Introduction
Acute superior mesenteric venous thrombosis (ASMVT)
is an insidious disease with high mortality, accounting for 1 in
5,000 to 15,000 inpatient admissions, 1 in 1,000 emergency
department admissions and 6–9% of all cases of acute mesenteric
ischemia (1–3). ASMVT was first reported by Elliot
(4), and subsequently Warren and
Eberhard indicated that it could be a cause of intestinal
infarction distinct from mesenteric arterial occlusion (5).
Although the prognosis has benefited from
improvements in diagnostic methods, particularly in
contrast-enhanced computed tomography (CT), the prognosis of ASMVT
is greatly improved by early diagnosis and early initiation of the
treatment. Overall, ASMVT remains an intractable disease with
unsatisfactory outcome. The requirement of laparotomy and the
mortality rate following therapy with traditional anticoagulant
medicines, including unfractionated heparin (UFH), low molecular
weight heparin (LMWH) and warfarin, remain high (2,6). The
application of these medications is limited due to the occurrence
of heparin-associated thrombocytopenia (7).
Thrombectomy is a useful procedure applied in acute
large vessel thrombosis (8,9). While transcatheter thrombolysis has
been recently considered to be another effective technique
(10,11), these procedures are invasive and not
substitutes for anticoagulation to diminish high rates of bleeding
(12). Prolonged transarterial
thrombolysis may even increase the potential risk of thrombosis or
embolization in the superior mesenteric artery (SMA), the SMA
branches and the common femoral artery near the arteriotomy site
(12). Therefore, these invasive
treatments are not substitutes for anticoagulation.
More effective and safe treatments are thus pursued.
Argatroban, a direct thrombin inhibitor, induces predictable
anticoagulant effects (13) and a
lower incidence of bleeding complications compared with heparin, as
observed in a rat model (14). In
addition, its hepatic clearance may be superior in ASMVT therapy
over other anticoagulants (15),
when adjusting the dose to achieve an activated partial
thromboplastin time (aPTT) to a target value. To the best of our
knowledge, only three case studies reporting the application of
argatroban in ASMVT and/or portal vein thrombosis (PVT) patients
have been published to date (16–18).
The present study presents 18 patients with early
initiation of argatroban therapy in the management of ASMVT over a
13-month period in a single institution. This is the first reported
series of argatroban therapy in ASMVT patients.
Patients and methods
Patients
The present retrospective study was approved by the
Institutional Review Board of the First Affiliated Hospital of
Chongqing Medical University (Chongqing, China). The medical
records of the Department of Vascular Surgery (the First Affiliated
Hospital of Chongqing Medical University) were searched to identify
patients with admitting diagnosis of ASMVT between March 2013 and
April 2014. A total of 18 consecutive adult patients with ASMVT who
received argatroban as the exclusive anticoagulant were evaluated
retrospectively. The selection of alternative anticoagulant agent
was at the discretion of the treating physician. The potential
risks and benefits of the anticoagulant were explained, and
informed consent was obtained from each patient and relatives.
Excluded cases included therapy with other anticoagulants or
transcatheter thrombolysis, incomplete medical records or imaging
data, incomplete follow-up, or allergy to anticoagulant or
thrombolytic agents. In total, 26 patients were excluded from the
current study.
Data collection
Information was collected regarding the age, gender,
past medical history, presenting symptoms and signs, anticoagulant
therapy details, operative details, CT scan, outcome of therapy,
complications, length of hospital stay and follow-up details of the
patients. The follow-up period was between discharge until April
2015.
Treatment, response assessment and
follow-up
Once the diagnosis was confirmed by
contrast-enhanced CT, all these patients began anticoagulant
therapy with argatroban. Continuous intravenous argatroban
(Novastan; Mitsubishi Pharma Co., Tokyo, Japan) was initiated at 2
µg/kg/min in patients without hepatic dysfunction, and the initial
argatroban dose was 0.5 µg/kg/min in hepatic dysfunction patients.
The aPTT was measured prior to and 2 h after argatroban
administration, and the dosage was adjusted until the aPTT was
1.5–3.0 times the baseline aPTT value. Next, the aPTT was measured
daily, at 2 h after each dosage adjustment. Hepatic dysfunction was
defined as a total serum bilirubin level of >25.5 µmol/l (1.5
mg/dl), aspartate aminotransferase level of >100 IU/l, or
alanine aminotransferase level of >100 IU/l (19).
In addition, all patients underwent physical
examination twice a day, along with evaluation of white blood cell
counts and hepatic function test. The non-operative therapy
included nasogastric suction, broad-spectrum prophylactic
antibiotics and total parenteral nutrition. If intestinal
infarction was suspected due to clinical manifestation or
radiologic findings, laparotomy was performed.
