Introduction
Heatstroke is a life-threatening illness that
results from exposure to a high environmental temperature or
vigorous exercise, which causes a rapid increase of body
temperature (to >40°C), with consequent abnormalities of the
central nervous system, including delerium, convulsions and coma
(1). Despite heatstroke being
prevalent in individuals in hot climates, it can also affect people
in warm climates during heat waves and sporadically in an epidemic
form (2). It is predicted that if no
effective measures are taken, increasingly hot climates will result
in prolonged periods of extremely hot temperatures, with a
subsequent increase in mortality rates (3).
Heatstroke is an illness with high morbidity and
mortality rates for which no methods of treatment have been clearly
defined due to the pathogenesis of cell apoptosis and tissue injury
associated with it not being well understood (1). Bouchama et al (4) suggested that tissue injury and cell
apoptosis were directly induced by heat. Extreme temperatures are
damaging to the majority of cellular structures, and cause
functional abnormalities, cell apoptosis and necrosis (5). Rats with moderate hyperthermia have
demonstrated accelerated apoptosis, which contributed to cell death
(6). However, whether cell death
occurs due to apoptosis in patients with heatstroke is not yet
understood.
Heatstroke directly induces cell injury as well as
the production of large numbers of inflammatory mediators, in
addition to cells revealing extensive biological activities that
contribute to a systemic inflammatory response and immune
dysfunction. In animal models and humans, an increase in
circulating pro-inflammatory cytokine levels has been found to
induce the development of progression of heatstroke (4,7,8). Cyclooxygenase (COX) is one of the most
important pro-inflammatory mediators. In addition, the protein
complex nuclear factor κB (NF-κB) regulates inflammation by
inducing the production of a variety of pro-inflammatory and
anti-inflammatory factors (9). In
the hypoxia-induced injury of HEI-OC1 cells, it was demonstrated
that NF-κB and hypoxia-inducible factor-1 were activated, thus
causing the upregulation of interleukin-6 release (10). However, whether heatstroke induces an
inflammatory response by activating a particular signaling pathway
remains unclear.
Ulinastatin, a typical Kunitz-type protease
inhibitor, has been widely used in the treatment of severe
heatstroke. Numerous studies have indicated that ulinastatin has
protective effects against acute lung injuries caused by endotoxins
as well as the mechanical damage resulting from Escherichia
coli lipopolysaccharides in rats (11–14).
However, there are few studies concerning the use of ulinastatin as
an intervention for heatstroke-induced injury in rats (15).
The aim of the present study was to investigate the
cellular pathological changes and the associated inflammatory
response of hippocampal tissues in rats with heatstroke and the
effects of treatment with ulinastatin at different doses. These
observations were performed with the aim of identifying the
mechanisms of action by which ulinastatin protects against
heatstroke in the clinic, as well as the importance of the dose of
application.
Materials and methods
Animals
A total of 48 specific pathogen-free Sprague Dawley
male rats (age, 4 weeks; weight, 180–220 g) were purchased from the
Shanghai Laboratory Animal Company (Shanghai, China) and housed in
the animal facility at 25°C (humidity, 60–70%) with a 12-h
light/dark cycle and free access to food and water. Rats were
randomly divided into four groups (12 rats per group). The four
groups were as follows: Τhe control group in which the rats were
maintained at 25°C, the heatstroke group where the rats were placed
at 42°C, ulinastatin treatment group 1 where 5,000 IU/kg
ulinastatin was administered 2 h prior to heating at 42°C and
ulinastatin treatment group 2 where 10,000 IU/kg ulinastatin was
administered 2 h prior to heating at 42°C. The present study was
performed in strict accordance with the Guide for the Care and Use
of Laboratory Animals of the National Institutes of Health (ninth
edition, 2010). The animal use protocol was approved by the
Institutional Animal Care and Use Committee of the General Hospital
of Jinan Military Command (Jinan, China).
Indicators and methods
Rats were anesthetized via intraperitoneal injection
of 3% sodium pentobarbital (45 mg/kg; Haoran Biological Technology
Co., Ltd., Shanghai, China). Subsequently, the rats, with the
exception of those in the control group, were placed into a heating
chamber (HD-80TST; Haida Equipment Co., Ltd. Shanghai, China) at
42°C with 60% humidity. Doses of 5,000 and 10,000 IU/kg ulinastatin
were administered 2 h prior to heating to rats in ulinastatin
treatment groups 1 and 2, respectively. Following 1 h of heating,
the rats were removed from the heating chamber and returned to
their housing conditions.
Histology
Rats were anesthetized with 3% sodium pentobarbital
(40 mg/kg; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) via
intraperitoneal injection prior to cervical dislocation.
Hippocampal tissues of rats were removed 1 h after heating and
fixed with 10% (v/v) neutral-buffered formalin. Specimens were
dehydrated and embedded in paraffin and 4-mm tissue sections were
cut using a Leica Biosystem Rotary Microtome (Leica Microsystem
Nussloch GmbH, Wetzlar, Germany). Next, sections of the tissues
were placed on slides, deparaffinized and sequentially stained with
hematoxylin and eosin (Richard-Allan Scientific, Kalamazoo, MI,
USA). Under an identical light microscope, the stained tissue
sections on slides were analyzed at magnification ×200.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) staining
TUNEL staining was performed using an in situ
cell death detection kit (POD (11684817910; Roche Diagnostics,
Shanghai, China) according to the manufacturer's instructions. The
fixed areas of each section were detected by microscopy and the
numbers of TUNEL-positive cells were counted at a magnification of
×200 for 30 fields per section.
