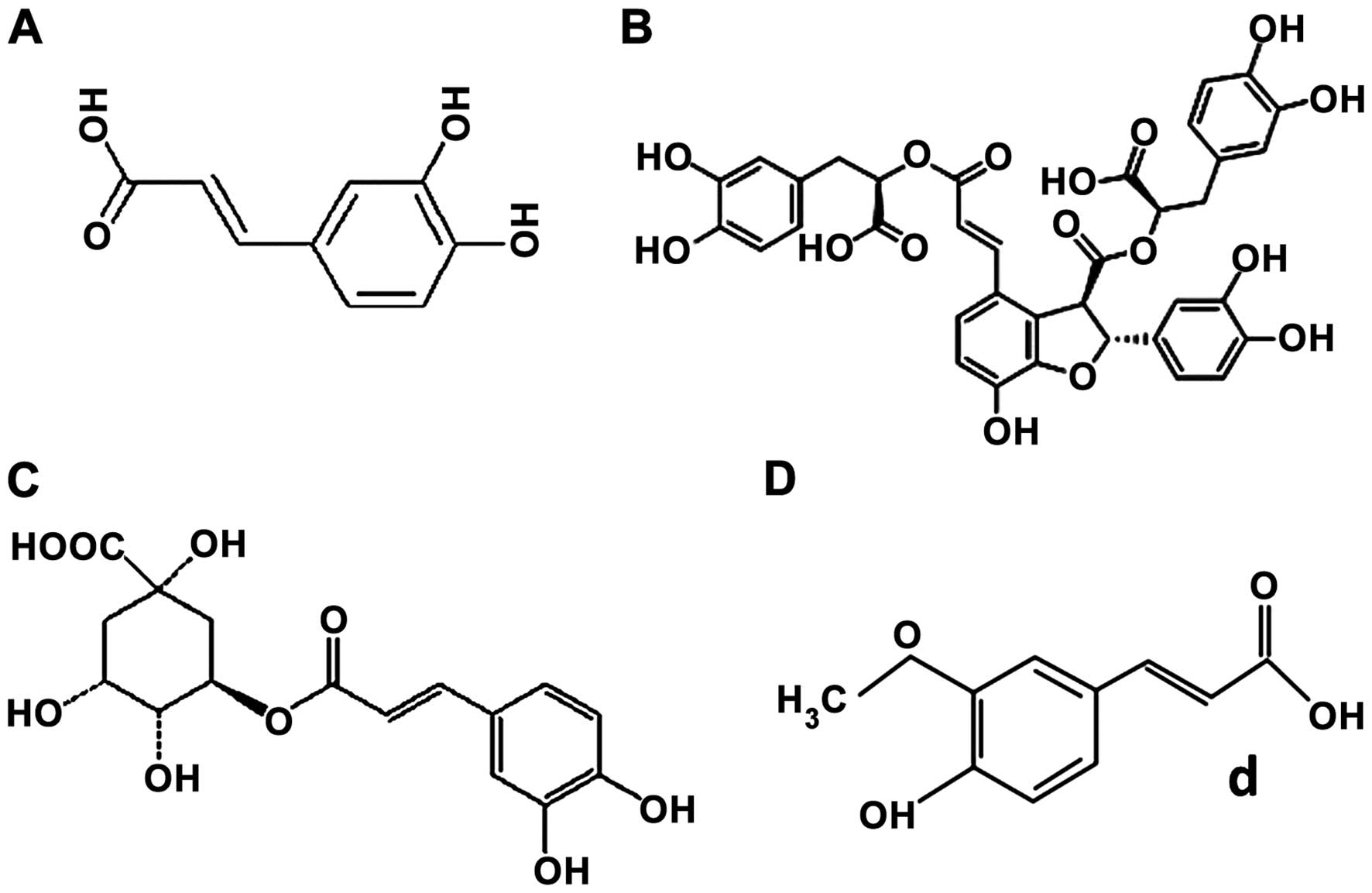
Introduction
Phenolic acids are widely used in phytotherapy
(1). Polyphenolic compounds commonly
found in Chinese medicinal preparations have been reported to have
several biological properties including antibacterial, detoxifying
and antiphlogistic properties. Polyphenolic compounds such as
chlorogenic acid (ChA), are commonly used as a characteristic
marker for quality control in traditional Chinese medicine
(2). However, there are studies on
the side-effects associated with the intravenous administration of
polyphenols in the clinical practice of Chinese herbal medicine
(3). These adverse effects range
from pruritus, asthma, shock, liver and kidney injury to death
(4). Dual effect (efficacy and
toxicity) of phenolic acid created major obstacles in the manner
these Chinese herbal injections are utilized. The sources of these
adverse effects and the mechanisms involved are creating increased
interest among the experts. However, little is known in detail, and
the current available data are inadequate.
In recent years, various biological and
pharmacological properties of phenolic acids have led to
researchers paying focus more attention on medical aspects of
polyphenolic compounds. One of these properties is the antioxidant
property of phenolic acids (5,6). There
are studies on antioxidants turning to pro-oxidants to accelerate
lipid peroxidation and/or induce damage to the DNA of the cell
(7,8). It is believed that the pro-oxidation
properties of plant-derived phenolics compounds may be important
due to their anticancer activities as well as their apoptotic
characteristics, rather than their antioxidation properties
(9). Therefore, identifying how an
antioxidant may turn into a pro-oxidant and play a biological role
is of interest. It has been reported that higher concentrations of
phenolic acid produced radicals, acting as a pro-oxidant, whereas
lower concentrations of phenolic acid scavenged superoxide and
hydroxyl radical in vitro (10).
