Introduction
Epstein-Barr virus (EBV) is a human gamma herpes
virus that can exist in humans for a long time without producing
any symptoms (1). A variety of human
malignancies such as Burkitt's lymphoma, nasopharyngeal carcinoma
and gastric cancer (GC) are reported to be associated with EBV
(2). EBV-associated gastric
carcinoma (EBVaGC) accounts for 10–18% of gastric carcinoma cases,
and there are estimated to be >75,000 new cases of EBVaGC each
year worldwide (3,4). As a type of GC, which is one of the
most common types of malignant tumors, EBVaGC is very difficult to
remove and the complete elimination of tumor cells by surgical,
radiotherapeutic and chemotherapeutic methods is challenging.
Therefore, the development of new therapeutic approaches for the
inhibition of tumor cell growth or survival to treat EBVaGC is of
critical importance.
EBVaGC is closely associated with EBV infection, and
EBVaGC cells have been found to express a well-defined set of
latent viral genes, including latent membrane protein 2A
(LMP2A) (5). It has been
revealed that ~40% EBVaGC expresses LMP2A, whose expression
closely correlates with a poor survival outcome (6). LMP2A consists of a 27-amino-acid
carboxy-terminal cytoplasmic domain, a 119-amino-acid tyrosine-rich
amino-terminal cytoplasmic domain and 12 hydrophobic transmembrane
domains, with the cytoplasmic domain playing a role as a signaling
effector (7). LMP2A has
various functions, one of which is to activate the phosphoinositide
3-kinase (PI3K)/Akt, nuclear factor (NF)-κB, β-catenin, signal
transducers and activators of transcription (STAT) and Syk tyrosine
kinase pathways in epithelial cells (8–10).
LMP2A also plays an important role in cell transformation
activities, such as the induction of cell growth, enhancement of
cell adhesion and cell motility, as well as the inhibition of
epithelial cell differentiation (11,12).
Thus, the aforementioned findings indicate that LMP2A may be
a potential target for gene therapy for EBVaGC treatment.
RNA interference (RNAi) is an efficient tool that
can cause post-transcriptional silencing of gene expression and
induce loss-of-function phenotypes (13). Lentivirus vectors have been developed
to be a powerful technology for the achievement of a significant
level of gene transfer in vitro (14). In the present study,
lentivirus-mediated RNAi was used to inhibit LMP2A gene
expression, and the effects of LMP2A-silencing on cell
growth, cell cycle and cell apoptosis of EBVaGC cell line GT38 were
studied.
Materials and methods
Cell line and culture
The EBV-positive human gastric carcinoma cell line
GT38 was bought from American Type Culture Collection (Manassas,
VA, USA). Cells were cultured in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; ExCell Bio, Shanghai, China), 50 U/ml penicillin
G and 50 U/ml streptomycin (Gibco) in a humidified atmosphere of 5%
CO2 at 37°C. The medium was changed every 2 days and the
cell line was passaged every 4–5 days.
Construction of lentivirus
vectors
In order to produce lentivirus expressing RNAi
specific for the LMP2A gene (GU979791), RNAi Designer
software (rnaidesigner.thermofisher.com/rnaiexpress/;
BLOCK-iTTM RNAi; Thermo Fisher Scientific, Inc.) was used to
identify the RNAi sequence for human LMP2A
(CTCCCAATATCCATCTGCT), and then a scrambled sequence
(TTCTCCGAACGTGTCACGT) was created as a negative control construct
(control RNAi) that should have no homology with the human genome.
DNA oligos with the target sequence were chemically synthesized,
annealed, double digested with AgeI and EcoRI, and inserted into
the pLenR-GPH expression vector (GeneChem Co., Ltd., Shanghai,
China) using T4 DNA ligase (Invitrogen; Thermo Fisher Scientific,
Inc.), following the manufacturer's guidelines. The ligated vector
was transformed into competent Escherichia coli DH5a cells
(Invitrogen; Thermo Fisher Scientific, Inc.). Restriction enzyme
analysis and DNA sequencing were performed to identify the correct
transformant. The sequences were cloned into the pGCSIL-Green
Fluorescent Protein (GFP) vector (GeneChem Co., Ltd.) to generate
lentivirus vectors. 293T cells (Shanghai Research Institute of
Chinese Academy of Sciences, Beijing, China) were used to generate
lentiviruses after their transfection into the expression vectors
and package vectors with the use of Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.). After 48 h, supernatants containing the
lentiviruses pGCSIL-LMP2A-shRNA-LV and pGCSIL-neg-shRNA-LV were
harvested and the remaining cells were removed by filtering with
0.45 µm filters. Ultracentrifugation (4,000 × g at 4°C for
10 min) was then performed to concentrate the lentiviruses and the
titer was finally determined by 293T cell infection assay.
Infection of lentivirus
In this assay, 5×103 GT38 cells in the
logarithmic growth phase were seeded in each well of a 96-well
microplate and cultured overnight. The lentiviruses were then
diluted with 0.2 ml RPMI complete medium containing Polybrene (10
µg/ml) and added to infect the seeded cells for 12 h at 37°C. The
virus-containing medium was then changed with fresh culture medium.