Biochemical measurements
Hippocampal tissue was homogenized with
physiological saline at a ratio of 1:9 (weight/volume) in a
homogenizer. The homogenate was then centrifuged at 1,000 × g for
10 min, and the supernatant was collected in order to determine the
activity of superoxide dismutase (SOD) and inducible nitric oxide
synthase (iNOS) using SOD and iNOS kits. To determine the
malondialdehyde (MDA) and reactive oxygen species (ROS) content,
MDA and ROS kits were used. The kits used in the present study were
purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China).
Protein extraction and western
blotting
Hippocampus tissues were harvested and lysed on ice
for 30 min in radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China) containing 1 mM
phenylmethylsulfonyl fluoride. Total proteins were isolated using
radioimmunoprecipitation buffer (Amyjet Scientific, Inc., Wuhan,
China) at 10 min at 95°C and centrifuged at 400 × g at 25°C
for 10 min. Equal amounts (50 µg) of cell lysates was subjected to
electrophoresis. Equal amounts of cell lysates were separated by
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto nitrocellulose membranes, followed by blocking in
fat-free milk overnight at 4°C. The blots were incubated with
primary antibodies targeting Bax (1:1,000; sc-493), Bcl-2 (1:200;
sc-492; both Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
caspase-3 (1:5,000; ab32351; Abcam, Cambridge, MA, USA), COX-2
(1:1,000; 12282; Cell Signaling Technology, Inc., Danvers, MA,
USA), iNOS (1:800; ab3523; Abcam), NF-κB p65 (1:1,000; 6956), H3
(1:1,000; 4499) and glyceraldehyde 3-phosphate dehydrogenase
(1:1,500; 1574; all Cell Signaling Technology, Inc.) at 4°C
overnight. Following washing, the membranes were subsequently
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG (1:1,000; A0208) and goat anti-mouse IgG (1:1,000; A0216; both
Beyotime Institute of Biotechnology) secondary antibodies for 1 h
at 37°C, and were washed three times with Tris-buffered saline with
Tween 20 (Amresco LLC, Solon, OH, USA). Chemiluminescence detection
was conducted using Western Lightning Chemiluminescence Reagent
Plus (PerkinElmer, Inc., Waltham, MA, USA) and signals were
quantified by densitometry (Quantity One software, version 4.62;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data were presented as the mean value ± standard
deviation. Paired, two-tailed Student's t-test and one-way analysis
of variance were used to analyze the significance of a difference
between groups. Survival analysis was performed by the Kaplan-Meier
method, and subjected to the log rank test. Data are presented as
the mean + standard deviation (n=12/group). P<0.05 was
considered to indicate a statistically significant difference.
Results
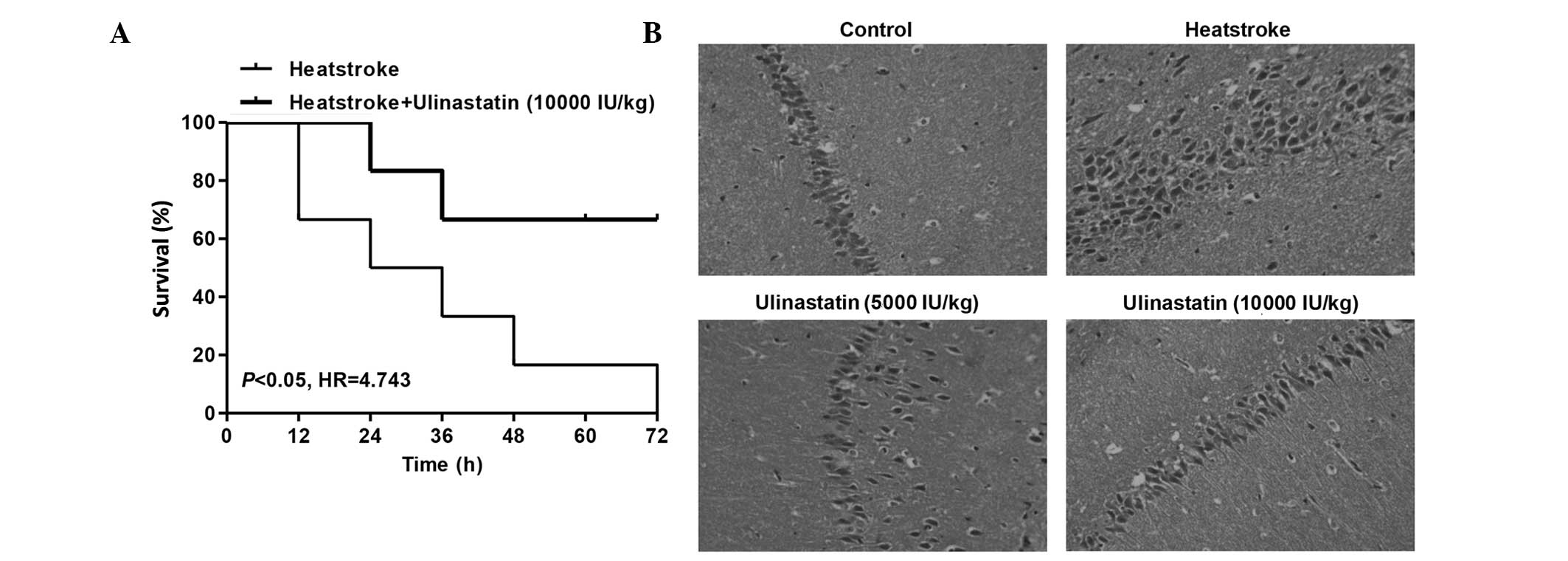
Ulinastatin protects rats against
heatstroke-induced injury
The survival times of rats subjected to heatstroke
with and without ulinastatin treatment were compared. The
cumulative survival rate was significantly lower in the heatstroke
group than in the rats with heatstroke treated with ulinastatin at
a dose of 10,000 IU/kg (Fig. 1A;
P<0.05). These results indicate that ulinastatin could improve
the prognosis of rats with heatstroke. Following the onset of
heatstroke, the damage to hippocampal neurons was greater in the
rats of the heatstroke group compared with the control group.
