Introduction
Ischemia-reperfusion injury (IRI) is a disease
caused by ischemia, reperfusion or revascularization to dredge by
vascular tissues and organs regain blood, in certain circumstances,
the reperfusion will result in more serious damage to tissues and
cells (1–3). The mechanism of ischemia reperfusion
injury is very complex, and so far has not been elucidated, but is
related to inflammation, endothelial cell injury, energy depletion
and production disorder, no reflow phenomenon, calcium overload and
oxygen free radical damage.
Lung IRI (LIRI) is one of the main causes of death
caused by pulmonary dysfunction in the early postoperative period
for patients who underwent double-sleeve lobectomy, lung
transplantation, or cardiopulmonary bypass (4–6). At
present, there are many methods to the prevention and treatment of
LIRI reported in the literature, such as reduction of LIRI by
inhibiting neutrophil aggregation and activation. The study found
that polyethylene glycol was added to the lungs in a low potassium
perfusion solution (7). It can
change the interaction between cell and cell, cell and protein as
well as with water, to reduce pulmonary edema and play a role of
lung protection. In the ischemia period, a large number of free
radicals are released in tissues, sulfur based compound free
radical causes membrane lipid peroxidation and severe cell damage,
and the most serious injury is in pulmonary vascular endothelial
cells (8). It has been reported that
the addition of N-acetyl cysteine in the perfusion solution can
alleviate lung reperfusion injury (9). It is also reported that the occurrence
of reperfusion injury can be reduced by reducing the infiltration
of inflammatory cells (10). In the
process of lung transplantation, the white blood cell filter is
used in the heart lung bypass loop to prevent the white blood cells
from entering the human pulmonary blood vessel. This experimental
method has also achieved some results (11).
Although in recent years, the LIRI has been widely
studied, the exact mechanism remains to be elucidated, various
measures of prevention and treatment effects are not ideal, and the
prevention of LIRI has still not improved. Clinical study found
that in lung transplantation due to LIRI pulmonary dysfunction rate
was very high, and the treatment was difficult and expensive, many
patients needed extra corporeal membrane oxygenator with high
mortality rate (12). Pulmonary IRI
has become one of the main factors that hinder the development of
lung transplantation. Therefore, it is still the focus of the
current research to identify the mechanism of LIRI and to search
for effective methods and drugs for the prevention and treatment of
ischemia reperfusion injury. It is necessary to screen out a more
accurate and appropriate lung protection method to prevent and
reduce the occurrence of IRI in lung transplantation.
Recently, many scholars have focused on the research
of IRI protection in traditional Chinese medicine. Danshensu,
ligustrazine and oxymatrine (OMT) have been demonstrated
significant against inflammation, antioxidant, scavenging oxygen
free radicals, to reduce reperfusion injury of tissue (13). OMT is a kind of compound of basic
structure of matridine-1-ketone, mainly alkaloids extracted from
sophora root or in sophora alopecuroides, and studies show that OMT
has a strong pharmacological effect of anti-inflammatory and
immunomodulatory, antioxidant and protecting heart and liver
ischemia reperfusion (14). However,
there is less research on the LIRI of the lungs. We observed the
effect of OMT on lung histopathology, levels of malondialdehyde
(MDA) and superoxide dismutase (SOD), as well as expression levels
of heat shock protein 90a (Hsp90a), to investigate the protective
mechanism against LIRI.
Materials and methods
Experimental animals
A total of 30 healthy, Japanese flap-eared white
rabbits, either male or female, weighing 1.8–2.5 kg were used.
Rabbits were provided by the Animal Laboratory of Chongqing Academy
of Chinese Materia Medica [Chongqing, China; animal certificate no.
SCXR(Yu)20070006]. Animals were randomly divided into the control
group (group C, n=10) and the experimental group (group E, n=20)
which was then further divided according to method of intervention:
Cervical vein administration group (group E1, n1=10) and
left pulmonary administration group (group E2, n2=10),
and they separately received OMT (100 mg/kg) via pump infusion (OMT
batch number: National Medicine Permission no. H20041843; Baoding
Sanjiu Jishi Biological Pharmaceutical Co., Ltd., Baoding, China)
from the distal heart side, 10 min before hilar blocking and moment
of pulmonary blocking.