A major source of the observed adverse side-effects
of phenolic acids is their ability to cause oxidative damage to
normal cells (11). This may explain
the side-effects reported from patients after the injection of
herbal medicines containing phenolic acids. These side-effects are
more intense when the phenolic compounds are injected
intravenously.
In the present study, we administered vayring
phenolic acid stress in rat microvascular endothelial cells in
vivo. By injecting high doses (enough to induce adverse
effects) we examined the potential role of the oxidative stress.
Phenolic acids investigated were as follows: i) caffeic acid (CA);
ii) salvianolic acid B (SAB); iii) ChA; and iv) ferulic acid (FA)
(Fig. 1).
Materials and methods
Animals
Thirty male Wistar rats, with a weight of 200–220 g,
were obtained from the Animal Center of Peking University Health
Science Center (certificate no. SCXK 2006–0008; Beijing, China).
The rats were randomly assigned to weight-matched groups (6 rats in
each group). The rats were housed in cages at the temperature of
22±2°C and with humidity equal to 40±5% under a 12-h light/dark
cycle. The rats received a standard diet and water ad
libitum. The rats were fasted for 12 h prior to the experiment.
The investigations conformed to the EU adopted Directive 2010/63/EU
and the Guide of Peking University Animal Research Committee.
Experiment protocols were approved by the Peking University
Biomedical Ethics Committee Experimental Animal Ethics Branch
(LA2011-38).
Experimental groups and drug
administration
The rats were randomly assigned to weight-matched
groups and anesthetized by intramuscular injection of 20% urethane
(1 ml/100 g BW). Saline or phenolic acid was continuously infused
via the left jugular vein catheter. CA, SAB, ChA and FA (National
Institutes for Food and Drug Control, Beijing, China) were
dissolved in the normal saline, and 7 mg/kg body weight was
administered at a speed of 8 ml/kg/h within 1 h. The concentration
of 7 mg/kg body weight was selected as it was 5-fold higher than
the recommended dose found in the instruction of Chinese herbal
medicine injection protocols. Additionally, most of the reported
adverse effects occurred around this dose. Animals in the control
group received an equivalent volume of saline within the same
period of time. In a series of experiments, the animals were
administered with drug or saline only once, and subsequently were
subjected to assessment of various parameters at the 120-min
time-point.
Microcirculatory observation
The surgical procedure was performed as previously
described (12). The rats were
anesthetized by intramuscular injection of 20% urethane (1 ml/100 g
BW). The abdomen was opened via incision of 25–30 mm in length. The
ileocecal portion of the mesentery (10–15 cm caudal) was gently
exteriorized and mounted on a transparent plastic stage designed
for the rat. The mesentery was kept warm and moist by continuous
superfusion with saline solution at 37°C. The mesenteric
microcirculation was observed under an inverted microscope (DM IRB;
Leica, Cologne, Germany) through the 20X objective lens. The
mesentery was transilluminated with a 12 V, 100 W,
direct-current-stabilized light source. The microscopic images were
obtained using a color video camera (JK-TU53H; Toshiba, Tokyo,
Japan) mounted on the microscope, and the images were transmitted
into a monitor (J2118A; TCL, Huizhou, China). The images were
recorded using a Digital Video Disk videocassette recorder
(DVR-R25; Malata, Xiamen, China). Single unbranched venules (30–50
µm in diameter; 200 µm in length) were selected for investigation
(12).
Microcirculation examinations were initiated after
10 min baseline observation. Adherent leukocytes were defined as
cells that attached to the same site for >10 sec as determined
from a replay of the video images. The number of adherent
leukocytes along venules (30–50 µm in diameter and 200 µm in
length) that were randomly selected from the videotape images were
counted at baseline (before infusion), and 40, 80 and 120 min after
the infusion and expressed as the number per 200 µm of venule
length (12).
To monitor the oxidant stress in the venular walls,
the oxidant-sensitive fluorescent probe dihydrorhodamine 123 (DHR
123; Molecular Probes, Eugene, OR, USA) was topically applied to
the mesenteric surface (10 µmol/l) just 5 min before the
observation. Fluorescence images were recorded at baseline (before
infusion), and 40, 80 and 120 min after infusion with an inverted
fluorescence microscope (DM IRB; Leica) under 455-nm excitation
light, and the fluorescence intensity of venular walls (Iv) and
extravenular interstices (Ie) was measured with Image-Pro Plus 5.0
software (Rockville, MD, USA), respectively. The differences
between Iv and Ie were determined for each time point, and the
ratio of each value to the baseline was calculated (12).
Western blot analysis of Nox4 and
P22phox protein expression
The terminal ileum tissues of rats were removed 120
min after infusion. Tissues were minced and homogenized on ice in
lysis buffer (150 mmol/l NaCl, 50 mM Tris-HCl, 1% Nonidet P-40
(NP-40) solution, and 0.1% sodium dodecyl sulfate (SDS), pH 7.4),
and centrifuged for 10 min at 12,000 × g. The supernatant
containing cytosol proteins was isolated and the total protein
levels in the homogenates were quantified with a bicinchoninic acid
(BCA) protein assay kit (Sun Biomedical Technology Co., Ltd.,
Beijing, China). The prepared samples in gel loading buffer [12.5
mmol/l Tris-HCl, 2% SDS, 10% glycerol, 1.56% dithiothreitol (DTT),
and 1% bromophenol blue, pH 6.8], were boiled for 5 min. Equal
amounts of proteins (50 µg) for each sample were separated on a 10%
SDS-polyacrylamide mini-gel at a constant voltage of 100 V for 2 h.