Histological examination revealed that heatstroke caused edema and
disappearance of cell nuclei in the hippocampus of rats (Fig. 1B). However, ulinastatin treatment at
doses of 5,000 and 10,000 IU/kg inhibited the effects induced by
heatstroke and thus exhibited neuroprotective effects.
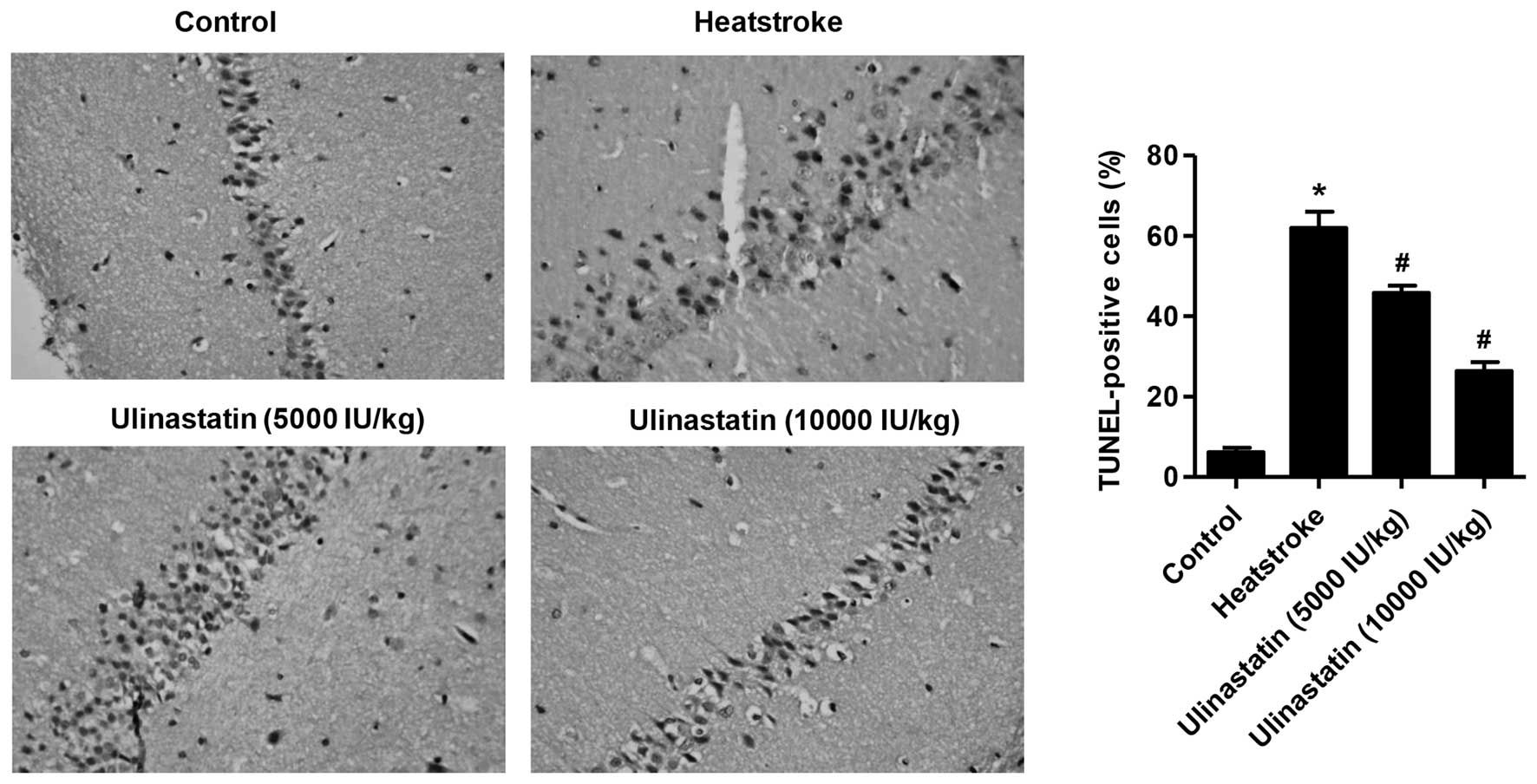
Ulinastatin protects rats against
heatstroke-induced apoptosis
Fig. 2 summarizes the
effects of heatstroke on the number of TUNEL-positive cells in the
hippocampus of the control, heatstroke and ulinastatin-treated
rats. Following the onset of heatstroke, the number of
TUNEL-positive cells of the hippocampus was increased in the
heatstroke group compared with the control group (Fig. 2; P<0.01). However, the increase in
the number of TUNEL-positive cells in the hippocampus of rats with
heatstroke was greatly attenuated by ulinastatin at doses of 5,000
and 10,000 IU/kg (P<0.01).
