Introduction
Nerve growth factor (NGF) is widely applied in the
clinic and plays a significant role in neuronal survival support,
peripheral nerve growth and nutrition adaptation, nerve
regeneration and fracture repair (1). Examination of fracture and the regional
callus tissue identified a high expression of NGF and its receptor
(2). Local injection of exogenous
NGF facilitated the complete healing of the fracture and reduced
the formation of heterotopic ossification (3). Therefore, NGF is a potential action
target for the prognosis of clinical fracture improvement. However,
its precise mechanism is not yet fully understood since NGF action
on bone tissue is multifaceted, multi-level and overlapping
(4).
Fracture healing process may require a variety of
cytokines, such as transforming growth factor-β, insulin-like
growth factor, bone morphogenetic protein and vascular endothelial
growth factor (5). However, whether
NGF plays a key role in the fracture healing process, a continuous
role in the process of callus formation, or whether there is any
association with osteoclast formation and cartilage differentiation
remains to be determined. In addition, exogenous NGF injection was
applied previously to observe its effect on fracture healing, thus
different conclusions may be drawn due to the differences in
appropriate dose and the injection site (6).
Non-interventional observation on stabilized
transgenic mice was used in the present study to identify the
specific mechanism involved in fracture healing of endogenous
NGF.
Materials and methods
Animals
A total of 48 NGF wild homozygotic mice (n=24) and
NGF transgenic homozygotic mice (n=24) were provided by JOINN
Laboratories Inc. (Suzhou, China). Quantitative polymerase chain
reaction (qPCR) amplification technology was utilized for gene
segment identification in agarose gel electrophoresis (AGE). The
mice were specific-pathogen-free male mice aged 6 weeks and
weighing 180±20 g. The animals were kept in cages with an ambient
temperature of 20–25°C and humidity 60%, were fed a normal diet and
had access to water ad libitum, and were subjected to a 12-h
dark/light cycle.
Main reagents and equipment
The reagents used for the study were
tartrate-resistant acid phosphatase (TRAP) staining kit (Sigma, St.
Louis, MO, USA), phosphate-buffered saline (PBS) (Gibco, Grand
Island, NY, USA), TRIzol (Invitrogen Life Technologies, Carlsbad,
CA, USA), RNA reverse transcription system (Takara Bio, Inc., Otsu,
Japan), X-ray film (Kodar, Rochester, NY, USA), safranin O and fast
green (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and
digoxin RNA labeling kit (Roche Diagnostics, Indianapolis, IN,
USA).
An animal trace X-ray machine (Faxitron X-ray Corp.,
Wheeling, IL, USA), MX3000P qPCR System (Stratagene, La Jolla, CA,
USA), gel imaging device (Bio-Rad, Berkeley, CA, USA), ultraviolet
(UV) spectrophotometer (Eppendorf AG, Hamburg, Germany), RM2135
microtome (Leica, Mannheim, Germany), Spot built-in digital color
camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA),
and −70°C cryogenic freezer (Sanyo, Tokyo, Japan) constituted the
equipment used.
Fracture models
Non-stabilized tibial fracture model of mice was
established as described in a previous study by Kim et al
(7). Intraperitoneal anesthetic of
2.5% Avertin 0.012–0.018 ml/g was injected into the mice. The skin
at middle tibia of its right lower limb was excised longitudinally,
with a cut of approximately 0.5 cm in length. The tibia was
transected with scissors following removal of the muscles at middle
tibia, and then the incision was sutured layer by layer. The
animals were sacrificed by cervical dislocation after 7, 14 and 21
days of the fracture. The animal skin was cut on the right limb to
expose knee joint and tibia. The femur was dissected at 0.5 cm
above the knee joint and the tibia was cut at 0.5 cm below the
fracture. The model was then rinsed with PBS, fixed, decalcified,
dewaxed, embedded and sectioned.