Fluorescence microscopy (IX-53; Olympus Corporation, Tokyo, Japan)
was used to detect GFP in the successfully infected cells, and the
percentage of GFP-positive cells was used to measure the infection
efficiency of the cells. At 5 days after the infection, analysis of
LMP2A expression, cell proliferation and cell apoptosis was
performed. GT38 cells treated differently were divided into three
groups in subsequent assays: Blank control group (CON group; cells
without infection), negative control (NC group; cells were infected
with pGCSIL-neg-shRNA-LV) and the LMP2A knockdown group (KD
group; cells were infected with pGCSIL-LMP2A-shRNA-LV).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Shanghai, China) and reverse transcribed using
the SuperScript III First-strand Synthesis System kit (cat. no.
18080051; Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. PCR amplification was carried out
using Platinum SYBR Green qPCR SuperMix UDG (Invitrogen). The
reaction volume contained DNA Polymerase 10X buffer (2 µl),
MgCl2 (1.5 mM), dNTP mix (2.5 mM), 10 pmol each primer,
DNA template (1 µg), Taq DNA Polymerase (2–3 µl) and nuclease-free
water to a final volume of 20 µl. The integrity of RNA was detected
by the parallel amplification of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) mRNA. The specific primer pairs were
as follows: LMP2A (280 bp), sense: ATGACTCATCTCAACACATA and
antisense: CATGTTAGGCAAATTGCAA; GAPDH (450 bp), forward:
5′-CTCAGACACCATGGGGAAGGTGA-3′ and reverse:
5′-ATGATCTTGAGGCTGTTGTCATA-3′. The following thermal cycling
conditions were used: 95°C for 5 min, followed by 40 cycles of 95°C
for 15 sec, 60°C for 15 sec and 72°C for 1 min, and the final
extension was 72°C for 5 min. The 2−ΔΔCq method was
applied to analyze the data (15).
Western blot analysis
Protein was extracted from the cells using lysis
buffer (150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet
P-40, and 50 mM Tris, pH 8.0), with the addition of 2 mM
phenylmethylsulfonyl fluoride. The six-well plates of
lentivirus-infected cells were put on ice, freshly prepared lysis
buffer (100 µl/well) was added and the cells were incubated for 10
min. A protein assay kit was used to determine the protein
concentration. Equal amounts of protein samples (10 µg per
condition) were prepared in loading buffer and boiled for 10 min,
then the boiled samples were loaded to 15% SDS-PAGE gels and
separated. Separated proteins were transferred from the gel to
polyvinylidene difluoride (PVDF) membranes at 100 V for 1 h. The
PVDF membrane with protein samples was blocked using blocking
buffer which was freshly prepared with PBS containing 5% skimmed
milk powder. After blocking, anti-LMP2A (cat. no. ab59026;
Abcam, Cambridge, UK) and anti-GAPDH (cat. no. 5174; Cell Signaling
Technology, Inc., Danvers, MA, USA) primary antibodies and
anti-rabbit horseradish-peroxidase (HRP)-conjugated IgG (cat. no.
7071; Cell Signaling Technology, Inc.) and anti-mouse
HRP-conjugated IgG (cat. no. 7072; Cell Signaling Technology, Inc.)
secondary antibodies were diluted to 1:1,000 concentration with PBS
and Tween 20 (PBST) buffer before they were successively incubated
with the PVDF membrane for 60 min at room temperature. Prior to
each step, the PVDF membrane was washed 3 times with PBST. Protein
bands were then detected using enhanced chemiluminescence (Pierce
ECL Substrate; Thermo Fisher Scientific, Inc.), and gel analysis
was performed using Image J software (version 1.8.0; National
Institutes of Health, Bethesda, MD, USA).
Proliferation assay
Cell proliferation was measured using a Cell
Counting kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). After lentivirus-infected cells had been seeded
in 96-well plates for 20, 44, 68, 92 or 116 h, 10 µl CCK-8 solution
was added to each well and incubated for 1 h at 37°C. Absorbance
was measured at 490 nm using an enzyme immunoassay analyzer (model
680; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Colony formation assay
A colony formation assay was performed to analyze
the effect of LMP2A silencing on the colony formation
ability of GT38 cells. In this assay, 8×102 cells were
seeded in 6-cm dishes and cultured with RPMI-1640 supplemented with
10% FBS in an atmosphere of 5% CO2 and 95% humidity at
37°C. After 2 weeks, the cell colonies were washed twice with PBS,
and were fixed with 4% paraformaldehyde for 15 min. The fixed
colonies were then stained with Giemsa for 20 min, and washed twice
with ddH2O. The colonies that consisted of ≥50 cells
were manually counted.
Cell cycle analysis
Cells were harvested with 0.25% trypsin, fixed with
70% ethanol, and then resuspended in 20 mg/ml propidium iodide
(PI). Flow cytometry (FCM) using a FACSCalibur™ flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) was used to detect DNA
content, and the FCM data were used to determine the relative
proportions of cells in the individual cell-cycle phases.
