Introduction
Duchenne muscular dystrophy (DMD), an X-linked
recessive disorder, is the most common muscular dystrophy that
affects approximately 1 in 3,500 newborn boys (1). Mutations of the dystrophin gene cause
an expression deficiency of dystrophin protein, and thus result in
muscle degeneration, necrosis and atrophy (2,3).
Furthermore, other mechanisms have been shown to serve key roles in
the process of muscle atrophy. Recently, an increasing number of
studies have focused on another prominent pathological feature of
DMD, the fibrosis of connective tissue, which was considered to be
compensatory for muscle cell loss (4–6).
Transforming growth factor-β (TGF-β) is a
multifunctional polypeptide factor that promotes tissue fibrosis,
cell growth and transformation. It is widespread in normal tissues,
particularly in the skeleton and platelets (7–9). In
humans, there are three subtypes of TGF-β, with TGF-β1 being the
most abundant subtype (10).
Connective tissue growth factor (CTGF) is also widespread in
endothelial, smooth muscle, fibroblast, cartilage and specific
tumor cells (11–13). It was revealed that TGF-β1 can
significantly increase the expression level of CTGF in human
foreskin fibroblasts, whereas CTGF can be important in the
proliferation of fibroblasts, chemotaxis, extracellular matrix
(ECM) production, vascular regeneration or other biological
activities (14–16). Previous studies have observed
overexpression of CTGF and TGF-β1 in patients with DMD (17–19).
Furthermore, the levels of TGF-β and CTGF were revealed to
correlate with fibrosis development in the skeletal muscle of DMD
patients or of X chromosome-linked muscular dystrophy (mdx) mouse
models of DMD (20,21). Inhibition of TGF-β1 or reduction of
CTGF expression levels can also reduce the fibrotic phenotype in
the mdx mouse model (22,23). Therefore, the present study aimed to
examine the association of the expression of CTGF and TGF-β1 with
the clinical severity of DMD in Chinese patients.
Subjects and methods
Subjects
Consecutive DMD patients admitted to the clinic of
the Department of Neurology at Xiangya Hospital (Central South
University, Changsha, China) between January 2013 and March 2014
were enrolled into the present study. A total of 35 suspected cases
were enrolled, of which 18 cases were confirmed to be DMD through
muscle biopsy and immunohistochemical methods. For pathological
diagnosis, immunohistochemical staining with monoclonal
anti-dystrophin antibody (1:10; Novocastra Laboratories Ltd.,
Newcastle, UK) was performed to establish whether dystrophin
protein expression in the muscle fiber membrane was severely or
completely absent (24).
In addition, 8 children who suffered from acute
trauma but did not present any neuromuscular diseases were
recruited from the Department of Orthopaedic Surgery as controls.
All controls were identified as healthy subsequent to routine
enzyme histochemical staining, as well as dystrophin protein
immunohistochemical examination. The present study was approved by
the Ethics Committee of Xiangya Hospital, and written informed
consent was obtained from the patients. All the muscle samples were
obtained from muscle biopsies and embedded in paraffin until
further use.
Clinical data
The medical history and physical examination details
of the DMD patients were recorded by two neurologists. The
following details were obtained: Onset age, symptoms, course of
disease, degree of muscle weakness and atrophy, pseudohypertrophy
signs, tendon reflexes, Gowers' sign, gait and family history.
ATPase and immunohistochemical
staining
ATPase (25,26) and immunohistochemical (27–29)
staining were conducted according to previously described methods,
using 8-µm sections from each sample. The primary antibodies used
were monoclonal anti-dystrophin (Novocastra; Leica Biosystems,
Wetzlar, Germany), polyclonal anti-CTGF and polyclonal anti-TGF-β1
(both from Beijing Biosynthesis Biotechnology Co., Ltd., Beijing,
China). The secondary antibody used was the horseradish
peroxidase-labeled goat anti-rabbit IgG antibody (P0448; Dako North
America, Inc., Carpinteria, CA, USA). An immunohistochemical kit
(Beijing Biosynthesis Biotechnology Co., Ltd.) and Image-Pro Plus
version 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA)
were used to quantitatively measure the integrated optical density
(IOD) value. The expression levels of CTGF and TGF-β1 in skeletal
muscle samples were quantitatively determined by an
streptavidin-peroxidase immunohistochemical method. Briefly, three
different visual fields were randomly selected under ×100
magnification light microscopy (30).
Assessment of the severity of DMD was conducted with
ATPase staining to observe muscle fiber pathology, according to
previously reported criteria (31,32).