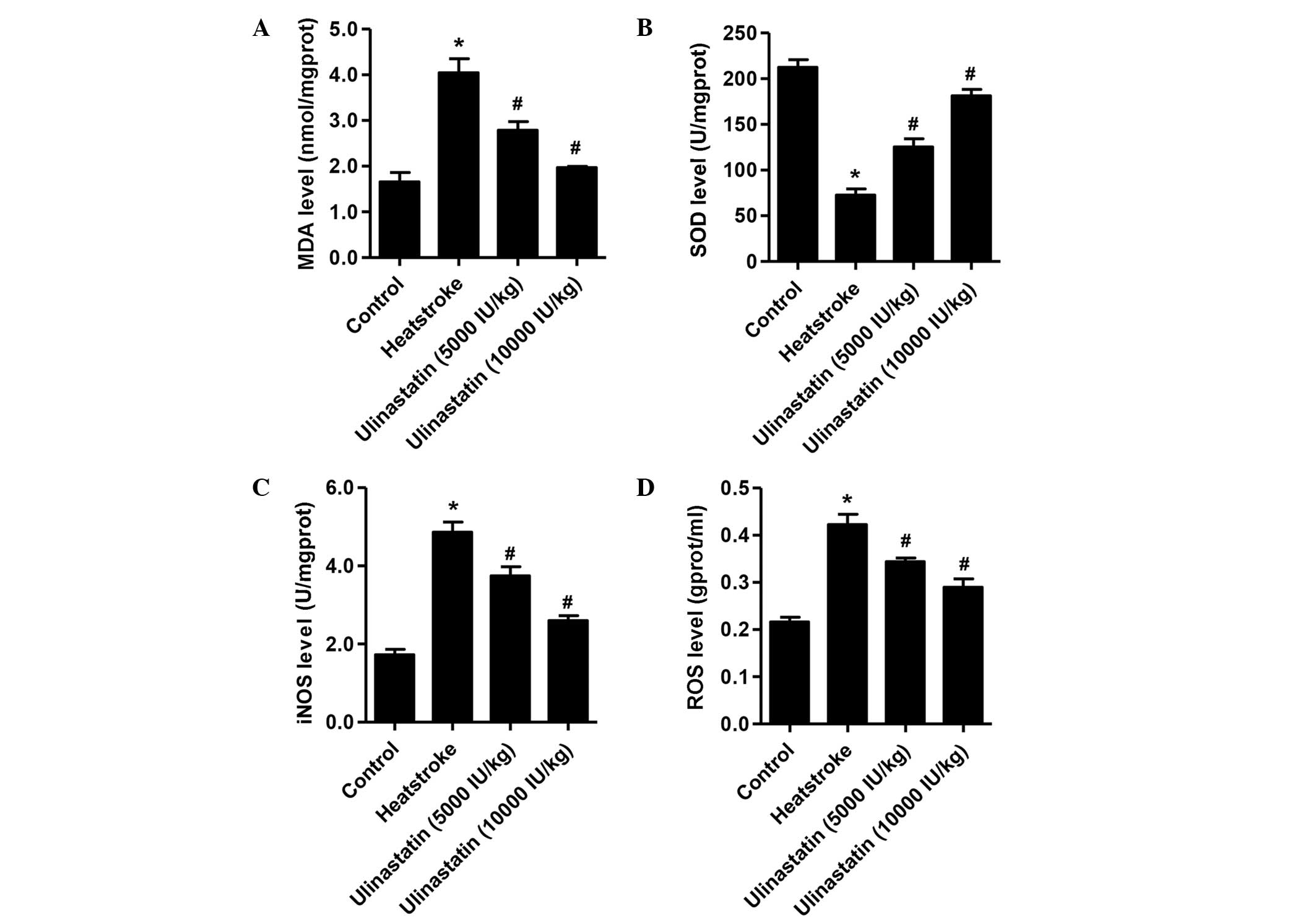
Ulinastatin treatment downregulates
MDA, iNOS and ROS levels but upregulates the SOD level in rats with
heatstroke
Fig. 3 shows the
levels of MDA, SOD, iNOS and ROS among the three experimental
groups and the control group. Compared with the control group,
following the onset of heatstroke, the rats had higher levels of
MDA, iNOS and ROS (P<0.01) and a lower level of SOD (P<0.01).
