Introduction
Wilson's disease (WD) is an autosomal genetic
disease in which the ATP7B gene mutation is localized on chromosome
13 (1,2). This is known to be the key gene that
leads to copper metabolism discharge barriers (1–3). Thus,
copper accumulates mainly in the liver, kidney, head and retina and
causes abnormal liver function, renal dysfunction and degeneration
of the nervous system (4,5). Moreover, widespread copper accumulation
in the central nervous system results in cognitive decline,
extrapyramidal change and also pyramidal dysfunction (6).
Brain magnetic resonance imaging (MRI) studies of WD
patients reveals that the lesions generally appeared to be
hyperintense on T2-weighted and hypointense on T1-weighted images
(7). It is associated with an
increased water content in the brain. Currently available MR
techniques, and susceptibility-weighted imaging (SWI) has been
demonstrated to be a more sensitive means of detecting mineral
deposits (of manganese, iron and copper compared to other
conventional MR imaging (8,9). Moreover, the lesions of WD patients
mainly include copper deposition. The present study reports a case
of newly diagnosed WD in order to characterize the patterns of
paramagnetic signal abnormalities by SWI.
Case report
A case of a 35-year-old woman was admitted to the
Fujian Medical University Union Hospital (Fuzhou, China) due to
drinking bucking (bulbar paralysis) and dysphagia in March 2012.
The patient was diagnosed with WD for nine years, and she had no
history of underlying disease.
Furthermore, the patient initially had auditory
hallucinations, delusions, disorganized speech and thinking. She
was initially diagnosed with schizophrenia in Fuzhou Fourth
Hospital (Fuzhou, China) in February 2001 and was the treated for
it. However, the patient gradually presented gait disturbance,
dysarthria, joint rigid dystonia and risus sardonicus, and she
visited Fuzhou Fourth Hospital again in July 2002 where she was
diagnosed with WD and was treated with penicillamine (PCA) for
three months. However, the patient's neurological symptoms did not
improve. Between 2003 and 2005, she had been hospitalized for two
months per year and had been treated with PCA (250 mg, four times
per day) and zinc (50 mg, three times per day). Furthermore, she
had also received Madopar (0.125 mg, three times per day) and
Artane (2 mg, three times per day) for gait disturbance and
dysdipsia aggratate.
Between 2006 and 2008, the patient was treated with
PCA, zinc, Madopar and Artane, but between 2009 and 2010, she did
not receive any medication. Moreover, the ceruloplasmin levels were
found to be normal in 2010. In 2011, the patient experienced
symptoms of drinking bucking and visited the Affiliated Union
Hospital, where she undertook a brain magnetic resonance imagine
(MRI) examination, and her ceruloplasmin levels were measured.
Physical examinations showed that she had a temperature of 36.5°C,
heart rate of 80 beats/min and blood pressure of 110/80 mmHg.
Furthermore, the patient presented mild dysarthria and rigid
dystonia. The patient's abdomen was also soft but not tender,
without organomegaly and no edema was identified. Laboratory
results are shown in Table I. The
results were negative for the serum antinuclear antibody, hepatitis
B surface antigen, hepatitis B core antibody and hepatitis C
antibody. In addition, the serum ceruloplasmin level was <2
mg/dl. An MRI revealed an abnormal hypointensity of the liver on
the T2-weighted images. Finally, the patient was diagnosed with WD,
and her gene was identified to have a mutation in the C-to-T
transition (Thr935Met) and the C-to-A transition (Pro992Leu).