The proteins were transferred by electrophoresis at 30 V for 16 h
to polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked for 1 h at room temperature in 5% (w/v) non-fat dry milk in
TBS-T (10 mmol/l Tris-HCl, 100 mmol/l NaCl, and 0.1 mmol/l
Tween-20, pH 7.4). The membranes were then incubated overnight with
rabbit polyclonal IgG against Nox4 [2 µg/ml; Abcam (Hong Kong)
Ltd., Hong Kong, China] and p22phox (1:200, cat. no.
sc-20781; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
After rinsing, the membranes were incubated with the horseradish
peroxidase-conjugated secondary antibody (1:3,000; Santa Cruz
Biotechnology, Inc.) for 2 h. Antibody labeling was detected by an
enhanced chemiluminescence system and subsequently exposed to
radiographic film. The optical density of bands were then
visualized and normalized to that of β-actin (13).
Statistical analysis
Data were presented as mean ± standard error (SE)
and analyzed with the SPSS 17.0 statistical software package (SPSS
Inc., Chicago, IL, USA). Differences were assessed with
single-factor analysis of variance (ANOVA) followed by LSD tests
and Tamhane's T2 tests. Statistically significant differences were
indicated at P<0.05.
Results
ChA and FA increases fluorescence
intensity of DHR in the venular walls
The fluorescence intensity of DHR in the venular
walls was examined in five groups at 120-min time-points. Fig. 2A shows the representative fluorescent