However, compared with the heatstroke group, rats treated with
ulinastatin at doses of 5,000 and 10,000 IU/kg had significantly
lower levels of MDA, iNOS and ROS, and higher levels of SOD
following the induction of heatstroke (P<0.01).
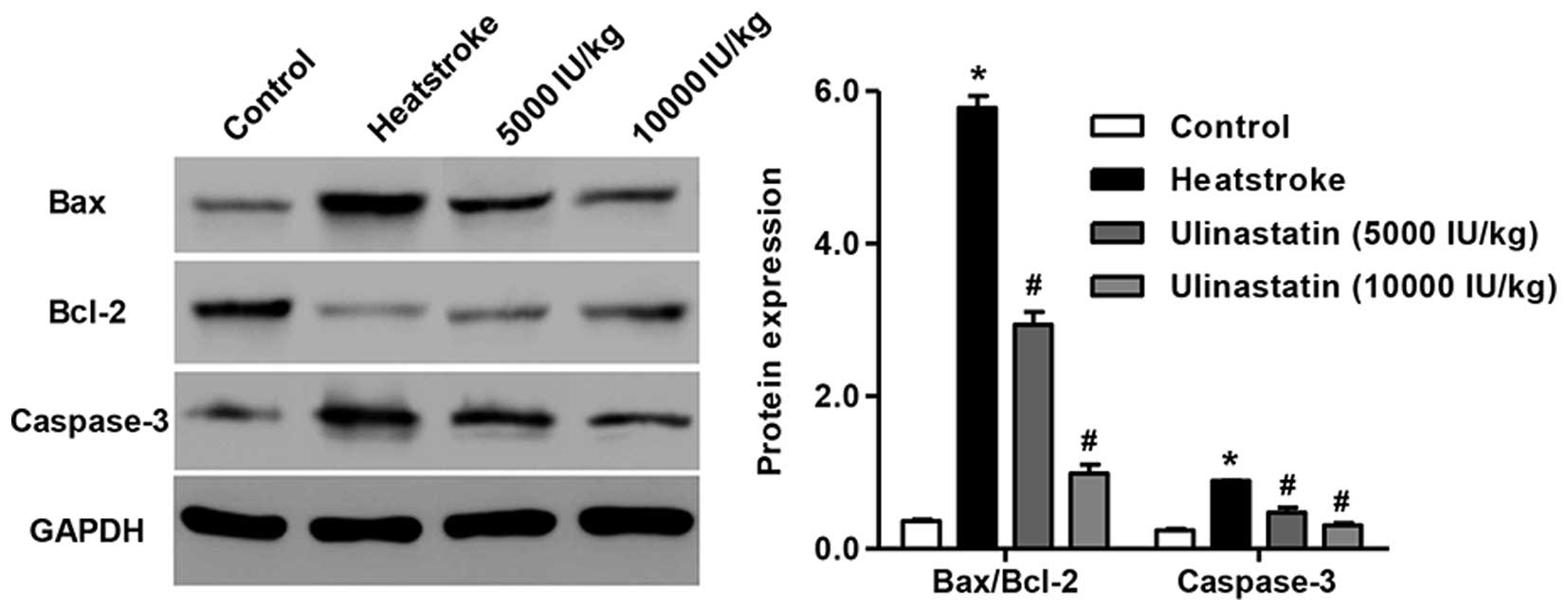
Ulinastatin decreases the Bax/Bcl-2
ratio and caspase-3 levels of rats with heatstroke
The Bcl-2 family contains pro-apoptotic (e.g., Bax)
and anti-apoptotic (e.g., Bcl-2) proteins for the regulation of
apoptosis and death progression (16–18).
Western blot analysis was performed to detect Bcl-2, Bax and
caspase-3 levels in heatstroke rats. Heatstroke resulted in a
significant reduction in the level of the anti-apoptotic protein
Bcl-2 accompanied by increases in the levels of the pro-apoptotic
proteins Bax and caspase-3 compared with those in the control group
(Fig. 4; P<0.01). However, the
rats treated with ulinastatin at doses of 5,000 and 10,000 IU/kg
had lower Bax/Bcl-2 ratios and caspase-3 levels compared with the
heatstroke rats without ulinastatin treatment. These data indicate
that heatstroke increases the Bax/Bcl-2 ratio and caspase-3 levels,
which may contribute to the increase of cell apoptosis, and
ulinastatin attenuates the apoptosis in heatstroke rats.
