Introduction
One of the basic functions carried out by the
stomatognathic system is the collection and grinding of food.
Mastication is considered to be the initial phase of food digestion
(1–3). Mastication occurs as a result of the
force-movement field, and is carried out via a complex interaction
between muscle systems, teeth, lips, cheeks, the palate, salivary
glands and the temporomandibular joints (4–7). Correct
mastication should proceed on both sides of the dental arch
openings, since one-sided mastication is the source of an uneven
load of the temporomandibular joints (8–10). An
important element of mastication which determines the appropriate
mechanical grinding of food are the teeth, located in the maxilla
and the mandible (11–14). From a mechanical point of view, the
predominant tasks of the teeth are biting, grinding and crushing.
Each type of tooth is adapted to different functions: The incisors
are used for bitting and cutting, the canines for tearing food
(15), and the premolars and molars
for crushing and chewing food. In the initial phase of mastication,
food introduced into the oral cavity is ground, mixed and moistened
with saliva, which is supplied to the oral cavity via salivary
glands. The duration of mastication proceeds until the moment when
food, adequately ground and moistened with saliva, is formed into
smaller bites and then swallowed. The ability to grind is an
individually variable feature and has a crucial impact on the later
phase of digestion, taking place in further sections of the
alimentary tract (16). A
significant influence on the efficacy of this physiological
activity is the stomatognathic muscle system, which occurs as a
result of the complex, periodical abduction and adduction movements
of the mandible. Trajectories made by the incisors in a single
mastication cycle resemble in their shape a deformed ellipse
(17–19). Additionally, in each cycle it is
possible to distinguish the abduction movement, the scope of which
depends on the size of the bite and the consistency of the
fragmented food (20–26). Lundeen and Gibbs (27) reported that if the fragmented food
was characterized by hardness, then the scope of the lateral shifts
of the mandible increase. In addition, the hardness of the bites
has a decisive impact on the number of mastication cycles; the
harder the food, the more cycles are required in order to obtain an
adequate consistency for swallowing (28). In the initial phase of crushing, the
alimentary bite decreases the distance between the antagonistic
teeth. When this distance is ~3 mm, the cusps of the mandibular
teeth are located nearly directly under the cusps of the maxillary
teeth. This is the starting point for the second phase, consisting
of trituration of the morsel of food. This stage depends strictly
on the topography of the cusps and the geometry of the tooth slopes
and furrows. Mandibular movement, responsible for grinding food,
proceeds until the cusps of the mandibular teeth are in contact
with the maxillary teeth furrows. Initial research suggested that
antagonistic teeth do not come into contact during mastication
(29), although in later
investigations the presence of contact was demonstrated (30,31). The
frequency of contact between the teeth increases with the gradual
grinding of a food morsel, and contact occurs in the final
mastication cycles immediately prior to swallowing (32).
For the purpose of the present study, an experiment
was conducted which aimed to identify a numeric reflection of the
movement of the mandible and of the stomatognathic muscle system
during mastication.
Materials and methods
Subject
An electronic facebow (Zebris JMA20, Zebris Medical
GmbH, Allgäu, Germany) was used to record spatial movements of the
mandible during food mastication. Recording of the mandible
movements, reflecting mastication, were carried out in a 47
year-old healthy person in whom, during clinical examination, no
functional disorders in the mastication muscle system were
observed. The study was conducted in the Laboratory Diagnosis and
Treatment of Dysfunction, the Department of Prosthodontics of the
Pomeranian Medical University. The patients involved were all
volunteers. Men with similar parameters (height and weight) aged
between 34 to 47 years old (average 39.83 years old) with full
dentition and without any dysfunction within the masticatory system
were selected. Only one 47 year-old healthy person, during clinical
examination, was observed to lack a single tooth mandibular tooth
no. 36. Routine clinical testing demonstrated the presence of
composite filling in all molars and insignificant abrasion of the
cusps and incising edges of the other teeth. The degree of loss of
dental hard tissues were determined to be I° and II° according to
the Martin scale (33). Contact
between the antagonistic teeth occurred in the correct triads with
the occlusion type protected by the canines. In addition, no
occlusion obstructions were observed, such as premature contact
during dynamic occlusion. The subject did not report any disorders
of the teeth or any other elements of the stomatognathic system.
The only reported disorder was periodical teeth occluding, which
occurred as a method for relieving stress in moments of emotional
excitement. The present study was approved by the Ethics committee
of the Pomeranian Medical University, Szczecin (no. KB-0012/30/13).
All patients gave written informed consent to testing.
The mandibular kinematics
The formal basis of carrying out model researches on
mandible kinematic movements were incisor and candylar process
trajectory Trajectories on the condyloid process and incisor heads
were recorded with an electronic Zebris JMA facebow following the
placement of a portion of food in the oral cavity. Completion of
the measurements was determined by the moment, in which total
fragmentation finalized by swallowing occurred. Clinical tests were
carried out 6 times per portion of bread (2 cm2 cube)
and hazelnuts (1.2/1.3 cm in diameter). Based on the trajectories
of the condyloid processes and incisors, the configuration
coordinates of the spatial model for the mandibular kinematics were
calculated on the basis of which length and spatial orientation the
mastication muscles were located. The first stage of the model
research is to calculate the configuration coordinates of the
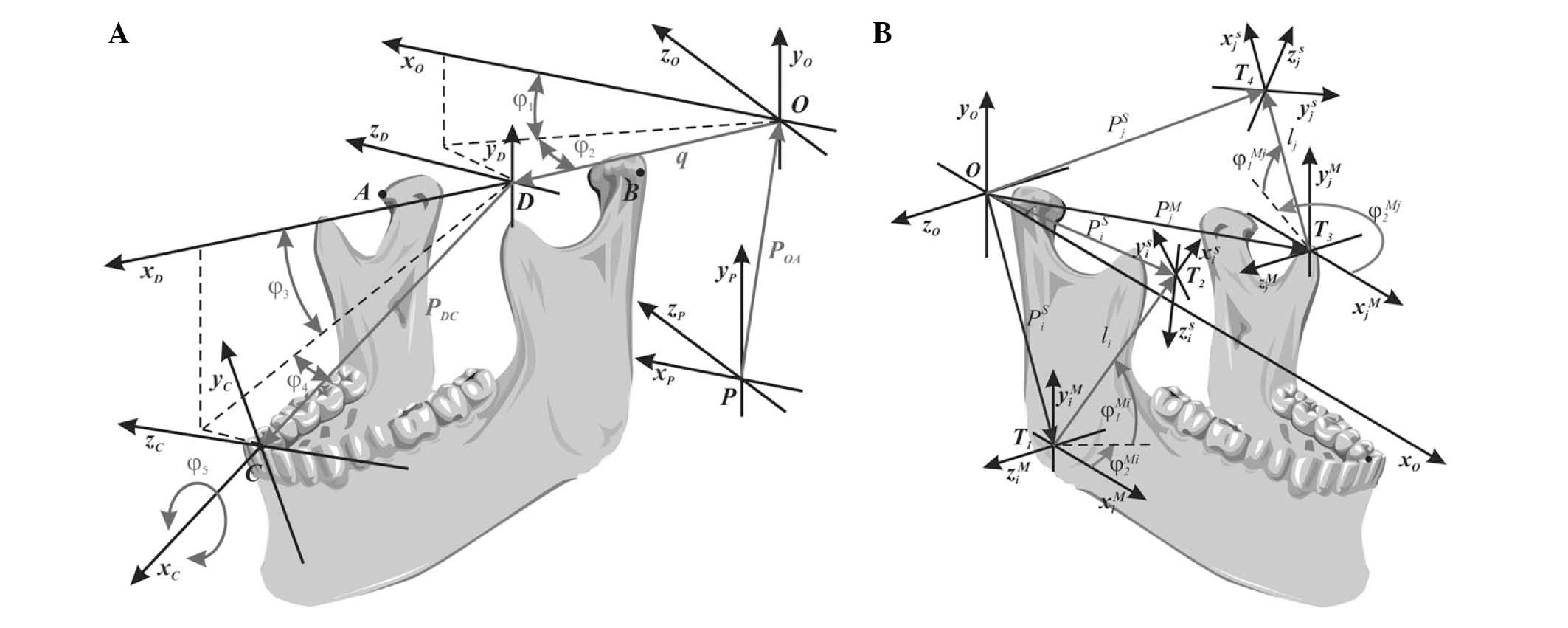
numerical model of the lower jaw (Fig.