The mean time for symptoms to be alleviated was
6.3±3.7 days, as presented in Table
I. Following the alleviation of symptoms, patients began
enteral nutrition support and contrast-enhanced CT was performed
for therapeutic effect evaluation. The degree of thrombus lysis was
divided into three levels based on CT examination: No lysis,
partial lysis and complete lysis. No lysis was defined as thrombus
removal <50% or worsening of the patient's condition. Partial
lysis indicated 50–90% clot removal. Complete lysis represented
>90% clot removal. The degree of lysis in each case was
determined by two radiologists independently (10). Once clinical and radiographic
improvement was noted, warfarin (Qilu Pharmaceutical Co., Ltd.,
Jinan, China) therapy was initiated at a dose of 2.5 mg/as a
long-term therapy, outside of hospital treatment (20). When the international normalized
ratio (INR) reached the target range of 2–3, argatroban was
discontinued and warfarin alone was continued. Subsequently, the
patient was discharged from hospital. The duration of oral
anticoagulation was ~6 months for patients with known reversible
factors, however this treatment was lifelong for patients who
suffered from the idiopathic or with prothrombotic states (20). Recurrence was defined as the symptoms
recurring due to the disease, while other causes were ruled out,
and when the CT scan indicated that the thrombus was not
significantly altered or was even worsened compared with the
observation at hospital discharge.
 | Table I.Clinical characteristics, disease
causes, CT scan details and disease course of 18 ASMVT
patients. |
Table I.
Clinical characteristics, disease
causes, CT scan details and disease course of 18 ASMVT
patients.
| Parameter | Value |
|---|
| Age, years | 50.9±13.9
(30–72) |
| Gender
(male/female), n | 12/6 |
| Thrombophilia,
n |
|
| Protein
C or S deficiency | 2 |
|
Antithrombin III
deficiency | 1 |
|
Malignancy | 2 |
|
Historical venous
thromboembolism | 3 |
| Local factors
causing vessel wall injury, n |
|
|
Intra-abdominal surgery | 6 |
|
Pancreatitis | 2 |
| Patients with
cirrhosis, n |
|
|
Hepatitis B virus | 4 |
|
Hepatitis C virus | 1 |
|
Alcohol | 1 |
| Clinical
presentation, n (%) |
|
|
Abdominal pain | 18 (100) |
|
Distention | 9 (50) |
|
Nausea | 4 (22) |
|
Melena | 3 (17) |
|
Ileus | 3 (17) |
|
Fever | 2 (11) |
|
Lumbodorsal pain | 1 (6) |
|
Emesis | 1 (6) |
|
Peritonitis | 1 (6) |
| Vessel with
thrombosis (CT scan), n |
|
|
SMV | 2 |
|
SMV+PV | 10 |
|
SMV+PV+SV | 6 |
| Symptom onset to
treatment, days | 5.0±3.5 |
| Surgery, n (%) | 1 (5.9) |
| Length of hospital
stay, days | 16.4±7.6 |
| Treatment to
symptom remission, days | 6.3±3.7 |
Complications
Bleeding events were recorded in all patients. Major
bleeding was defined as: i) Fatal bleeding; and/or ii) symptomatic
bleeding in a critical area or organ, such as intracranial,
intraspinal, intraocular, retroperitoneal, intraarticular or
pericardial, or intramuscular with compartment syndrome; and/or
iii) bleeding causing a reduction in hemoglobin level of at least
20 g/l (1.24 mmol/l), or leading to the transfusion of two or more
units of whole blood or red cells (21). Minor bleeding was any overt bleeding
not fitting the major bleeding definition (22,23).
When major bleeding occurred, the administration of argatroban was
terminated immediately and was followed by transfusion therapy
according to the degree of anemia. When minor bleeding occurred,
the administration of argatroban was terminated at the
investigator's discretion. The aPTT, INR and hemoglobin level were
monitored in both situations.
Statistical analysis
A descriptive retrospective study was conducted. The
present study did not include tests of statistical significance due
to the limited number of patients. All collected variables were
used in descriptive statistical analysis. Numerical data were
summarized by means of standard statistics (i.e. mean, standard
deviation, minimum, median and maximum). Data were analyzed using
SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Etiology and clinical
presentation
A total of 18 ASMVT patients (6 females and 12
males), with a mean age of 50.9±13.9 years (between 30 and 72
years) and a mean weight of 60.7±8.2 kg, were included in the
present study. The etiology of ASMVT and the presenting symptoms of
the 18 patients are displayed in Table
I. In addition, the mean time for symptom remission was 6.3±3.7
days, and the mean length of hospital stay was 16.4±7.6 days.
Dose-response of argatroban
In total, 16 patients without hepatic dysfunction
underwent anticoagulant therapy with argatroban for a mean dose of
1.57±0.34 µg/kg/min and mean treatment duration of 12.2±3.7 days
(between 7 and 18 days), and the aPTT was elevated 1.95±0.26 times
over the baseline value. In addition, 2 hepatic dysfunction
patients underwent therapy with an initial dose of 0.5 µg/kg/min,
and a mean dose of 0.41 µg/kg/min for 9 days and 0.45 µg/kg/min for
6 days, achieving an aPTT of 1.68 and 1.62 times higher than the
baseline value, respectively (Table
II). In these 2 patients, the serum bilirubin level was
increased to 108.1 µmol/l and 39.3 µmol/l prior to treatment, thus
the initial dose was adjusted to 0.5 µg/kg/min.
 | Table II.Dose-response of argatroban. |
Table II.