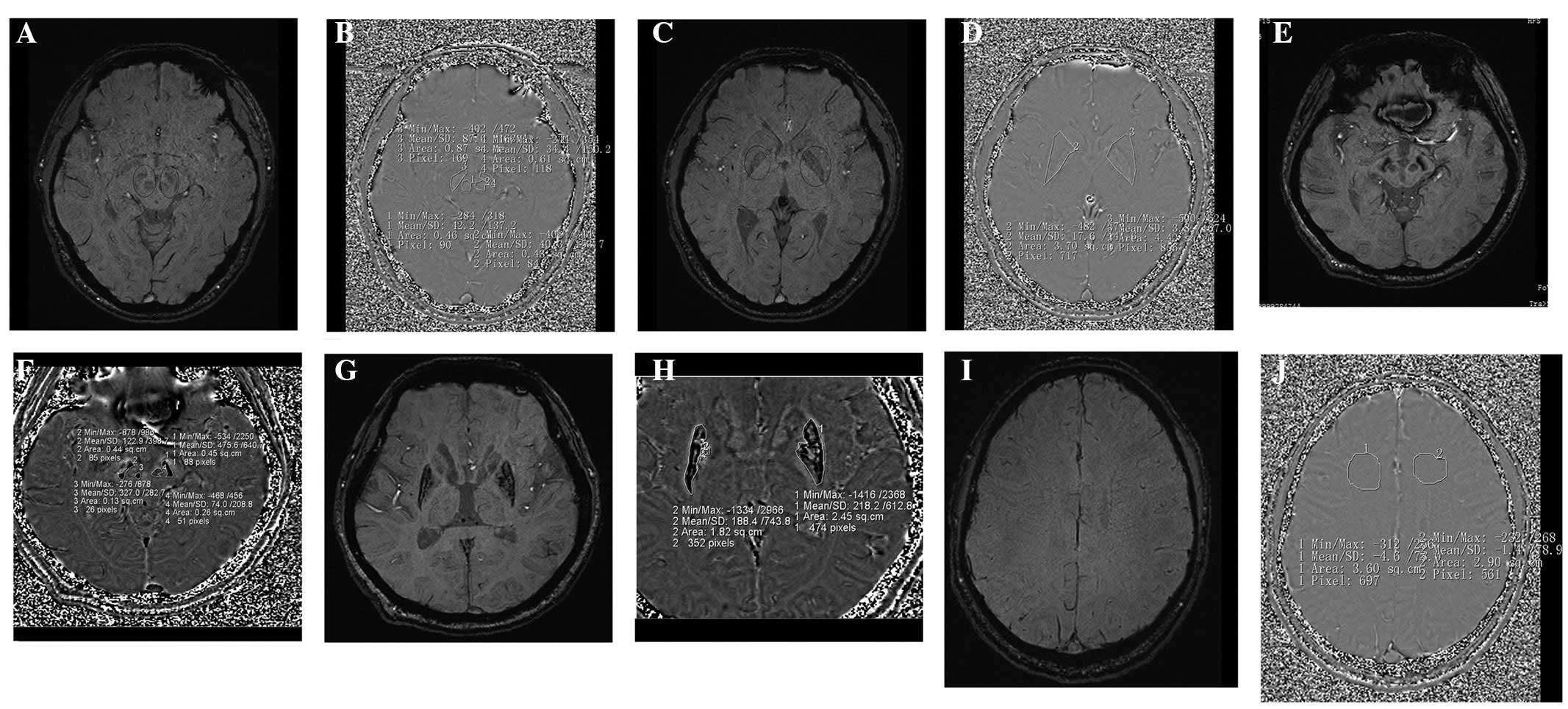
Furthermore, an SWI of the brain was captured and high signal
intensities were apparent in the bilateral substantia nigra and
lenticular nuclei (Fig. 1). Informed
consent was obtained from patients.
 | Table I.Laboratory data on admission. |
Table I.
Laboratory data on admission.
| Characteristic | Value |
|---|
| Hemoglobin (g/l) | 128.0 |
| White blood cells
(cells/l) |
3.8×109 |
| Neutrophils (%) | 70.1 |
| Blood platelets
(platelets/l) |
175×109 |
| Ceruloplasmine
(g/l) | 0.23 |
| Proteinuria | 1+ |
| Albuminuria | (−) |
| Fibrinogen (g/l) | 4.23 |
| AST (IU/l) | <40 |
| ALT (IU/l) | <40 |
Discussion
SWI is used to demonstrate the magnetic
susceptibility difference between the magnetic material tissue and
non-containing magnetic material tissue (10,11).
More specifically, the corrected phase imaging of the filter can
directly reflect the local magnetic field inhomogeneity that is
caused by the change of the proton spin phase (10,12).
Recently, SWI imaging has been applied to mostly study Parkinson's
disease (PD), and the increase of iron deposition in the substantia
nigra in PD patients (13,14). The magnetic susceptibility of the
substantia nigra was likely to change due to the high content of
iron. Furthermore, the signal of SWI was low in healthy individuals
(15). Therefore,a SWI signal may
indicate changes in regional cerebral iron. However, there is not
much research on the of correlation between SWI and WD. To the best
of our knowledge, the present WD patient who had the
Thr935Met/Pro992Leu compound heterozygote mutation in the ATP7B
gene is the first to show intra-cerebral paramagnetric signal
abnormalities on SWI.
SWI is very sensitive to mineral deposits and has
been used as a relatively simple, safe, noninvasive in vitro
examination of the deposition of iron metabolism (10,16).
Moreover, an SWI scan of the brain is not only used to observe the
iron deposition, but also as a sensitive method for quantification
(9,15). Abnormal iron deposition is detected
before the appearance of clinical symptoms, and it provides
information on the pathogenesis (11,12,16).
Furthermore, it is an important and novel method for detecting
abnormal iron metabolism in a high-risk population (11,16). WD
patients are known to have an abnormal copper metabolism but
whether they also have iron metabolism abnormalities has not yet
been determined (17).