Apoptosis assay
In order to differentiate intact cells from
apoptotic cells, the cells were stained with Annexin V-fluorescein
isothiocyanate (FITC) and PI. Briefly, a total of
1.0×106 cells were washed twice with ice-cold PBS and
put into binding buffer (5 µl Annexin V-FITC and 5 µl PI) for a 30
min incubation. Finally, FCM was used to analyze the Annexin
V-FITC/PI staining.
Statistical analysis
All experiments were performed in triplicate, and
data are shown as the mean ± standard deviation where applicable.
Statistically significant differences between the control and
treatment groups were determined by one-way analysis of variance
followed by Tukey's test using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA), and P<0.05 was considered to indicate a
statistically significant result.
Results
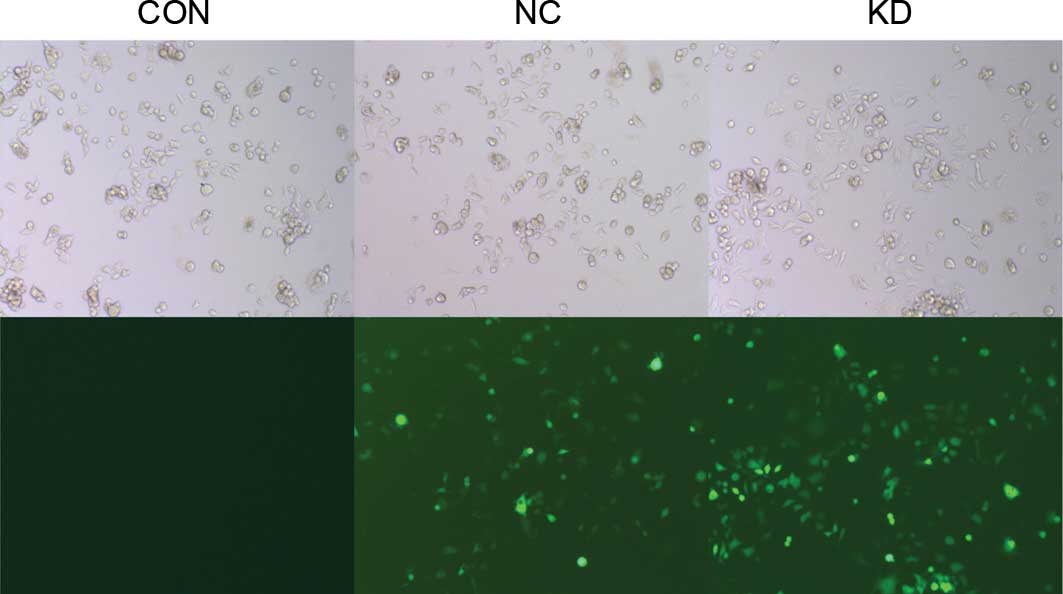
Successfully construction of
LMP2A-RNAi-LV
A lentivirus vector system derived from HIV-1 was
used to knock down the LMP2A gene by expressing short
hairpin RNAs (shRNAs) directed against LMP2A.
pGCSIL-LMP2A-shRNA-LV and pGCSIL-neg-shRNA-LV were
constructed successfully, carrying GFP as a reporter gene. When
GT38 cells were infected once with the constructed lentiviruses,
>95% of the infected GT38 cells expressed GFP 72 h after the
infection (Fig. 1), indicating that
highly efficient and stable lentivirus vectors had been
successfully constructed.
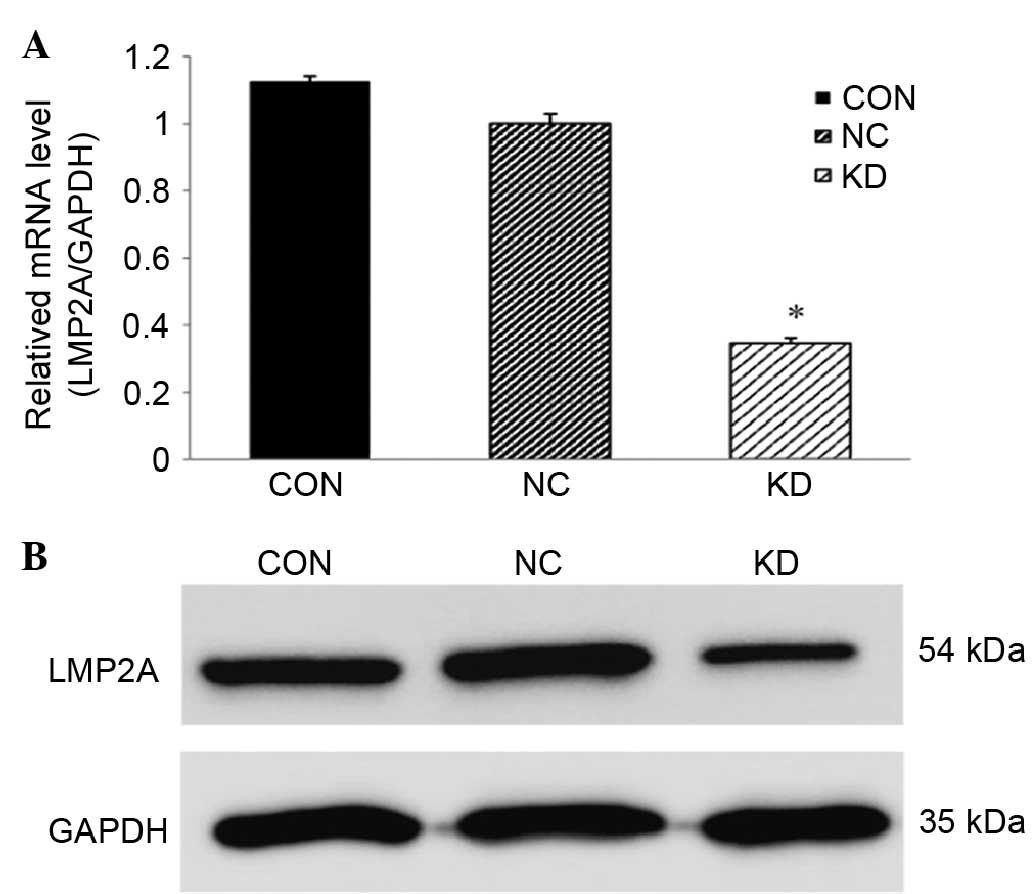