1A). These calculations are carried out on the basis of data
recorded by an electronic facebow Zebris JMA. Formulated
verification criteria, confirming compliance trajectory of the
incisors (pts. C Fig. 1A) and the
heads of the condylar process (pts. AiB, Fig. 1A), calculated numerically and
registered in a clinical trial confirms the correctness of the
mandible numerical model. A model verified in this way is still
used for the numerical calculation of the average change in
stomatognathic system muscles length. A numerically calculation of
the average change in length of masticatory muscles can be
experimentally verified only by taking measurements on pictures
taken using X-ray imaging techniques: magnetic resonance imaging or
computer tomography. Such verification can be useful for planning
the therapy for patients with disabilities, for instance after
condylar jaw fracture.
Mathematical and Engineering techniques such as
automation and robotics were used. As a result configuration
coordinates of the mathematical jaw model were calculated (Equation
1). In addition, the spatial configuration of the jaw was
calculated (Equation 2) and it was used as a reference for the
identification of accidental points of the jaw representing the
movable muscle attachments in the stomatognathic system (Equation
3). The knowledge of the Cartesian coordinates imitating (at any
point in time) the spatial position of the movable muscle
attachments allowed us to determine the average change in length
(Equation 4) and the spatial orientation of the muscle fibres
(Equation 5). It should be emphasized that the authors of the
present study did not come across any other similar approach to the
kinematics of the lower jaw and the muscles during the review of
the Literature.
In order to improve the numeric calculations,
original Kinematics 3D software version 10.5.60 (WinJaw
Evaluation software, Zebris Medical GmbH) was developed, in which
mastication was mathematically reflected for the bread and
hazelnuts. This program provides all the necessary information
about the kinematics of the mandible and masticatory muscles
registered in clinical trials, which can be verified by
experimental research. For example, we can see how changing one
factor, (eg. muscle length) affects other movement parameter
characteristics for the patients lower jaw. The first stage of the
examination was done on the basis of the registered incisors
trajectory (point C, Fig. 1A) and
the mandibular condyles (points A and B, Fig. 1A) that calculates the coordinates of
the configuration of the jaw. On this basis it is possible to
further assess the changes in length of the muscle fibers of the
masseters (Fig. 1B).
In computer simulations for the mathematical
description of the human body movement, kinematic chains with an
open structure are most often used. Movement of the biomechanism
reflecting the functioning of the mastication muscles may be
modelled using the Cartesian or polar coordinates. In addition, as
is the case with technical systems, the position and orientation of
particular biosegments is described with respect to an immovable
reference system. From a mechanical point of view, two kinematic
tasks may be distinguished: A simple task, which is the
identification of trajectories on which typical biomechanical
points move based on present configuration coordinates, and the
so-called reverse task, which consists in the identification of the
configuration coordinates (Cartesian coordinates) that reflect the
trajectories of characteristic biomechanical points. No matter
which kinematic task is the object of the model tests, during their
solution, it is necessary to have a kinematic model that is
formulated accordingly. In the present study, spatial mandibular
movement was projected using an open kinematic chain with a
configuration of variable in time.
Identification of configuration coordinates. The
xO, yO, zO
reference system in respect of which calculations are conducted may
be assumed in any manner, and can be located for instance in the
central part of the section connecting the condyloid process heads
of the mandible (point D; Fig. 1A).
However, such a location results in specific difficulties,
including uncertainties during the calculations, such as the
substitution averaging points that define the location of the
muscles in the three dimensional space. For this reason and to
eliminate these issues, it is necessary to introduce an additional
xP, yP, zP
coordinate system. This mathematical procedure does not complicate
the numeric calculations and may be interpreted as an expansion of
the model by an additional segment, the orientation of which
remains constant during the mandibular movements.
The numerical calculations were used with the
application of the algorithm of Fourier fast transformation.
Fourier spectral analysis is one of the most popular tools to judge
the parameters and properties of the signal by spreading it on the
harmonic amplitude frequency spectrum. Spectral analysis of signals
is widely used as a result of the computer technology development.
Getting faster and optimized algorithms in numerical methods allow
you to perform any analysis of the spectral signal in a fast and
precise way without the knowledge of the explicit representation of
the analytical record. Direct calculation of the discrete Fourier
transform (DFT)requires N2 multiplication and addition, where N
defines the number of samples analyzed signal. In order to speed up
the calculation, the so-called Fast Fourier Transform (FFT) is used
with the Cooley and Tukey algorithms. Another approved procedure is
a modification of the FFT algorithms, Cooley and Tukey, which uses
the radix-2 algorithm. For an even number of samples N, it allows
the breakdown of DFT on the two interleaved smaller size N/2,
resulting in a number of necessary mathematical operations being
halved compared to the classical DFT. Currently, the radix-2
algorithm is the most common numerical procedure whereby it is
possible to reduce the necessary number of arithmetic operations
(N-ln(N)). The shorter computation time of harmonic components
achieved is caused by the elimination of unnecessary intermediate
records performed in the computer's memory.
In the model adopted for the numeric tests, three
coordinates define the spatial orientation of the mandible
(Ф3, Ф4, Ф5), and the others
reflect the lifting movement (q, Ф1,
Ф2). The first step in forming a mathematical model of
the kinematics of the jaw is a solution to the simple equation.
This stage is necessary since it provides information with regard
to the structure of the equations, which is used to derive
analytical associations describing the relationships between the
configuration coordinates of the model and the recorded
trajectories during clinical examination. The present study is
limited only to specifying the final equations, describing the
association between configuration and Cartesian coordinates
(equation 1):
q=(xD–x0)2+(yD–y0)2+(zD–z0)2,φ1=arctan(yD–y0xD–x0),φ3=arctan(yC–yDxC–xD),φ2=arctan(–(zD–z0)(cosφ1+sinφ1)(xD–x0)+(yD–y0)),φ4=arctan(–(zC–zD)(cosφ3+sinφ3)(xC–xD)+(yC–yD)),φ5=arctan(((xA–xD)+(yA–yD))cosφ4(zA–zD)(sinφ3–cosφ3)–sinφ4(cosφ3+sinφ3)(sinφ3–cosφ3)).
Identification of Cartesian
coordinates
The factor that determines the mastication organ
functioning is the muscular system controlled by the central
nervous system. In order to determine the numeric projection of the
motor activity of particular muscle fibres, appropriate
mathematical associations must be defined. These associations are
derived on the basis of the identified configuration coordinates
(equation 1). In order to determine, using theoretical
considerations, the scope of the changes in length and spatial
orientation of specific groups of muscles, the Cartesian
coordinates typical of attachment locations must be determined
using the following formula, in which PiT is
the vector defining the location of the muscle attachment ‘i’
associated with the mandible, for the selected orientation and
location of the mandible; PiT is the vector
defining the location of the muscle attachment ‘i’ associated with
the mandible at the resting position; PD is the vector
defining the location of point D (Fig.
1); and R the orientation matrix of the mandible (equation
2):
PiT=PD+R·Pi0,
The model assumes the resting position of the
mandible, which is a characteristic of opening dental arches. A
characteristic feature in the rest position is a jaw-jaw distance,
or alternatively the lack of occlusal contact between opposing
dental arches. A characteristic feature of the resting position is
the downwards shift of the jaw, which results in the opening of
occlusion surfaces of the dental arches. Following the measurement
of the distance between the teeth in the resting position, the
resting gap in normal occlusion conditions was previously
determined to be 2–4 mm (34). In
addition, in the resting position the muscle adductors and
abductors are in balance, therefore, muscle function is only
balancing the weight of the lower jaw and bioelectric muscle
activity is minimal (35–40). A shifting mandible changes its
orientation and position, and specific muscle groups become longer
or shorter. The length and spatial orientation of the muscle fibres
can only be approximately determined. This approximation is caused
by the large surface space of the muscle attachments to the
mandibular and cranial bones. Lack of precise criteria that would
ensure the clear projection of the location of the muscle
attachments results in the majority of model tests offering
approximate solutions. Their advantage is the possibility of
reducing the attachment surfaces to a single point. Therefore, this
approach causes the lengths of specific groups of muscle fibres to
be averaged.