Briefly, in each pathological section of the ATPase staining, a
semi-quantitative method was used to calculate the percentage of
opaque muscle fibers and degeneration (32) The samples were classified according
to the percentage of muscle fibers presenting necrosis, which
indicates the pathological severity of DMD, as follows: <10%
necrosis was classified as level 0, 10–40% as level 1, 40–70% as
level 2 and >70% as level 3 (32)
The degree of dystrophin protein expression loss was
determined compared with that in the control samples, using
immunohistochemical staining. The results were divided into the
following levels (32): Grade 0, no
expression; grade 1, low protein expression (<10%); grade 2,
small number of dystrophin-positive fibers (10–30%); grade 3,
diffuse expression (30–70%); grade 4, positive muscle fibers with
partial deletion, or mosaic distribution with dystrophin-positive
or -negative muscle fibers (70–99%); grade 5, normal expression
(100%); and grade 5+, enhanced expression (>100%).
Statistical analysis
SPSS version 19.0 software (IBM SPSS, Armonk, NY,
USA) was used for data analysis. IOD values between the groups were
compared with the Wilcoxon test. P<0.05 was used to indicate a
statistically significant difference. The association among CTGF
expression, TGF-β1 expression, pathological grading of severity and
grading of clinical severity was analysed by the Spearman rank
correlation analysis.
Results
Clinical data of DMD patients
All 18 cases of DMD patients were male, with an age
distribution between 3 and 13 years and a mean age of 6.88±2.33
years. There was an onset age distribution between 0 months and 6
years and a mean onset age of 2.24±1.58 years. Furthermore, the
course of disease distribution was between 2 and 7 years, and the
average course was 4.63±1.65 years. Amongst all the patients, there
were 3 children with a DMD family history (16.7%), 15 sporadic
cases (83.3%) and parents of 2 cases with a consanguineous marriage