LMP2A-RNAi-LV downregulates LMP2A
expression in GT38 cells
The expression of LMP2A in GT38 cells was
examined by RT-qPCR and western blot analysis following infection
with LMP2A-specific RNAi-expressing lentivirus. Compared
with the NC group, the mRNA and protein levels of LMP2A in
the KD group were significantly reduced (P<0.05, n=6; Fig. 2). The mRNA level was decreased by
65.4%, while the protein level was reduced by 50.8%. The CON group
exhibited no significant difference compared with the NC group.
These data show that the expression of the LMP2A gene in
GT38 cells was efficiently downregulated following the lentivirus
infection.
Knockdown of LMP2A inhibits the
proliferation and clonogenicity of GT38 cells
To determine the effect of downregulation of
LMP2A on cell growth in vitro, a cell proliferation
assay was performed after LMP2A RNAi using the CCK-8 method.
The results show that cell proliferation in the KD group was
inhibited (Fig. 3A), compared with
that in the CON and NC groups. A colony formation assay was then
performed to confirm the inhibitory effect of LMP2A RNAi.
The results show that when compared with the CON and NC groups, the
colony formation ability of LMP2A RNAi cells was
significantly decreased (P<0.05, n=6; Fig. 3B and C), suggesting that knockdown of
the LMP2A gene results in the inhibition of cell growth.
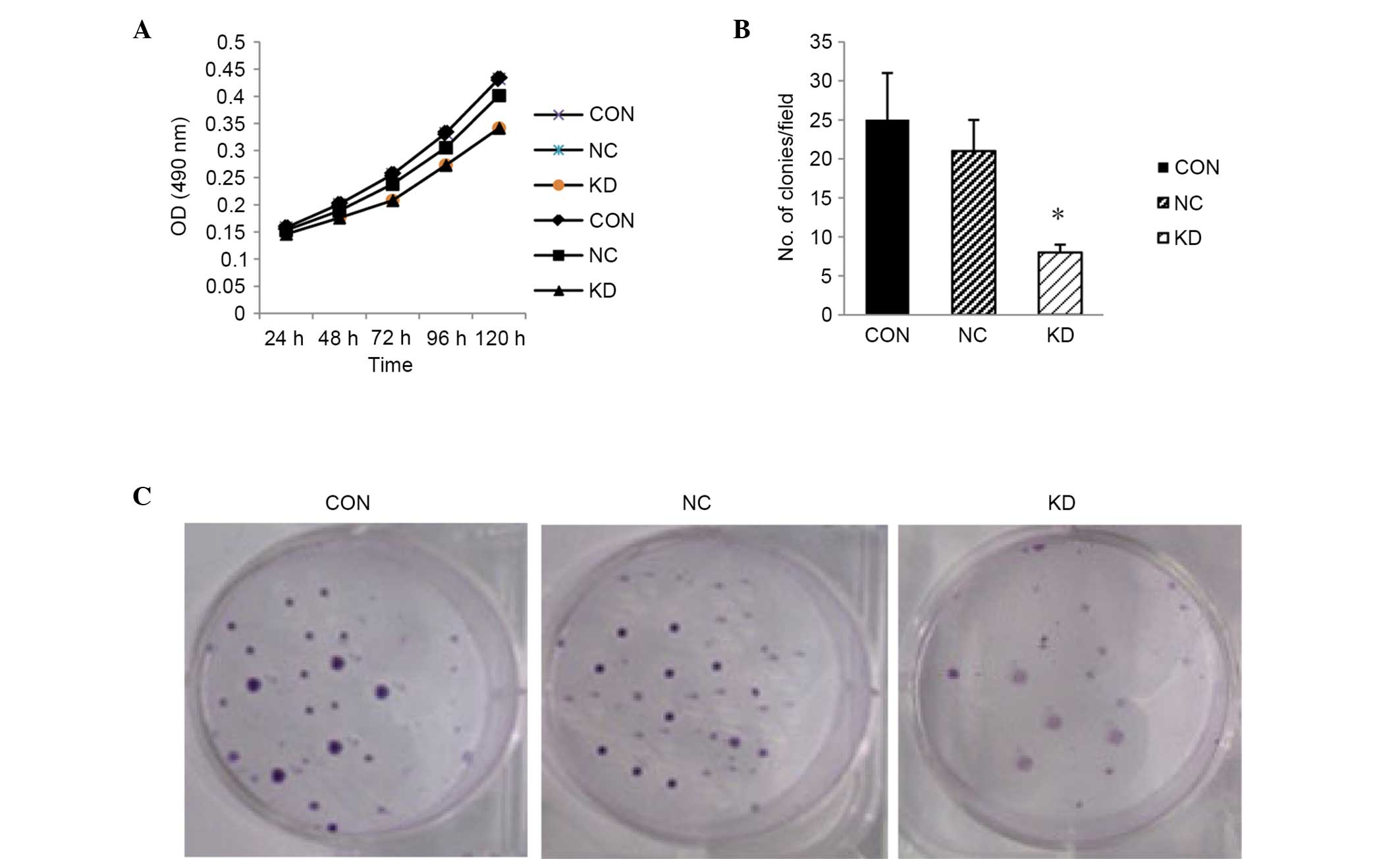 | Figure 3.Suppression of cell growth in