General analytic association
The movement of muscles in the stomatognathic system
was calculated in relation to a fixed coordinate system (point O,
Fig. 1B). The local coordinate
system T2 represents the position of the muscle attachment
‘i’ to the skull, and this system is associated with the fixed
reference system O. On the other hand, the local variable
coordinate system T1 is associated with the attachment of
the mandible muscle ‘i’. In order to simplify the calculations, the
same spatial orientation is specified for both the T1 and O
reference systems, given that this simplification has no effect on
the identified averaged lengths or orientation of the muscle
fibres. On the basis of the schematic diagram (Fig. 1B), a general analytical association
was derived, which simultaneously considered the length of the
mandibular muscle and its spatial orientation. In this formula,
PiS is the vector defining the position of
the muscle attachment ‘i’ associated with the skull;
PiM the position of the muscle attachment ‘i’
associated with the mandible; RiZ and
RiY the rotation matrixes relative to the
axes z and y; and Li = [li 0 0]T is
the vector defining the distance between local coordinate systems
T1 and T2 (equation 3):
PiS=PiM+RiZ·RiY·Li,
Calculation of the average muscle length. The
average length of the muscle is calculated based on the average of
Cartesian coordinates that define the position of muscle trailers
to the mandible and the skull (equation 4):
li=(xiM–xiS)2+(yiM–yiS)2+(ziM–ziS)2.
In the above equation, the xi,
yi, zi coordinates represent
the Cartesian coordinates of the muscle attachments, given that the
superscripts marked with the symbol ‘S’ correspond to the
fixed attachments to the cranial bones, whereas symbol ‘M’
corresponds to the variable incidental attachments to the mandible.
The change in the length of the mandibular muscle, manifested by
the shortening or elongation Δl, is calculated as a
difference between the resting length of the muscle
li 0 and its length at any given time
of the mandibular movement li. The resting length
of the functioning mastication muscles is associated with the
position of the mandible at rest. Note that positive Δl
difference values may be interpreted as extensions of the
mandibular muscle, whereas negative values as shortening of the
mandibular muscle. Angles φ1Mi and
φ2Mi defining the spatial orientation
of the muscle ‘i’ may be obtained by transformations of equation 3
(equation 5):
φ1Mi=arctan(yiC–yiZxiC–xiZ),φ2Mi=arctan(–(ziC–ziZ)·(cos(φ1Mi)+sin(φ1Mi))(xiC–xiZ)+(yiC–yiZ)).
Analytical associations based on data derived from
equations 1–5 supplemented by data recorded during mandible
movement on clinical examination are the formal basis for
conducting numerical calculations on the activity of mastication
muscle functioning.
Results
Trajectories of the incisors and
Cartesian coordinates
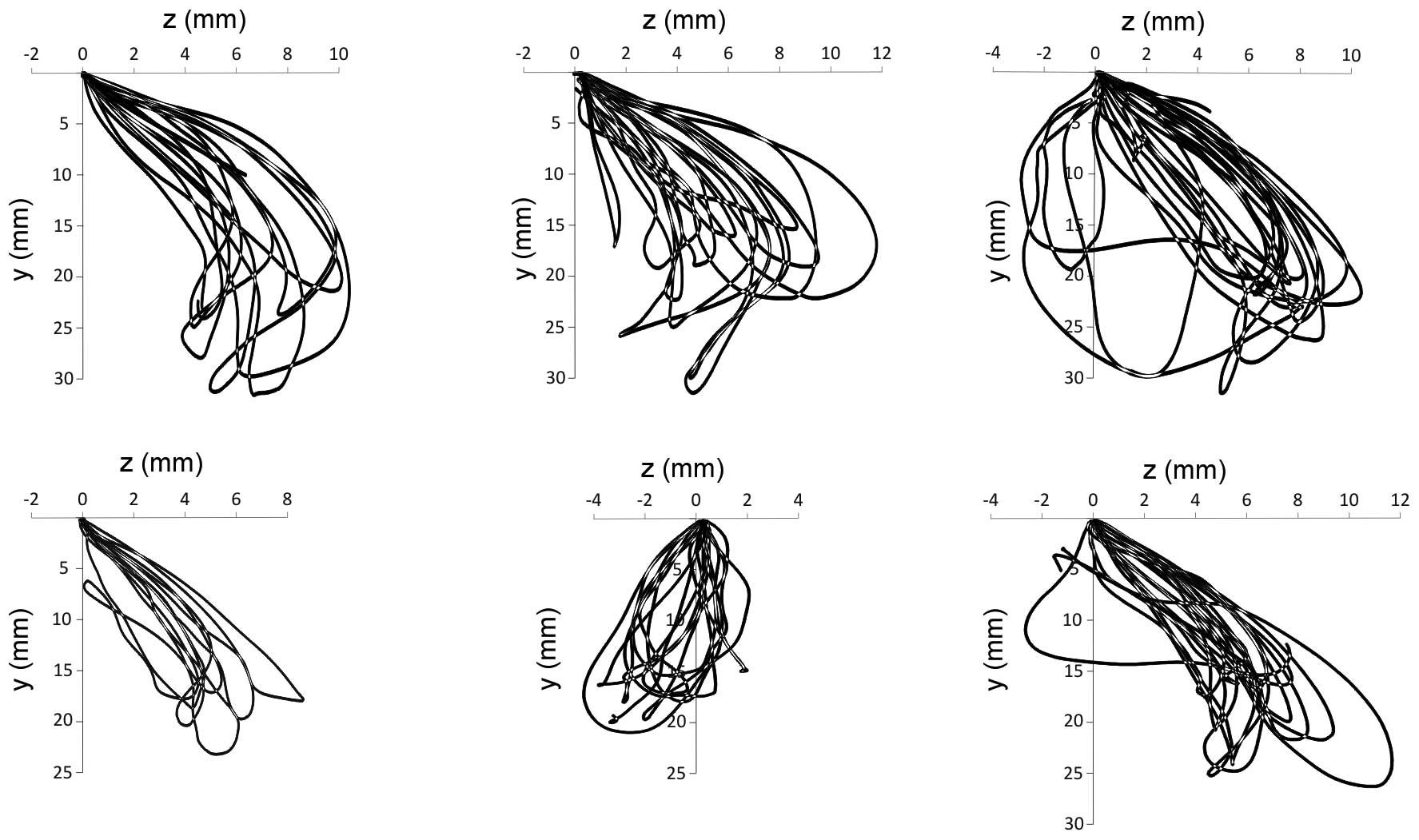
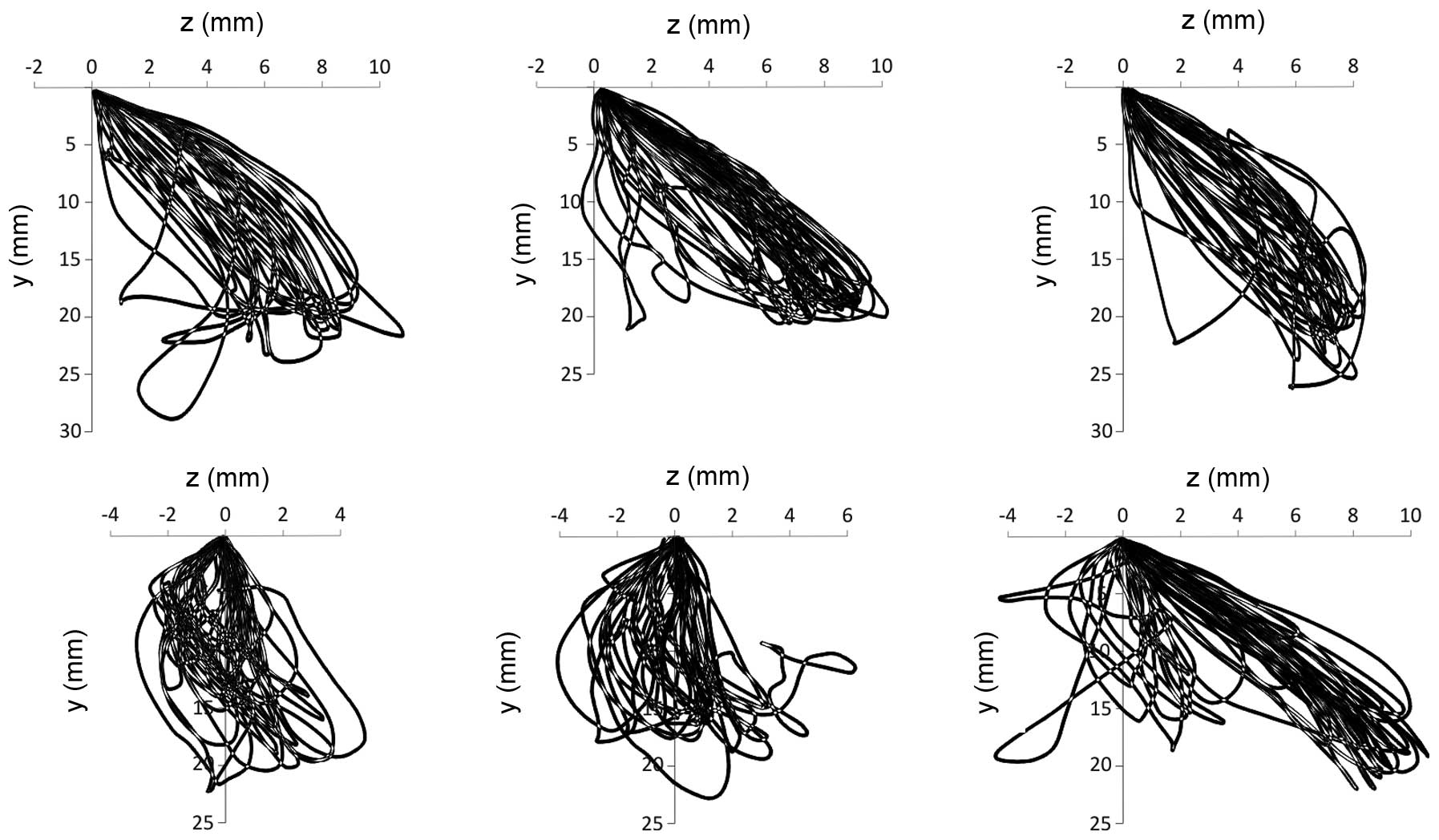
The trajectories along which the incisors move in
the front plane, reflecting the mastication of the bread and
hazelnuts, are presented in Figs. 2
and 3. The first cycle was omitted,
since the trajectories projected at this time are associated with
the abduction movement, which has a negligent effect on the further
course of mastication. The average minimum and maximum values of
the shifts of the Cartesian coordinates, recorded using the
electronic facebow, are shown in Table
I.