images of venules in various conditions with the respective
quantitative results shown in Fig.
2B. No DHR fluorescence was observed in rat mesenteric venular
walls prior to infusion in the five groups (Fig. 2A-a1-e1), and low fluorescence
intensity remained over the observation in the control group
(Fig. 2A-a1-a4), as well as in the
CA group (Fig. 2A-b1-b4) and SAB
group (Fig. 2A-c1-c4). By contrast,
ChA induced a pronounced DHR fluorescence in venular walls after
infusion for 40 min (Fig. 2A-d2), 80
min (Fig. 2A-d3) and 120 min
(Fig. 2A-d4) with significant
differences as compared to the control group (Fig. 2B). Similarly, FA induced an obvious
DHR fluorescence after infusion of 80 min (Fig. 2A-e3) and 120 min (Fig. 2A-e4) compared to the control group
and slightly below the levels found in the ChA group. This
indicated that ChA and FA were potentially able to induce the
production of reactive oxygen species (ROS) from venules.
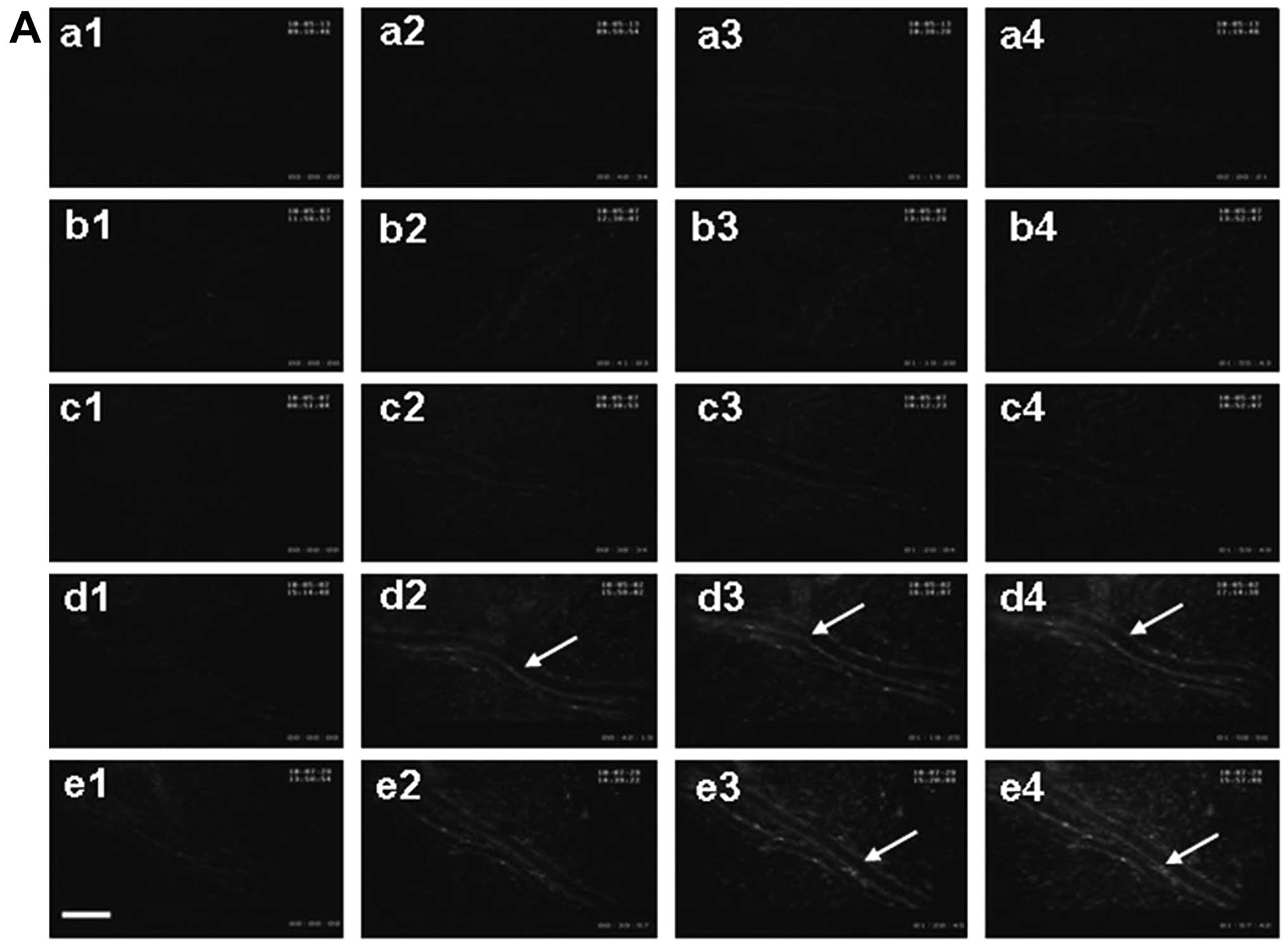 | Figure 2.Effect of phenolic acids on DHR
fluorescence intensity in rat mesenteric venular wall. (A)
Representative images of the changes in DHR fluorescence intensity
of the H2O2-sensitive probe DHR in the
mesenteric venular wall in the (a) control, (b) CA, (c) SAB, (d) CA
and (e) FA groups at (1) baseline,
(2) 40, (3) 80 and (4)
120 min, respectively. The arrow indicates DHR fluorescence on the