Fracture healing situations
X-ray radiography and safranin-fast green staining
were applied to observe fracture healing. Each group had 8 mice
samples at the 7th, 14th and 21st day, respectively. Giotto FFDM
system (Broomfield, CO, USA) was applied in the X-ray photography
of the fracture. The main procedures of safranin-fast green
staining included, staining with hematoxylin for 20–30 sec, rinsing
in running water for 8 min, 1% hydrochloric acid-ethanol color
separation for 40 sec, rinsing in running water for 5 min, 2% fast
green staining for 3 min, fast rinse in 1% acetic acid, rinsing in
clean water, 0.1% safranin O staining for 2.5–3 min, rinsing in
clean water, and 10 sec in 95% alcohol for 2 min in 100% alcohol.
Mounting in neutral balsam was initiated after xylene became
transparent. The results showed the cartilage as red stained and
the bone tissue as green stained.
In situ hybridization to examine the
expression of NGF mRNA in tibia
A Digoxin RNA labeling kit was used to prepare the
RNA probe which was complementary to Digoxin labeling in accordance
with the manufacturer's instructions. The main steps of in
situ hybridization included sequential treatment with 0.2 mol/l
HCl at room temperature for 10 min, 1X PBST solution for 5 min × 2
times, 20 µg/ml proteinase K solution digestion at 37°C for 15 min,
and washing in PBST solution for 5 min × 2 times. The probe was
fixed in 4% paraformaldehyde for 10 min, 10 min for 0.1 mol/l
acetic anhydride/triethanolamine, and finally 50°C
pre-hybridization for 1 h. The RNA probe was denatured after 85°C,
pre-hybridized, dropped and hybridized at 50°C for overnight. The
hybridisation buffer sample without the probe was considered the
negative control. The film was washed, and confined in confining
liquid at room temperature for 1 h. Primary rabbit polyclonal
anti-digoxin antibody (dilution: 1/1000; Abcam, Cambridge, MA, USA;
catalog no.: ab30512) was used for incubation at 37°C for 2 h, and
the sample with TBST only was used as the negative control. After
rinsing, the NBT/BCIP liquid was kept in the dark to develop color
for 4 h, and then counterstained with methyl green, dehydrated and
mounted. It was found that positive cells of in situ
hybridization were cytoplasm or nucleus, which were stained in
purplish red or black blue.
Callus tissue TRAP staining
Osteoclast formation was observed through callus
tissue TRAP staining and the TRAP mRNA expression levels. TRAP
staining kit was applied in strict accordance with operating
instructions.
Quantitative fluorescent PCR method
used to detect chondrocyte differentiation-related genes (COL2A1
and SOX9)
According to the TRIzol reagent protocol, the one
step method was applied to extract total RNA of tissues. AGE was
used to examine its integrity, and the total RNA concentration and
purity were measured using a UV lamp and UV spectrophotometer. The
primers used in the study were: COL2A1 (5′-CTGGTGGAGCAGCAAGAGCAA-3′
and 5′-CAGTGGACAGTAGACGGAGGAAAG-3′), SOX9
(5′-GGGCTCTACTCCACCTTCACT-3′ and 5′-AAGATCAGCTCGGTCACCATA-3′),
β-actin cyclophillin A (5′-CGAGCTCTGAGCACTGGAGA-3′ and
5′-TGGCGTGTAAAGTCACCACC-3′). The amplification conditions used
were: 95°C for 30 sec, 95°C for 5 sec, 57°C for 20 sec, 72°C for 15
sec, 40 cycles (amplification); 95°C for 30 sec, 57°C for 30 sec,
95°C for 30 sec (dissociation curve). The value of c (q) and the
relative content of the target gene were provided using MX3000P
qPCR instrument software (Stratagene).
