Introduction
Intervertebral disc degeneration is characterized by
a reduced number and impaired function of intervertebral disc
cells, and manifests as nucleus dehydration, decreased proteoglycan
content, particularly in aggregation states, and changes in
collagen type and distribution (1).
These changes weaken or remove the tension and pressure on
intervertebral discs, and the changes in histology eventually
results in changes of intervertebral disc biomechanics (1). Therefore, it is evident that a decrease
in the number of active cells in intervertebral discs will result
in the reduction in the synthesis of extracellular matrix and
alterations in the cell composition (2,3). This is
the pathological basis of intervertebral disc degeneration, and
excessive apoptosis is the direct cause of the decrease in
intervertebral disc cells (2,3).
Apoptosis serves an important role in the
development of the body, as well as in a number of physiological
and pathological processes (3). It
has been suggested that apoptosis may be involved in the
pathophysiologic changes experienced during intervertebral disc
tissue degeneration, and it is noted that apoptosis serves an
important role in the intervertebral disc degeneration process
(4). Excessive apoptosis of
intervertebral disc cells results in the reduction of active cells,
s well as changes of cell composition, which is the pathological
basis of intervertebral disc degeneration (5). However, studies have demonstrated that
oxidative stress resulting from reactive oxygen species is the
primary cause of apoptosis (6).
Reactive oxygen species include superoxide anion,
hydrogen peroxide and hydroxyl radicals. Various antioxidant
defense mechanisms exist in vivo, including antioxidant
enzymes, such as superoxide dismutase (SOD) and catalase (3–5). When
these defense mechanisms cannot prevent the generation of excessive
reactive oxygen species, oxidative stress will occur, which causes
the degeneration of cells or protein tissue, lipid oxidation, DNA
damage and other physiological dysfunctional processes, leading to
apoptosis (7). However, only a
limited number of studies have focused on intervertebral disc
degeneration and the apoptosis of nucleus pulposus cells due to
oxidative stress (8–10).
Aloperine is a novel type of alkaloid drug with a
significant inhibitory effect on acute inflammation, Type III and
IV hypersensitivity, and adjuvant arthritis caused by multiple
proinflammatory agents (11). The
chemical structure of aloperine is presented in Fig. 1. Aloperine is a herb that has been
found to have significant inhibitory effects on T cells and B
cells, and on the production of interleukin (IL)-2 according to
preliminary results (12).
In the present study, hydrogen peroxide
(H2O2) was used as a stimulus to study the
effect of oxidative stress on nucleus pulposus cell injury and
underlying mechanisms (8). The study
determined that the protective effect of aloperine attenuates
H2O2-induced injury via its activation of AKT
and suppression of the nuclear factor-κB (NF-κB) pathway.
Materials and methods
Compounds
Dulbecco's modified Eagle's medium/Ham's F-12 medium
(DMEM/F-12) and fetal bovine serum (FBS) were acquired from Hyclone
(GE Healthcare Life Sciences, Little Chalfont, UK). Collagenase II
(0.2%; C0014) was purchased from Sunshine Biotechnology (Nanjing)
Co., Ltd. (Nanjing, China).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
ST316) was purchased from Beyotime Institute of Biotechnology
(Haimen, China). Tumor necrosis factor-α (TNF-α; R019), IL-6
(R016), SOD (A001-3) and glutathione peroxidase (GSH-Px; A005)
commercial kits were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Aloperine (purity,
>99%) was acquired from Yanchi Dushun Biological and Chemical
Co., Ltd. (Ningxia, China).
Experimental animals and cell
culture
Ethical approval for this study was provided by the
Animal Ethical and Welfare Committee of Hebei Province (approval
no. Hb14-3012). Adult male Sprague-Dawley rats (n=24, 9–10 weeks),
weighing 250±30 g, were obtained from the Animal Resource Center of
the First Central Hospital of Baoding (Baoding, China). They were
housed at 22–23°C with 55–60% humidity and a 12:00/12:00 light/dark
cycle. The rats were euthanized by an overdose of pentobarbital
[100 mg/kg body weight; Sunshine Biotechnology (Nanjing) Co.,
Ltd.]. The spinal column was removed at the L1-L6 lumbar
intervertebral discs, and then the gel-like nucleus pulposus was
separated from the samples under aseptic conditions. The nucleus
pulposus tissue samples were immediately placed into DMEM/F-12 and
FBS, and were digested with 0.01% trypsin (Beyotime Institute of
Biotechnology) at 37°C for 0.5–1 h. The trypsin was absorbed and
removed, and the nucleus pulposus tissue samples were washed with
phosphate-buffered saline (PBS) and digested with 0.2% collagenase
II at 37°C for 4 h. Following digestion, nucleus pulposus cells
were harvested using a 200 µm mesh strainer. Next, the cells
(1×107 cells) were seeded into a new culture bottle and
incubated with DMEM/F-12 and 10% FBS containing 1%
penicillin/streptomycin (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) in a humidified atmosphere at 37°C and 5% CO2.
Subsequent to incubation, nucleus pulposus cells were washed with
PBS.
Cell viability determined by MTT
assay
Nucleus pulposus cells (5×103 cells/well)
were seeded into a 96-well plate, and incubated with fresh medium
containing 200 µM H2O2 alone or with
aloperine (0.1–1,000 µM) for 24 h. After incubation, 10 µl MTT was
added into each well and incubated in a humidified atmosphere at
37°C and 5% CO2. Subsequently, 150 µl dimethyl sulfoxide
was added to each well and agitated for 20 min. The absorbance was
measured using Labsystems Multiskan MS plate Reader (Synergy2;
BioTek Instruments, Inc., Winooski, VT, USA) at 540 nm.