history (11.1%). DMD patients demonstrated motor delay (50.0%),
floppy infant symptoms (11.1%), difficulty in standing up (5.6%),
frequent falling down (16.7%) and an abnormal gait (16.7%; Table I).
 | Table I.Clinical data of DMD patients. |
Table I.
Clinical data of DMD patients.
| Patient | Age (years) | Onset (years) | Course (years) | Family history | First symptom | Muscular force | Amyotrophy | Waddling gait | EMG |
|---|
| 1 | 3 | 0.7 | 2.3 | Y | Floppy infant | 4/4 | N | Y | – |
| 2 | 7.7 | 1.3 | 6.4 | N | Motor delay | 4/4- | Y | Y | M |
| 3 | 5.6 | 1.4 | 4.2 | N | Difficulty in
standing up | 4/4- | Y | Y | M |
| 4 | 6 | 1 | 5 | N | Frequent falling
down | 4/4 | Y | Y | M |
| 5 | 7 | 3 | 4 | N | Motor delay | 4/3 | Y | Y | M |
| 6 | 7.6 | 4 | 3.6 | N | Abnormal gait | 4/3 | Y | Y | M |
| 7 | 8 | 3 | 5 | N | Frequent falling
down | 4-/4- | Y | Y | M |
| 8 | 5 | 2 | 3 | N | Motor delay | 5/4 | Y | Y | M |
| 9 | 9 | 2 | 7 | N | Frequent falling
down | 4/4 | Y | Y | M |
| 10 | 6 | 3 | 3 | N | Abnormal gait | 4/4- | Y | Y | M |
| 11 | 10 | 4 | 6 | N | Motor delay | 4/4 | Y | Y | M |
| 12 | 6 | 2 | 4 | Y | Motor delay | 4/4- | Y | Y | M |
| 13 | 3 | 0 | 3 | Y | Floppy infant | 5/5 | N | Y | – |
| 14 | 7 | 2 | 5 | N | Motor delay | 4/4 | Y | Y | M |
| 15 | 7 | 1 | 6 | N | Abnormal gait | 4/4- | Y | Y | M |
| 16 | 13 | 6 | 7 | N | Motor delay | 4/4 | Y | Y | M |
| 17 | 6 | 4 | 2 | N | Motor delay | 4/4- | Y | Y | M |
| 18 | 7 | 0 | 7 | N | Motor delay | 3/3+ | Y | Y | M |
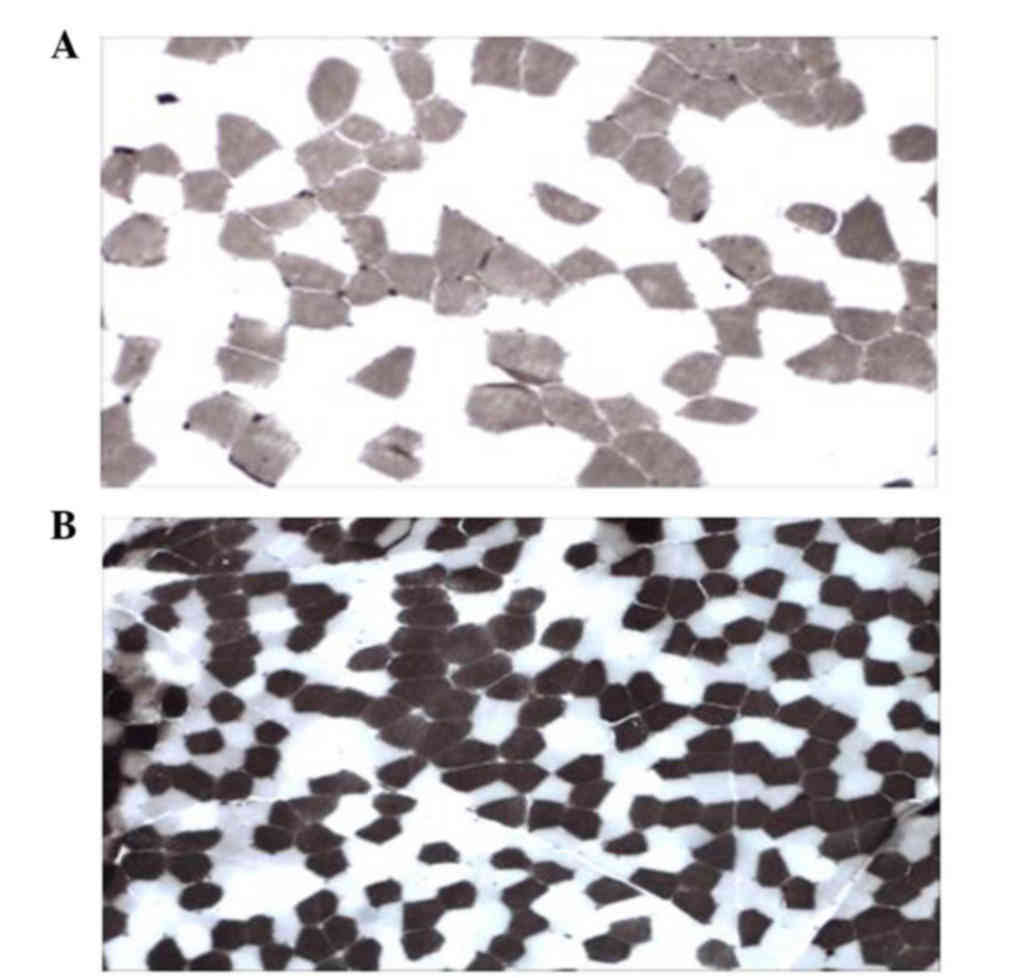
ATPase staining
The results of ATPase staining displayed two types
of muscle fibers, type I and II, which were visible with a mosaic
distribution in the DMD group (Fig.
1A) and the control (Fig. 1B)
groups. The fiber type grouping phenomenon in muscular fiber type I
or II was not identified. According to the aforementioned
classification, there were 2 cases with level 0, 5 cases with level
1, 6 cases with level 2 and 5 cases with level 3 in the 18 DMD
patients.