 | Table I.Extent of the shifts in the
measurement points of the mandible presented as the mean ± standard
error. |
Table I.
Extent of the shifts in the
measurement points of the mandible presented as the mean ± standard
error.
| A, Mastication of
bread (n=6). |
|
| Right condyloid
process | Left condyloid
process | Incisors |
|---|
|
|
|
|
|
|---|
| Item | x (mm) | y (mm) | z (mm) | x (mm) | y (mm) | z (mm) | x (mm) | y (mm) | z (mm) |
|---|
| min | −1.2±0.4 | −7.4±0.7 | −1.1±0.2 | −1.1±0.6 | −7.9±0.9 | −1.0±0.3 | −3.0±0.4 | −25.3±2.2 | −2.0±0.7 |
| max | 7.3±0.5 | 1.2±0.5 | 0.6±0.1 | 13.2±1.3 | 1.2±0.5 | 1.0±0.2 | 2.9±0.2 | 2.4±1.2 | 9.2±1.4 |
|
|---|
| B, Mastication of
hazelnuts (n=6). |
|
|---|
|
| Right condyloid
process | Left condyloid
process | Incisors |
|
|
|
|
|
| Item | x (mm) | y (mm) | z (mm) | x (mm) | y (mm) | z (mm) | x (mm) | y (mm) | z (mm) |
|
|---|
| min | −1.9±0.4 | −7.4±1.0 | −1.3±0.4 | −1.2±0.3 | −9.4±0.6 | −1.2±0.4 | −3.9±0.9 | −21.9±1.1 | −1.7±1.0 |
| max | 5.7±0.4 | 1.7±0.6 | 1.6±0.1 | 11.2±1.3 | 1.4±0.5 | 2.0±0.2 | 2.1±0.4 | 2.2±0.8 | 9.6±0.7 |
Length of the muscle fibers
Bearing in mind the evaluation of the effect of
particular groups of muscles on the process of occlusion, changes
in their length in each cycle were calculated. The numeric values
that were obtained were used to determine the extent of the changes
in muscle fibre length. The average parameters, illustrating
maximum shortening Δlmin or elongation
Δlmax of the muscle fibres during mastication of
the bread and hazelnuts, are shown in Table II.
 | Table II.Maximum extent of the changes in
muscle fibre length during mastication. |
Table II.
Maximum extent of the changes in
muscle fibre length during mastication.
|
| Mastication of
bread (n=6) | Mastication of
hazelnuts (n=6) |
|---|
|
|
|
|
|---|
|
| On the right
side | On the left
side | On the right
side | On the left
side |
|---|
|
|
|
|
|
|
|---|
| Item | Δlmin
(mm) | Δlmax
(mm) | Δlmin
(mm) | Δlmax
(mm) | Δlmin
(mm) | Δlmax
(mm) | Δlmin
(mm) | Δlmax
(mm) |
|---|
| MSA | −1.2±0.6 | 20.0±1.1 | −1.1±0.6 | 18.9±0.9 | −1.3±0.4 | 15.8±0.7 | −1.4±0.5 | 16.1±0.6 |
| MSP | −1.0±0.5 | 14.7±0.7 | −0.9±0.4 | 13.5±0.6 | −1.2±0.3 | 11.7±0.6 | −1.2±0.3 | 11.9±0.4 |
| MDA | −0.9±0.5 | 14.2±0.7 | −0.9±0.4 | 14.0±0.8 | −1.1±0.4 | 11.5±0.5 | −0.9±0.2 | 12.7±0.4 |
| MDP | −0.9±0.4 | 11.8±0.6 | −0.8±0.4 | 11.3±0.6 | −1.2±0.4 | 10.0±0.5 | −0.9±0.2 | 10.9±0.3 |
| PA | −1.0±0.5 | 17.2±1.1 | −0.9±0.4 | 15.9±0.6 | −1.1±0.3 | 13.7±0.7 | −1.3±0.4 | 12.8±0.6 |
| PP | −0.9±0.4 | 12.4±0.8 | −0.8±0.3 | 11.6±0.4 | −0.9±0.2 | 10.6±0.6 | −1.2±0.3 | 9.9±0.5 |
| LU | −2.7±0.3 | 1.5±0.3 | −5.9±0.7 | 0.8±0.3 | −3.9±0.3 | 3.2±0.5 | −5.5±0.9 | 1.2±0.5 |
| LP | −3.4±0.3 | 1.1±0.3 | −6.3±0.7 | 0.7±0.3 | −4.4±0.3 | 2.7±0.5 | −6.1±0.9 | 0.8±0.4 |
| LL | −7.2±0.4 | 0.7±0.4 | −9.4±0.6 | 0.8±0.5 | −7.9±0.3 | 1.4±0.3 | −9.4±0.6 | 0.5±0.1 |
| TV | −1.4±0.7 | 22.4±1.2 | −1.4±0.7 | 22.9±1.3 | −1.2±0.5 | 18.7±0.7 | −1.3±0.4 | 21.0±0.6 |
| TA | −1.3±0.7 | 22.0±1.1 | −1.4±0.8 | 24.1±1.6 | −1.1±0.6 | 19.4±0.6 | −0.9±0.3 | 23.2±0.7 |
| TP | −1.1±0.6 | 16.9±0.8 | −1.2±0.8 | 19.7±9.2 | −1.1±0.5 | 15.9±0.5 | −0.6±0.2 | 19.9±0.6 |
| D | −9.1±0.7 | 1.9±0.6 | −8.4±0.6 | 2.8±0.88 | −8.8±0.7 | 2.0±1.14 | −7.4±0.8 | 2.4±0.9 |
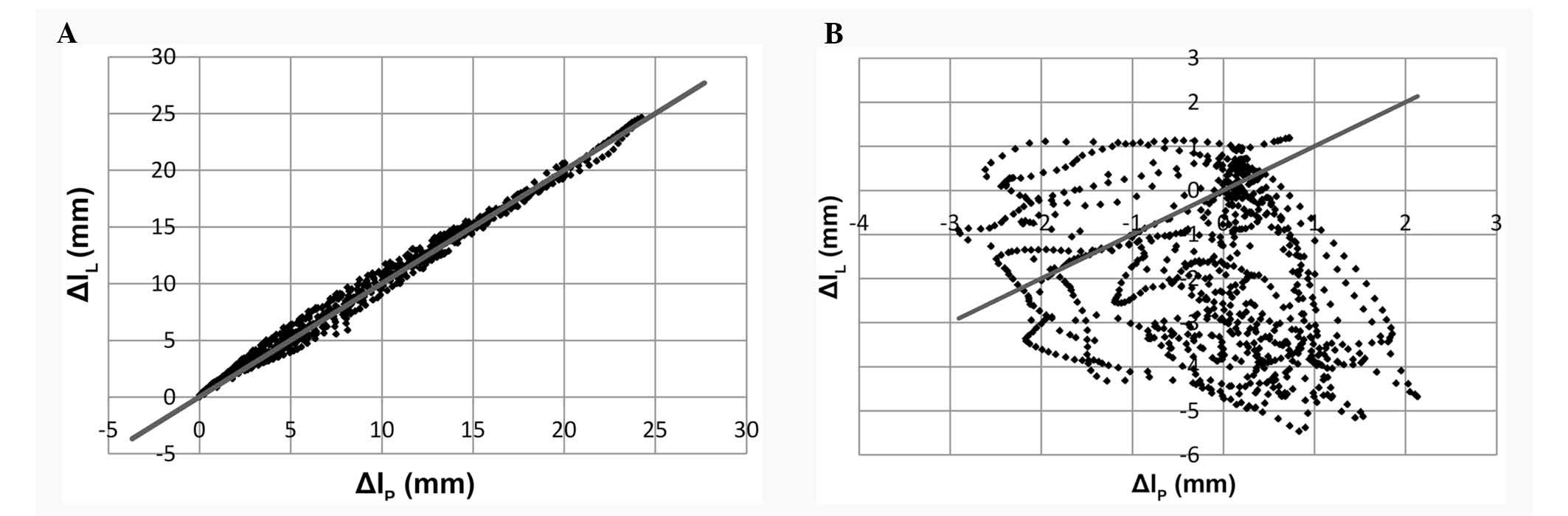
The data presented in Table I do not clearly describe the degree
of asymmetric function of the muscles located on either side of the
mandible. For this reason, individual axes were assigned to changes
in length Δl of the muscles on the right and left side of