Cell apoptosis determined by flow
cytometry
Nucleus pulposus cells (2×106 cells/well)
were seeded into a 6-well plate and incubated with fresh medium
containing 200 µM H2O2 alone or with
aloperine (1, 10 and 100 nM) for 24 h. Following incubation,
nucleus pulposus cells were washed twice with cold PBS and
incubated with 500 µl binding buffer (BestBio, Shanghai, China).
Subsequently, 5 µl Annexin V-FITC and 5 µl propidium iodide
(BestBio) were added and the cells were incubated for 30 min at 4°C
in the dark. Cell apoptosis was analyzed on a FACScan flow
cytometer (BD Biosciences, San Jose, CA, USA) with CellQuest
software (BD Biosciences).
Determination of inflammation and
oxidation activity
Nucleus pulposus cells (5×103 cells/well)
were seeded into a 96-well plate, and incubated with fresh medium
containing 200 µM H2O2 alone or plus
aloperine (1, 10 and 100 nM) for 24 h. After incubation, TNF-α,
IL-6, SOD and GSH-Px were measured using commercial ELISA kits
according to the manufacturer's instructions and a Labsystems
Multiskan MS Plate Reader was used to measure the absorbance at 450
nm.
Measurement of caspase-9 activity
Nucleus pulposus cells (2×106 cells/well)
were seeded into a 6-well plate, and incubated with fresh medium
containing 200 µM H2O2 alone or with
aloperine (1, 10 and 100 nM) for 24 h. Subsequently, nucleus
pulposus cells were incubated with 100 µl tissue lysis buffer
(Beyotime Institute of Biotechnology) for 30 min on ice.
Homogenates were centrifuged at 12,000 × g for 10 min at 4°C
and the supernatant was collected to assess the protein
concentration using a BCA assay kit, according to the
manufacturer's protocol (KeyGen Biotech Co., Ltd., Nanjing, China).
Equal protein (10 mg) was incubated with substrate
(Ac-DEVD-pNA-caspase-9; BestBio) for 2 h in the dark at room
temperature. The absorbance was then measured using a Labsystems
Multiskan MS plate reader at 405 nm.
Western blot analysis of NF-κB and
phosphorylated AKT (p-AKT) levels
Nucleus pulposus cells (2×106 cells/well)
were seeded into a 6-well plate, and incubated with fresh medium
containing 200 µM H2O2 alone or with
aloperine (1, 10 and 100 nM) for 24 h. Next, the cells were