The model of LIRI was established according to the
method by Yamashita et al (15). Heparin (1.0 mg/kg) was injected via
the jugular vein. At 60 min after ischemia, continuous perfusion
was performed and lasted for 180 min. This study was approved by
the Animal Ethics Committee of Chongqing Medical University Animal
Center.
Biochemical analyses of lung
tissue
Lung tissues were collected at the time of chest
opening [0 min (T0)], after ischemia [60 min
(T1)], and following reperfusion [180 min
(T3)] and [240 min (T4)]. Lung tissue
specimens were used to prepare 10% homogenates, and samples were
preserved at −20°C until further use. The aldehyde shrinkage method
was used to determine tissue protein content, and after determining
SOD and MDA by the xanthine oxidase method, protein content was
determined with a protein quantification kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
instructions.
Measurement of lung tissue wet/dry
weight ratio
At T2, T3 and T4,
we removed roughly 2 g of left lung tissue as the wet weight, and
the weight after 10 h of baking in an electric tachometer indicator
thermostatic drying oven, was taken as the dry weight. The ratio of
the weights was determined as wet/dry (W/D).
Observation of lung tissue
morphology
At T2, T3 and T4,
roughly 1×1×1 cm pieces of tissue from the lower lobe of left lungs
were removed and fixed in 10% formaldehyde, and conventional
processing and sectioning of tissue were performed. Tissue sections
underwent H&E staining, immunohistochemical staining, and
observation by light microscopy. Additionally, pieces of lung
(0.1×0.1×0.1 cm) from the lower left lobe (three for each group)
were fixed with 2.5% osmic acid and post fixed with 1% osmic acid.
Tissue was sectioned and treated with glutaraldehyde for electron
microscopic observation.
Immunohistochemistry
The non-apoptotic cell detection kit was from Roche
Diagnostics (Basel, Switzerland). Hsp90a detection reagent was from
Fuzhou Maixin Biotech. Co., Ltd. (Fuzhou, China), and were both
used according to the manufacturer's instructions.
Apoptosis-positive cell nuclei appeared brown-yellow, and the
cytoplasmic expression of Hsp90a expression was brown-yellow. For
each tissue section, five random visual fields were observed by
light microscopy (20×10 times). We enumerated the apoptosis index
(AI) as positive cell number/total cell number × 100%. The same was
done for the Hsp90a expression index.
Statistical analysis
Data were analyzed with SPSS 15.0 software (SPSS,
Inc., Chicago, IL, USA). Data are expressed as mean ± standard
deviation. One way ANOVA, LSD and t-test were used for comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Measurement of SOD activity and MDA
levels
At T1 and T3, SOD and MDA
levels in the three groups (group C, E1 and E2) were not
significantly different. After T2 and during the LIR
period, in group C the MDA levels progressively increased, while
SOD activity remained the same, and compared with groups E1 and E2,
the corresponding values were significantly different (E1,
P<0.05; E2, P<0.01). MDA levels in E1 were increased over E2,
whereas SOD activity was lower compared with E2. The differences
were all statistically significant (P<0.05; Table I).
 | Table I.Comparison of lung tissue homogenate
SOD (U/mg prot) and MDA (nmol/mg prot). |
Table I.
Comparison of lung tissue homogenate
SOD (U/mg prot) and MDA (nmol/mg prot).