Dose-response of argatroban.
|
|
|
| aPTT (sec) | INR |
|
|
|---|
|
|
|
| aPTT (sec) | INR |
|
|
|---|
| Patient | Cirrhosis | Hepatic
dysfunction | Prior to argatroban
therapy | Following
argatroban therapy | Mean value during
therapy | Prior to argatroban
therapy | Following
argatroban therapy | Starting dose
(µg/kg/min) | Mean dose
(µg/kg/min) | Duration of therapy
(days) |
|---|
| 1 | − | − | 32.6 | 58.7 | 65.7 | 1.17 | 1.31 | 2 | 1.19 | 18 |
| 2 | − | − | 26.7 | 63.2 | 61.8 | 0.99 | 1.55 | 2 | 2 | 12 |
| 3 | − | − | 24.5 | 68.3 | 60.0 | 1.26 | 1.61 | 2 | 1.52 | 12 |
| 4 | + | + | 40.5 | 84.7 | 68.1 | 1.39 | 2.32 | 0.50 | 0.41 | 9 |
| 5 | − | − | 32.6 | 92.1 | 64.0 | 1.07 | 1.76 | 2 | 1.33 | 14 |
| 6 | − | − | 43.3 | 71.1 | 72.0 | 1.21 | 1.55 | 2 | 1.05 | 16 |
| 7 | − | − | 34.9 | 66.5 | 69.9 | 1.15 | 1.66 | 2 | 2 | 8 |
| 8 | − | − | 28.2 | 66.1 | 67.3 | 1.28 | 1.64 | 2 | 1.61 | 14 |
| 9 | − | − | 41.4 | 67.8 | 73.6 | 1.18 | 1.63 | 2 | 2 | 12 |
| 10 | + | − | 44.2 | 76.4 | 72.2 | 1.48 | 1.82 | 2 | 1.13 | 7 |
| 11 | − | − | 36.4 | 79.1 | 75.8 | 1.28 | 2.49 | 2 | 1.54 | 10 |
| 12 | + | + | 42.1 | 59.4 | 68.4 | 1.31 | 1.5 | 0.50 | 0.45 | 6 |
| 13 | − | − | 39.9 | 62.4 | 77.9 | 0.98 | 1.58 | 2 | 1.53 | 15 |
| 14 | − | − | 45.3 | 68.3 | 74.4 | 1.08 | 1.48 | 2 | 2 | 7 |
| 15 | − | − | 41 | 76.9 | 74.8 | 1.42 | 1.52 | 2 | 1.21 | 7 |
| 16 | + | − | 39 | 76.2 | 78.9 | 1.22 | 2.57 | 2 | 1.58 | 13 |
| 17 | + | − | 45.4 | 78.7 | 74.7 | 1.08 | 1.48 | 2 | 1.41 | 18 |
| 18 | + | − | 39.8 | 77.1 | 70.5 | 1.32 | 1.78 | 2 | 2 | 12 |
| Total | 6+/12- | 2+/16- | 37.7±6.4 | 71.8±9.0 | 70.6±5.4 | 1.22±0.14 | 1.74±0.36 | 1.83±0.49 | 1.44±0.49 | 11.8±3.6 |
Details of in-hospital surgery
Intestinal resection surgery was performed in 2/18
patients (11%). One of these patients had been suspected to have
intestinal infarction upon admission to our hospital, and underwent
laparotomy within 12 h of admission. Extensive intestinal necrosis
was found during the surgical procedure, and thus resection of 130
cm of the small bowel and an end-to-end intestinal anastomosis were
performed. The other patient had peritonitis on the 4th day after
admission, and underwent laparotomy and resection of 90 cm of the
jejunum for localized bowel necrosis (Table III). As the intestinal necrosis of
the first patient was not ascribed to the failure of argatroban
therapy, the incidence of laparotomy during hospitalization was
considered to be 6% (1/17 patients).
 | Table III.Therapeutic evaluation, complications
and follow-up data of argatroban treatment. |
Table III.
Therapeutic evaluation, complications
and follow-up data of argatroban treatment.
