45:119–124. 1984.PubMed/NCBI
|
|
21
|
Stoeck A, Gast D, Sanderson MP, Issa Y,
Gutwein P and Altevogt P: L1-CAM in a membrane-bound or soluble
form augments protection from apoptosis in ovarian carcinoma cells.
Gynecol Oncol. 104:461–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harris RE: Cyclooxygenase-2 (cox-2) and
the inflammogenesis of cancer. Subcell Biochem. 42:93–126. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turini ME and DuBois RN: Cyclooxygenase-2:
A therapeutic target. Annu Rev Med. 53:35–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson AB, Das SK, Bruegger DE, Hoover
LA and Sanford TR: Characterization of cyclooxygenase in laryngeal
papilloma by molecular techniques. Laryngoscope. 109:1137–1141.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banerjee AG, Gopalakrishnan VK,
Bhattacharya I and Vishwanatha JK: Deregulated cyclooxygenase-2
expression in oral premalignant tissues. Mol Cancer Ther.
1:1265–1271. 2002.PubMed/NCBI
|
|
26
|
Chan G, Boyle JO, Yang EK, Zhang F, Sacks
PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, et al:
Cyclooxygenase-2 expression is up-regulated in squamous cell
carcinoma of the head and neck. Cancer Res. 59:991–994.
1999.PubMed/NCBI
|
|
27
|
Ranelletti FO, Almadori G, Rocca B,
Ferrandina G, Ciabattoni G, Habib A, Galli J, Maggiano N, Gessi M
and Lauriola L: Prognostic significance of cyclooxygenase-2 in
laryngeal squamous cell carcinoma. Int J Cancer. 95:343–349. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kulkarni S, Rader JS, Zhang F, Liapis H,
Koki AT, Masferrer JL, Subbaramaiah K and Dannenberg AJ:
Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin
Cancer Res. 7:429–434. 2001.PubMed/NCBI
|
|
29
|
Golijanin D, Tan JY, Kazior A, Cohen EG,
Russo P, Dalbagni G, Auborn KJ, Subbaramaiah K and Dannenberg AJ:
Cyclooxygenase-2 and microsomal prostaglandin E synthase-1 are
overexpressed in squamous cell carcinoma of the penis. Clin Cancer
Res. 10:1024–1031. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arun B and Goss P: The role of COX-2
inhibition in breast cancer treatment and prevention. Semin Oncol.
31(2): Suppl 7. S22–S29. 2004. View Article : Google Scholar
|
|
32
|
Tsujii M, Kawano S and DuBois RN:
Cyclooxygenase-2 expression in human colon cancer cells increases
metastatic potential. Proc Natl Acad Sci USA. 94:3336–3340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krysan K, Dalwadi H, Sharma S, Põld M and
Dubinett S: Cyclooxygenase 2-dependent expression of surviving is
critical for apoptosis resistance in non-small cell lung cancer.
Cancer Res. 64:6359–6362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Subbmaramiah K and Dannenberg AJ:
Cyclooxygenase 2: A molecular target for cancer prevention and
treatment. Trends Pharmacol Sci. 24:96–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Loop T, Dovi-Akue D, Frick M, Roesslein M,
Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, et al:
Volatile anesthetics induce caspase-dependent,
mitochondria-mediated apoptosis in human T lymphocytes in vitro.
Anesthesiology. 102:1147–1157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aravindan N, Cata JP, Hoffman L, Dougherty
PM, Riedel BJ, Price KJ and Shaw AD: Effects of isoflurane,
pentobarbital and urethane on apoptosis and apoptotic signal
transduction in rat kidney. Acta Anaesthesiol Scand. 50:1229–1237.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp
A, Yue Y, Xu T and Xie Z: The mitochondrial pathway of anesthetic
isoflurane induced apoptosis. J Biol Chem. 285:4025–4037. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Orrenius S, Zhivotovsky B and Nicotera P:
Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol
Cell Biol. 4:552–565. 2003. View Article : Google Scholar : PubMed/NCBI
|