incubated with 100 µl tissue lysis buffer for 30 min on ice.
Homogenates were centrifuged at 12,000 × g for 10 min at 4°C
and the supernatant was collected to assess the protein
concentration using a BCA kit, according to the manufacturer's
protocol. Equal protein (50–60 mg) was separated with 8–12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto a nitrocellulose membrane using an electroblotting apparatus.
The nitrocellulose membrane was incubated with anti-NF-κB (sc-8008,
1:500), anti-p-AKT (sc-7985; 1:1,000) and anti-AKT (sc-8312;
1,1,000) antibodies all from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) overnight at 4°C. Subsequently, the
nitrocellulose membrane was incubated with the appropriate
horseradish peroxidase (HRP)-conjugated IgG secondary antibody
(sc-358915, sc-2008; 1:5000; Santa Cruz Biotechnology, Inc.)
followed by incubation with an enhanced chemiluminescence
substrate. The relative quantity of each protein was measured using
AlphaEase FC (FluorChem FC2) software (ProteinSimple, Santa Clara,
CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 18.0 software (SPSS, Inc., Chicago, IL, USA) and data are
presented as the mean ± standard deviation from at least three
experiments. Comparisons between two groups were performed using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
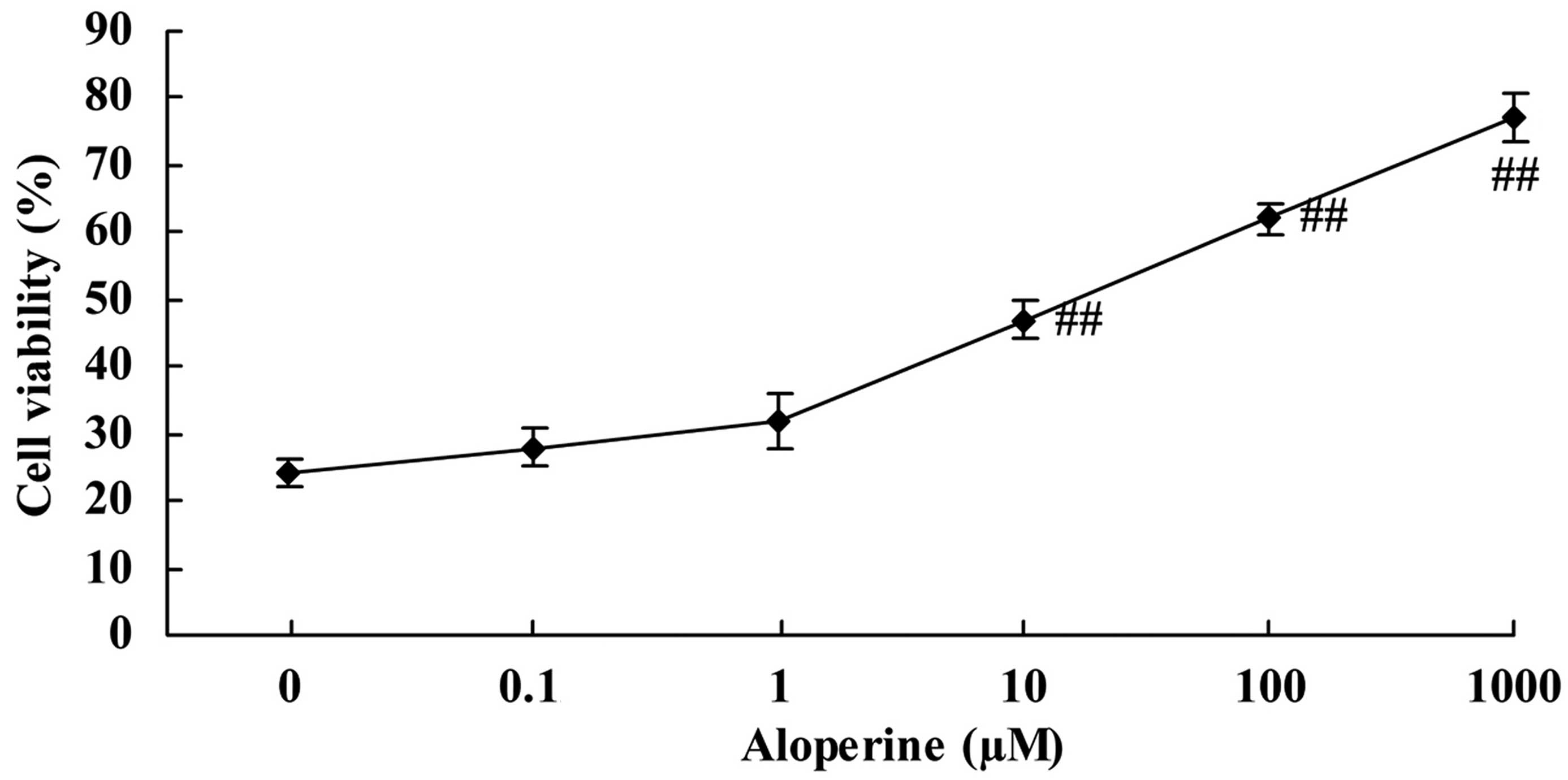
Aloperine increases the viability of
H2O2-treated nucleus pulposus cells
Treatment of nucleus pulposus cells with
H2O2 alone (0 nM aloperine) resulted in low
cell viability, as determined by MTT assay. However, aloperine
(0.1–1,000 µM) promoted the cell viability of
H2O2-treated nucleus pulposus cells in a
concentration-dependent manner. In particular, aloperine
significantly increased the cell viability of
H2O2-treated nucleus pulposus cells at doses
of 10, 100 and 1,000 µM (P<0.01; Fig.
2).
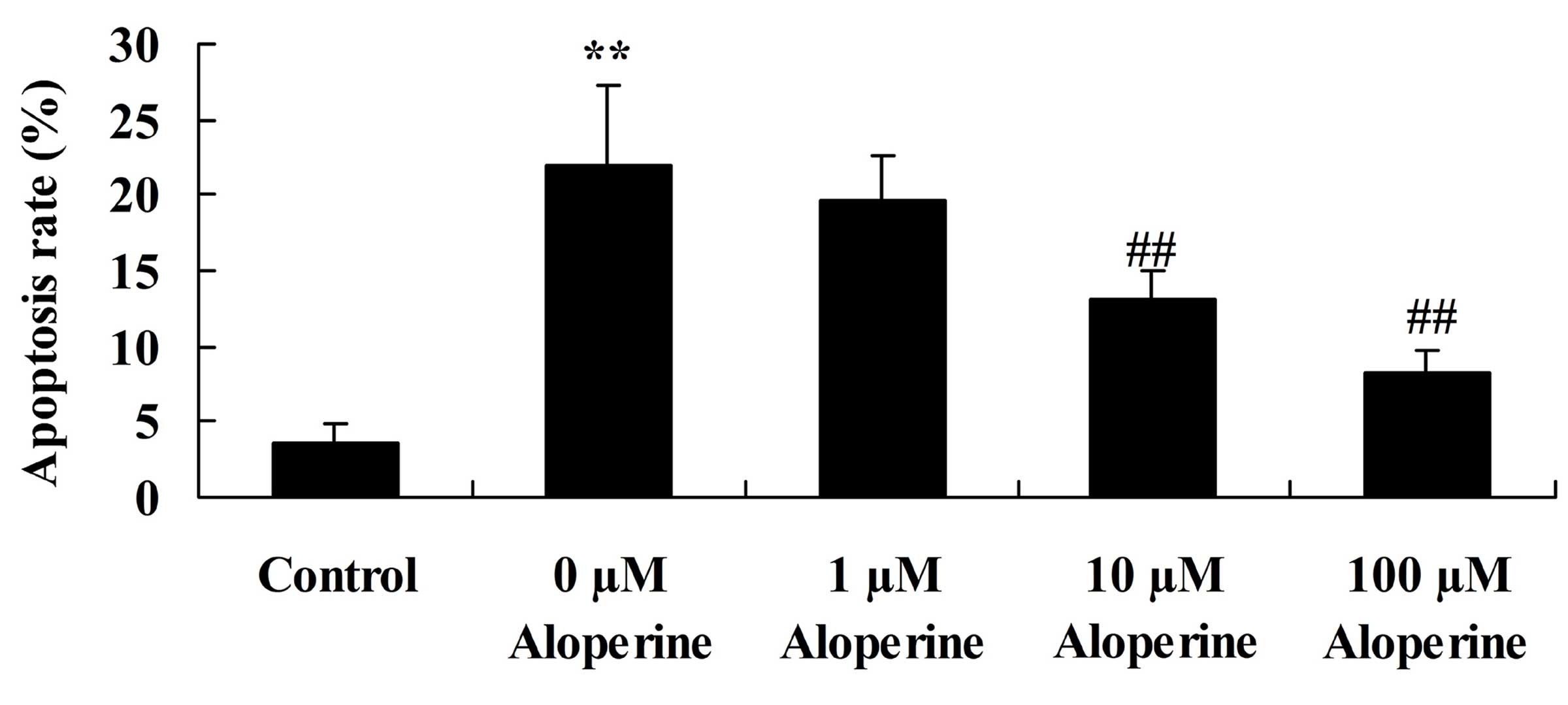
Aloperine suppresses
H2O2-induced apoptosis
The effects of aloperine on
H2O2-induced apoptosis in nucleus pulposus
cells were determined using flow cytometry. Treatment with 10 and
100 nM aloperine significantly suppressed the
H2O2-induced apoptosis (P<0.01; Fig. 3).