| Index | Group | T0 | T1 | T2 | T3 | T4 |
|---|
| SOD | C |
14.32±1.27 |
15.38±2.24 |
13.88±1.61 |
13.62±1.82 |
12.50±1.31 |
|
| E1 |
14.04±1.42 |
15.42±5.00 |
18.00±1.81a |
19.26±6.57a |
22.28±3.11b |
|
| E2 |
14.12±1.14 |
17.10±4.31 |
19.94±6.06b |
21.10±5.42b |
24.38±3.61b |
| MDA | C |
1.06±0.19 |
1.21±0.41 |
1.50±0.30 |
1.54±0.31 |
1.76±0.42 |
|
| E1 |
1.05±0.23 |
1.21±0.19 |
1.38±0.31a |
1.05±0.33a |
1.17±0.19c |
|
| E2 |
1.08±0.21 |
1.19±0.16 |
1.31±0.21b |
1.02±0.31b |
1.12±0.15b |
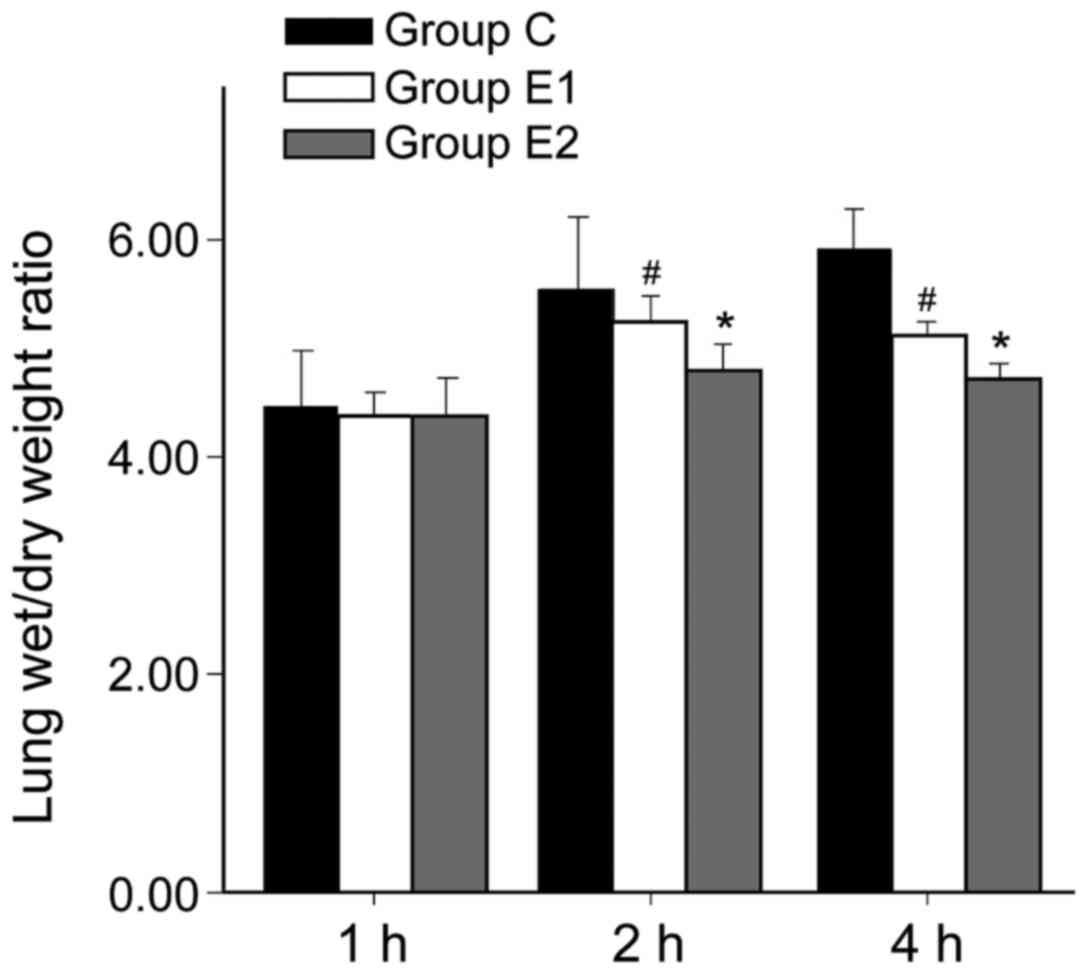