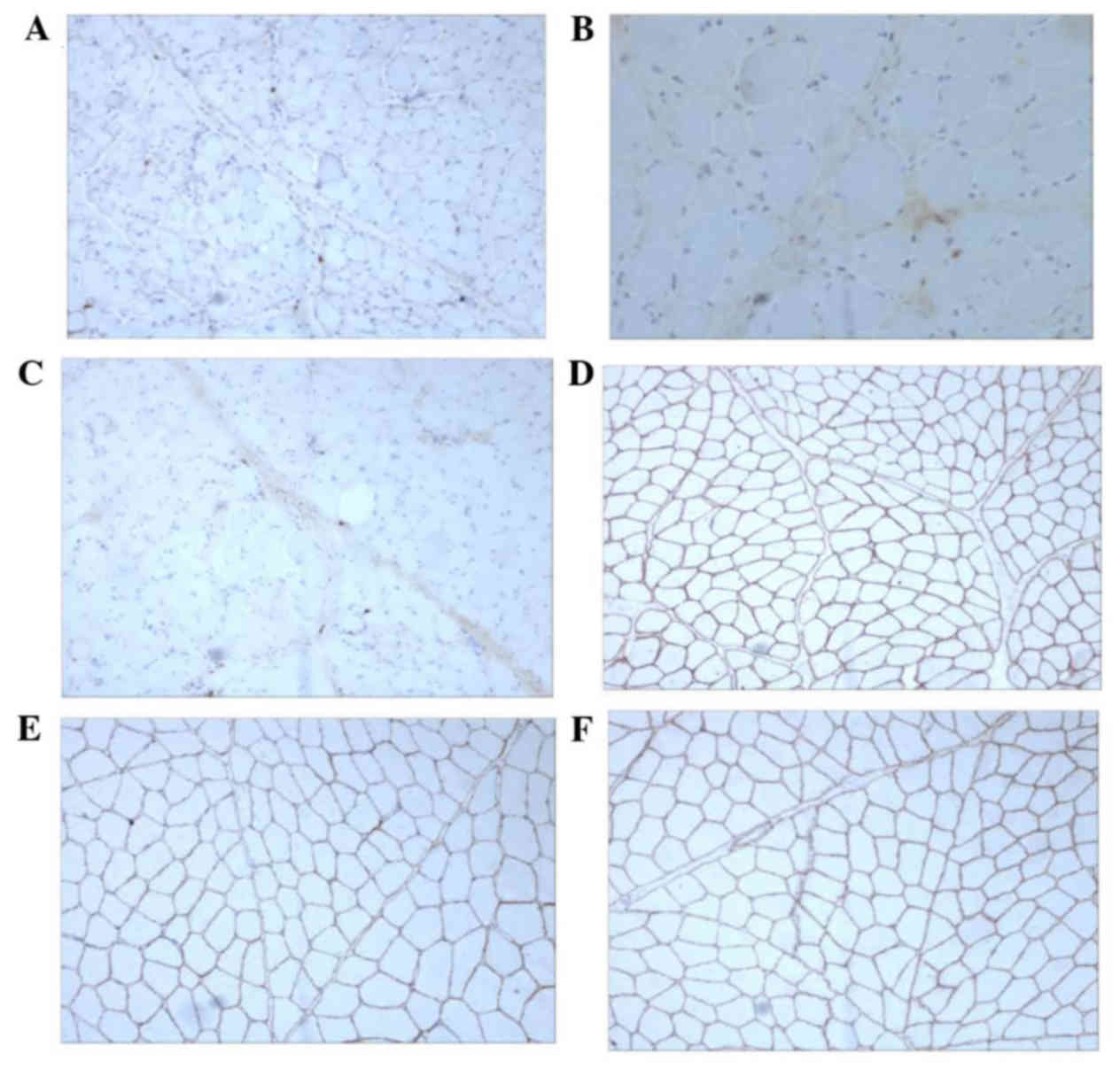
Dystrophin immunohistochemical
staining
For all 18 patients with DMD, the expression of
three antibodies against the Rod domain (DYS-1), C terminus
(DYS-2), and N terminus (DYS-3) of dystrophin (Fig. 2A-C, respectively) in the muscle fiber
membrane was severely lost or almost no expression was observed
(grades 0–2, including 13 cases with grade 0 and 5 cases with grade
1–2). By contrast, the control group presented a normal expression
(all 8 cases were classified as grade 5), with a consistent and
uniformly brown muscle fiber membrane (Fig. 2D-F).