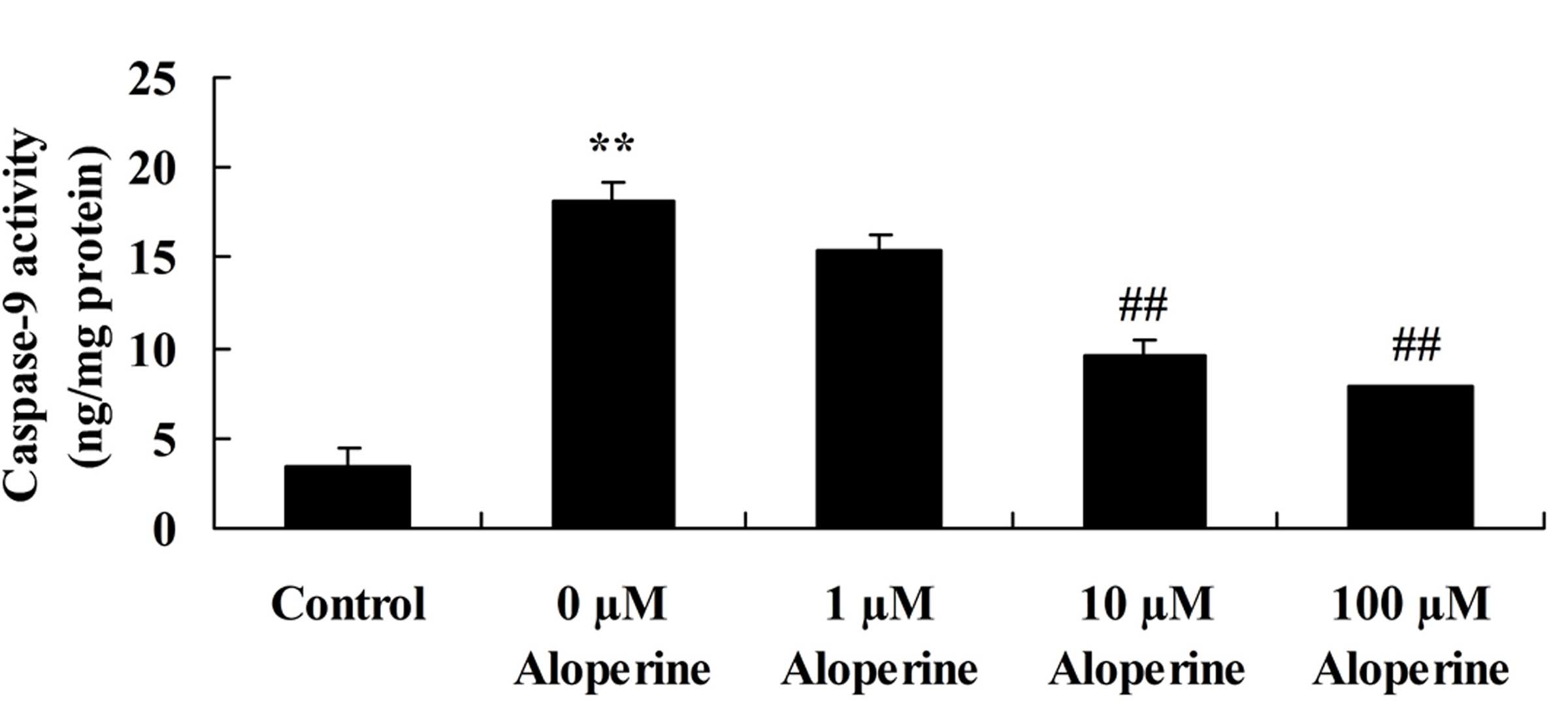
Aloperine decreases
H2O2-induced caspase-9 activity
In nucleus pulposus cells, the caspase-9 activity in
the H2O2 model group was significantly
increased compared with that in the control group (P<0.01).
Compared with the H2O2 model group, the
caspase-9 activity was significantly decreased by 10 and 100 nM
aloperine in H2O2-induced nucleus pulposus
cells (P<0.01; Fig. 4).