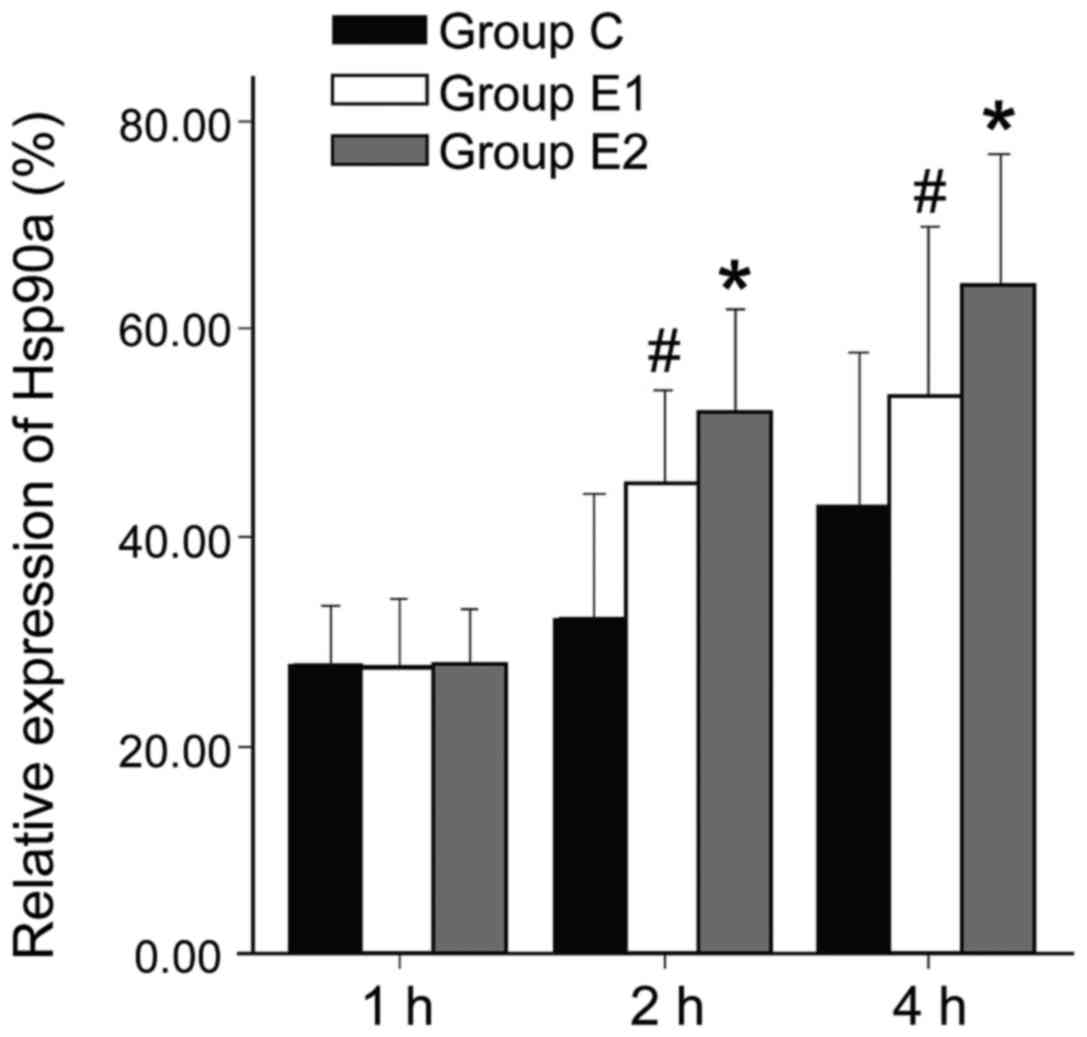
Measurement of W/D ratio, AI and
Hsp90a
W/D ratio and AI of group C was higher than in
groups E1 or E2, and the differences were all statistically
significant (P<0.05, P<0.01). Hsp90a expression was higher in
groups E1 and E2 compared with group C, and the differences were
statistically significant (P<0.05, P<0.01). The W/D ratio and
AI of group E2 were significantly lower than in E1 (P<0.05,
P<0.01). Hsp90a expression in group E2 was significantly higher
than in E1 (P<0.05; Figs.
1–3).