Statistical analysis
SPSS 19.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was applied for data entry and analysis. Measurement data
were presented as mean ± standard deviation. Group comparisons were
made using single-factor ANOVA. Countable data were expressed as a
percentage (%) and group comparisons were tested using the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
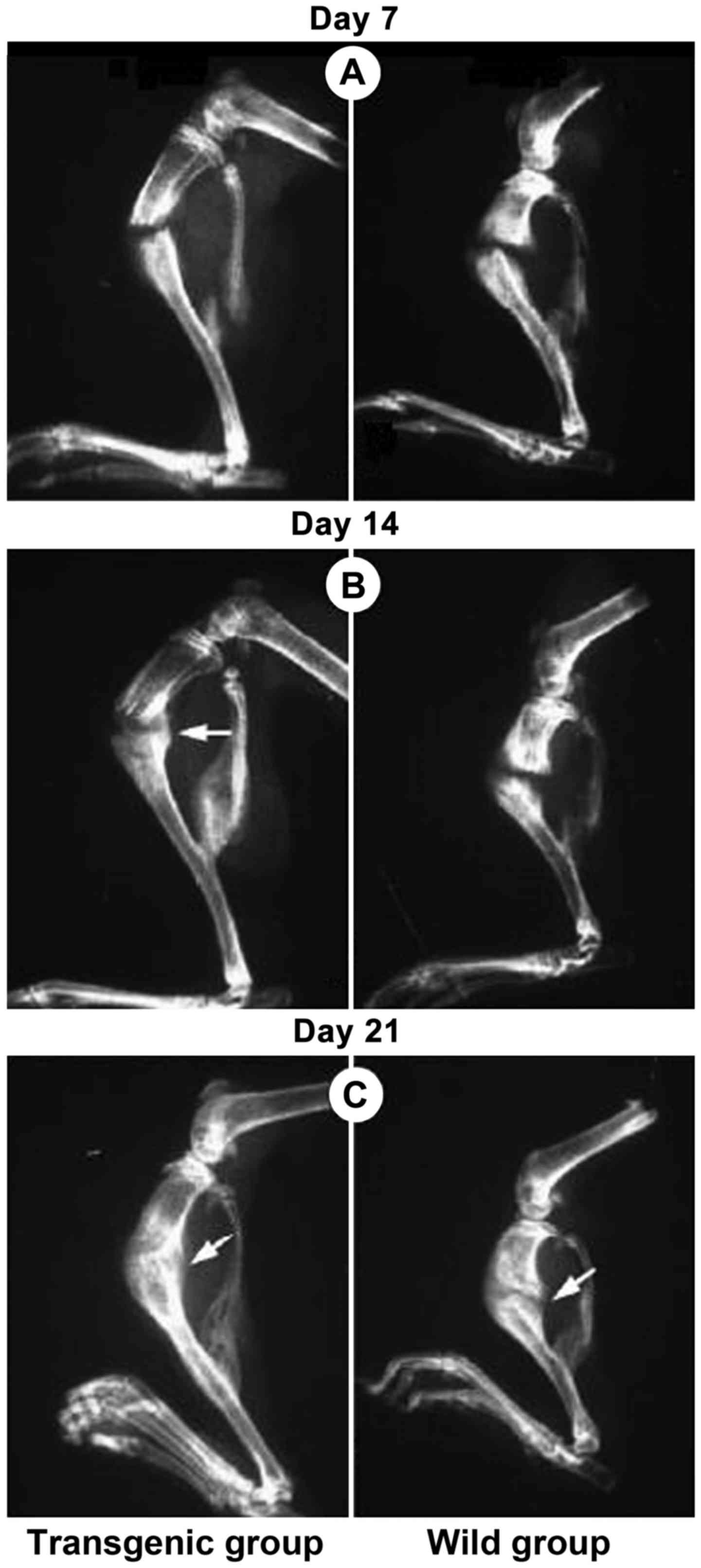
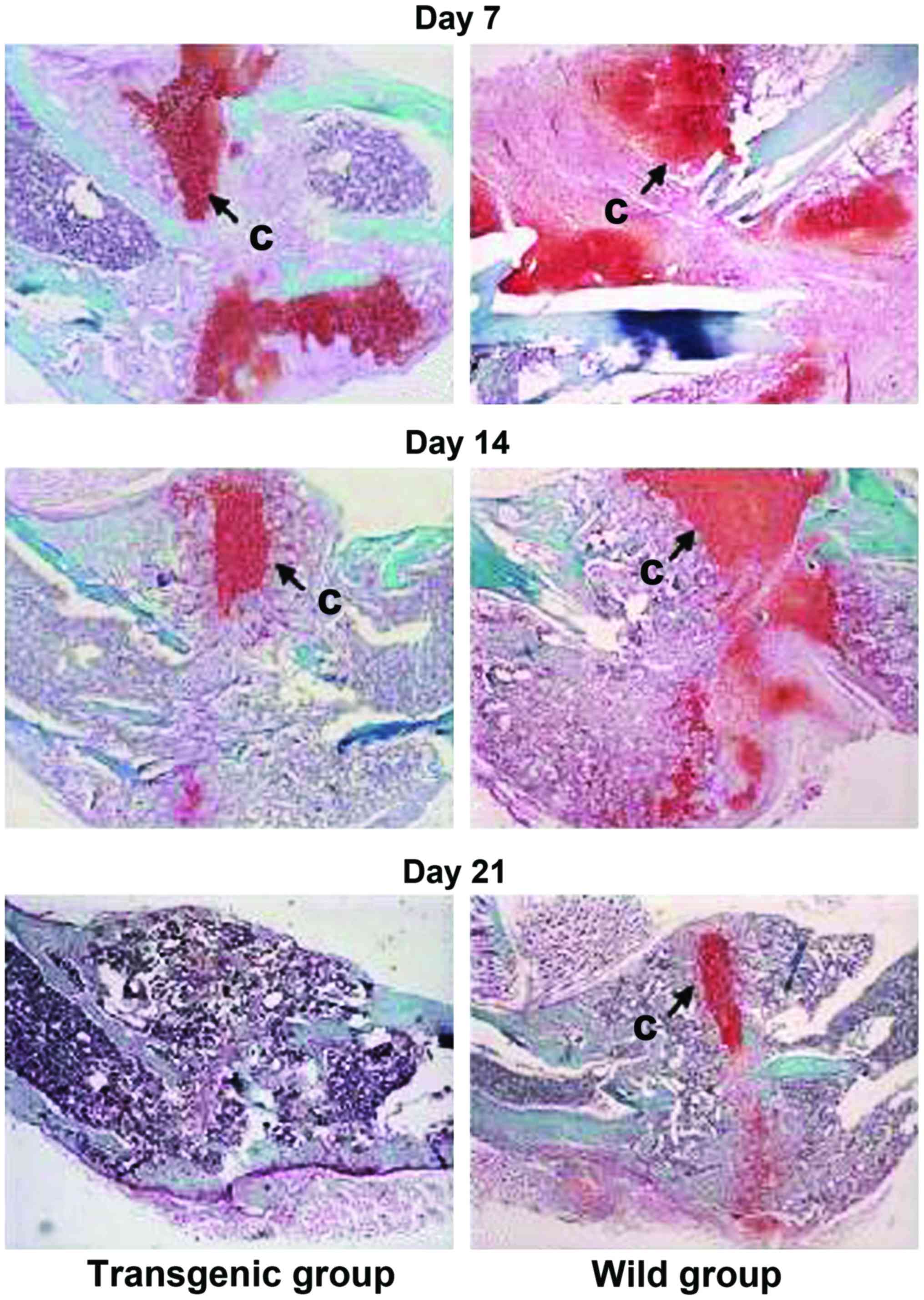
X-ray radiography and safranin fast
green for fracture healing observation
Mice fracture models in each group were successfully
established. Under X-ray radiography observation, the fracture of
NGF transgenic homozygotic mice healed in advance (Fig. 1). Cartilage and bone tissue were
expressed by safranin and fast green staining. The residual
cartilage on callus of NGF transgenic homozygotic mice had
decreased significantly (Fig.
2).