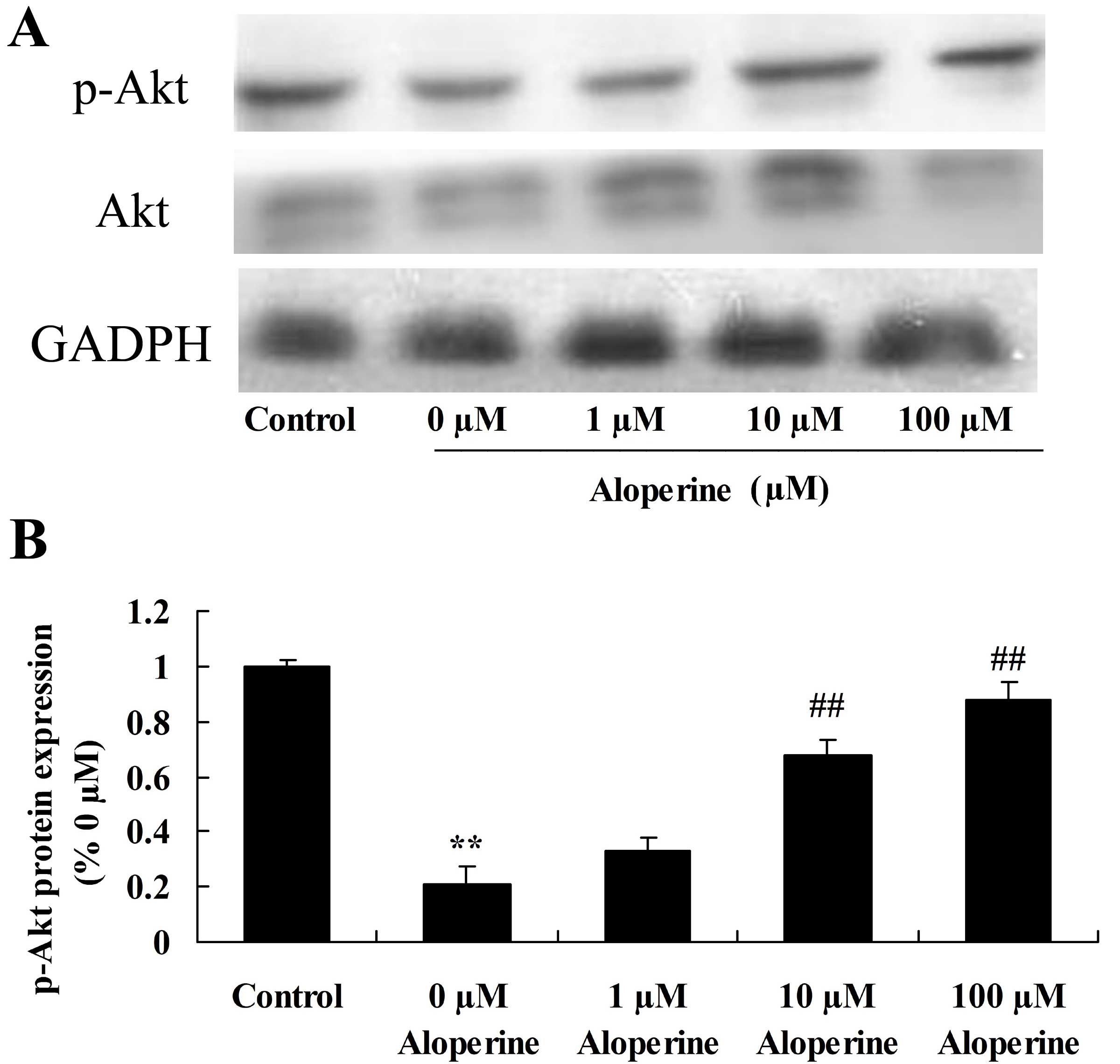
Effect of aloperine on p-AKT
expression in H2O2-treated nucleus pulposus
cells
To examine the mechanism of action of aloperine on
H2O2-induced cell apoptosis in nucleus
pulposus cells, p-AKT expression was analyzed using western
blotting. The p-Akt expression of the H2O2
model group was reduced compared with that of the control group
(P<0.01). p-AKT was significantly upregulated upon treatment
with 10 and 100 nM aloperine in H2O2-treated
nucleus pulposus cells, compared with the
H2O2 alone treatment group (P<0.01;
Fig. 5).
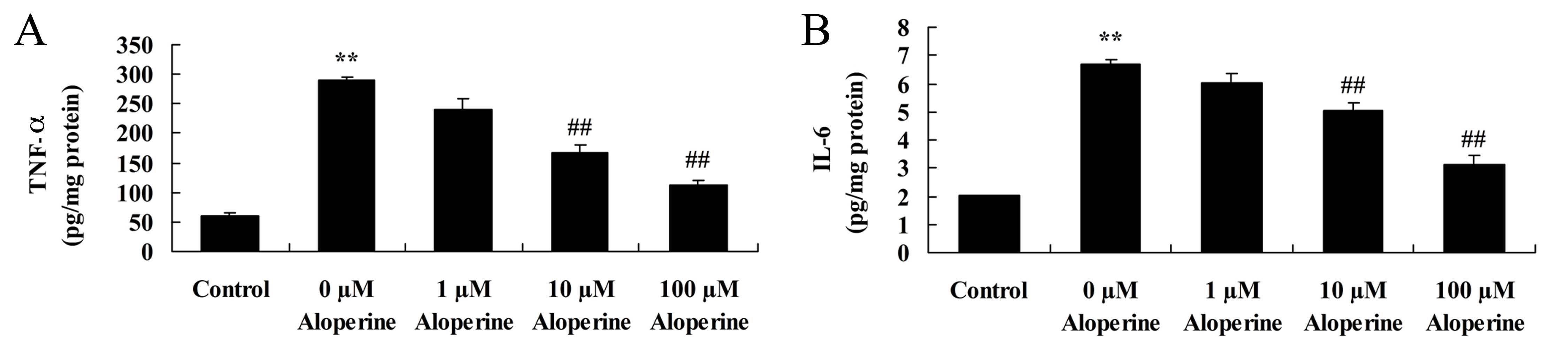
Aloperine reduces TNF-α and IL-6
expression levels in H2O2-treated cells
H2O2 significantly increased
TNF-α and IL-6 levels in nucleus pulposus cells (P<0.01).