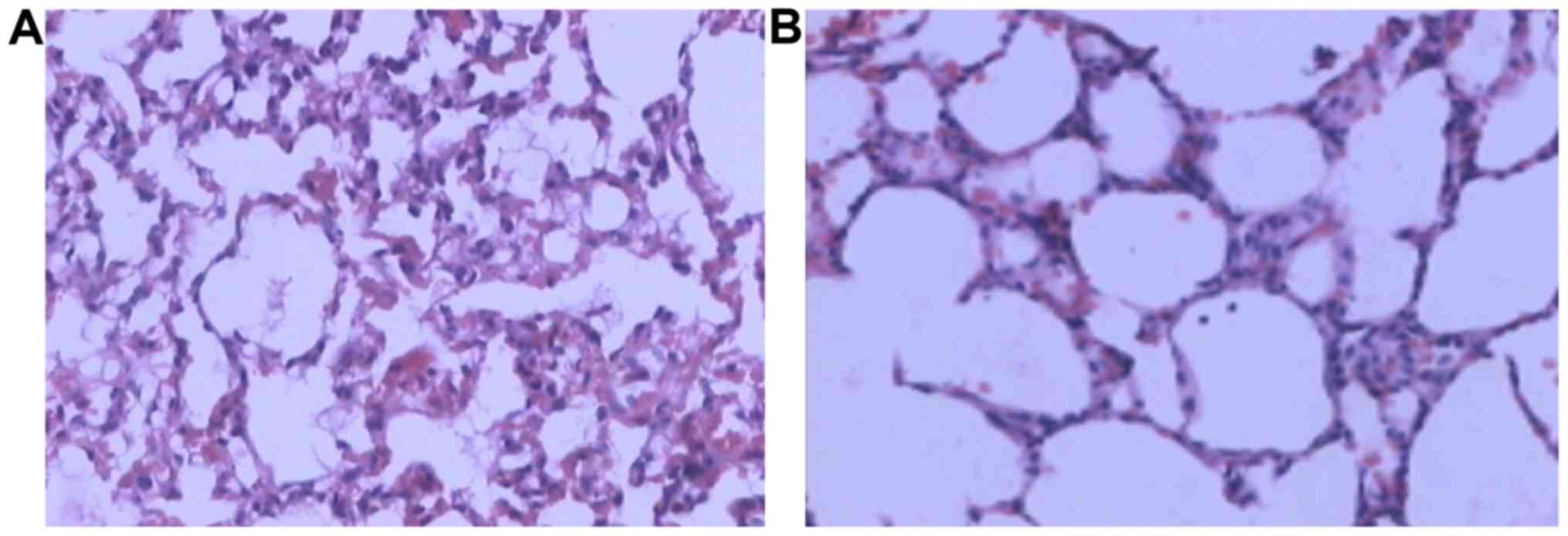
Lung tissue structural changes by
light microscopy
In group C, there was noticeable lung atelectasis,
pulmonary interstitial edema, inflammatory cell infiltration, and
alveolar exudate (Fig. 4A). In group
E, lung injury was significantly reduced, with less inflammatory
cell infiltration and complete alveoli (Fig. 4B).
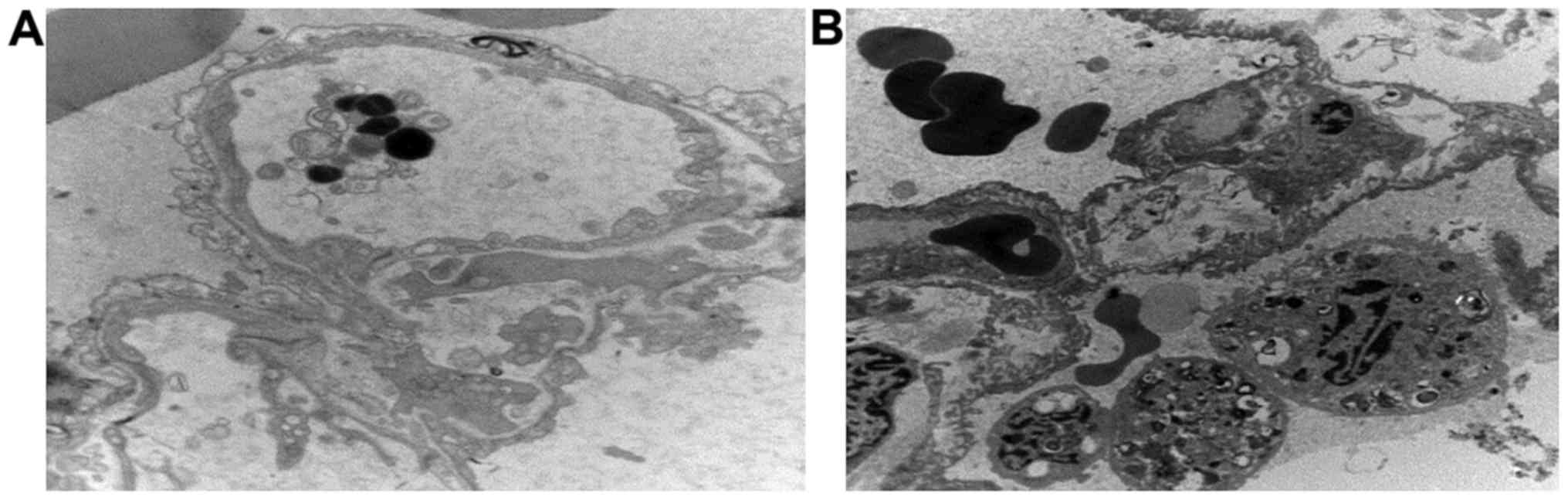
Lung tissue examination by electron
microscopy
In group C, endocytic vesicles inside endothelial
cells of small pulmonary arteries increased, and mitochondria
appeared swollen and vacuolized. The underlying basement membrane
of endometrial cells appeared with edema and vacuolar degeneration.
There were capillary transluminal polymorphonuclear blocks, type II
epithelial cell mitochondria were swollen, and apoptotic bodies
were visible (Fig. 5A). In group E,
endothelial morphology of small pulmonary arteries was mostly
normal, the external basement membrane of endothelial cells was
complete, and the morphology of type II epithelial cells was not
significantly abnormal (Fig.
5B).
Discussion
The present study is based on the rabbit LIRI
experimental model. Histomorphology after LIRI, and observations of
tissue biochemistry have shown that LIRI causes increased
permeability of the alveolar-capillary barrier, pulmonary
interstitial edema, slurry exudation in alveoli, and neutrophil
infiltration. Neutrophil accumulation in lung tissue leads to the
phenomenon of ‘respiratory burst’, during which several toxic
factors are released including oxygen free radicals, and cause
tissue injury (15,16). During reperfusion, oxygen-rich blood
provides reactive oxygen species and inflammatory mediators. This
produces oxygen free radicals and other reactive oxygen species.
Furthermore, neutrophils interact with ischemic endothelial cells,