| Patient | Surgery
in-hospital | Clinical
outcome | Thrombolysis
degree | Complications | Follow-up duration
(months) | Outcome of
follow-up |
|---|
| 1 | N/A | Elimination of
symptoms | Partial | N/A | 18 | Surgical treatment
for jejunum perforation, but no recurrence |
| 2 | N/A | Clinical
improvement | Partial | N/A | 18 | No recurrence |
| 3 | N/A | Clinical
improvement | Partial | N/A | 15 | No recurrence |
| 4 | N/A | Elimination of
symptoms | None | Hematochezia | 18 | Recurrence |
| 5 | N/A | Elimination of
symptoms | Partial | N/A | 14 | No recurrence |
| 6 | N/A | Clinical
improvement | Partial | N/A | 15 | No recurrence |
| 7 | N/A | Elimination of
symptoms | Partial | N/A | 12 | No recurrence |
| 8 | Resection of 130 cm
of the small bowel | Clinical
improvement | Partial | N/A | 12 | No recurrence |
| 9 | N/A | Clinical
improvement | Partial | N/A | 12 | No recurrence |
| 10 | N/A | Clinical
improvement | Partial | N/A | 15 | No recurrence |
| 11 | N/A | Clinical
improvement | Complete | N/A | 24 | No recurrence |
| 12 | N/A | No improvement | None | Hematochezia | 6 | Recurrence and
mortality |
| 13 | Resection of 90 cm
of the jejunum | Clinical
improvement | Partial | N/A | 20 | No recurrence |
| 14 | N/A | Clinical
improvement | Partial | N/A | 20 | No recurrence |
| 15 | N/A | Elimination of
symptoms | Partial | N/A | 20 | No recurrence |
| 16 | N/A | Elimination of
symptoms | Partial | N/A | 18 | No recurrence |
| 17 | N/A | Clinical
improvement | Partial | N/A | 15 | Surgical treatment
for intestinal obstruction, but no recurrence |
| 18 | N/A | Clinical
improvement | Partial | N/A | 20 | No recurrence |
Clinical improvement and bleeding
episodes
Clinical symptom improvement or elimination was
observed in 94% (17/18) of the patients, and no mortality occurred
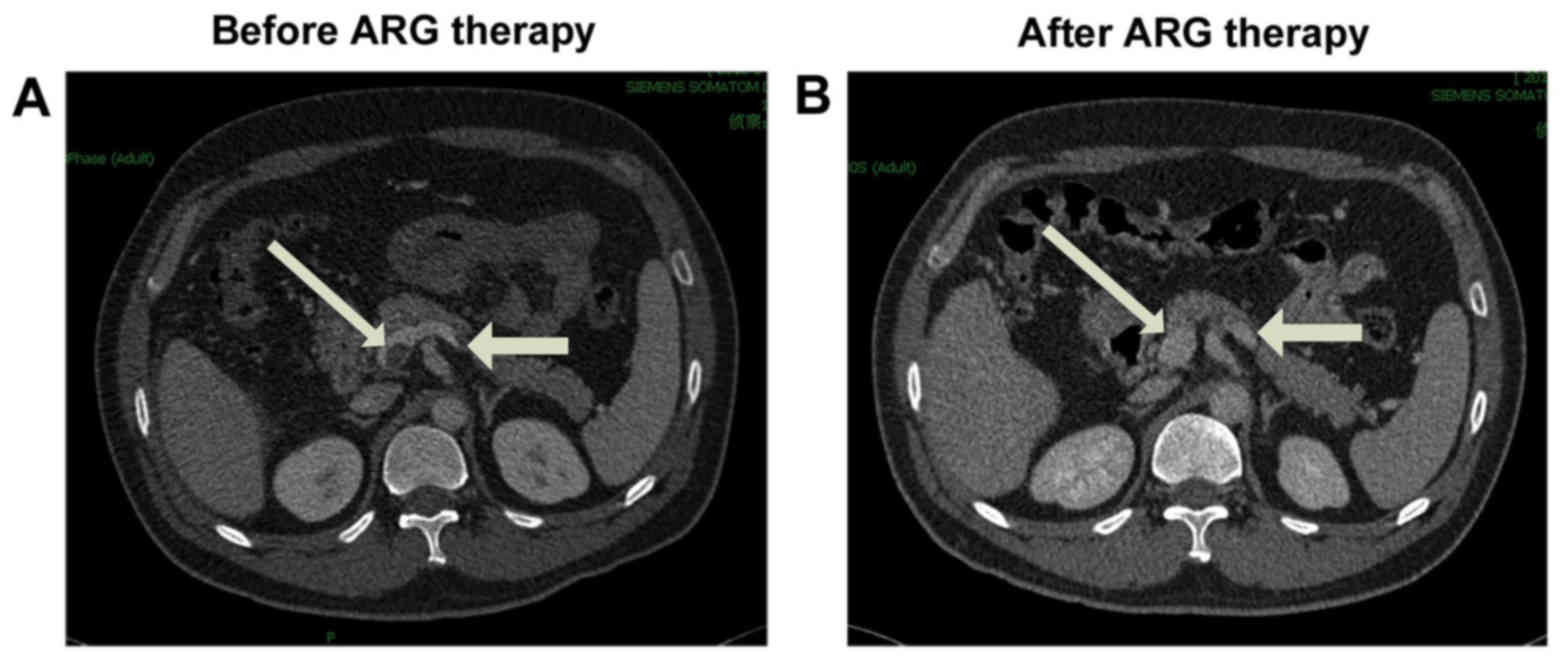
during the hospitalization period. Contrast-enhanced CT images
indicated that the thrombus was partially dissolved when compared
with the previous CT scan in 83% (15/18) of patients, dissolved
completely in 6% (1/18) of patient (Fig.
1), and was not significantly altered in the remaining 11%
(2/18) of patients (Table
III).
No major bleeding or mortality secondary to bleeding
occurred. However, minor bleeding complications occurred in 2
patients (11%) during anticoagulation therapy with argatroban
(Table III). Both cases presented
hematochezia, thus argatroban treatment was terminated, and aPTT,
INR and hemoglobin levels were monitored daily. No transfusion was
required in these patients. When the hemorrhage stopped, argatroban
therapy was continued, with no further bleeding episodes observed
during the remaining of the treatment.