LMP2A RNAi-treated GT38 cells. (A) Cell proliferation was
determined by CCK-8 assay. Cell proliferation in the KD group was
inhibited at each time point (24, 48, 72, 96 and 120 h) compared
with the CON and NC groups. (B) Colony formation assay was
performed, and the cell proliferation inhibitory effect of
LMP2A RNAi was confirmed (*P<0.05 vs. CON and NC),
indicating that the clone number in the KD group was greatly
reduced after LMP2A RNAi. Data are presented as the mean
plus standard deviation (n=6). (C) Colonies of GT38 cells that were
cultured in 6-cm dishes for 2 weeks and stained with Giemsa.
LMP2A, latent membrane protein 2A; RNAi, RNA interference;
CCK-8, Cell Counting kit-8; CON, blank control; NC, negative
control; KD, LMP2A knockdown; OD, optical density. |
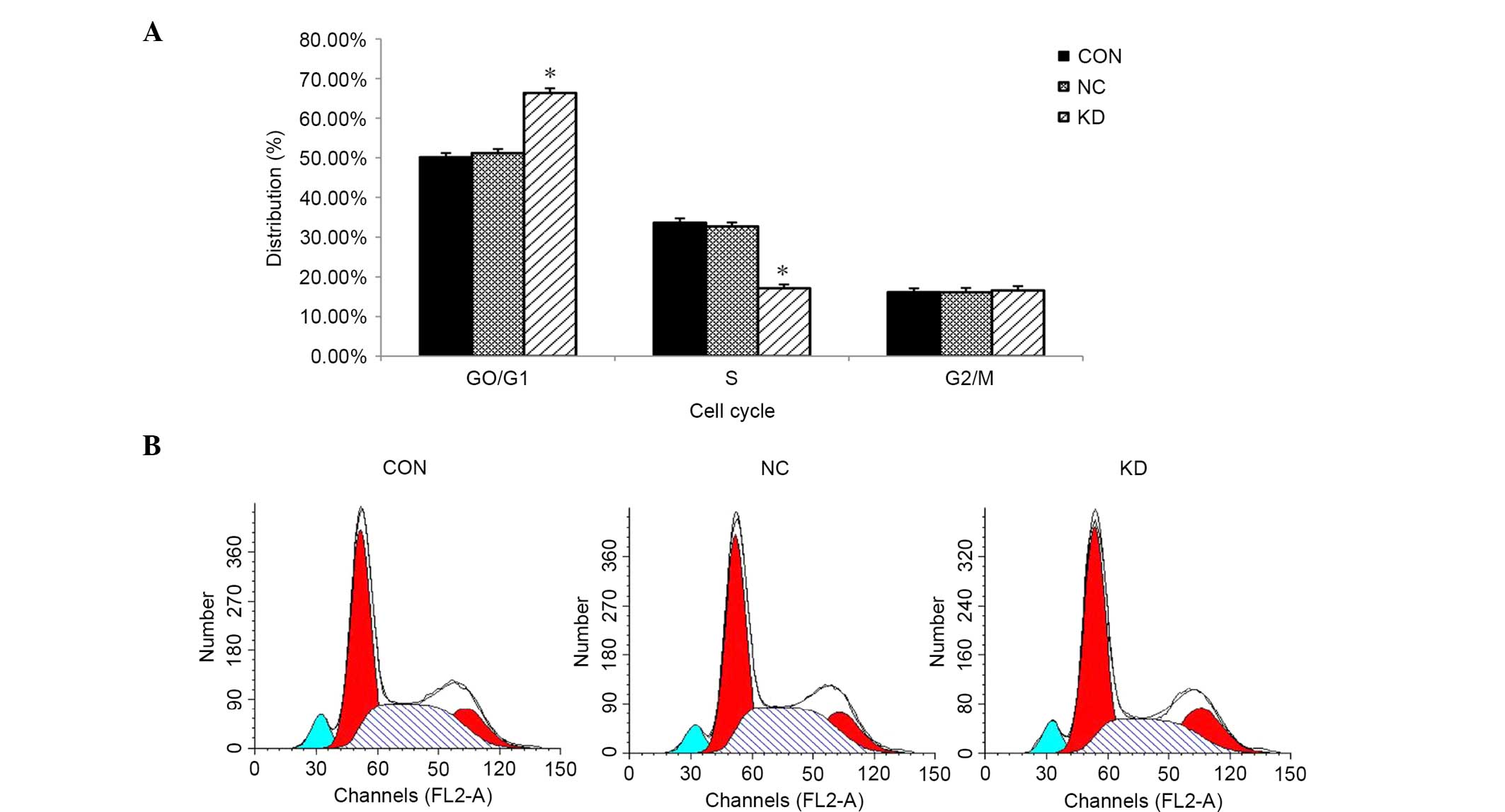
GT38 cells are arrested in the G0/G1
phase after knockdown of LMP2A
FCM analysis was conducted to evaluate whether
downregulation of LMP2A affects the cell cycle of GT38
cells. The results show an increase in the proportion of cells in
the G0/G1 phase in the KD group compared with the NC group
(66.35±1.18 vs. 51.19±1.07%, respectively; P<0.05, n=6; Fig. 4). Compared with the control group, a
greater proportion of LMP2A RNAi cells remained in the G0/G1
phase while fewer cells were in the S phase. The results indicate
that downregulation of LMP2A expression in GT38 cells
arrests their cell cycle in the G0/G1 phase.