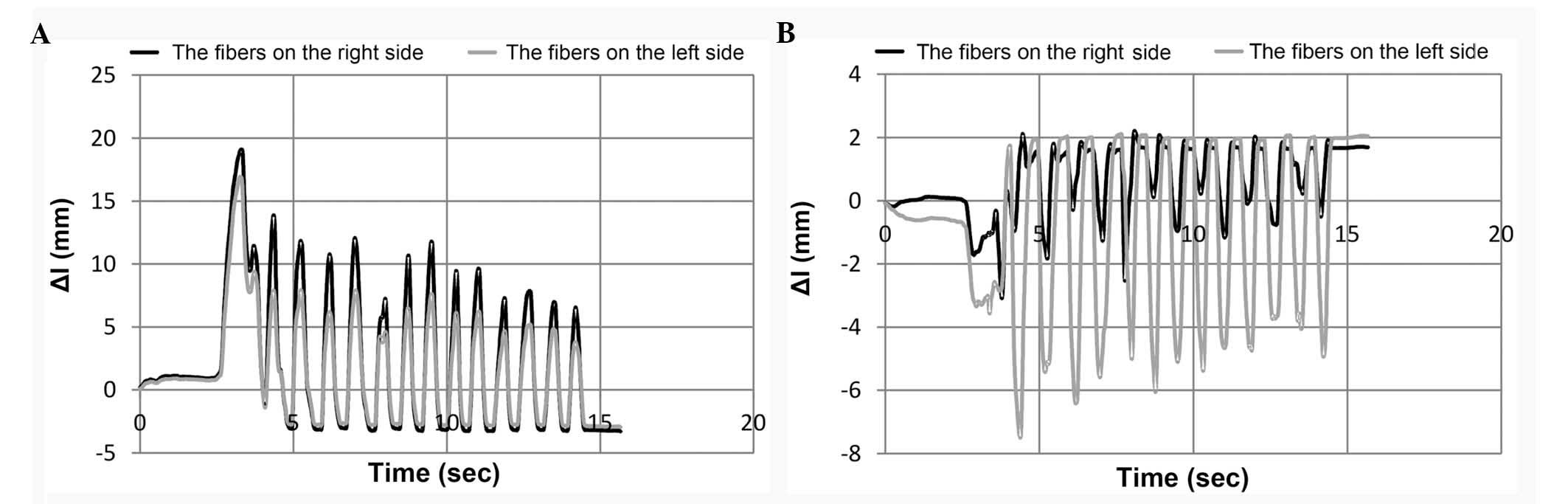
the mandible (Fig. 4).
Coefficients of muscular work
Numerical data specified in this way, are referred
to idealized situations (a straight line traversing the beginning
of the coordinate system), which occurs when the muscles on both
sides of the mandible function symmetrically. On this basis the
identified muscle function coefficients wP can be
interpreted as a measure of their asymmetric or symmetric
operation. With the assumption that muscle fibres are not elongated
with constant speed dΔl/dt and permanent acceleration
d2Δ/dt2, the resultant
operation was calculated as an arithmetic average from three
kinematic sizes.
Length of the muscles in the act of
chewing
Data presented in Table III should be interpreted as
follows: If the ratio wP has a value close to
homogeneity, the muscle fibres function symmetrically; if however
the ratio wP is close to zero, the fibres
function asymmetrically.
 | Table III.Ratios (wP) of
muscle mastication function presented as the mean ± standard
error. |
Table III.
Ratios (wP) of
muscle mastication function presented as the mean ± standard
error.
|
| Mastication of
bread (n=6) | Mastication of
hazelnut (n=6) |
|---|
|
|
|
|
|---|
| Item | Δl (mm) | dΔl/dt (mm/s) |
d2Δl/dt2
(mm/s2) | Mean | Δl (mm) | dΔl/dt (mm/s) |
d2Δl/dt2
(mm/s2) | Mean |
|---|
| MSA | 0.87±0.07 | 0.81±0.09 | 0.55±0.31 | 0.74±0.10 | 0.97±0.01 | 0.96±0.01 | 0.89±0.03 | 0.94±0.02 |
| MSP | 0.70±0.16 | 0.56±0.23 | 0.25±0.32 | 0.50±0.13 | 0.94±0.01 | 0.91±0.02 | 0.78±0.06 | 0.88±0.05 |
| MDA | 0.94±0.01 | 0.84±0.06 | 0.48±0.29 | 0.75±0.14 | 0.83±0.05 | 0.91±0.01 | 0.80±0.02 | 0.84±0.03 |
| MDP | 0.92±0.02 | 0.81±0.07 | 0.45±0.28 | 0.73±0.14 | 0.85±0.05 | 0.92±0.01 | 0.80±0.03 | 0.86±0.03 |
| PA | 0.74±0.13 | 0.72±0.14 | 0.58±0.22 | 0.68±0.05 | 0.58±0.12 | 0.76±0.06 | 0.73±0.05 | 0.69±0.06 |
| PP | 0.71±0.16 | 0.69±0.16 | 0.48±0.31 | 0.63±0.07 | 0.53±0.12 | 0.73±0.07 | 0.68±0.06 | 0.65±0.06 |
| LU | 0.00±0.00 | 0.04±0.09 | 0.00±0.00 | 0.01±0.01 | 0.00±0.00 | 0.03±0.03 | 0.01±0.01 | 0.01±0.01 |
| LP | 0.02±0.06 | 0.08±0.14 | 0.00±0.01 | 0.04±0.02 | 0.00±0.00 | 0.05±0.05 | 0.03±0.03 | 0.03±0.02 |
| LL | 0.46±0.14 | 0.56±0.06 | 0.28±0.17 | 0.44±0.08 | 0.14±0.09 | 0.47±0.04 | 0.30±0.08 | 0.30±0.10 |
| TV | 0.99±0.00 | 0.98±0.01 | 0.83±0.21 | 0.93±0.05 | 0.95±0.02 | 0.98±0.00 | 0.96±0.00 | 0.96±0.01 |
| TA | 0.90±0.04 | 0.89±0.02 | 0.75±0.11 | 0.85±0.05 | 0.74±0.06 | 0.88±0.01 | 0.83±0.01 | 0.82±0.04 |
| TP | 0.80±0.09 | 0.82±0.04 | 0.61±0.13 | 0.74±0.06 | 0.59±0.09 | 0.81±0.02 | 0.74±0.01 | 0.71±0.06 |
| D | 0.64±0.20 | 0.59±0.19 | 0.72±0.11 | 0.65±0.04 | 0.29±0.19 | 0.58±0.12 | 0.76±0.05 | 0.54±0.14 |
In the initial phase of mastication, the lateral
pterygoid muscles are extended to their maximum lengths. Following
increasingly broad dental arch opening, the lateral pterygoid
muscles are supported by the suprahyoid muscles. Such a situation,
in which the lateral pterygoid muscles have a dominant role, is a
feature variable between individuals. The results of the computer
simulations conducted as a part of this investigation indicate that
the suprahyoid muscles initiate the abduction movement. Deviation
of the trajectories of the incisors from the medial plane is
associated with the asymmetrical functioning of medial- and
lateral-pterygoid muscles, suprahyoid muscles and transitional and
back temporal muscle fibres. The mean change in the length fibres
of the masticator muscle and a lateral-pterygoid muscle are shown
in Fig. 5A and B, respectively.