which causes apoptosis and lung injury (15).
OMT is one of many alkaloids extracted from the
sophora root or bitter beans. Modern research has shown that OMT
has potent anti-inflammatory, immune regulatory, and antioxidant
functions and can protect the heart and liver from IRI (17,18). Xu
et al (19) reported that OMT
may inhibit protein kinase activity and the proinflammatory effect
of TNF-α by blocking phosphorylation, and thus protect against
acute lung injury. Tian et al (20) reported that OMT can effectively clear
oxhydryl. OMT reacts with free radicals, forming OMT radicals,
which break the chain reaction of lipid peroxidation, and improve
the ability of cells to resist oxidation-mediated injury. The
present study shows that during lung ischemia and hypoxia, MDA
values in groups E1 and E2 are not significantly different with
group C. At 2 h after reperfusion, MDA levels of the control group
were significantly higher than in the experimental group, and SOD
activity was significantly lower. The data show that oxygen free
radicals increase and endogenous antioxidant enzymes are inhibited.
After intervention with OMT, the concentration of MDA decreased
compared with the group that received no intervention, and SOD
activity increased significantly, which suggests that OMT can
indirectly inhibit xanthine oxidase, and decrease the production of
superoxide anion, or make xanthine oxidase combine with superoxide
anion, forming toxic metabolites. OMT can also lower the levels of
oxygen free radicals, thus protecting lung tissue from injury
caused by oxygen free radicals, and reduce LIRI.
Hsp90a is a member of the heat-shock protein family,
which is activated by external stimuli. Under stress, such as in
high fever, ischemia, hypoxia, and trauma, it will also increase.