venular wall; scale bar, 50 µm. (B) Time course of changes in the
ratio of DHR fluorescence on the venular walls in different groups.
Data are presented as means ± SE of six animals. *P<0.05 vs.
control group. DHR, dihydrorhodamine 123; CA, caffeic acid; SAB,
salvianolic acid B; FA, ferulic acid. |
No change in the number of leukocytes
adhering to the venular walls
Images of leukocytes adhering to the venular walls
of the control (a), CA (b), SAB (c), ChA (d) and FA (e) groups were
taken at baseline 0, 40, 60 and 120 min after infusion (Fig. 3A). No adherent leukocytes were
observed prior to the infusion in each group (Fig. 3A-a1-e1). Only a small amount of
adherent leukocytes were observed along the venular walls in the
control group over the period of infusion (Fig. 3A-a2-a4). No significant differences
were observed in the number of adherent leukocytes in the other
groups of rats compared with the control group at each time-point
(Fig. 3A-b2-e4).
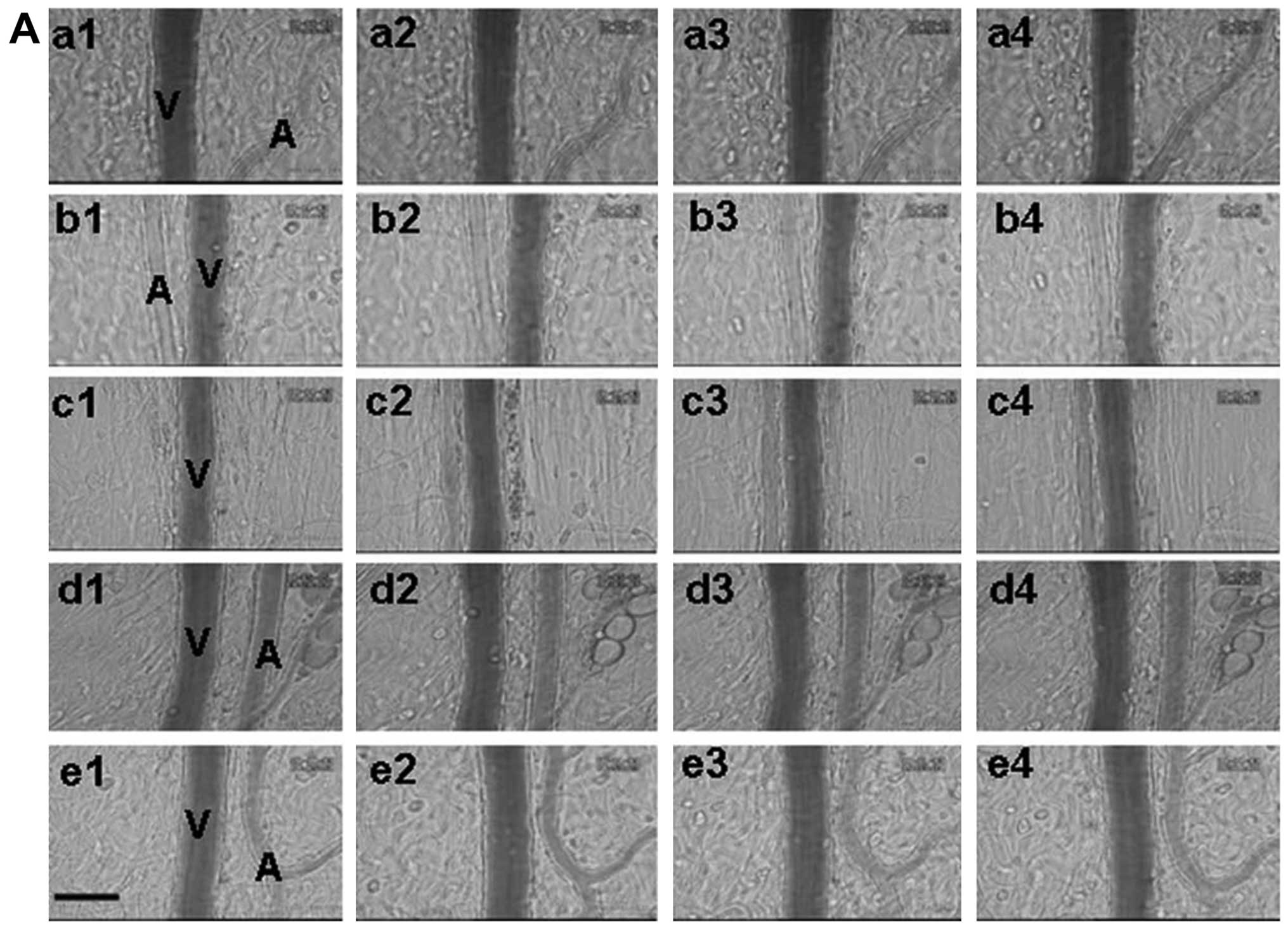 | Figure 3.Effect of phenolic acids on leukocyte
adhesion to the rat mesenteric venular wall. (A) Representative
images showing the effect of phenolic acids on leukocyte adhesion
to the wall of a mesentery venule in the (a) control, (b) CA, (c)
SAB, (d) CA and (e) FA groups at (1)
baseline, (2) 40, (3) 80 and (4)
120 min, respectively. V, mesenteric venule; A, mesenteric