Follow-up results
The median duration of follow-up was 16.2 months
(range, 6–24 months). The contrast-enhanced CT examination as the
main method was applied in evaluating the recurrence. Overall,
recurrence was observed in 11% (2/18) of patients, with 1 patient
succumbing to the disease during follow-up; thus, the mortality
rate was 6% (1/18 patients). These 2 recurrence cases had received
the lower adjusted initial dose of argatroban therapy for hepatic
dysfunction, and 1 of these patients received a shorter duration of
anticoagulant therapy due to minor bleeding, which may have
resulted in an unsatisfactory thrombolysis degree at hospital
discharge.
The patient who succumbed 6 months after treatment
did not achieve optimal therapy effect during the hospitalization
period; however, the abdominal symptoms were relieved gradually
after hospital discharge. However, recurrence occurred, which
resulted in intestinal infarction and septic shock, leading to
mortality.
Another patient required further surgery 1 month
following discharge for intestinal obstruction. A segmental
inflammatory structured intestine ~10 cm in length with extensive
inflammatory adhesion was detected during the surgery. Resection of
30 cm of the small bowel was thus performed. No ASMVT recurrence
was indicated by the contrast-enhanced CT scanning, but these
inflammatory changes were likely to be a result of SMVT.
Furthermore, another patient received jejunum perforation repair
after 1 month. It is difficult to determine whether the jejunum
perforation was secondary to SMVT or an independent event, since
the contrast-enhanced CT imaging showed no recurrence (Table III).
Discussion
ASMVT is an insidious disease that is difficult to
diagnose. The most common symptoms include abdominal pain,
distention and nausea, which are not specific symptoms and easily
mimic other diseases. The duration of symptoms prior to treatment
initiation significantly influences the outcome in patients with
AMVT, including the mortality rate, possibility of needing a
laparotomy and the long-term prognosis (9,24). With
the assistance of contrast-enhanced CT in recent years, the time
from symptom presentation to diagnosis has decreased from an
average of 1 week during 1978–1995 to ~1 day during 1995–2003
(5). However, due to the lack of a
standard diagnosis and treatment strategy and the imbalance
distribution of medical resources amongst other issues in China,
the interval between symptom presentation and treatment is even
longer. This interval was found to be 5.0±3.5 days in the present
study study.
Even when ASMVT is diagnosed earlier, treatment does
not necessarily result in a more satisfactory outcome. Currently,
anticoagulation is widely accepted as the first-choice therapy in
ASMVT patients (25). Immediate
heparinization using UFH or LMWH upon diagnosis of SMVT is
considered to be the current standard therapy even in certain
patients with bleeding due to surgery or SMVT-induced ischemia.
However, the majority of the relevant data published to date
demonstrate that UFH or LMWH as traditional anticoagulant medicine
present certain limitations in SMVT-PVT therapy, such as
heparin-associated thrombocytopenia, high incidence of laparotomy
requirement and high mortality rate (2,5). As
presented in Table IV, which
summarizes the findings of the present and selected previous
studies, the incidence of laparotomy is as high as 0–56%, and the
30-day in-hospital mortality ranges between 0 and 25%.
 | Table IV.Selected studies reporting the
treatment and outcome of acute mesenteric venous thrombosis. |
Table IV.
Selected studies reporting the
treatment and outcome of acute mesenteric venous thrombosis.
| First author | Median study
year | N | Mean age
(years) | Mean hospital stay
(days) | Surgery (%) | Resection (%) | Anticoagulation
therapy (%) | Anticoagulant | Mortality (%) | Ref. |
|---|
| Morasch | 1992 | 31 | 49.1 | N/A | 35.5 | 32.3 | 71.0 | UFH | 22.6%a | 30 |
| Alvi | 1996 | 20 | 55.6 | N/A | 40 | 40 | 100 | UFH | 20% | 24 |
| Brunaud | 1997 | 12 | 56.7 | 23.2±8.4 | 8.3 | 8.3 | 100 | UFH | 25.0% | 2 |
| Zhang | 1999 | 28 | 45 | 12.6±4.6 | 32.1 | 17.9 | 100 | UFH+LMWH | 11.0% | 6 |
| Joh | 1999 | 6 | 45 | 31 | 0 | 0 | 100 | UFH or LMWH | 0% | 31 |
| Muñoz | 2000 | 13 | 68 | N/A | 38.5 | N/A | 69.2 | UFH | 38.5%b | 32 |
| Cenedese | 2005 | 9 | 57 | N/A | 55.6 | 55.6 | 100 | N/A | 0% | 33 |
| Present study | 2014 | 18 | 50.9 | 16.4±7.6 | 11.1 | 11.1 | 100 | ARG | 0% | − |
Transcatheter thrombolysis has been recently
reported to be a more effective alternative in the management of
ASMVT (10,11). The catheter can be inserted via
percutaneous transhepatic (26),
transfemoral (12), transjugular
(27), or transarterial (in the SMA)
approaches (12,28). However, numerous hospitals do not
have the ability to apply these techniques due to the high
technical requirement. In addition, transjugular intrahepatic
portosystemic shunt is only recommended when anticoagulation is
unsuccessful and the clinical condition worsens (20), due to the technical difficulties in
constructing the shunt in the absence of a normal anatomy of the
portal and the hepatic vein systems in hepatic and portal vein
thrombosis patients (29). The
indications include extensive thromboses, severe symptoms, and
persistent or worsening symptoms despite anticoagulation (11), and thus the use of this technique is
limited and can not be administered to all patients. Furthermore,
despite published data only available from specific case reports
and small case series, a remarkable high rate of bleeding was
noticed, especially via the transhepatic route. For instance, the
series reported by Hollingshead et al revealed that 75% of
patients (n=15) had partial or complete clot resolution; however,
60% of patients (n=12) developed a major complication, with
bleeding the most common complication (12). Therefore, a more effective and safe
anticoagulation method is pursued.