The majority of the muscles during mastication of
the bread were extended from 11.8–24.1 mm, with the exception of
the lateral pterygoid muscle fibres and suprahyoid muscle, the
shortening of which remained at 5.5–9.4 mm. Conversely, during the
adduction movement the fibres of the lateral pterygoid and the
suprahyoid muscles were elongated by 0.7–1.9 mm. Shortening of the
other muscles was <2 mm. The nature of the changes in muscle
fibre length did not significantly depend on the consistency of the
masticated food. However, during mastication of hard food, the
muscles were marginally less elongated and shortened, as compared
with the food with a soft consistency. The activity of the
mastication muscles need to be considered with respect to the
maximum range of changes in length in association with resting
lengths. The activity ratio defined in this way demonstrated that
the greatest activity in the working mandible was carried out by
the front fibres of the medial-pterygoids and front and temporary
(middle) fibres of the temporal muscles. Conversely, the lowest
activity was typically observed in fibres of the lateral-pterygoid
muscles, back superficial fibres of masticators and suprahyoid
muscles. The results were similar during both the mastication of
the bread and the hazelnuts. One of the analyses conducted was the
numerical assessment of the asymmetric operation of muscle fibres
during mastication. Computer simulation (Table II) demonstrated that the symmetric
activity of the front temporal muscle fibres was
wP >0.9. The greatest asymmetry of activity
was observed in the group of lateral-pterygoids
(wP <0.44). Furthermore, the model test
results suggested that the hardness of consumed food manifests
itself in increased asymmetrical muscle activity, specifically
visible in the superficial and deep fibres of masticators.
Discussion
In clinical tests, the stomatognathic system should
be considered as a unit, individually assigned to a patient and
characteristic of that patient. The notion of the stomatognathic
system, or the mastication organ, implies a system in which
particular elements combine into a functional whole, both
physiologically and pathologically. To examine the events taking
place in the system, consideration of their mutual associations is
important. A comprehensive approach of psychogenic phenomena,
physiological and pathological causes of the stomatognathic system,
are treated as a chain of cause and effect. (40,41).
These associations result from the fact that slight loss of
function may cause disorders, and as a consequence may create
morphological changes throughout the masticatory system. One of the
symptoms of masticatory organ dysfunction is acoustic signals
recorded within the temporomandibular joints (42–44).
Their source is the incorrect cooperation of transport disks with
the heads of condyloid processes and the acetabula (19). Loss of proper functioning of the
stomatognathic system is a severe limitation that manifests itself
in difficulties in biting and grinding food or sound articulation
(45,46). However, the complexity of the
muscular-skeletal system as well as its compensating capabilities
enables the restoration or reduction of lost movement functions
(47).
Previous studies have assessed and measured the
movements of condyloid process heads and mandibular incisors
(48–50). Other studies investigated the forces
generated by particular groups of muscle fibres (51–56,34). It
should be noted that direct measurement of muscle forces is
difficult to achieve. To date, no technology has been developed
that would permit the direct recording of muscular forces.
Measurements of potential function can be carried out but only to a
limited extent. These limitations result primarily from the need to
breach the continuity of the skin. For this reason, novel methods
are sought that will provide information regarding the activity of
the mastication muscles (35–39,57–60).
The results of clinical tests supplemented with model experiments
may provide the basis for formulating the criteria that would
enable the evaluation of forces generated by particular groups of
mastication muscles. Data obtained from clinical tests does not
provide clear results on the biomechanical states of the
mastication muscle. In addition, kinematic models of the
mastication muscle are limited to the projection of the movement of
the mandible itself, ignoring the impact of changes in muscle
fibres length (61–66). For this reason, the present study
examined a spatial model of kinematics. Verification criteria were
formulated as conformity of trajectories calculated numerically
with those registered in the clinical setting. The results obtained
in this way demonstrated the efficacy and suitability of the
numeric model of the mastication muscle presented in the present
investigation.
The model test results indicated that the maximum
range of the dental arch opening during mastication of the bread
was ~25.3 mm, and this value was ~21.9 mm for the hazelnut. These
scopes are close to those obtained by Hannam et al (67). The recorded trajectories of the
incisors were subject to deviation from the medial plane by a ≤9.2
mm during the consumption of bread, and this deviation was slightly
higher at ~9.6 mm in the case of the hazelnut, the direction of the
deviations were turned towards the load influencing the dental
arches. The results of the tests did not show any significant
impact of food consistency on the deviation of trajectories from
the medial plane like, results which were concordant with those of
Lundeen and Gibbs (3). It is also
worth taking into consideration that in particular mastication
cycles, the trajectories of the incisors were not always subject to
deviation by the aforementioned values. This variable scope of
trajectory deviations from the medial plane may be assigned to the
individual habits of the subject (68), as well as the degree of food
grinding. The frontal movement of the condyloid process head,
situated on the balancing side was somewhat higher compared with
the condyloid process head located on the working part (~6.5 mm for
bread). Conversely, during the course of hazelnut mastication, this
difference was determined to be ~5.5 mm. Vertically, the maximum
displacement of condyloid process heads, by principle, assumes
similar values. Insignificant differences of ~0.5 mm for soft food
(bread) and ~2 mm for hard nuts to be associated with the
positioning of dental arches of the mandible when biting the food.
The aforementioned numeric values of the shifts of the incisors and
condyloid process heads characterize the abduction phase of the
dental arches. When grinding a portion of food, dental arches come
into contact with each other. Regardless of the food consistency,
the recorded values of the shifts was ~2.3 mm. In addition, this
value corresponds approximately to the distance between the teeth
at resting position. At the time of contact of the dental arches,
condyloid process heads withdraw into the articular space by and
average of ~1.2 mm. Nut grinding results in the condyloid process
head, located at the working side, withdrawing by a maximum of ~1.9
mm. The vertical movement of the condyloid processes is limited by
the anatomical structure of the temporomandibular joint and the
biomechanical properties of the transport disks (69–74).
Mastication is a biomechanical process that depends on numerous
factors, including the shape of the dental cusps, or the preferred
side of mastication in specific people (1). For this reason, it is difficult to
compare the results of the present study to those obtained by
previous investigators. This is because comparing results without
ensuring that the same conditions were used may result in false
conclusions.
The dominant componential harmonics of the
amplitude-frequency spectrum were identified based on the
calculated time courses and configuration coordinates. These
components determine the frequency of the repetitions of the
mastication cycles. Appropriate numerical calculations were used
with the application of the algorithm of Fourier fast
transformation. From a theoretical point of view, the majority of
the information regarding the harmonic components is contained
within the signal of the configuration coordinate Ф3,
since its scope of variability is the greatest. The results of the
present study determined that the average value of the dominant
frequency of bread mastication was ~1.16±0.06 Hz. In the case of
the hazelnut, this value was nearly twice as large at ~1.84±0.07
Hz. The identified frequencies of bread and hazelnut mastication
demonstrated the impact of food consistency on the course of
mastication, results which were concordant with those of Horio and
Kawamura (29).