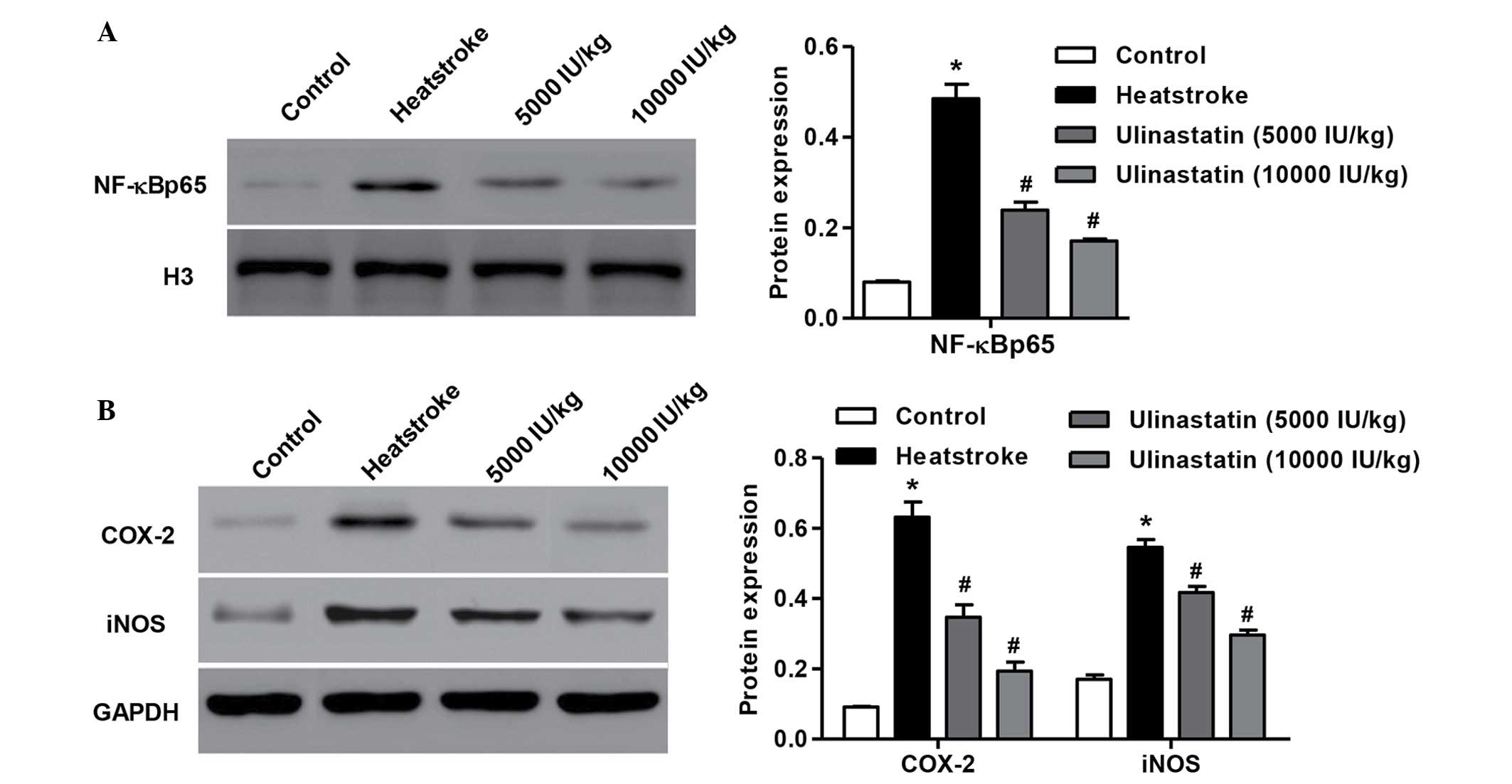
Ulinastatin inhibits NF-κB p65, COX-2
and iNOS levels of rats with heatstroke
Rats exposed to heat for 1 h exhibited significantly
increased the intranuclear NF-κB p65 level compared with the
control, and since the expression of NF-κB p65 is the central step
of NF-κB activation, this indicated that heatstroke may cause NF-κB
activation (P<0.01; Fig. 5A).
Pretreatment of rats with 5,000 or 10,000 IU/kg ulinastatin 2 h
prior to heat exposure significantly suppressed NF-κB p65 subunit
nuclear translocation (P<0.01). Furthermore, pretreatment with
ulinastatin abrogated not only heatstroke-induced COX-2
upregulation but also the protein level of iNOS (Fig. 5B; P<0.01). These observations
indicate that the protective effects of ulinastatin against
inflammatory response caused by heatstroke are associated with the
attenuation of NF-κB activation in rats.
Discussion
The aim of the present study was to elucidate the
pathogenic and molecular mechanisms that underlie tissue injury and
cell apoptosis in heatstroke using a rat model. One of the
principal observations of the present study was that the survival
time of heatstroke rats pretreated with ulinastatin at a dose of
10,000 IU/kg was notably longer than that of heatstroke rats
without ulinastatin treatment. Furthermore, heatstroke induced
edema and the loss of nuclei from hippocampal neurons in rats and
induced cell apoptosis by increasing the Bax/Bcl-2 ratio and
caspase-3 protein levels. Secondly, heatstroke also upregulated
MDA, iNOS and ROS levels but downregulated SOD levels, and induced
inflammatory responses via increasing the expression of NF-κB p65,
COX-2 and iNOS. Thirdly, pretreatment with ulinastatin
significantly attenuated the injury induced by heatstroke.
There are numerous different mechanisms by which
heatstroke induces damage in the body (19). Previous studies have demonstrated
that high temperature and humidity are two principal factors in the
induction of a hyperthermic animal model of heatstroke (4,20). The
histological examination conducted in the present study revealed
that the hippocampal neurons of the rats in the heatstroke group
presented extensive pathological changes compared with those of the
control group. Light microscopy revealed the presence of edema in
and the loss of nuclei from hippocampal neurons in the
heatstroke-induced rats (Fig. 1B).