In recent years, argatroban, a direct thrombin
inhibitor, has been successfully used in numerous thrombotic
diseases. Compared with other anticoagulants, argatroban has unique
attributes that contribute to its safety and efficacy, including
its small molecular weight, peptidomimetic structure, reversible
binding to thrombin and nonimmunogenic nature (7). Furthermore, argatroban can be
differentiated from other anticoagulants by its hepatic (not renal)
clearance (15). In consideration of
these characteristics, argatroban may be considered to have certain
superiorities in ASMVT therapy over other anticoagulants.
To the best of our knowledge, this is the first
reported series to highlight the effectiveness and safety of
argatroban therapy in ASMVT patients. The incidence of surgery and
bowel resection, and the in-hospital mortality in the current study
were found to be 6 (excluding a case presenting intestinal necrosis
upon admission, as mentioned earlier) and 0%, respectively. These
rates are lower compared with those reported in recent studies
involving anticoagulant therapy initiated with UFH or/and LMWH in
ASMVT patients within the last few years (Table IV) (2,6,24,30–33).
This indicates that argatroban can effectively avoid bowel
infarction and mortality in ASMVT patients. Furthermore, the mean
time from treatment initiation to symptom remission in the current
study was 6.3±3.7 days, which indicated the timely onset of the
anticoagulation effect induced by argatroban.
Only 1 patient (5%) had complete thrombolysis in the
present study, which is lower than the 15.0% rate reported by
Hollingshead et al (12), or
the 53.8 and 62.5% rates reported by Yang et al (10,11)
conducted via transcatheter thrombolysis and aspiration
thrombectomy therapies, respectively. However, if partial
thrombolysis cases are considered, then 89% patients in the current
study exhibited complete or partial thrombolysis, with 17 (94%)
cases having clinical improvement or elimination of symptoms;
therefore, this rate is not markedly different from previous
studies that similarly presented conclusions which considered
partial and complete thrombolysis. However, thrombolysis results in
significantly higher risk of bleeding. For instance, 60% of
patients in the study by Hollingshead et al (12) experienced bleeding or decreased
hematocrit level, while 23.1 and 25% of patients experienced
bleeding complications in the studies by Yang et al
(10,11).
Bleeding is the primary adverse effect associated
with anticoagulation therapy. The incidence of major bleeding in
previous studies involving argatroban and UFH used in
heparin-induced thrombocytopenia (HIT; 6.9 vs. 6.7%) (23), in HIT with thrombosis syndrome (5.7
vs. 7.0%) (34), in percutaneous
coronary intervention (0% vs. 3.0%) (35), and in acute myocardial infarction
(19% vs. 20%) (36) do not reveal
apparent differences in major bleeding; however, in accordance with
various studies, argatroban improves the clinical outcome (23,34,35,36).
The recommendation for the initial dose of
argatroban in HIT is 2 µg/kg/min (0.5 µg/kg/min in hepatic
dysfunction patients), adjusted to achieve an aPTT value 1.5–3
times of the patient's baseline aPTT (23,34).
However, limited data exist regarding dosing patterns, course and
safety of argatroban therapy in ASMVT patients. In the current
study, the most commonly used initial dose was administered,
adjusted to maintain the aPTT at 1.5–3 times the baseline value,
and the results presented were satisfactory. Only 2 patients (11%)
experienced bleeding complication, and no major bleeding occurred
in the current study.
Certain patients included in the current series also
suffered from cirrhosis and pancreatitis. Whether cirrhosis
patients with portomesenteric venous thrombosis require
anticoagulation therapy is controversial due to the high risk of
bleeding (37,38). It was also reported that a patient
with PVT and acute pancreatitis developed severe hematemesis due to
UFH (39). In the present study,
there was also a patient with evidently elevated serum bilirubin
level, who presented minor bleeding in spite of the initial dose
being administered at 0.5 µg/kg/min and adjusted to 0.41 µg/kg/min.
The elimination half-life of argatroban is increased when other
cofactors are present, such as hepatic dysfunction, renal
insufficiency and critical illness (13). Further studies have proven that lower
initial dosage is indicated for specific patient populations. An
initial dose of 0.2 µg/kg/min in critically-ill patients with
multiple organ dysfunction was sufficient and safe for achieving
effective anticoagulation (40).
However, the extent of association among dose, therapeutic effect
and bleeding risk in hepatic dysfunction patients may not be simply
concluded owing to the limited sample size in the current study,
and thus further studies are required.