Bruehlmeier et al (18) demonstrates that the uptake of
radioactive 52Fe significantly increased in the pia mater of PD
patients from plasma into the brain tissue compared to the uptake
in healthy individuals. However, the result is not clear and needs
further investigation on WD patients with abnormal brain iron
metabolism mechanism. In the present case, the lenticular nucleus,
substantia nigra and red nucleus have an abnormal SWI signal, and
the phase value of corrected phase imaging in WD (Fig. 1F and H) is more than that of a
healthy patient (Fig. 1B and D). The
results indicate that in LN/SN/RN there were paramagnetic mineral
deposits.
Finally, the case of the present study demonstrates
that the SWI hyperintense area lenticular nucleus, substantia nigra
and red nucleus in combination with the patient's symptoms
indicated a possibility of diagnosing WD when the gene had not been
detected. Moreover, it demonstrates that systemic mineral removal
treatment, including manganese, iron and copper, may be successful
for the primary treatment of WD.
As it is only one case presented here, the
conclusions of the present study are limited and further
complementary studies involving a larger number of cases are
required.
Acknowledgements
The present study was supported by the Fujian
Provincial Health and Family Planning Commission Youth Research
Project (grant no. 2015-1-32) and the Key Clinical Specialty
Discipline Construction Program of Fujian and Nation in China.
References
|
1
|
Tanzi RE, Petrukhin K, Chernov I,
Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L,
Brzustowicz LM, et al: The Wilson disease gene is a copper
transporting ATPase with homology to the Menkes disease gene. Nat
Genet. 5:344–350. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
La Fontaine S and Mercer JFB: Trafficking
of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis.
Arch Biochem Biophys. 463:149–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
La Fontaine S, Ackland ML and Mercer JF:
Mammalian copper-transporting P-type ATPases, ATP7A and ATP7B:
Emerging roles. Int J Biochem Cell Biol. 42:206–209. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosencrantz R and Schilsky M: Wilson
disease: Pathogenesis and clinical considerations in diagnosis and
treatment. Semin Liver Dis. 31:245–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu F, Wang J, Pu C, Qiao L and Jiang C:
Wilson's disease: a comprehensive review of the molecular
mechanisms. Int J Mol Sci. 16:6419–6431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loudianos G, Lepori MB, Mameli E, Dessì V
and Zappu A: Wilson's disease. Pril (Makedon Akad Nauk Umet Odd Med
Nauki). 35:93–98. 2014.PubMed/NCBI
|
|
7
|
Kozic D, Svetel M, Petrovic B, Dragasevic
N, Semnic R and Kostic VS: MR imaging of the brain in patients with
hepatic form of Wilson's disease. Eur J Neurol. 10:587–592. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mittal S, Wu Z, Neelavalli J and Haacke
EM: Susceptibility-weighted imaging: Technical aspects and clinical
applications, part 2. AJNR Am J Neuroradiol. 30:232–252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haacke EM, Cheng NY, House MJ, Liu Q,
Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W and Obenaus A:
Imaging iron stores in the brain using magnetic resonance imaging.
Magn Reson Imaging. 23:1–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haacke EM, Xu Y, Cheng YC and Reichenbach
JR: Susceptibility weighted imaging (SWI). Magn Reson Med.
52:612–618. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barnes SR and Haacke EM:
Susceptibility-weighted imaging: clinical angiographic
applications. Magn Reson Imaging Clin N Am. 17:47–61. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sehgal V, Delproposto Z, Haacke EM, Tong
KA, Wycliffe N, Kido DK, Xu Y, Neelavalli J, Haddar D and
Reichenbach JR: Clinical applications of neuroimaging ith
susceptibility-weighted imaging. J Magn Reson Imaging. 22:439–450.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Zhang Y, Wang J, Cai P, Luo C,
Qian Z, Dai Y and Feng H: Characterizing iron deposition in
Parkinson's disease using susceptibility-weighted imaging: An in
vivo MR study. Brain Res. 1330:124–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wallis LI, Paley MN, Graham JM, Grünewald
RA, Wignall EL, Joy HM and Griffiths PD: MRI assessment of basal
ganglia iron deposition in Parkinson's disease. J Magn Reson
Imaging. 28:1061–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haacke EM, Ayaz M, Khan A, Manova ES,
Krishnamurthy B, Gollapalli L, Ciulla C, Kim I, Petersen F and
Kirsch W: Establishing a baseline phase behavior in magnetic
resonance imaging to determine normal vs. abnormal iron content in
the brain. J Magn Reson Imaging. 26:256–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haddar D, Haacke E, Sehgal V, Delproposto
Z, Salamon G, Seror O and Sellier N: Susceptibility weighted
imaging: Theory and applications. J Radiol. 85:1901–1908. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hingwala DR, Kesavadas C, Thomas B and
Kapilamoorthy TR: Susceptibility weighted imaging in the evaluation
of movement disorders. Clin Radiol. 68:e338–e348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bruehlmeier M, Leenders KL, Vontobel P,
Calonder C, Antonini A and Weindl A: Increased cerebral iron uptake
in Wilson's disease: A 52Fe-citrate PET study. J Nucl Med.
41:781–787. 2000.PubMed/NCBI
|