Both the results of the present study and those of
Throckmorton et al (75) led
to the hypothesis that the asymmetrical action of the
stomatognathic system muscles results in an uneven load to the
temporomandibular joint. Unbalanced muscular action may cause
byorthognathic defects; these defects were reported by previous
studies that used electromyography prior to and following
orthognathic surgery (76,77). The present study presents a
mathematical approach to the model assessment of the activity of
the human mastication muscle. The analytical associations
characterizing the variability of the configuration coordinates
were presented in a general equation. This enabled the numeric
evaluation of the activity of the stomatognathic muscle system, not
only with respect to the mastication cycle, but also with that of
the free articulatory movements. The method presented in the
current investigation may serve as a useful tool in the diagnosis
of human mastication disorders.
In summary, the model and clinical tests of the
present study led to the following general conclusion:
Configuration coordinates calculated as a result of the solution to
a reverse task of kinematics are the basis for conducting model
tests, in the scope of assessment of the activity of the
mastication muscles. In addition, kinematics of muscle activity can
be analyzed with respect to the Cartesian coordinates, defining the
location of the muscle attachments of the mandibular and cranial
bones.
The spatial model of mandibular kinematics included
in the present study contributes qualitatively novel information,
and furthered our knowledge of the functioning of the mastication
muscle. The results obtained from the computer simulations
indicated that the numerical modelling of the mastication muscle is
an effective tool, and may help dentists to formulate a diagnosis.
Information on the spatial orientation of the muscle fibres is
required in order to determine the directions of the forces
generated by the muscles. Having estimated the muscle forces,
future studies will strive to identify the loads affecting the
structure of the temporomandibular joint.
References
|
1
|
Okeson JP: Neuroanatomy and physiology of
the masticatory locomotor system from the functional sideManagement
of temporomandibular disorders and occlusion. 1. 5th. Mosby
Elsevier; St. Louis, MO: pp. 40–48. 2005
|
|
2
|
Ahlgren J: Mechanism of mastication. Acta
Odontol Scand. 24:(Suppl 44). 1–109. 1966.
|
|
3
|
Wickwire NA, Gibbs CH, Jacobson AP and
Lundeen HC: Chewing patterns in normal children. Angle Orthod.
51:48–60. 1981.PubMed/NCBI
|
|
4
|
Gibbs CH, Messerman T, Reswick JB and
Derda HJ: Functional movements of the mandible. J Prosthet Dent.
26:604–620. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gibbs CH and Lundeen HC: Jaw movement and
forces during chewing and swallowing and their clinical
significanceGibbs CH and Lundeen HC: Advances in occlusion. John
Wright-PSG, Inc; Littleton, Mass: 1. pp. 2–32. 1982
|
|
6
|
Miyaoka Y, Ashida I, Iwamori H, Kawakami
S, Tamaki Y, Yamazaki T and Ito N: Synchronization of masseter
activity patterns between the right and left sides during chewing
in healthy young males. J Med Eng Technol. 38(1-5): 2812014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crane EA, Rothman ED, Childers D and
Gerstner GE: Analysis of temporal variation in human masticatory
cycles during gum chewing. Arch Oral Biol. 58:1464–1474. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slavicek G, Soykher M, Soykher M, Gruber H
and Siegl P: Relevance of a standard food model in combination with
electronic jaw movement recording on human mastication pattern
analysis. Advances in Bioscience and Biotechnology. 1:68–78. 2010.
View Article : Google Scholar
|
|
9
|
Ogawa T, Ogawa M and Koyano K: Different
responses of masticatory movements after alteration of occlusal
guidance related to individual movement pattern. J Oral Rehabil.
28:830–841. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mongini F and Tempia-Valenta G: A graphic
and statistical analysis of the chewing movements in function and
dysfunction. J Craniomandib Pract. 2:125–134. 1984. View Article : Google Scholar
|
|
11
|
Hashii K, Tomida M and Yamashita S:
Influence of changing the chewing region on mandibular movement.
Aust Dent J. 54:38–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henrikson T, Ekberg EC and Nilner M:
Masticatory efficiency and ability in relation to occlusion and
mandibular dysfunction in girls. Int J Prosthodont. 11:125–132.
1998.PubMed/NCBI
|
|
13
|
Youssef RE, Throckmorton GS, Ellis E and
Sinn DP: Comparison of habitual masticatory cycles and muscle
activity before and after orthognathic surgery. J Oral Maxillofac
Surg. 55:699–707. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
English JD, Buschang PH and Throckmorton
GS: Does malocclusion affect masticatory performance? Angle Orthod.
72:21–27. 2002.PubMed/NCBI
|
|
15
|
Slavicek G: Human mastication.
International journal of stomatology & occlusion medicine.
3:29–41. 2010. View Article : Google Scholar
|
|
16
|
Kim BI, Jeong SH, Cho KH, Chung YK, Kwon
HK and Choi CH: Subjective food intake ability in relation to
maximal bite force among Korean adults. J Oral Rehabil. 36:168–175.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Proeschel P: An extensive classification
of chewing patterns in the frontal plane. Cranio. 5:55–63. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pröschel P and Hofmann M: Frontal chewing
patterns of the incisor point and their dependence on resistance of
food and type of occlusion. J Prosthet Dent. 59:617–624. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyauchi S, Nakaminami T, Nishio K and
Maruyama T: Chewing pattern in posterior crossbite. Classification
of chewing pattern in the frontal plane. Nippon Hotetsu Shika
Gakkai Zasshi. 33:938–951. 1989.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dan H and Kohyama K: Interactive
relationship between the mechanical properties of food and the
human response during the first bite. Arch Oral Biol. 52:455–464.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reed DA and Ross CF: The influence of food
material properties on jaw kinematics in the primate, Cebus. Arch
Oral Biol. 55:946–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson K, Throckmorton GS, Buschang PH
and Hayasaki H: The effects of bolus hardness on masticatory
kinematics. J Oral Rehabil. 29:689–696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Horio T and Kawamura Y: Effect of texture
of food on chewing patterns in the human subject. J Oral Rehabil.
16:177–183. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Der Bilt A, Engelen L, Abbink J and
Pereira LJ: Effects of adding fluids to solid foods on muscle
activity and number of chewing cycles. Eur J Oral Sci. 115:198–205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiozawa M, Taniguchi H, Hayashi, Hori,
Tsujimura, Nakamura, Ito K and Inoue M: Differences in chewing
behavior during mastication of foods with different textures. J
Text Stud. 44:44–45. 2013. View Article : Google Scholar
|
|
26
|
Wintergerst AM, Throckmorton GS and
Buschang PH: Effects of bolus size and hardness on within-subject
variability of chewing cycle kinematics. Arch Oral Biol.
53:369–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lundeen HC and Gibbs CH: Jaw movements and
forces during chewing and swallowing and their clinical
significanceAdvances in occlusion. 1. 5th. John Wright-PSG Inc.;
Bristol: pp. 2–32. 1982
|
|
28
|
Horio T and Kawamura Y: Effects of texture
of food on chewing patterns in the human subject. J Oral Rehabil.
16:177–183. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jankelson D, Hoffman GM and Hendron AJ:
The psysiology of the stomatognathic system. The Journal of the
American Dental Association. 46:375–386. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson DJ and Picton DC: Tooth contact
during chewing. J Dent Res. 36:21–26. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahlgren J: Mechanism of mastication; a
quantitative cinematographic and electromyographic study of
masticatory movements in children with special reference to
occlusion of the teeth. Acta Odontolologica Scandinavica. 44:44–45.
1966.
|
|
32
|
Adams SH and Zander HA: Functional tooth
contacts in lateral and centric occlusion. J Am Dent Assoc.