Hsp90a acts as both a functional protease and hormone receptor, and
can adjust the activity and involvement of transcription of heat
shock protein genes, to reduce inflammation, promote
anti-oxidation, and inhibit apoptosis (21–23).
DeMeester et al (24) used
Hsp90a to induce IκB-α expression and observed the function of
inhibiting endothelial cell apoptosis (25). The present study shows that Hsp90a
was expressed in all groups. However, 2 h after LIRI in group E,
expression levels increased significantly and W/D ratio and AI
index decreased. In group E2, Hsp90a was significantly higher than
in groups E1 and C, and W/D ratio and AI index were significantly
lower than in groups E1 and C. Abnormal morphological changes to
lung tissue were reduced significantly, suggesting that OMT can
increase Hsp90a expression. At the early stage of ischemia and
hypoxia, Hsp90a is involved in endogenous protection from injury,
inhibiting cell apoptosis and reducing LIRI.
In conclusion, OMT can protect rabbit lung tissue
from experimental IRI, through the combined effects of clearing
oxygen free radicals, exerting its anti-inflammatory and immune
regulatory effects, increasing Hsp90a expression, and inhibiting
apoptosis. The results also suggest that the protective effect of
OMT is better in the partial pulmonary perfusion group (group E2)
than in the systemic administration group (group E1), which
provides new possibilities for the protection of lung ischemia
reperfusion injury.
Acknowledgements
This study was supported by the National Natural
Sciences Foundation of China (grant no. 30471984), the Foundation
of Bureau of Public Health of Chongqing (grants nos. 2006-2-001,
2006-B-26 and 2010-2-127) and the project Innovation of Science and
Technology, Chongqing Science and Technology Commission (project
no. cstc2013jcyjA10108).
References
|
1
|
Cuzzocrea S, Riley DP, Caputi AP and
Salvemini D: Antioxidant therapy: A new pharmacological approach in
shock, inflammation, and ischemia/reperfusion injury. Pharmacol
Rev. 53:135–159. 2001.PubMed/NCBI
|
|
2
|
Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera
JS, Halkos ME, Kerendi F, Guyton RA and Vinten-Johansen J:
Postconditioning attenuates myocardial ischemia-reperfusion injury
by inhibiting events in the early minutes of reperfusion.
Cardiovasc Res. 62:74–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mori K, Lee HT, Rapoport D, Drexler IR,
Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, et al:
Endocytic delivery of lipocalin-siderophore-iron complex rescues
the kidney from ischemia-reperfusion injury. J Clin Invest.
115:610–621. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
den Hengst WA, Gielis JF, Lin JY, Van
Schil PE, De Windt LJ and Moens AL: Lung ischemia-reperfusion
injury: A molecular and clinical view on a complex
pathophysiological process. Am J Physiol Heart Circ Physiol.
299:H1283–H1299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimamoto A, Pohlman TH, Shomura S,
Tarukawa T, Takao M and Shimpo H: Toll-like receptor 4 mediates
lung ischemia-reperfusion injury. Ann Thorac Surg. 82:2017–2023.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ng CS, Wan S, Arifi AA and Yim AP:
Inflammatory response to pulmonary ischemia-reperfusion injury.