Treatment with 10 and 100 nM aloperine was found to significantly
inhibit the expression levels of TNF-α and IL-6 compared with those
in the cells treated only with H2O2
(P<0.01; Fig. 6).
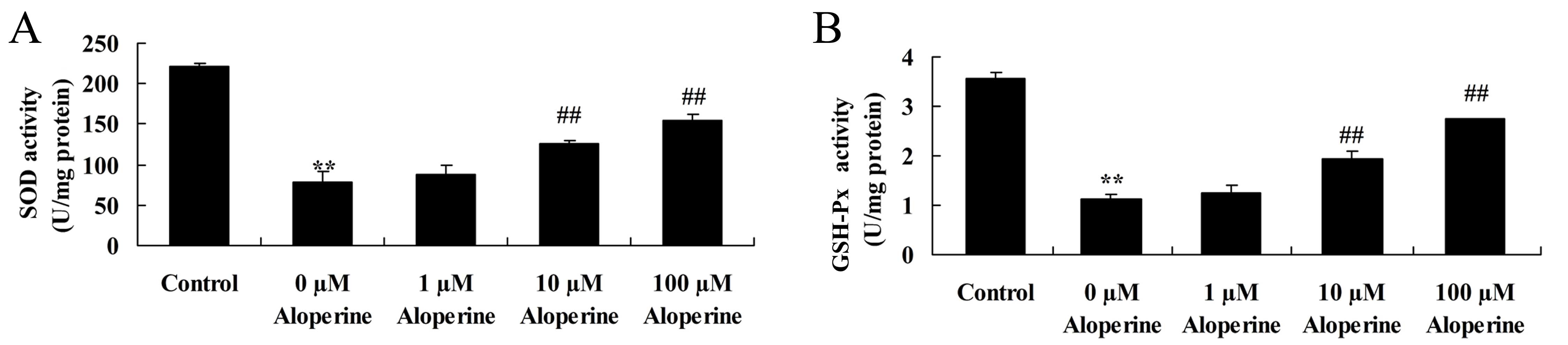
Effect of aloperine on
H2O2-induced oxidation activity
The effect of aloperine on the SOD and GSH-Px
activities in H2O2-treated nucleus pulposus
cells is presented in Fig. 7.
H2O2 significantly inhibited SOD and GSH-Px
activities in the model group compared with the control group
(P<0.01). Following treatment with 10 and 100 nM aloperine,
there were significant increases in the SOD and GSH-Px activities
in nucleus pulposus cells compared with those in the cells treated
only with H2O2 (P<0.01; Fig. 7).
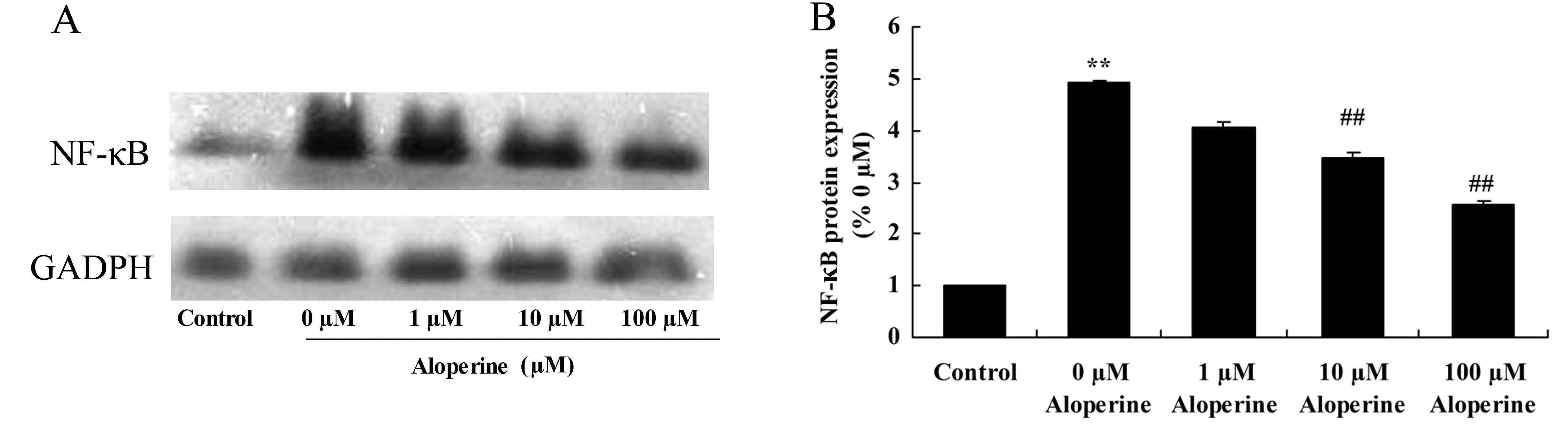
Effect of aloperine on
H2O2-induced NF-κB protein expression
To determine the protective mechanism of aloperine
against H2O2-induced inflammation in nucleus
pulposus cells, NF-κB protein expression levels were analyzed using
western blotting. The results showed that NF-κB protein expression
of model group was higher than that of control group (Fig 8). Treatment with 10 and 100 nM
aloperine significantly inhibited NF-κB protein expression in
H2O2-induced nucleus pulposus cells compared
with the expression in cells treated with
H2O2 alone (P<0.01; Fig. 8).