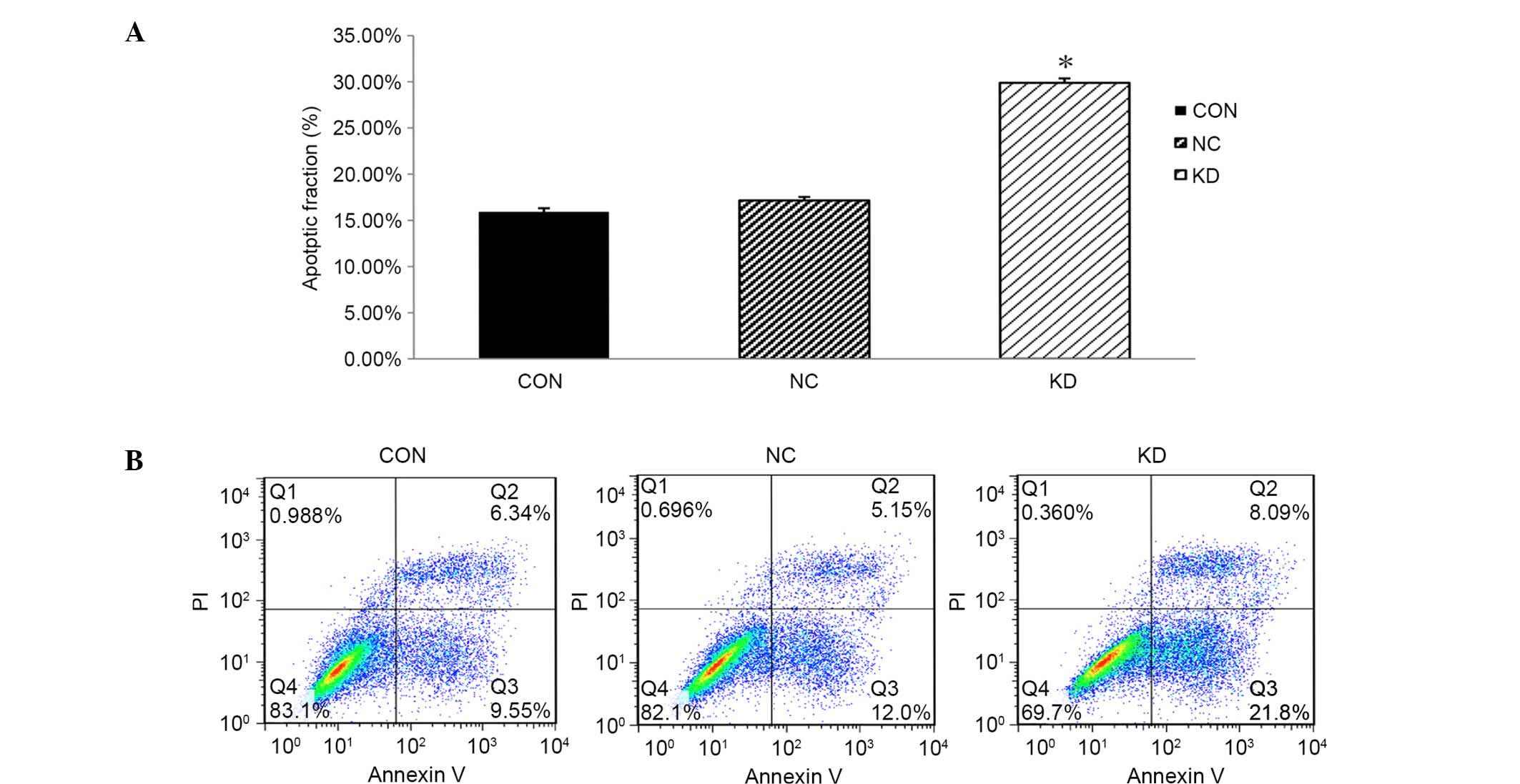
Knockdown of LMP2A induces apoptosis
of GT38 cells
FCM analysis was applied to evaluate the rate of
apoptosis, aiming to further investigate the effects of
downregulation of LMP2A expression on the cell death of GT38
cells. Apoptosis rates in the CON and NC groups were 15.89±0.41 and
17.15±0.39%, respectively, while the apoptosis rate of the KD group
was 29.89±0.48%; the percentage of apoptosis in the KD group was
significantly increased compared with that in the CON and NC groups
(P<0.05, n=6; Fig. 5). These data
indicate that downregulation of LMP2A significantly
increases the apoptosis rate of GT38 cells compared with that in
the control group, demonstrating that suppression of cell
proliferation may be caused by cell death resulting from
LMP2A RNAi.