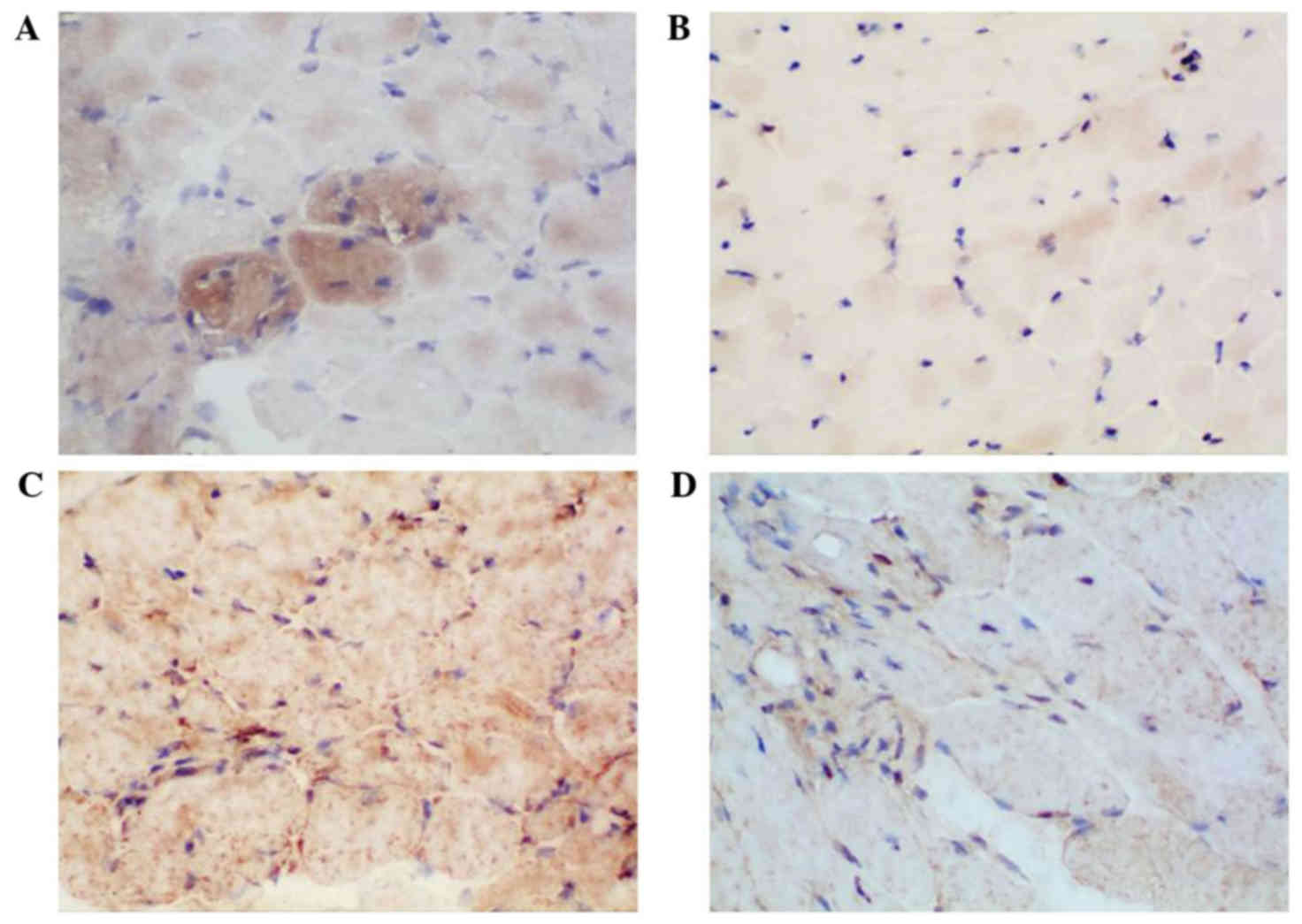
CTGF and TGF-β1 immunohistochemical
staining
As shown in Fig. 3,
the results of CTGF and TGF-β1 immunohistochemical staining in the
18 DMD patients indicated upregulated expression of CTGF and TGF-β1
in the muscle cell plasma and myenteric interstitium, with
yellowish brown staining of the cells observed, when compared with
the expression in the normal control group (Fig. 3B and D). The IOD values of those two
proteins demonstrated a statistically significant difference
between the two groups (P<0.05; Table II).
 | Table II.Expression levels of CTGF and TGF-β1
between groups. |
Table II.
Expression levels of CTGF and TGF-β1
between groups.
| Parameter | DMD (n=18) | Control (n=8) | P-value |
|---|
| CTGF |
35,596.80±21,653.86 | 312.71±243.72 |
0.0001a |
| TGF-β1 |
40,110.80±22,410.68 | 319.98±289.40 |
<0.0001a |
Correlation analysis of CTGF and
TGF-β1 expression levels with clinical manifestation
Spearman rank correlation analysis was conducted to
investigate the association of CTGF and TGF-β1 expression levels
with the age, clinical severity and pathological severity in DMD
patients. The CTGF and TGF-β1 expression levels were significantly
correlated with the degree of clinical and pathological severity
(P<0.05); however, no significant correlation of the CTGF and
TGF-β1 expression levels with the age of the patients was observed
(P>0.05; Table III).
 | Table III.Spearman rank correlation
analysis. |
Table III.