All 18 patients were followed up for a median time
of 16.2 months, and received at least 6 months of
oral-anticoagulant therapy with warfarin, as the majority of
studies recommend (3). However,
during the follow-up performed in the present study, 2 patients
(11%) presented SMVT recurrence, including 1 patient (5%) who
succumbed to the disease. This recurrence may be due to various
reasons. First, the 2 recurrence patients underwent therapy with an
initial dose of 0.5 µg/kg/min lasting for 9 and 6 days; thus, the
anticoagulation time was slightly shorter compared with that in
other patients in the current study, which may result in
insufficient anticoagulation therapy and unsatisfactory thrombus
dissolution upon hospital discharge. In addition, both recurrence
patients presented ASMVT combined with cirrhosis and hepatic
dysfunction. Whether such ASMVT patients have a higher risk of
recurrence remains unknown.
The present study has several limitations. Firstly,
the study does not provide an answer to the question of whether
argatroban is a better option for ASMVT patients due to the absence
of a control group, such as a group receiving interventional
treatment or anticoagulant therapy with another medicine. A
single-center randomized clinical trial on argatroban and LMWH in
ASMVT therapy is currently conducted. Furthermore, as a result of
the retrospective and cross-sectional nature, and the small number
of patients included in the current study, the dosage, course and
target value of argatroban therapy remains unknown. Further
investigation is required to improve the understanding and
management of argatroban therapy in ASMVT patients.
In conclusion, argatroban therapy is effective and
safe in patients with ASMVT. It may be another feasible
anticoagulant in ASMVT therapy, which is beneficial in that it can
rapidly improve symptoms, with low incidence of bowel resection or
bleeding complication, and a low mortality rate. However,
random-controlled trials on the use of argatroban and other
anticoagulants or interventional treatment are needed. In addition,
the optimal dose, course and target value of argatroban need to be
further researched.
References
|
1
|
Rhee RY and Gloviczki P: Mesenteric venous
thrombosis. Surg Clin North Am. 77:327–338. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brunaud L, Antunes L, Collinet-Adler S,
Marchal F, Ayav A, Bresler L and Boissel P: Acute mesenteric venous
thrombosis: Case for nonoperative management. J Vasc Surg.
34:673–679. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harnik IG and Brandt LJ: Mesenteric venous
thrombosis. Vasc Med. 15:407–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elliot JW: II. The operative relief of
gangrene of intestine due to occlusion of the mesenteric vessels.
Ann Surg. 21:9–23. 1895. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Warren S and Eberhard TP: Mesenteric
venous thrombosis. Surg Gynecol Obstet. 61:102–121. 1935.
|
|
6
|
Zhang J, Duan ZQ, Song QB, Luo YW, Xin SJ
and Zhang Q: Acute mesenteric venous thrombosis: A better outcome
achieved through improved imaging techniques and a changed policy
of clinical management. Eur J Vasc Endovasc Surg. 28:329–334. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeske WP, Fareed J, Hoppensteadt DA, Lewis
B and Walenga JM: Pharmacology of argatroban. Expert Rev Hematol.
3:527–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar S, Sarr M and Kamath PS: Mesenteric
venous thrombosis. N Engl J Med. 345:1683–1688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brandt LJ and Boley SJ: AGA technical
review on intestinal ischemia. American Gastrointestinal
Association. Gastroenterology. 118:954–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang S, Liu B, Ding W, He C, Wu X and Li
J: Acute superior mesenteric venous thrombosis: Transcatheter
thrombolysis and aspiration thrombectomy therapy by combined route
of superior mesenteric vein and artery in eight patients.
Cardiovasc Intervent Radiol. 38:88–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang SF, Liu BC, Ding WW, He CS, Wu XJ and
Li JS: Initial transcatheter thrombolysis for acute superior
mesenteric venous thrombosis. World J Gastroenterol. 20:5483–5492.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hollingshead M, Burke CT, Mauro MA, Weeks
SM, Dixon RG and Jaques PF: Transcatheter thrombolytic therapy for
acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol.
16:651–661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swan SK and Hursting MJ: The
pharmacokinetics and pharmacodynamics of argatroban: Effects of
age, gender, and hepatic or renal dysfunction. Pharmacotherapy.
20:318–329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berry CN, Girard D, Lochot S and Lecoffre
C: Antithrombotic actions of argatroban in rat models of venous,
‘mixed’ and arterial thrombosis, and its effects on the tail
transection bleeding time. Br J Pharmacol. 113:1209–1214. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hursting MJ, Alford KL, Becker JC, Brooks
RL, Joffrion JL, Knappenberger GD, Kogan PW, Kogan TP, McKinney AA
and Schwarz RP Jr: Novastan (brand of argatroban): A
small-molecule, direct thrombin inhibitor. Semin Thromb Hemost.
23:503–516. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muta T, Okamura T, Kawamoto M, Ichimiya H,
Yamanaka M, Wada Y, Urata M, Kayamori Y, Hamasaki N, Kato K, et al:
Successful therapy with argatroban for superior mesenteric vein
thrombosis in a patient with congenital antithrombin deficiency.
Eur J Haematol. 75:167–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dager WE, Gosselin RC and Owings JT:
Argatroban therapy for antithrombin deficiency and mesenteric
thrombosis: Case report and review of the literature.