Discussion
LMP2A has been detected in the majority of
EBVaGC samples (16). EBVaGC has
been reported constitute 10–18% of gastric carcinoma cases and has
a very poor survival outcome (17).
LMP2A has been found to play important roles in epithelial
cells, such as the enhancement of cell adhesion and cell motility,
inhibition of epithelial cell differentiation and induction of
anchorage-independent growth; these functions are reported to be
correlated with the activation of the PI3K/Akt, NF-κB, β-catenin,
STAT and Syk tyrosine kinase pathways (18–20). In
addition, LMP2A is also reported to be involved in the
regulation of the proliferation, apoptosis and self-renewal ability
of cancer stem cells (21). Through
the findings outlined above, LMP2A can be considered as a
potential target of gene therapy for EBVaGC treatment. However, the
detailed mechanism by which LMP2A expression downregulation
inhibits EBVaGC cell growth has not been clarified.
In the present study, an LMP2A-specific
RNAi-expressing lentivirus was constructed and used to infect the
human gastric carcinoma cell line GT38. This was conducted to
downregulate LMP2A expression, in order to investigate the
effects of LMP2A silencing on cell growth, the cell cycle
and cell apoptosis of EBVaGC cells. The results indicate that when
LMP2A is downregulated, the proliferation of GT38 cells is
significantly decreased, while the cell apoptosis is markedly
increased, and a greater proportion of GT38 cells are arrested in
the G0/G1 phase of the cell cycle. The inhibition of cell growth
may be caused by cell apoptosis resulting from LMP2A RNAi.
Although, the mechanisms by which downregulation of the
LMP2A gene results in growth inhibition and apoptosis
promotion in the GT38 cell line are not clear, there is evidence
that when LMP2A is activated, gastric carcinoma cells would
be protected from apoptosis and their proliferating ability
promoted, all of which are regulated by a variety of signaling
pathways (22–25) Thus, it is hypothesized that
LMP2A gene silencing suppresses cell proliferation of the
EBVaGC cell line GT38 following such mechanisms, and LMP2A
can be considered as a potential therapeutic target in the
treatment of EBVaGC. For further investigation, future studies may
be conducted to attempt to clarify the mechanisms by which
LMP2A modulate those pathways to affect cell biological
functions, which may indicate new methods for creating novel
therapies against EBVaGC.
RNAi has become a powerful tool for molecular
biological research with efficient ability for gene functional
analysis, and also can be considered as a potential therapeutic
strategy for various diseases, including cancers (26). RNAi is considered to be a novel
method for gene therapy against cancer, particularly when combined
with lentivirus. The exploration of target genes that when
downregulated could efficiently inhibit EBVaGC cell growth may
increase the possibility of clinical application of gene-silencing
therapy. During the present study, a highly efficient and stable
lentivirus vector system was successfully constructed, which
efficiently knocked down the LMP2A gene in the infected cell
line GT38. The results also revealed that after LMP2A was
knocked down by lentivirus-mediated RNAi, the cell proliferation
and growth potency of EBVaGC cells were decreased significantly.
Thus, a new strategy involving reduction of the expression of
LMP2A by lentivirus-mediated RNAi is provided with the
potential for treating human EBVaGC.
In conclusion, the results of the present study show
that the knockdown of LMP2A by lentivirus-mediated RNAi
significantly suppresses cell growth and induces apoptosis in the
EBVaGC cell line GT38. This provides an attractive anticancer
therapeutic strategy for the treatment of human EBVaGC.
Acknowledgements
This study was supported by the Key Scientific
Research Program of Wuxi Municipal Health Bureau (grant no.
Z201509), the Natural Science Foundation of Jiangsu Province (cat.
no. BK20161152), the National Natural Science Foundation of China
(grant nos. 31670857, 81672975, 81522039 and 31400720) and the Key
Research and Development Program of China (grant no.
2016YFC0904702).
References
|
1
|
Kieff E: Epstein-Barr virus and its
replicationFields Virology. Fields BN, Knipe DM and Howley PM: 3rd.