arteriole; scale bar, 50 µm. (B) Time course of changes in the
number of leukocytes adhering to the mesenteric venules. The number
of adherent leukocytes was expressed as the number of cells per 200
µm of venule. Data are presented as means ± SE of six animals.
*P<0.05 vs. control group. CA, caffeic acid; SAB, salvianolic
acid B; FA, ferulic acid. |
A quantitative analysis of the number of leukocytes
adherent to venular walls at 120 min demonstrated that the number
of adherent leukocytes in the control group was (1.5±0.43 per
200-µm venule), CA group (1.33±0.42 per 200-µm venule), SAB group
(1.5±0.5 per 200-µm venule), ChA group (1.67±0.49 per 200-µm
venule) and FA group (1.33±0.33 per 200-µm venule) (Fig. 3B).
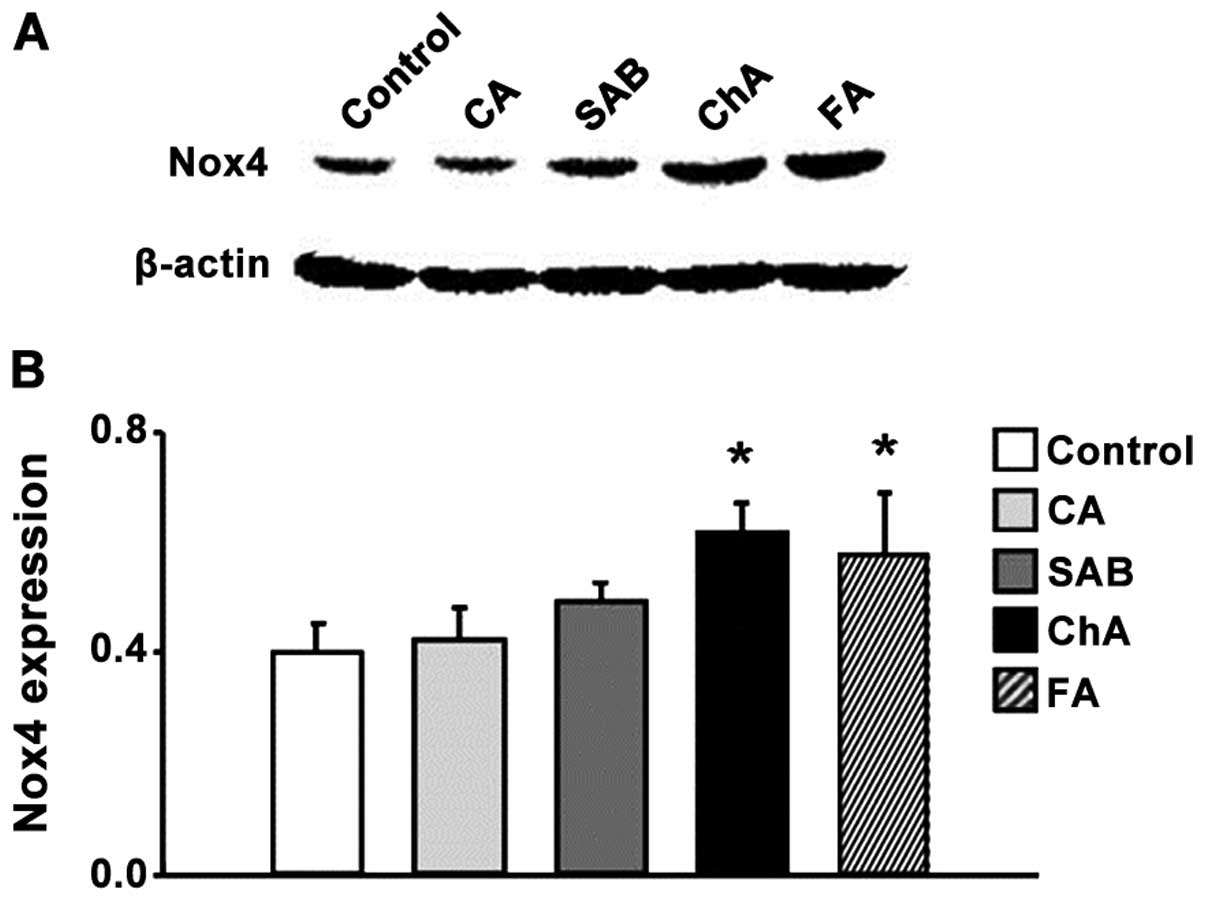
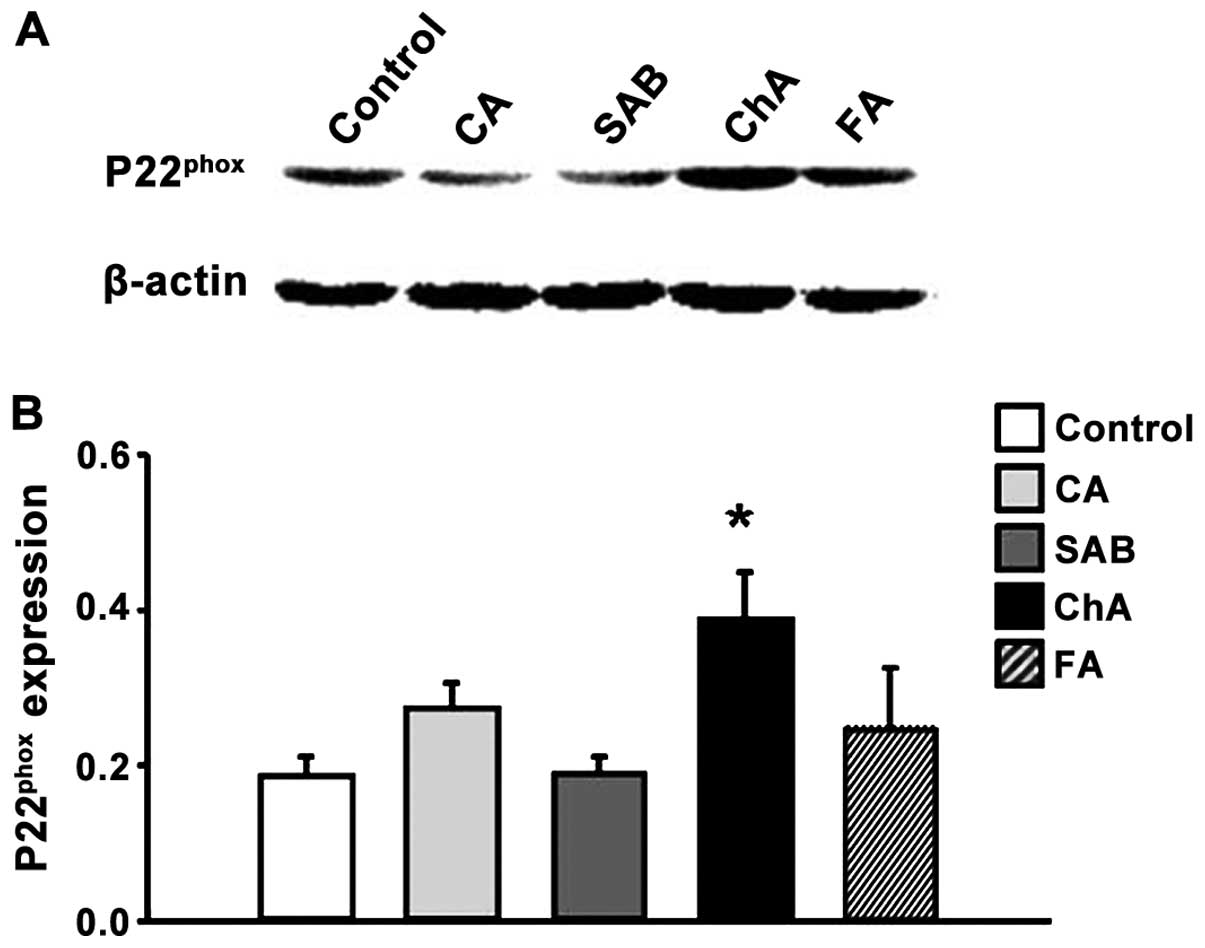
ChA and FA increase Nox4 and P22phox
protein expression
We evaluated the role of NADPH oxidase in high-dose
phenolic acid-induced ROS production from vasculature. We employed
western blotting to determine the expression levels for the two
subunits of NADPH oxidase, Nox4 and p22phox (Figs. 4 and 5).
The expression of Nox4 and p22phox
revealed no significant differences between the control, CA and SAB
groups at the 120-min time-points examined. ChA increased the
levels of protein expression (for both proteins) compared to the
control group, while FA only boosted the Nox4 expression and not
the expression of p22phox (Figs. 4 and 5).