Surg Today. 36:205–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jayle C, Hauet T, Menet E, Hébrard W,
Hameury F, Eugene M, Carretier M and Corbi P: Beneficial effects of
polyethylene glycol combined with low-potassium solution against
lung ischemia/reperfusion injury in an isolated, perfused,
functional pig lung. Transplant Proc. 34:834–835. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hatachi G, Tsuchiya T, Miyazaki T,
Matsumoto K, Yamasaki N, Okita N, Nanashima A, Higami Y and
Nagayasu T: The poly(adenosine diphosphate-ribose) polymerase
inhibitor PJ34 reduces pulmonary ischemia-reperfusion injury in
rats. Transplantation. 98:618–624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weinbroum AA, Rudick V, Ben-Abraham R and
Karchevski E: N-acetyl-L-cysteine for preventing lung reperfusion
injury after liver ischemia-reperfusion: A possible dual protective
mechanism in a dose-response study. Transplantation. 69:853–859.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakano H, Nagasaki H, Barama A, Boudjema
K, Jaeck D, Kumada K, Tatsuno M, Baek Y, Kitamura N, Suzuki T, et
al: The effects of N-acetylcysteine and anti-intercellular adhesion
molecule-1 monoclonal antibody against ischemia-reperfusion injury
of the rat steatotic liver produced by a
choline-methionine-deficient diet. Hepatology. 26:670–678. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halldorsson A, Kronon M, Allen BS, Bolling
KS, Wang T, Rahman S and Feinberg H: Controlled reperfusion after
lung ischemia: implications for improved function after lung
transplantation. J Thorac Cardiovasc Surg. 115:415–424; discussion
424–425. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ali HS, Hassan IF and George S: Extra
corporeal membrane oxygenation to facilitate lung protective
ventilation and prevent ventilator-induced lung injury in severe
Pneumocystis pneumonia with pneumomediastinum: A case report and
short literature review. BMC Pulm Med. 16:522016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Wan Z, Yan X, Wang DG, Leng Y,
Liu Y, Zhang Y, Zhang H and Han X: Protective effect of Shenfu
injection preconditioning on lung ischemia-reperfusion injury. Exp
Ther Med. 12:1663–1670. 2016.PubMed/NCBI
|
|
14
|
Zheng P, Niu FL, Liu WZ, Shi Y and Lu LG:
Anti-inflammatory mechanism of oxymatrine in dextran sulfate
sodium-induced colitis of rats. World J Gastroenterol.
11:4912–4915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamashita H, Akamine S, Sumida Y, Inoue M,
Sawada T, Nagayasu T and Oka T: Inhaled nitric oxide attenuates
apoptosis in ischemia-reperfusion injury of the rabbit lung. Ann
Thorac Surg. 78:292–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haddad JJ, Olver RE and Land SC:
Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and
NF-kappa B redox sensitivity. Evidence for inhibition by
glutathione oxidation in alveolar epithelial cells. J Biol Chem.
275:21130–21139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu XH, Qiu YD, Shi MK and Ding YT:
Effects of matrine on cold ischemia and reperfusion injury during
orthotopic liver transplantation in rats. World Chin J Digestology.
14:1675–1680. 2006. View Article : Google Scholar
|
|
18
|
Shang L, Wang L, Sun H and Yang B:
Protective effects of oxymatrine on acute myocardial ischemia in
rats. J Harbin Med Univ. 39:124–126, 129. 2005.
|
|
19
|
Xu GL, Yao L, Rao SY, Gong ZN, Zhang SQ
and Yu SQ: Attenuation of acute lung injury in mice by oxymatrine
is associated with inhibition of phosphorylated p38
mitogen-activated protein kinase. J Ethnopharmacol. 98:177–183.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian X, Cong J and Sun C: SER Study on
oxidation sophora clear radical radical and protective effect of
thymidylate padiation. J Prev Med Chin People's Liberation Army.
14:412–415. 1996.(In Chinese). http://www.cnki.com.cn/Article/CJFDTotal-JYYX606.006.htm
|
|
21
|
Lammi MJ, Elo MA, Sironen RK, Karjalainen
HM, Kaarniranta K and Helminen HJ: Hydrostatic pressure-induced
changes in cellular protein synthesis. Biorheology. 41:309–313.
2004.PubMed/NCBI
|
|
22
|
Kamal A, Boehm MF and Burrows FJ:
Therapeutic and diagnostic implications of Hsp90 activation. Trends
Mol Med. 10:283–290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blagosklonny MV: Hsp-90-associated
oncoproteins: Multiple targets of geldanamycin and its analogs.
Leukemia. 16:455–462. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DeMeester SL, Buchman TG, Qiu Y, Jacob AK,
Dunnigan K, Hotchkiss RS, Karl I and Cobb JP: Heat shock induces
IkappaB-alpha and prevents stress-induced endothelial cell
apoptosis. Arch Surg. 132:1283–1287; discussion 1287–1288. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang YY, Peng YZ and Zhao XH: The research
of heat shock protein 90's expression in hypoxic rat myocardial
cells. Program Mod Biomed. 7:486–488. 2007.(In Chinese). http://www.cnki.com.cn/Article/CJFDTotal-SWCX200704003.htm
|