Spearman rank correlation
analysis.
|
| CTGF | TGF-β1 |
|---|
|
|
|
|
|---|
| Characteristic | Spearman
correlation coefficient | P-value | Spearman
correlation coefficient | P-value |
|---|
| Age | −0.089 | 0.589 | −0.114 | 0.378 |
| Degree of
pathological severity | 0.767 | 0.001a | 0.465 | 0.018a |
| Degree of clinical
severity | 0.622 | 0.004a | 0.487 | 0.022a |
Discussion
The results of the present study demonstrated that
an upregulated expression of TGF-β1 and CTGF was observed in the
cytosolic and myenteric interstitia of the DMD skeletal muscle.
There were significant differences in the expression of those two
proteins between the DMD and control groups. The expression levels
of CTGF and TGF-β1 were positively correlated with the pathological
and clinical severity, which can provide important information for
the evaluation of DMD severity. Furthermore, CTGF and TGF-β1 were
involved in the fibrosis process, which is known to be one of key
pathogenesis factors in DMD.
Nevertheless, further research is required on the
involvement of TGF-β1 and CTGF in skeletal muscle fibrosis. In
vitro experiments confirmed that TGF-β1 can downregulate
myogenic protein to induce fibrosis-associated proteins (33). In addition, it has been shown that
TGF-β1 can promote a skeletal muscle fiber cascade and induce
differentiation from myogenic to fiber cells in vivo
(33). In the fibrosis process of
the skin, liver or kidney, the upregulated expression of CTGF can
directly promote cell matrix adhesion, ECM deposition, and
synthesis of collagen I and III, integrin-β1 and fibronectin
(34–36). Furthermore, the inflammatory response
is capable of activating macrophages or fibroblasts in order to
increase TGF-β1 secretion, which then activates CTGF (37). TGF-β1 and CTGF are considered to
participate in the promotion of ECM synthesis and fibroblast
chemotaxis through the following signaling pathways: Smad, cyclic
adenosine monophosphate-protein kinase A, mitogen-activated protein
kinases and c-Jun N-terminal kinase-dependent signaling (38–40). In
the mdx animal models, overexpressed CTGF can directly result in a
muscular dystrophy phenotype, whereas anti-CTGF may reverse the
muscular dystrophy phenotype and improve the effect of cell therapy
(23). It has been hypothesized that
myenteric interstitial fibrosis causes shortage of blood supply by
surrounding muscle cells, which then inhibits the regeneration of
muscle satellite cells (20).
CTGF and TGF-β1 are important in the fibrosis
process of DMD, and thus may assist in the development of novel
treatments. The results of the present study indicated that, as
common pathogenic factors, CTGF and TGF-β1 are important in the
fibrosis processes of DMD. TGF-β1 receptors are distributed widely.
In animal models, blocking of TGF-β1 can inhibit CTGF release, as
well as inhibit the carcinogenic and pro-inflammatory response and
other side effects (41). In
addition, CTGF is mainly confined to the connective tissue to take
function, with low expression observed in normal physiological
conditions; thus, blocking CTGF expression in order to antagonize
fibrosis may be safe (6). Therefore,
the TGF-β1/CTGF signaling pathway may also be promising in the
search for therapeutic agents against DMD.
In conclusion, in the present study, the upregulated
expression of CTGF and TGF-β1 was identified in the skeletal muscle
of DMD patients, which were in positive correlation with the degree
of pathology and clinical severity. Finally, these two factors may
be involved in the pathophysiology of fibrosis in DMD patients.
Acknowledgements
The study was supported by the Science and
Technology Planning of Hunan Province (grant no. 2012SK3207) and
the Youth Fund of Xiangya Hospital (2015Q06).
References
|
1
|
Emery AE: Population frequencies of
inherited neuromuscular diseases - a world survey. Neuromuscul
Disord. 1:19–29. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deconinck N and Dan B: Pathophysiology of
duchenne muscular dystrophy: Current hypotheses. Pediatr Neurol.
36:1–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Worton RG: Duchenne muscular dystrophy:
Gene and gene product; mechanism of mutation in the gene. J Inherit
Metab Dis. 15:539–550. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pessina P, Cabrera D, Morales MG, Riquelme
CA, Gutiérrez J, Serrano AL, Brandan E and Muñoz-Cánoves P: Novel
and optimized strategies for inducing fibrosis in vivo: Focus on
duchenne muscular dystrophy. Skelet Muscle. 4:72014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gibertini S, Zanotti S, Savadori P, Curcio
M, Saredi S, Salerno F, Andreetta F, Bernasconi P, Mantegazza R and
Mora M: Fibrosis and inflammation are greater in muscles of
beta-sarcoglycan-null mouse than mdx mouse. Cell Tissue Res.