Discussion
The present findings have demonstrated that the
intravenous injection of phenolic acids (ChA and FA) in high doses
may create an imbalance in the oxidant/antioxidant mechanism,
leading to oxidant stress and increased production of ROS in
venular walls. High levels of Nox4 and p22phox
expression were observed in response to ChA and FA injections,
indicating the involvement of NADPH oxidase in phenolic
acid-induced oxidant stress. On the other hand, the injection of
phenolic acids, used in this study, did no affect leukocyte
recruitment in microvessels. This suggests that the phenolic
acid-induced oxygen-free radicals are produced by endothelial cells
and not neutrophils. Notably, pro-oxidation occurred only after the
injection of high doses of ChA and FA, but not CA and SAB (with the
same concentration) at 120 min.
The H2O2-sensitive fluorescent
probe DHR has been successfully employed for monitoring the
oxidative stress and the measurement of dynamic alterations in
intracellular H2O2 and other types of ROS
levels in endothelial cell cultures. Similar results were obtained
in other cell types (14–16). Previously, we established that low
concentrations of ChA (0.336 and 1.68 mg/kg) were not capable of
inducing any DHR fluorescence intensity in rat microvasculature
endothelial cells (17). The results
of the present study show that oxidative stress in microvasculature
was enhanced in the high-dose ChA and FA groups (7 mg/kg) starting
from 40 and 80 min post-injection until 120 min. This result
suggested that phenolic acid-induced oxidative stress is
dose-dependent. The finding was consistent with those of other
reports (10,18). The dose used in this study (7 mg/kg
body weight) was 5-fold higher than the recommended dose identified
in the instructions for the ChA dose in Qingkailing injection (a
Chinese herb medicine). Available data indicated that in the
majority of cases reporting adverse effects of ChA, victims were
injected with a quantity close to this dose (4). In addition, the results showed that
exposure to the same dose of CA and SAB did not trigger generation
of ROS. Thus, the order of pro-oxidant effectiveness for phenolic
acid family is ChA > FA > CA and SAB.
The excessive generation of ROS is considered a
pathological mechanism responsible for cell damage and organ
dysfunction (10). Possible sources
for the generation of ROS, include NAD(P)H oxidase, xanthine
oxidase, and the uncoupling of endothelial nitric oxide synthase
(eNOS) (19). In view of the
critical importance of NADPH oxidase in generating the oxidative
stress of dysregulated vascular redox environment (20), we investigated the members of NADPH
oxidase family and explored the potential of this enzyme as the
source of phenolic acids-induced ROS. It was found that high doses
of ChA significantly increased the expression levels of Nox4 and
p22phox proteins in ileum tissue at 120 min after
infusion. The FA group revealed a similar outcome for the
expression levels of Nox4. Nox4 is present in all vascular cell
walls and is significantly more abundant than any other Nox enzyme
(21). Nox4 heterodimerizes with
p22phox and causes enzyme functions (13,22). The
high levels of Nox4 and p22phox expression suggest that
NADPH oxidase is involved in phenolic acid-induced vascular ROS
production. Previously, it was shown that the nutritional ChA led
to antioxidant effects by inhibiting the NADPH oxidase activity in
aorta (23). The possibility of
NADPH oxidase being involved in phenolic acid-induced redox
reaction supports our findings. Results obtained from the
intravenous injection of high doses of ChA or FA demonstrated that
the pro-oxidant activity of the enzyme was significantly boosted
while the antioxidant activity remained unchanged. Additionally,
phenolic acids played a pro-oxidant role by mobilizing endogenous
copper to increase the production of ROS (17,24).
Leukocyte adhesion to the venules may be the source
of oxygen-free radicals released from the leukocytes (25). Nevertheless, leukocytes were not the
primary source of ROS release since we did not detect any
leukocytes adhering to the venular walls in the course of our
observations. Thus, there is a possible involvement of other
peroxidases and other mechanisms in vascular cells induced by
phenolic acids.
In conclusion, the present study findings show that
intraveinal injection of high doses of phenolic acids particularly
ChA and FA induced excessive ROS release from the venules, which
suggests the double-edged sword property of phenolic acids as a
drug. Our results provide new insight into understanding the
reasons behind the observed adverse effects after the injection of
phenolic acids. Clinicians are therefore required to be more
diligent especially when a Chinese herbal injection containing high
levels of phenolic acid content is to be applied by intravenous
injection.
References
|
1
|
Huang WY, Cai YZ and Zhang Y: Natural
phenolic compounds from medicinal herbs and dietary plants:
potential use for cancer prevention. Nutr Cancer. 62:1–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang FH, Zhang XY, Zhang LY, Li Q, Ni B,
Zheng XL and Chen AJ: Mast cell degranulation induced by
chlorogenic acid. Acta Pharmacol Sin. 31:849–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Zhang XY and Chen GS: Adverse effect
and mechanism of chlorogenic acid in clearing heat and detoxication
traditional Chinese medicine injections. Chin JMAP. 26:555–558.
2009.
|
|
4
|
Yuan Q, Wang L, Cheng L, Cui XH, Zhong DK,
Li YY, Shang HC, Zang Bl and Li YP: Adverse drug reactions and
adverse events of 33 varieties of Traditional Chinese Medicine
Injections on the National Essential Drugs List (2004 edition) of
China: An Overview on published literatures. Chin J Evid-based Med.