NGF mRNA expression in each callus
formation phase
NGF mRNA expression levels in each callus formation
phase in NGF transgenic homozygotic mice was significantly higher
than that of the wild group (Fig.
3).
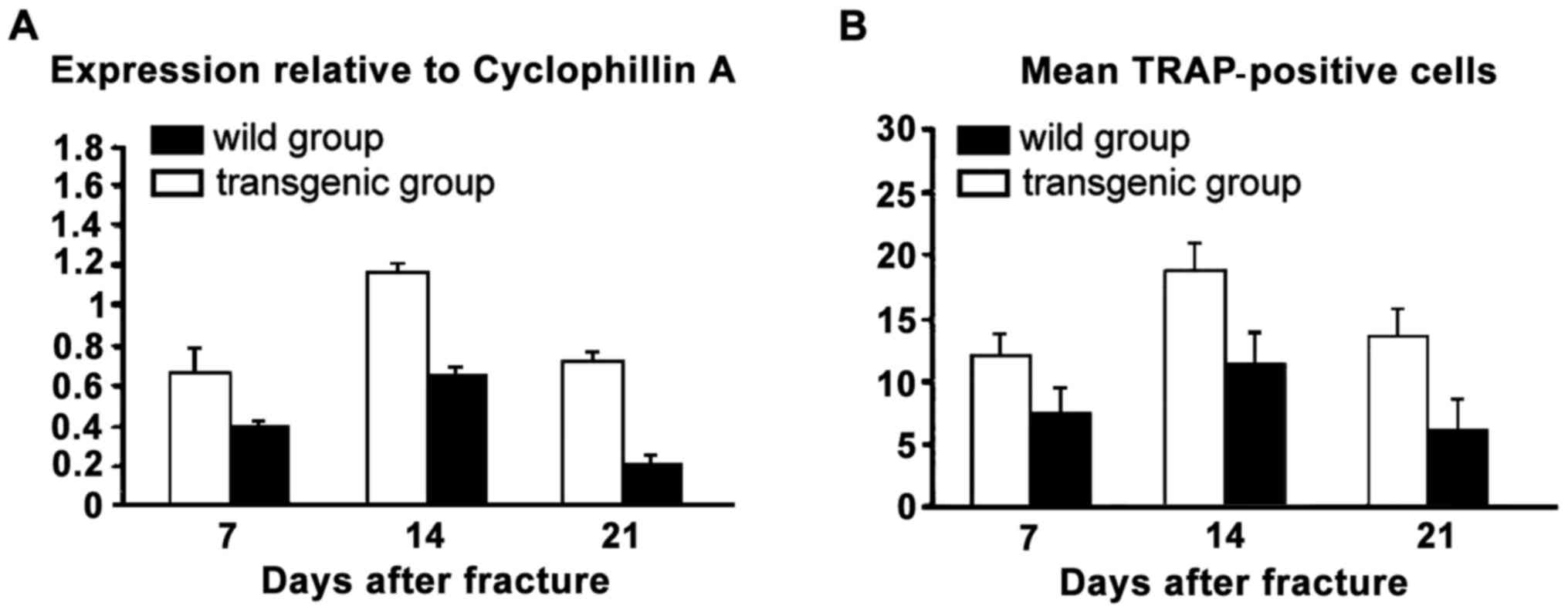
TRAP mRNA expression levels
The number of positive cells in NGF-TRAP staining at
each time point after the fracture and the expression level of NGF
mRNA were significantly higher than that of the wild group
(Fig. 4).