356:427–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou L and Lu H: Targeting fibrosis in
Duchenne muscular dystrophy. J Neuropathol Exp Neurol. 69:771–776.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rifkin DB, Kojima S, Abe M and Harpel JG:
TGF-beta: Structure, function, and formation. Thromb Haemost.
70:177–179. 1993.PubMed/NCBI
|
|
8
|
Samarakoon R, Overstreet JM and Higgins
PJ: TGF-β signaling in tissue fibrosis: Redox controls, target
genes and therapeutic opportunities. Cell Signal. 25:264–268. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moses HL, Arteaga CL, Alexandrow MG,
Dagnino L, Kawabata M, Pierce DF Jr and Serra R: TGF beta
regulation of cell proliferation. Princess Takamatsu Symp.
24:250–263. 1994.PubMed/NCBI
|
|
10
|
Wenner CE and Yan S: Biphasic role of
TGF-beta1 in signal transduction and crosstalk. J Cell Physiol.
196:42–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luft FC: CCN2, the connective tissue
growth factor. J Mol Med (Berl). 86:1–3. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanaan RA, Aldwaik M and Al-Hanbali OA:
The role of connective tissue growth factor in skeletal growth and
development. Med Sci Monit. 12:RA277–RA281. 2006.PubMed/NCBI
|
|
13
|
Leask A and Abraham DJ: The role of
connective tissue growth factor, a multifunctional matricellular
protein, in fibroblast biology. Biochem Cell Biol. 81:355–363.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grotendorst GR: Connective tissue growth
factor: A mediator of TGF-beta action on fibroblasts. Cytokine
Growth Factor Rev. 8:171–179. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Winter P, Leoni P and Abraham D:
Connective tissue growth factor: Structure-function relationships
of a mosaic, multifunctional protein. Growth Factors. 26:80–91.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi-Wen X, Leask A and Abraham D:
Regulation and function of connective tissue growth factor/CCN2 in
tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev.
19:133–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bernasconi P, Di Blasi C, Mora M, Morandi
L, Galbiati S, Confalonieri P, Cornelio F and Mantegazza R:
Transforming growth factor-beta1 and fibrosis in congenital
muscular dystrophies. Neuromuscul Disord. 9:28–33. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun G, Haginoya K, Wu Y, Chiba Y,
Nakanishi T, Onuma A, Sato Y, Takigawa M, Iinuma K and Tsuchiya S:
Connective tissue growth factor is overexpressed in muscles of
human muscular dystrophy. J Neurol Sci. 267:48–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vial C, Zúñiga LM, Cabello-Verrugio C,
Cañón P, Fadic R and Brandan E: Skeletal muscle cells express the
profibrotic cytokine connective tissue growth factor (CTGF/CCN2),
which induces their dedifferentiation. J Cell Physiol. 215:410–421.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morales MG, Cabello-Verrugio C, Santander
C, Cabrera D, Goldschmeding R and Brandan E: CTGF/CCN-2
over-expression can directly induce features of skeletal muscle
dystrophy. J Pathol. 225:490–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andreetta F, Bernasconi P, Baggi F, Ferro
P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R and Confalonieri P:
Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective
tissue proliferation in diaphragm but increases inflammatory
response: Implications for antifibrotic therapy. J Neuroimmunol.
175:77–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taniguti AP, Pertille A, Matsumura CY,
Neto H Santo and Marques MJ: Prevention of muscle fibrosis and
myonecrosis in mdx mice by suramin, a TGF-β1 blocker. Muscle Nerve.
43:82–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morales MG, Gutierrez J, Cabello-Verrugio
C, Cabrera D, Lipson KE, Goldschmeding R and Brandan E: Reducing
CTGF/CCN2 slows down mdx muscle dystrophy and improves cell
therapy. Hum Mol Genet. 22:4938–4951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miranda AF, Bonilla E, Martucci G, Moraes
CT, Hays AP and Dimauro S: Immunocytochemical study of dystrophin
in muscle cultures from patients with Duchenne muscular dystrophy
and unaffected control patients. Am J Pathol. 132:410–416.
1988.PubMed/NCBI
|
|
25
|
Momma K, Noguchi S, Malicdan MC, Hayashi
YK, Minami N, Kamakura K, Nonaka I and Nishino I: Rimmed vacuoles
in Becker muscular dystrophy have similar features with inclusion
myopathies. PLoS One. 7:e520022012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan Z, Wang J, Ahn M, Shiloh-Malawsky Y,
Chahin N, Elmore S, Bagnell CR Jr, Wilber K, An H, Lin W, et al:
Characteristics of magnetic resonance imaging biomarkers in a
natural history study of golden retriever muscular dystrophy.