Lippincott-Raven; Philadelphia, PA: pp. 2343–2396. 1996
|
|
2
|
Shah KM and Young LS: Epstein-Barr virus
and carcinogenesis: Beyond Burkitt's lymphoma. Clin Microbiol
Infect. 15:982–988. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin JY, Kim JO, Lee SK, Chae HS and Kang
JH: LY294002 may overcome 5-FU resistance via down-regulation of
activated p-AKT in Epstein-Barr virus-positive gastric cancer
cells. Bmc Cancer. 10:4252010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takada K: Epstein-Barr virus and gastric
carcinoma. Mol Pathol. 53:255–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo JS, Jun SM, Kwon SW, Oh IH, Kim TG and
Lee SK: Establishment and characterization of gastric carcinoma
cell clones expressing LMP2A of Epstein-Barr virus. Int N Mol Med.
25:11–16. 2010.
|
|
6
|
Iizasa H, Nanbo A, Nishikawa J, Jinushi M
and Yoshiyama H: Epstein-Barr virus (EBV)-associated gastric
carcinoma. Viruses. 4:3420–3439. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukuda M and Longnecker R: Epstein-Barr
virus latent membrane protein 2A mediates transformation through
constitutive activation of the Ras/PI3-K/Akt Pathway. J Virol.
81:9299–9306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Lin WH, Chen SY, Longnecker R, Tsai
SC, Chen CL and Tsai CH: Syk tyrosine kinase mediates Epstein-Barr
virus latent membrane protein 2A-induced cell migration in
epithelial cells. J Biol Chem. 281:8806–8814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morrison JA and Raab-Traub N: Roles of the
ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A
in the inhibition of epithelial cell differentiation and activation
of {beta}-catenin signaling. J Virol. 79:2375–2382. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan YR, Vatsyayan J, Chang YS and Chang
HY: Epstein-Barr virus latent membrane protein 2A upregulates
UDP-glucose dehydrogenase gene expression via ERK and PI3K/Akt
pathway. Cell Microbiol. 10:2447–2460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allen MD, Young LS and Dawson CW: The
Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote
epithelial cell spreading and motility. J Virol. 79:1789–1802.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fotheringham JA, Coalson NE and Raab-Traub
N: Epstein-Barr virus latent membrane protein-2A induces ITAM/Syk-
and Akt-dependent epithelial migration through αV-integrin membrane
translocation. J Virol. 86:10308–10320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fire A, Xu SQ, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Rourke JP, Newbound GC, Kohn DB, Olsen
JC and Bunnell BA: Comparison of gene transfer efficiencies and
gene expression levels achieved with equine infectious anemia
virus- and human immunodeficiency virus type 1-derived lentivirus
vectors. J Virol. 76:1510–1515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han J, Chen JN, Zhang ZG, Li HG, Ding YG,
Du H and Shao CK: Sequence variations of latent membrane protein 2A
in Epstein-Barr virus-associated gastric carcinomas from Guangzhou,
southern China. PloS One. 7:e342762012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukayama M and Ushiku T: Epstein-Barr
virus-associated gastric carcinoma. Pathol Res Pract. 207:529–537.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda M and Longnecker R: Epstein-Barr
virus latent membrane protein 2A mediates transformation through
constitutive activation of the Ras/P13-K/Akt pathway. J Virol.
81:9299–9306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scholle F, Bendt KM and Raab-Traub N:
Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell
differentiation and activates Akt. J Virol. 74:10681–10689. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stewart S, Dawson CW, Takada K, Curnow J,
Moody CA, Sixbey JW and Young LS: Epstein-Barr virus-encoded LMP2A
regulates viral and cellular gene expression by modulation of the
NF-kappaB transcription factor pathway. Proc Natl Acad Sci USA.
101:15730–15735. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH,
Liang Y, Xiong D, Guan S, Guo BH, Mai HQ, et al: Epstein-Barr
virus-encoded LMP2A induces an epithelial-mesenchymal transition
and increases the number of side population stem-like cancer cells
in nasopharyngeal carcinoma. PloS Pathog. 6:e10009402010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lan YY, Hsiao JR, Chang KC, Chang JS, Chen
CW, Lai HC, Wu SY, Yeh TH, Chang FH, Lin WH, et al: Epstein-Barr
virus latent membrane protein 2A promotes invasion of
nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction
of matrix metalloproteinase 9. J Virol. 86:6656–6667. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Portis T and Longnecker R: Epstein-Barr
virus (EBV) LMP2A mediates B-lymphocyte survival through
constitutive activation of the Ras/PI3K/Akt pathway. Oncogene.
23:8619–8628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pal AD, Basak NP, Banerjee AS and Banerjee
S: Epstein-Barr virus latent membrane protein-2A alters
mitochondrial dynamics promoting cellular migration mediated by
Notch signaling pathway. Carcinogenesis. 35:1592–1601. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pang MF, Lin KW and Peh SC: The signaling
pathways of Epstein-Barr virus-encoded latent membrane protein 2A
(LMP2A) in latency and cancer. Cell Mol Biol Lett. 14:222–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Izquierdo M: Short interfering RNAs as a
tool for cancer gene therapy. Cancer Gene Ther. 12:217–227. 2005.
View Article : Google Scholar : PubMed/NCBI
|