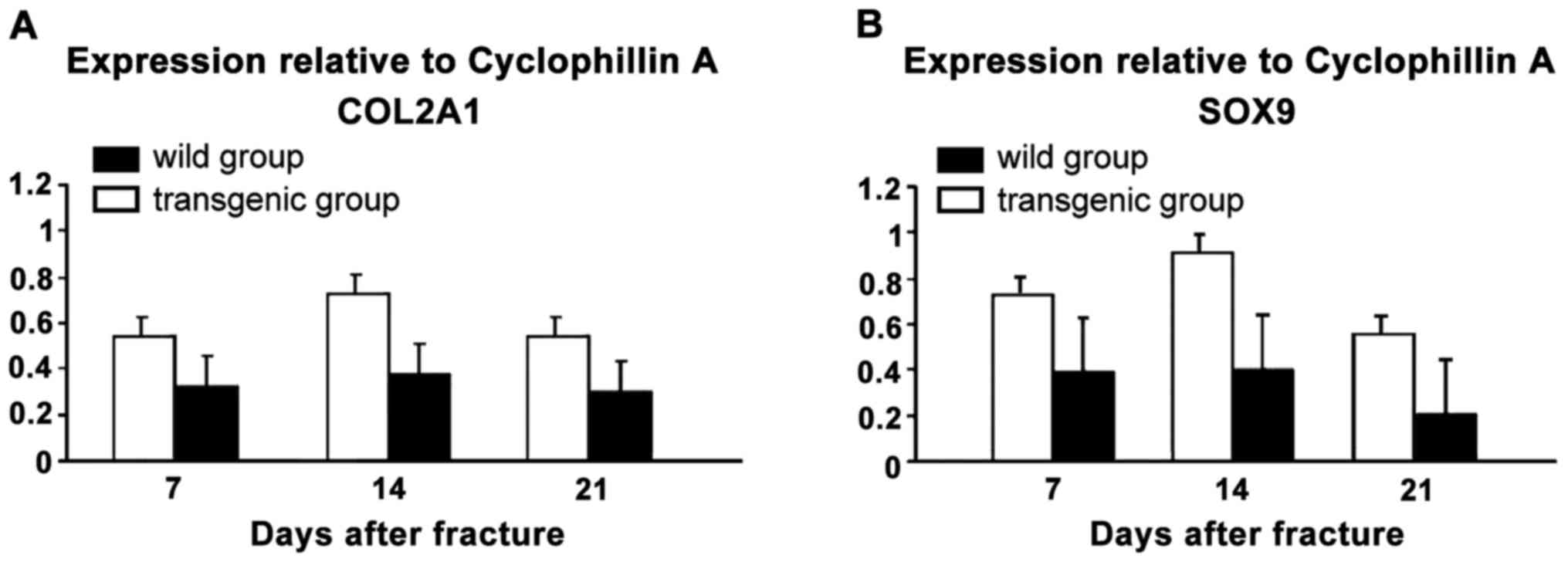
COL2A1 and SOX9 mRNA expression
levels
The COL2A1 and SOX9 mRNA expression levels of
NGF-TRAP stained mice at each time point after the fracture were
markedly higher than that of the wild group (Fig. 5).
Discussion
NGF is a polypeptide protein that exists widely in
various organs in animals. It is mainly produced by target tissue
that is dominated by neurons in the neural crest. Neuron axon
uptake is retrogradely transported to soma. NGF regulates the gene
transcription of neuron cells in various ways, thus exerting a
biological effect (8). In the 1990s,
it was found that NGF may be produced in callus and immature
tissues, which would induce nerve fibers growing into embryonic
bone tissues and callus (9). Thus,
NGF was capable of adjusting the growth and development of bone
tissues and callus (9). Neuropeptide
Y with sympathetic nerve component, peptidergic nerve fibre in
intestinal vascular and alcitonin gene-related peptide with sensory
nerve component, p substance and other peptidergic nerve fibers
were present in callus (10).
Various peptidergic nerve fibers existing in bone tissues are
mainly distributed in metabolically active bone tissues, fracture
sections, in particular. These fibers can significantly increase
the expression of NGF and its receptor (11). Among the various osteoblast lineages
cultured in vitro, there were not only NGF mRNA but also
protein expression (12).