10:132–139. 2010.
|
|
5
|
Taubert D, Breitenbach T, Lazar A,
Censarek P, Harlfinger S, Berkels R, Klaus W and Roesen R: Reaction
rate constants of superoxide scavenging by plant antioxidants. Free
Radic Biol Med. 35:1599–1607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roche M, Dufour C, Mora N and Dangles O:
Antioxidant activity of olive phenols: mechanistic investigation
and characterization of oxidation products by mass spectrometry.
Org Biomol Chem. 3:423–430. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azmi AS, Bhat SH and Hadi SM:
Resveratrol-Cu(II) induced DNA breakage in human peripheral
lymphocytes: Implications for anticancer properties. FEBS Lett.
579:3131–3135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuhara K, Nagakawa M, Nakanishi I,
Ohkubo K, Imai K, Urano S, Fukuzumi S, Ozawa T, Ikota N, Mochizuki
M, et al: Structural basis for DNA-cleaving activity of resveratrol
in the presence of Cu(II). Bioorg Med Chem. 14:1437–1443. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hadi SM, Asad SF, Singh S and Ahmad A:
Putative mechanism for anticancer and apoptosis-inducing properties
of plant-derived polyphenolic compounds. IUBMB Life. 50:167–171.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang Y, Kusama K, Satoh K, Takayama E,
Watanabe S and Sakagami H: Induction of cytotoxicity by chlorogenic
acid in human oral tumor cell lines. Phytomedicine. 7:483–491.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rakshit S, Mandal L, Pal BC, Bagchi J,
Biswas N, Chaudhuri J, Chowdhury AA, Manna A, Chaudhuri U, Konar A,
et al: Involvement of ROS in chlorogenic acid-induced apoptosis of
Bcr-Abl+ CML cells. Biochem Pharmacol. 80:1662–1675.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang MX, Liu YY, Hu BH, Wei XH, Chang X,
Sun K, Fan JY, Liao FL, Wang CS, Zheng J, et al: Total salvianolic
acid improves ischemia-reperfusion-induced microcirculatory
disturbance in rat mesentery. World J Gastroenterol. 16:5306–5316.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collins-Underwood JR, Zhao W, Sharpe JG
and Robbins ME: NADPH oxidase mediates radiation-induced oxidative
stress in rat brain microvascular endothelial cells. Free Radic
Biol Med. 45:929–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan Q, Liu YY, Sun K, Chen CH, Zhou CM,
Wang CS, Li A, Zhang SW, Ye ZL, Fan JY, et al: Improving effect of
pretreatment with yiqifumai on LPS-induced microcirculatory
disturbance in rat mesentery. Shock. 32:310–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han JY, Miura S, Akiba Y, Higuchi H, Kato
S, Suzuki H, Yokoyama H and Ishii H: Chronic ethanol consumption
exacerbates microcirculatory damage in rat mesentery after
reperfusion. Am J Physiol Gastrointest Liver Physiol.
280:G939–G948. 2001.PubMed/NCBI
|
|
16
|
Han JY, Horie Y, Fan JY, Sun K, Guo J,
Miura S and Hibi T: Potential of 3,4-dihydroxy-phenyl lactic acid
for ameliorating ischemia-reperfusion-induced microvascular
disturbance in rat mesentery. Am J Physiol Gastrointest Liver
Physiol. 296:G36–G44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang C, Li WH, Li PT, Wang F, Wu JR and
Du WY: Methods for testing of chlorogenic acid safty involving in
traditional Chinese medicine. CN patent 102,200,505. March 23–2011,
issued September 28, 2011.
|
|
18
|
Zheng LF, Dai F, Zhou B, Yang L and Liu
ZL: Prooxidant activity of hydroxycinnamic acids on DNA damage in
the presence of Cu(II) ions: mechanism and structure-activity
relationship. Food Chem Toxicol. 46:149–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Touyz RM: Reactive oxygen species,
vascular oxidative stress, and redox signaling in hypertension:
what is the clinical significance? Hypertension. 44:248–252. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai DZ and Dai Y: Role of endothelin
receptor A and NADPH oxidase in vascular abnormalities. Vasc Health
Risk Manag. 6:787–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haurani MJ, Cifuentes ME, Shepard AD and
Pagano PJ: Nox4 oxidase overexpression specifically decreases
endogenous Nox4 mRNA and inhibits angiotensin II-induced
adventitial myofibroblast migration. Hypertension. 52:143–149.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katsuyama M: NOX/NADPH oxidase, the
superoxide-generating enzyme: its transcriptional regulation and
physiological roles. J Pharmacol Sci. 114:134–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki A, Yamamoto N, Jokura H, Yamamoto
M, Fujii A, Tokimitsu I and Saito I: Chlorogenic acid attenuates
hypertension and improves endothelial function in spontaneously
hypertensive rats. J Hypertens. 24:1065–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azmi AS, Bhat SH, Hanif S and Hadi SM:
Plant polyphenols mobilize endogenous copper in human peripheral
lymphocytes leading to oxidative DNA breakage: a putative mechanism
for anticancer properties. FEBS Lett. 580:533–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurose I, Suematsu M, Miura S, Fukumura D,
Sekizuka E, Nagata H, Oshio C and Tsuchiya M: Oxyradical generation
from leukocytes during endotoxin-induced microcirculatory
disturbance in rat mesentery - attenuating effect of cetraxate.
Toxicol Appl Pharmacol. 120:37–44. 1993. View Article : Google Scholar : PubMed/NCBI
|