However, the brain injury induced by heatstroke in the rats was
attenuated by ulinastatin treatment. This is consistent with a
previous study in which Qin et al (21) identified that the early application
of ulinastatin relieved edema in the lung tissue of rats subjected
to hyperthermia.
Another interesting observation of the present study
was the critical role of apoptosis as a mechanism of cell death in
heatstroke. An increase in apoptosis was observed in
heatstroke-induced rats using the TUNEL assay (Fig. 2). These observations were similar to
those in baboons subjected to heatstroke (22). Furthermore, to further understand the
mechanism of cell apoptosis, the expression of Bax, Bcl-2 and
caspase-3 levels in heatstroke-induced rats was detected. As shown
in Fig. 4, the Bax/Bcl-2 ratio and
the protein level of caspase-3 were increased in heatstroke-induced
rats. Importantly, heatstroke rats treated with ulinastatin
revealed a reduction in the number of TUNEL-positive cells and an
attenuated increase of Bax/Bcl-2 ratio and caspase-3 protein level,
compared with rats without ulinastatin treatment. These results
demonstrate that apoptosis is associated with the pathophysiology
of heat stress. In the present study, the results also demonstrated
that ulinastatin suppressed the MDA, iNOS and ROS upregulation and
SOD downregulation induced by heatstroke in rats (Figs. 3 and 5). These data are consistent with results
of other studies, which demonstrated that ulinastatin
administration inhibited the increase of MDA, iNOS and ROS levels,
and increased SOD levels in various types of damage in animal
models (23–26).
In humans and animals subjected to heat stress, an
inflammatory response has been shown to contribute to the damage of
various tissues and organs (8).
COX-2, a pro-inflammatory mediator, promotes the production of a
number of inflammatory factors in multiple cells and tissues.
Tsutakawa et al (27)
identified that COX-2 mRNA expression is upregulated in rat skin
with ischemia/reperfusion (I/R)-induced lesions, and that NS-398, a
COX-2 inhibitor, protects against nicotine exacerbated
I/R-triggered skin apoptosis and necrosis. The present study
indicated that exposure of rats to heat caused the expression level
of COX-2 to increase, and that pretreatment with ulinastatin for 2
h suppressed heatstroke-induced COX-2 upregulation. NF-κB is an
inducible transcription factor that increases COX-2 expression
levels (28). The p65 protein is the
most abundant subunit of NF-κB, with p50, p52, REL and REL-B being
the others, and promotes the activation of NF-κB. A previous study
identified that ulinastatin inhibited the translocation of the
NF-κB p65 subunit from the cytoplasm to the nucleus in rats with
smoke inhalation-induced injury (29). In agreement with this previous study,
the exposure of rats to heat in the present study resulted
increased NF-κB p65 expression, which was notably reversed by
pretreatment with ulinastatin. The results demonstrate the
association between NF-κB and inflammation, and suggest that
ulinastatin protects against heatstroke-induced inflammatory
responses by inhibiting the NF-κB/COX-2 pathway in rats.
Overall, to the best of our knowledge the present
study is the first to suggest that ulinastatin exhibits a
protective effect against brain injury and inflammatory responses
induced by heatstroke through the inhibition of cell apoptosis and
blockage of the NF-κB/COX-2 pathway in rats. Furthermore, the
present study provides novel evidence concerning the mechanism by
which ulinastatin reduces heatstroke-induced brain injury.
Therefore, administration of ulinastatin may be a novel therapeutic
strategy for brain injury induced by heatstroke.
References
|
1
|
Bouchama A and Knochel JP: Heat stroke. N
Engl J Med. 346:1978–1988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vandentorren S, Bretin P, Zeghnoun A,
Mandereau-Bruno L, Croisier A, Cochet C, Ribéron J, Siberan I,
Declercq B and Ledrans M: August 2003 heat wave in France: Risk
factors for death of elderly people living at home. Eur J Public
Health. 16:583–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patz JA, Campbell-Lendrum D, Holloway T
and Foley JA: Impact of regional climate change on human health.
Nature. 438:310–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bouchama A, Roberts G, Al Mohanna F,
El-Sayed R, Lach B, Chollet-Martin S, Ollivier V, Al Baradei R,
Loualich A, Nakeeb S, et al: Inflammatory, hemostatic and clinical
changes in a baboon experimental model for heatstroke. J Appl
Physiol (1985). 98:697–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buckley IK: A light and electron
microscopic study of thermally injured cultured cells. Lab Invest.
26:201–209. 1972.PubMed/NCBI
|
|
6
|
Sakaguchi Y, Stephens LC, Makino M, Kaneko
T, Strebel FR, Danhauser LL, Jenkins GN and Bull JM: Apoptosis in
tumors and normal tissues induced by whole body hyperthermia in
rats. Cancer Res. 55:5459–5464. 1995.PubMed/NCBI
|
|
7
|
Chen CM, Hou CC, Cheng KC, Tian RL, Chang
CP and Lin MT: Activated protein C therapy in a rat heat stroke
model. Crit Care Med. 34:1960–1966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu KC, Wang JY, Lin SH, Chu P and Lin YF:
Role of circulating cytokines and chemokines in exertional
heatstroke. Crit Care Med. 32:399–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abd-El-Aleem SA, Ferguson MW, Appleton I,
Bhowmick A, McCollum CN and Ireland GW: Expression of
cyclooxygenase isoforms in normal human skin and chronic venous
ulcers. J Pathol. 195:616–623. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeong HJ, Hong SH, Park RK, Shin T, An NH
and Kim HM: Hypoxia-induced IL-6 production is associated with
activation of MAP kinase, HIF-1 and NF-kappaB on HEI-OC1 cells.