Discussion
Intervertebral disc degeneration is considered to be
closely associated with apoptosis of nucleus pulposus cells
(1). Although numerous studies have
investigated apoptosis, few studies have examined how oxidative
stress results in nucleus pulposus cell apoptosis and
intervertebral disc degeneration (10,13,14).
Therefore, the current study used H2O2 to
establish an oxidative stress model in nucleus pulposus cells in
order to identify the underlying mechanisms (15). In addition, the current study aimed
to explore the role of apoptosis in intervertebral disc
degeneration, and to clarify the signal transduction pathway
involved in intervertebral disc apoptosis, which may help to
achieve the prevention of intervertebral disc degeneration by
interfering with apoptosis (15).
Following the primary culture of rat nucleus pulposus cells, 200 µM
H2O2 was used to stimulate the cells for 24
h. H2O2 treatment resulted in significant
apoptosis, which confirms that oxidative stress can lead to
apoptosis (16). In the present
study, it was demonstrated that treatment with aloperine
significantly increased cell viability and inhibited cell apoptosis
of H2O2-treated nucleus pulposus cells in a
dose-dependent manner.
Caspase exists in cells as an inactive zymogen form,
and becomes an active fragment following proteolytic processing,
and thus is used in the investigation of cell apoptosis (17). In neuronal apoptosis induced by
various stimuli, caspase acts as a modulating agent (18). With increasing doses of
H2O2, the expression of caspase-9 is
increased (19). The caspase family
serves an important role in the process of mediating apoptosis, in
which caspase-9 is a critical executioner molecule, acting in
numerous apoptosis signaling transduction pathways (20). In the current study, aloperine
treatment significantly suppressed the
H2O2-induced caspase-9 activity of nucleus
pulposus cells via the upregulation of the AKT signaling pathway.
Wang et al (21) suggested
that aloperine exerts antitumor effects against multiple myeloma
through the caspase-9/p-PTEN/p-AKT-dependent apoptotic
pathways.
NF-κB is a protein with multidirectional
transcriptional regulation functions, which is widely distributed,
adjusts the transcriptional regulation of numerous genes and is
involved in a number of important physiological and pathological
processes, such as inflammatory reactions, the immune response,
cell proliferation, transformation and apoptosis (22). The most common form of NF-κB is a
heterodimer consisting of two protein subunits, P50 and RelA/P65.
It has been demonstrated that an increase in
H2O2 concentration, which is known to induce
apoptosis, results in increased NF-κB P65 expression and activity
(23). The results of the present
study suggest that treatment with aloperine significantly reduced
TNF-α and IL-6 activities, and enhanced SOD and GSH-Px activities
in H2O2-treated nucleus pulposus cells
through the downregulation of the NF-κB signaling pathway. Zhou
et al (24) reported that the
anti-inflammatory and anti-oxidative action of aloperine
significantly inhibited the effects of allergic reactions. In
addition, Xu et al (25)
demonstrated that administration of aloperine attenuated
neuropathic pain through its anti-oxidation activity and
suppression of the NF-κB signaling pathway.
In the present study, aloperine increased the
viability and inhibited the apoptosis of
H2O2-treated nucleus pulposus cells in a
dose-dependent manner. In addition, aloperine exerted
anti-inflammatory, anti-oxidative and anti-apoptotic effects,
upregulated p-AKT expression and downregulated the NF-κB signaling
pathway in H2O2-treated nucleus pulposus
cells. In particular, aloperine at concentrations of 10 and 100 nM
exerted significant effects. In conclusion, aloperine attenuated
H2O2-induced nucleus pulposus cell injury via
anti-apoptotic activity and suppression of the NF-κB signaling
pathway.
References
|
1
|
Wang HQ and Samartzis D: Clarifying the
nomenclature of intervertebral disc degeneration and displacement:
From bench to bedside. Int J Clin Exp Pathol. 7:1293–1298.
2014.PubMed/NCBI
|
|
2
|
Liang QQ, Ding DF, Xi ZJ, Chen Y, Li CG,
Liu SF, Lu S, Zhao YJ, Shi Q and Wang YJ: Protective effect of
ligustrazine on lumbar intervertebral disc degeneration of rats
induced by prolonged upright posture. Evid Based Complement
Alternat Med. 2014:5084612014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Presciutti SM, Paglia DN, Karukonda T,
Soung do Y, Guzzo R, Drissi H and Moss IL: PDGF-BB inhibits
intervertebral disc cell apoptosis in vitro. J Orthop Res.
32:1181–1188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kermani H Reihani, Pourghazi M and Mahani
SE: Effects of pulsed electromagnetic field on intervertebral disc
cell apoptosis in rats. Electromagn Biol Med. 33:246–249. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ariga K, Yonenobu K, Nakase T, Hosono N,
Okuda S, Meng W, Tamura Y and Yoshikawa H: Mechanical
stress-induced apoptosis of endplate chondrocytes in organ-cultured
mouse intervertebral discs: An ex vivo study. Spine (Phila Pa
1976). 28:1528–1533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heyde CE, Tschoeke SK, Hellmuth M,
Hostmann A, Ertel W and Oberholzer A: Trauma induces apoptosis in
human thoracolumbar intervertebral discs. BMC Clin Pathol. 6:52006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gruber HE, Watts JA, Hoelscher GL, Bethea
SF, Ingram JA, Zinchenko NS and Hanley EN Jr: Mitochondrial gene
expression in the human annulus: In vivo data from annulus cells
and selectively harvested senescent annulus cells. Spine J.