Fracture healing involves the stages of organization
of hematoma, original callus formation and callus transformation
and shaping. NGF is a bone resorption inhibitor that can increase
bone reconstruction to avoid bone losses after fracture by reducing
the excretion of proline and calcium (3). It can facilitate the process of callus
chondrification and vascularization, and accelerate fracture
healing through early cartilage mineralization of fracture ends
(13). Previous findings have shown
that, fractures treatment may be one of the diseases that are most
appropriate for gene therapy (14).
Grills et al found that callus formation, its stiffness and
anti-bending strength were significantly increased in a local
injection of NGF used to treat the fractured rib end of the mice
(3). That result showed that a local
injection of NGF facilitated the speed and efficiency of fracture
healing, and played a significant role in each stage of fracture
healing.
Through the high expression of endogenous NGF in
transgenic mice, the present study has concluded that NGF mRNA
expression levels in each callus formation phase were higher than
those of the wild group on average. Fracture healing was completed
in advance, residual cartilage decreased greatly, osteoclast
formation increased and cartilage differentiation accelerated. The
strain of NGF homozygous transgenic mice was relatively stable
after 9 passages on average with rare gene mutation recovery.
Additionally, compared with purely supplemented exogenous NGF,
highly expressed NGF and its receptor could improve the NGF effect,
avoid inadequate or excessive NGF doses of exogenous supplement, as
well as allergic or other adverse reactions of the body (15). Findings of the current study showed
that delayed fracture healing may be associated with an increase of
the residual chondrocytes, chondrocyte differentiation barrier and
slow cartilage matrix degradation. TRAP is an osteoclast
characteristic enzyme. Degradation of the cartilage matrix is
closely associated with the osteoclast absorption effect on the
cartilage matrix (16). A large
number of cartilage and mesenchymal tissues were evident in the
middle and late stage of fracture healing because the COL2A1
expression of the chondrocyte marker gene in the wild group was
significantly lower than that of the transgenic group (17). SOX9 gene, an important
transcription factor for chondrocyte genesis and differentiation,
expressed from the cartilage progenitor cell of mesenchyme, reached
its expression peak at differentiated chondrocyte, and was
significantly downregulated in hypertrophic chondrocytes (18). Previous studies were less involved in
the relationship of NGF between chondrocytes in fracture healing
(19). Current investigations on the
promotion of NGF in fracture healing and newly-generated capillary
are not comprehensive. Recently identified neurotrophins such as
neuritin may constitute a downstream factor of neural activity and
whether it would play a role in the promotion of NGF of the
fracture healing process remains to be investigated (20).
In summary, NGF can promote cartilage
differentiation, increase osteoclast formation and promote the
healing of tibial fractures by increasing the levels of COL2A1 and
SOX9 mRNA expression. The wide application of NGF in bone
non-union, delayed healing and the prevention and treatment of
osteoporosis greatly reduced morbidity and improved quality of
life, providing greater understanding of the theory of the fracture
healing mechanism.
References
|
1
|
Turner JE and Bosch JA: Closing the Border
on a New Frontier: The problem with salivary nerve growth factor.
Psychosom Med. 78:114–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Majuta LA, Longo G, Fealk MN, McCaffrey G
and Mantyh PW: Orthopedic surgery and bone fracture pain are both
significantly attenuated by sustained blockade of nerve growth
factor. Pain. 156:157–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grills BL, Schuijers JA and Ward AR:
Topical application of nerve growth factor improves fracture
healing in rats. J Orthop Res. 15:235–242. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Zhou S, Liu B, Lei D, Zhao Y, Lu C
and Tan A: Locally applied nerve growth factor enhances bone
consolidation in a rabbit model of mandibular distraction
osteogenesis. J Orthop Res. 24:2238–2245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhuang YF and Li J: Serum EGF and NGF
levels of patients with brain injury and limb fracture. Asian Pac J
Trop Med. 6:383–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rapp AE, Kroner J, Baur S, Schmid F,
Walmsley A, Mottl H and Ignatius A: Analgesia via blockade of
NGF/TrkA signaling does not influence fracture healing in mice. J
Orthop Res. 33:1235–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SJ, Shin SJ, Choi NH and Cho SK:
Arthroscopically assisted treatment of avulsion fractures of the
posterior cruciate ligament from the tibia. J Bone Joint Surg Am.