Hear Res. 207:59–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inoue K, Takano H, Sato H, Yanagisawa R
and Yoshikawa T: Protective role of urinary trypsin inhibitor in
lung expression of proinflammatory cytokines accompanied by lethal
liver injury in mice. Immunopharmacol Immunotoxicol. 31:446–450.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang W, Huang W, Chen S, Li Z, Wang W and
Wang M: Changes of tumor necrosis factor-alpha and the effects of
ulinastatin injection during cardiopulmonary cerebral
resuscitation. J Huazhong Univ Sci Technolog Med Sci. 24:269–271.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bae HB, Jeong CW, Li M, Kim HS and Kwak
SH: Effects of urinary trypsin inhibitor on
lipopolysaccharide-induced acute lung injury in rabbits.
Inflammation. 35:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Liu F, Liu H, Cheng H, Wang W,
Wen Q and Wang Y: Urinary trypsin inhibitor attenuates
lipopolysaccharide-induced acute lung injury by blocking the
activation of p38 mitogen-activated protein kinase. Inflamm Res.
60:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng CY, Zhang W and Liang YG:
Inflammatory factor level and ulinastatin intervention in the early
stage of heat stress in rats. J Med Postgraduates. 24:25–28.
2011.
|
|
16
|
Adams JM and Cory S: Life-or-death
decisions by the Bcl-2 protein family. Trends Biochem Sci.
26:61–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hetz C: BCL-2 protein family: Essential
regulators of cell death. Preface. Adv Exp Med Biol. 687:vii–viii.
2010.PubMed/NCBI
|
|
18
|
Reed JC: Regulation of apoptosis by bcl-2
family proteins and its role in cancer and chemoresistance. Curr
Opin Oncol. 7:541–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hernandez JF, Secrest JA, Hill L and
McClarty SJ: Scientific advances in the genetic understanding and
diagnosis of malignant hyperthermia. J Perianesh Nurs. 24:19–34.
2009. View Article : Google Scholar
|
|
20
|
Wang HM, Bodenstein M and Markstaller K:
Overview of the pathology of three widely used animal models of
acute lung injury. Eur Surg Res. 40:305–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin ZS, Tian P, Wu X, Yu HM and Guo N:
Effects of ulinastatin administered at different time points on the
pathological morphologies of the lung tissues of rats with
hyperthermia. Exp Ther Med. 7:1625–1630. 2014.PubMed/NCBI
|
|
22
|
Roberts GT, Ghebeh H, Chishti MA,
Al-Mohanna F, El-Sayed R, Al-Mohanna F and Bouchama A:
Microvascular injury, thrombosis, inflammation and apoptosis in the
pathogenesis of heatstroke: A study in baboon model. Arterioscl
Throm Vasc Biol. 28:1130–1136. 2008. View Article : Google Scholar
|
|
23
|
Xu M, Wen X, Chen S, An X and Xu H:
Addition of ulinastatin to preservation solution promotes
protection against ischemia-reperfusion injury in rabbit lung. Chin
Med J (Engl). 124:2179–2183. 2011.PubMed/NCBI
|
|
24
|
Hua G, Haiping Z, Baorong H and Dingjun H:
Effect of ulinastatin on the expression of iNOS, MMP-2 and MMP-3 in
degenerated nucleus pulposus cells of rabbits. Connect Tissue Res.
54:29–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tong Y, Tang Z, Yang T, Yang Y, Yang L,
Shen W and Chen W: Ulinastatin preconditioning attenuates
inflammatory reaction of hepatic ischemia reperfusion injury in
rats via high mobility group box 1 (HMGB1) inhibition. Int J Med
Sci. 11:337–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu JB and Yao SL: Protective effects of
hemin pretreatment combined with ulinastatin on septic shock in
rats. Chin Med J (Engl). 121:49–55. 2008.PubMed/NCBI
|
|
27
|
Tsutakawa S, Kobayashi D, Kusama M, Moriya
T and Nakahata N: Nicotine enhances skin necrosis and expression of
inflammatory mediators in a rat pressure ulcer model. Brit J
Dermatol. 161:1020–1027. 2009. View Article : Google Scholar
|
|
28
|
Kang YJ, Wingerd BA, Arakawa T and Smith
WL: Cyclooxygenase-2 gene transcription in a macrophage model of
inflammation. J Immunol. 177:8111–8122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiu X, Ji S, Wang J, Li H, Xia T, Pan B,
Xiao S and Xia Z: The therapeutic efficacy of Ulinastatin for rats
with smoking inhalation injury. Int Immunopharmacol. 14:289–295.
2012. View Article : Google Scholar : PubMed/NCBI
|