Neuromuscul Disord. 24:178–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Confalonieri P, Oliva L, Andreetta F,
Lorenzoni R, Dassi P, Mariani E, Morandi L, Mora M, Cornelio F and
Mantegazza R: Muscle inflammation and MHC class I up-regulation in
muscular dystrophy with lack of dysferlin: An immunopathological
study. J Neuroimmunol. 142:130–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wahab NA, Yevdokimova N, Weston BS,
Roberts T, Li XJ, Brinkman H and Mason RM: Role of connective
tissue growth factor in the pathogenesis of diabetic nephropathy.
Biochem J. 359:77–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun G, Haginoya K, Dai H, Chiba Y, Uematsu
M, Hino-Fukuyo N, Onuma A, Iinuma K and Tsuchiya S: Intramuscular
renin-angiotensin system is activated in human muscular dystrophy.
J Neurol Sci. 280:40–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mezzano V, Cabrera D, Vial C and Brandan
E: Constitutively activated dystrophic muscle fibroblasts show a
paradoxical response to TGF-beta and CTGF/CCN2. J Cell Commun
Signal. 1:205–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Werneck LC, Scola RH, Maegawa GH and
Werneck MC: Comparative analysis of PCR-deletion detection and
immunohistochemistry in Brazilian Duchenne and Becker muscular
dystrophy patients. Am J Med Genet. 103:115–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuo QH: Nervous system disorders in
children. 2nd. Beijing: People's Medical Publishing House, Beijing;
2002
|
|
33
|
Li Y, Foster W, Deasy BM, Chan Y, Prisk V,
Tang Y, Cummins J and Huard J: Transforming growth factor-beta1
induces the differentiation of myogenic cells into fibrotic cells
in injured skeletal muscle: A key event in muscle fibrogenesis. Am
J Pathol. 164:1007–1019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang JC, Sonnylal S, Arnett FC, De
Crombrugghe B and Zhou X: Attenuation of expression of
extracellular matrix genes with siRNAs to Sparc and Ctgf in skin
fibroblasts of CTGF transgenic mice. Int J Immunopathol Pharmacol.
24:595–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Falke LL, Dendooven A, Leeuwis JW, Nguyen
TQ, van Geest RJ, van der Giezen DM, Broekhuizen R, Lyons K, Stoop
R, Kemperman H, et al: Hemizygous deletion of CTGF/CCN2 does not
suffice to prevent fibrosis of the severely injured kidney. Matrix
Biol. 31:421–431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tzeng JI, Chen MF, Chung HH and Cheng JT:
Silymarin decreases connective tissue growth factor to improve
liver fibrosis in rats treated with carbon tetrachloride. Phytother
Res. 27:1023–1028. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ambrosio F, Ferrari RJ, Distefano G,
Plassmeyer JM, Carvell GE, Deasy BM, Boninger ML, Fitzgerald GK and
Huard J: The synergistic effect of treadmill running on stem-cell
transplantation to heal injured skeletal muscle. Tissue Eng Part A.
16:839–849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Black SA Jr, Palamakumbura AH, Stan M and
Trackman PC: Tissue-specific mechanisms for CCN2/CTGF persistence
in fibrotic gingiva: Interactions between cAMP and MAPK signaling
pathways, and prostaglandin E2-EP3 receptor mediated activation of
the c-JUN N-terminal kinase. J Biol Chem. 282:15416–15429. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Black SA Jr and Trackman PC: Transforming
growth factor-beta1 (TGFbeta1) stimulates connective tissue growth
factor (CCN2/CTGF) expression in human gingival fibroblasts through
a RhoA-independent, Rac1/Cdc42-dependent mechanism: statins with
forskolin block TGFbeta1-induced CCN2/CTGF expression. J Biol Chem.
283:10835–10847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tian J, Yang F and Liu H: TGF-Beta and
CTGF mediated signal transduction pathway and fibrosis. Proceedings
of the 2012 international conference on biomedical engineering and
biotechnology. 913–916. 2012. View Article : Google Scholar
|
|
41
|
Frazier K, Williams S, Kothapalli D,
Klapper H and Grotendorst GR: Stimulation of fibroblast cell
growth, matrix production, and granulation tissue formation by
connective tissue growth factor. J Invest Dermatol. 107:404–411.
1996. View Article : Google Scholar : PubMed/NCBI
|