83-A:698–708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Zhao D, Wang W, Wang B, Liu Z,
Zhang Y and Li Z: Nerve growth factor modulates bone morphogenetic
protein expression in rabbit fracture. Zhonghua Yi Xue Za Zhi.
94:1825–1828. 2014.(In Chinese). PubMed/NCBI
|
|
9
|
Frenkel SR, Guerra LA, Mitchell OG and
Singh IJ: Nerve growth factor in skeletal tissues of the embryonic
chick. Cell Tissue Res. 260:507–511. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo TZ, Wei T, Li WW, Li XQ, Clark JD and
Kingery WS: Immobilization contributes to exaggerated neuropeptide
signaling, inflammatory changes, and nociceptive sensitization
after fracture in rats. J Pain. 15:1033–1045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghilardi JR, Freeman KT, Jimenez-Andrade
JM, Mantyh WG, Bloom AP, Bouhana KS, Trollinger D, Winkler J, Lee P
and Andrews SW: Sustained blockade of neurotrophin receptors TrkA,
TrkB and TrkC reduces non-malignant skeletal pain but not the
maintenance of sensory and sympathetic nerve fibers. Bone.
48:389–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yasui M, Shiraishi Y, Ozaki N, Hayashi K,
Hori K, Ichiyanagi M and Sugiura Y: Nerve growth factor and
associated nerve sprouting contribute to local mechanical
hyperalgesia in a rat model of bone injury. Eur J Pain. 16:953–965.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bei C, Lin Z, Yang Z, Zhao J, Su W, Sha K,
Wei Q, Hua Q and Bo Z: Study on effect of NGF on fracture healing.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 23:570–576. 2009.(In
Chinese). PubMed/NCBI
|
|
14
|
Ishihara A and Bertone AL: Cell-mediated
and direct gene therapy for bone regeneration. Expert Opin Biol
Ther. 12:411–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mo Y, Yang Z, Zhao J, Su W, Sha K, Wei Q,
Yang F, Hua Q and Ding X: Preliminary study on appropriate
concentration gradient of nerve growth factor in promoting fracture
healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 25:575–581.
2011.(In Chinese). PubMed/NCBI
|
|
16
|
Wang T, Wang Y, Menendez A, Fong C, Babey
M, Tahimic CG, Cheng Z, Li A, Chang W and Bikle DD:
Osteoblast-Specific Loss of IGF1R Signaling Results in Impaired
Endochondral Bone Formation During Fracture Healing. J Bone Miner
Res. 30:1572–1584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang RC, Chen MH, Chen PY, Chen CY, Tsai
SF, Cheng CK and Sun JS: A mutation of the Col2a1 gene (G1170S)
alters the transgenic murine phenotype and cartilage matrix
homeostasis. J Formos Med Assoc. 113:803–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Liang DC, Bai JY, Kang N, Feng JY
and Yang ZQ: Overexpression of Sox9 gene by the lentiviral vector
in rabbit bone marrow mesenchymal stem cells for promoting the
repair of cartilage defect. Zhongguo Gu Shang. 28:433–440. 2015.(In
Chinese). PubMed/NCBI
|
|
19
|
Pecchi E, Priam S, Gosset M, Pigenet A,
Sudre L, Laiguillon MC, Berenbaum F and Houard X: Induction of
nerve growth factor expression and release by mechanical and
inflammatory stimuli in chondrocytes: Possible involvement in
osteoarthritis pain. Arthritis Res Ther. 16:R162014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Liu C, Xu F, Cui L, Tan S, Chen R,
Yang L and Huang J: Effects of neuritin on the migration,
senescence and proliferation of human bone marrow mesenchymal stem
cells. Cell Mol Biol Lett. 20:466–474. 2015. View Article : Google Scholar : PubMed/NCBI
|