Pharmacotherapy. 24:659–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Young SK, Al-Mondhiry HA, Vaida SJ,
Ambrose A and Botti JJ: Successful use of argatroban during the
third trimester of pregnancy: Case report and review of the
literature. Pharmacotherapy. 28:1531–1536. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine RL, Hursting MJ and McCollum D:
Argatroban therapy in heparin-induced thrombocytopenia with hepatic
dysfunction. Chest. 129:1167–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singal AK, Kamath PS and Tefferi A:
Mesenteric Venous Thrombosis. Mayo Clin Proc. 88:285–294. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schulman S and C; Subcommittee on Control
of Anticoagulation of the Scientific and Standardization Committee
of the International Society on Thrombosis and Haemostasis Kearon:
Definition of major bleeding in clinical investigations of
antihemostatic medicinal products in non-surgical patients. J
Thromb Haemost. 3:692–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doepker B, Mount KL, Ryder LJ, Gerlach AT,
Murphy CV and Philips GS: Bleeding risk factors associated with
argatroban therapy in the critically ill. J Thromb Thrombolysis.
34:491–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis BE, Wallis DE, Berkowitz SD, Matthai
WH, Fareed J, Walenga JM, Bartholomew J, Sham R, Lerner RG, Zeigler
ZR, et al: Argatroban anticoagulant therapy in patients with
heparin-induced thrombocytopenia. Circulation. 103:1838–1843. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alvi AR, Khan S, Niazi SK, Ghulam M and
Bibi S: Acute mesenteric venous thrombosis: Improved outcome with
early diagnosis and prompt anticoagulation therapy. Int J Surg.
7:210–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kearon C, Akl EA, Comerota AJ, Prandoni P,
Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali
F, et al: Antithrombotic therapy for VTE disease: Antithrombotic
Therapy and Prevention of Thrombosis(9th ed). American college of
chest physicians evidence-based clinical practice guidelines.
Chest. 141 2 Suppl:e419S–e496S. 2012.
|
|
26
|
Takahashi N, Kuroki K and Yanaga K:
Percutaneous transhepatic mechanical thrombectomy for acute
mesenteric venous thrombosis. J Endovasc Ther. 12:508–511. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakayama S, Murashima N and Isobe Y:
Superior mesenteric venous thrombosis treated by direct aspiration
thrombectomy. Hepatogastroenterology. 55:367–370. 2008.PubMed/NCBI
|
|
28
|
Poplausky MR, Kaufman JA, Geller SC and
Waltman AC: Mesenteric venous thrombosis treated with urokinase via
the superior mesenteric artery. Gastroenterology. 110:1633–1635.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riggio O, Ridola L, Lucidi C and Angeloni
S: Emerging issues in the use of transjugular intrahepatic
portosystemic shunt (TIPS) for management of portal hypertension:
Time to update the guidelines? Dig Liver Dis. 42:462–467. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morasch MD, Ebaugh JL, Chiou AC, Matsumura
JS, Pearce WH and Yao JS: Mesenteric venous thrombosis: A changing
clinical entity. J Vasc Surg. 34:680–684. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joh JH and Kim DI: Mesenteric and portal
vein thrombosis: Treated with early initiation of anticoagulation.
Eur J Vasc Endovasc Surg. 29:204–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muñoz S, Cubo P, González-Castillo J,
Nuevo JA, García-Lamberechts EJ and Sanz A: Superior mesenteric
venous thrombosis: A retrospective study of thirteen cases. Rev Esp
Enferm Dig. 96:385–394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cenedese A, Monneuse O, Gruner L, Tissot
E, Mennesson N and Barth X: Initial management of extensive
mesenteric venous thrombosis: Retrospective study of nine cases.
World J Surg. 33:2203–2208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lewis BE, Wallis DE, Leya F, Hursting MJ
and Kelton JG: Argatroban-915 Investigators: Argatroban
anticoagulation in patients with heparin-induced thrombocytopenia.
Arch Intern Med. 163:1849–1856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rössig L, Genth-Zotz S, Rau M, Heyndrickx
GR, Schneider T, Gulba DC, Desaga M, Buerke M, Harder S and Zeiher
AM: ARG-E04 study group: Argatroban for elective percutaneous
coronary intervention: The ARG-EO4 multi-center study. Int J
Cardiol. 148:214–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vermeer F, Vahanian A, Fels PW, Besse P,
Müller E, Van de Werf F, Fitzgerald D, Darius H, Puel J, Garrigou
D, et al: Argatroban and alteplase in patients with acute
myocardial infarction: The ARGAMI study. J Thromb Thrombolysis.
10:233–240. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Franchis R and V Faculty Baveno:
Revising consensus in portal hypertension: Report of the Baveno V
consensus workshop on methodology of diagnosis and therapy in
portal hypertension. J Hepatol. 53:762–768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
DeLeve LD, Valla DC and Garcia-Tsao G;
American Association for the Study Liver Diseases: Vascular
disorders of the liver. Hepatology. 49:1729–1764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park WS, Kim HI, Jeon BJ, Kim SH and Lee
SO: Should anticoagulants be administered for portal vein
thrombosis associated with acute pancreatitis? World J
Gastroenterol. 18:6168–6171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Beiderlinden M, Treschan TA, Görlinger K
and Peters J: Argatroban anticoagulation in critically ill
patients. Ann Pharmacother. 41:749–754. 2007. View Article : Google Scholar : PubMed/NCBI
|