69:465–473. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin R and Saller K: Textbook of
anthropology in systematic representation with special emphasis on
anthropological methods. Fischer Publishing Company; 3. Stuttgart:
1957
|
|
34
|
Ichim I, Kieser JA and Swain MV:
Functional significance of strain distribution in the human
mandible under masticatory load: Numerical predictions. Arch Oral
Biol. 52:465–473. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bérzin F: Surface electromyography in the
diagnosis of syndromes of the cranio-cervical pain. Braz J Oral
Sci. 10:484–491. 2004.
|
|
36
|
Coelho-Ferraz MJP, Bérzin F and Amorim C:
Electromyography Evaluations of the masticator muscles during the
maximum bite force. Revista Espanola de Cirugia Oral y
Maxilofacial. 6:420–427. 2008.
|
|
37
|
Koolstra JH: Dynamics of the human
masticatory system. Crit Rev Oral Biol Med. 13:366–376. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Castroflorio T, Icardi K, Becchino B,
Merlo E, Debernardi C, Bracco P and Farina D: Reproducibility of
surface EMG variables in isometric sub-maximal contractions of jaw
elevator muscles. J Electromyogr Kinesiol. 16:498–505. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santana-Mora U, Cudeiro J, Mora-Bermúdez
MJ, Rilo-Pousa B, Ferreira-Pinho JC, Otero-Cepeda JL and
Santana-Penín U: Changes in EMG activity during clenching in
chronic pain patients with unilateral temporomandibular disorders.
J Electromyogr Kinesiol. 19:e543–e549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu F and Steinkeler A: Epidemiology,
diagnosis and treatment of temporomandibular disorders. Dent Clin
North Am. 57:465–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karibe H, Goddard G, Aoyagi K, Kawakami T,
Warita S, Shimazu K, Rudd PA and McNeill C: Comparison of
subjective symptoms of temporomandibular disorders in young
patients by age and gender. Cranio. 30:114–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zimmer B: Correlations between the loss of
acoustic TMJ symptoms and alterations in mandibular advancement.
Eur J Orthod. 15:229–234. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Motoyoshi M, Matsumoto Y, Ohnuma M,
Arimoto M, Takahashi K and Namura S: A study of temporomandibular
joint sounds. Part 2. Acoustic characteristics of joint sounds. J
Nihon Univ Sch Dent. 37:47–54. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prinz JF: Autocorrelation of acoustic
signal from the temporomandibular joint. J Oral Rehabil.
25:635–644. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Roda R Poveda, Bagan JV, Fernandez JM
Diaz, Hernández Bazán S and Jiménez Soriano Y: Review of
temporomandibular joint pathology. Part I. Classification,
epidemiology and risk factors. Med Oral Patol Oral Cir Bucal.
12:E292–E298. 2007.PubMed/NCBI
|
|
46
|
Vanderas AP and Papagiannoulis L:
Multifactorial analysis of the aetiology of craniomandibular
dysfunction in children. Int J Paediatr Dent. 12:336–346. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chladek W: System of modeling the selected
mechanical states of human mandible. Scientific Papers of Silesian
University of Technology. 59:1082000.(In Polish).
|
|
48
|
Zhang X, Ashton-Miller JA and Stohler CS:
Three-dimensional unilateral method for the bilateral measurement
of condylar movements. J Biomech. 28:1007–1011. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zuccari AG, Andres CJ and Simpson GW: A
color-enhanced aid for location of the transverse horizontal
opening and closing axis of the mandible. J Prosthet Dent.
76:181–186. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peck CC, Murray GM, Johnson CW and
Klineberg IJ: Trajectories of condylar points during nonworking
side and protrusive movements of the mandible. J Prosthet Dent.
82:322–331. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Osborn J and Baragar F: Predicted pattern
of human muscle activity during clenching derived from a computer
assisted model: Symmetric vertical bite force. J Biomech.
18:599–612. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Korioth TW and Hannam AG: Deformation of
the human mandible during simulated tooth clenching. J Dent Res.
73:56–66. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meyer C, Kahn JL, Lambert A, Boutemy P and
Wilk A: Development of a static simulator of the mandible. J
Craniomaxillofac Surg. 28:278–286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peck CC, Langenbach GE and Hannam AG:
Dynamic simulation of muscle and articular properties during human
wide jaw opening. Arch Oral Biol. 45:963–982. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sellers WI and Crompton RH: Using
sensitivity analysis to validate the predictions of a biomechanical
model of bite forces. Ann Anat. 186:89–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Peck CC and Hannam AG: Human jaw and
muscle modeling. Arch Oral Biol. 52:300–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu ZJ, Yamagata K, Kasahara Y and Ito G:
Electromyographic examination of jaw muscles in relation to
symptoms and occlusion of patients with temporomandibular joint
disorders. J Oral Rehabil. 26:33–47. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Al-Saleh M, Armijo-Olivo S, Flores-Mir C
and Thie N: Electromyography in diagnosing temporomandibular
disorders. J Am Dent Assoc. 143:351–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Manfredini D, Cocilovo F, Favero L,
Ferronato G, Tonello S and Guarda-Nardini L: Surface
electromyography of jaw muscles and kinesiographic recordings:
Diagnostic accuracy for myofascial pain. J Oral Rehabil.
38:791–799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ferrario VF, Sforza C, Colombo A and Ciusa
V: An electromyographic investigation of masticatory muscles
symmetry in normo-occlusion subjects. J Oral Rehabil. 27:33–40.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Leader JK, Boston J, Debski R and Rudy T:
Mandibular kinematics represented by a non-orthogonal floating axis
joint coordinate system. J Biomech. 36:275–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Daumas B, Xu WL and Bronlund JE: Jaw
mechanism modelling and simulation. Mech Mach Theory. 7:821–833.
2005. View Article : Google Scholar
|
|
63
|
Slavicek G and Schimmer C: Analysis of
human mastication behavior: a new approach using planar
calculations of fragmented chewing sequences. International journal
of stomatology & occlusion medicine. 3:61–67. 2010. View Article : Google Scholar
|
|
64
|
Hayashi K, Mizoguchi I, Lee SP and Reich
B: Development of a novel statistical model for mandibular
kinematics. Med Eng Phys. 32:423–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Slavicek G, Soykher M, Gruber H, Siegl P
and Oxtoby M: A novel standard food model to analyze the individual
parameters of human mastication. International journal of
stomatology & occlusion medicine. 2:163–174. 2009. View Article : Google Scholar
|
|
66
|
Χie D, Xu WL, Foster KD and Bronlund J:
Object-oriented knowledge framework for modelling human mastication
of foods. Expert Systems with Applications. 36:4810–4821. 2009.
View Article : Google Scholar
|
|
67
|
Hannam AG, Stavness I, Lloyd JE and Fels
S: A dynamic model of jaw and hyoid biomechanics during chewing. J
Biomech. 41:1069–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pond LH, Barghi N and Barnwell GM:
Occlusion and chewing side preference. J Prosthet Dent. 55:498–500.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tanne K, Tanaka E and Sakuda M: The
elastic modulus of the temporomandibular joint disc from adult
dogs. J Dent Res. 70:1545–1548. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chin LP, Aker FD and Zarrinnia K: The
viscoelastic properties of the human temporomandibular joint disc.
J Oral Maxillofac Surg. 54:315–318; discussion 318–319. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lai WF, Bowley J and Burch JG: Evaluation
of shear stress of the human temporomandibular joint disc. J Orofac
Pain. 12:153–159. 1998.PubMed/NCBI
|
|
72
|
Tanaka E, Shibaguchi T, Tanaka M and Tanne
K: Viscoelastic properties of the human temporomandibular joint
disc in patients with internal derangement. J Oral Maxillofac Surg.
58:997–1002. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tanaka E, Tanaka M, Jattori Y, Aoyama J,
Watanabe M, Sasaki A, Sugiyama M and Tanne K: Biomechanical
behaviour of bovine temporomandibular articular disc with age. Arch
Oral Biol. 46:997–1003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tanaka E, Tanaka M, Miyawaki Y and Tanne
K: Viscoelastic properties of canine temporomandibular joint disc
in compressive load-relaxation. Arch Oral Biol. 44:1021–1026. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Throckmorton GS, Groshan GJ and Boyd SB:
Muscle activity patterns and control of temporomandibular joint
loads. J Prosthet Dent. 63:685–695. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Di Palma E, Gasparini G, Pelo S, Tartaglia
GM and Chimenti C: Activities of masticatory muscles in patients
after orthognathic surgery. J Craniomaxillofac Surg. 37:417–420.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Di Palma E, Gasparini G, Pelo S, Tartaglia
GM and Sforza C: Activities of masticatory muscles in patients
before orthognathic surgery. J Craniofac Surg. 21:724–726. 2010.
View Article : Google Scholar : PubMed/NCBI
|