11:782–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Rong Z, Zeng M, Cao Y, Gong X, Lin
L, Chen Y, Cao W, Zhu L and Dong W: Pyrroloquinoline quinone
protects nucleus pulposus cells from hydrogen peroxide-induced
apoptosis by inhibiting the mitochondria-mediated pathway. Eur
Spine J. 24:1702–1710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu H, Yu H, Ren D, Zhu K and Huang H:
Plumbagin exerts protective effects in nucleus pulposus cells by
attenuating hydrogen peroxide-induced oxidative stress,
inflammation and apoptosis through NF-κB and Nrf-2. Int J Mol Med.
37:1669–1676. 2016.PubMed/NCBI
|
|
10
|
Chen J, Hou C, Chen X, Wang D, Yang P, He
X, Zhou J and Li H: Protective effect of cannabidiol on hydrogen
peroxide-induced apoptosis, inflammation and oxidative stress in
nucleus pulposus cells. Mol Med Rep. 14:2321–2327. 2016.PubMed/NCBI
|
|
11
|
Yuan XY, Ma HM, Li RZ, Wang RY, Liu W and
Guo JY: Topical application of aloperine improves
2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin
lesions in NC/Nga mice. Eur J Pharmacol. 658:263–269. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan XY, Ma HM, Li RZ, Wang RY, Liu W and
Guo JY: Topical application of aloperine improves
2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin
lesions in NC/Nga mice. Eur J Pharmacol. 658:263–269. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miao D and Zhang L: Leptin modulates the
expression of catabolic genes in rat nucleus pulposus cells through
the mitogen-activated protein kinase and Janus kinase 2/signal
transducer and activator of transcription 3 pathways. Mol Med Rep.
12:1761–1768. 2015.PubMed/NCBI
|
|
14
|
Chen JW, Ni BB, Li B, Yang YH, Jiang SD
and Jiang LS: The responses of autophagy and apoptosis to oxidative
stress in nucleus pulposus cells: implications for disc
degeneration. Cell Physiol Biochem. 34:1175–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nerlich AG, Schleicher ED and Boos N: 1997
Volvo Award winner in basic science studies. Immunohistologic
markers for age-related changes of human lumbar intervertebral
discs. Spine (Phila Pa 1976). 22:2781–2795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Zhang HL, Gu GF, Ding Y, Jia JB,
Fu QS and He SS: significant In Vitro Cell Dev. Biol Anim.
49:279–286. 2013. View Article : Google Scholar
|
|
17
|
Park C, Jin CY, Kim GY, Jeong YK, Kim WJ
and Choi YH: Induction of apoptosis by ethanol extract of Prunus
mume in U937 human leukemia cells through activation of caspases.
Oncol Rep. 26:987–993. 2011.PubMed/NCBI
|
|
18
|
He Q, Bao L, Zimering J, Zan K, Zhang Z,
Shi H, Zu J, Yang X, Hua F, Ye X and Cui G: The protective role of
(−)-epigallocatechin-3-gallate in thrombin-induced neuronal cell
apoptosis and JNK-MAPK activation. Neuroreport. 26:416–423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu TT, Li LF, Du R, Jiang L and Zhu YQ:
Hydrogen peroxide induces apoptosis in human dental pulp cells via
caspase-9 dependent pathway. J Endod. 39:1151–1155. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park WH: Anti-apoptotic effect of caspase
inhibitors on H2O2-treated HeLa cells through
early suppression of its oxidative stress. Oncol Rep. 31:2413–2421.
2014.PubMed/NCBI
|
|
21
|
Wang H, Yang S, Zhou H, Sun M, Du L, Wei
M, Luo M, Huang J, Deng H, Feng Y, et al: Aloperine executes
antitumor effects against multiple myeloma through dual apoptotic
mechanisms. J Hematol Oncol. 8:262015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun BZ, Chen L, Wu Q, Wang HL, Wei XB,
Xiang YX and Zhang XM: Suppression of inflammatory response by
flurbiprofen following focal cerebral ischemia involves the NF-κB
signaling pathway. Int J Clin Exp Med. 7:3087–3095. 2014.PubMed/NCBI
|
|
23
|
Jia WC, Liu G, Zhang CD and Zhang SP:
Formononetin attenuates hydrogen peroxide
(H2O2)-induced apoptosis and NF-κB activation
in RGC-5 cells. Eur Rev Med Pharmacol Sci. 18:2191–2197.
2014.PubMed/NCBI
|
|
24
|
Zhou CC, Gao HB, Sun XB, Shi HB, Liu W,
Yuan HN and Wang ZX: Anti-inflammatory and anti-allergic action of
aloperine. Zhongguo Yao Li Xue Bao. 10:360–365. 1989.(In Chinese).
PubMed/NCBI
|
|
25
|
Xu YQ, Jin SJ, Liu N, Li YX, Zheng J, Ma
L, Du J, Zhou R, Zhao CJ, Niu Y, et al: Aloperine attenuated
neuropathic pain induced by chronic constriction injury via
anti-oxidation activity and suppression of the nuclear factor kappa
B pathway. Biochem Biophys Res Commun. 451:568–573. 2014.
View Article : Google Scholar : PubMed/NCBI
|