Introduction
The implant infection treatment is still
problematic, and the new antibacterial drug endogenous antibiotic
peptide, human β-defensin 3 (HBD-3), plays an important role in the
inhibition of biofilm infection and inflammation as well as the
immune regulation (1). The early
in vitro studies found (2)
that the killing role of HBD-3 in the methicillin-resistant
Staphylococcus aureus (S. aureus) (MRSA) was significantly
enhanced and it could inhibit the expression of the film formation
gene icaAD and drug resistance gene MecA; and after the stimulation
of the S. aureus against the mouse osteoblasts, the mouse
β-defensin-14 (MBD-14) congenetic to the osteoblast secreted HBD-3
in the structure and function could be promoted by activating the
film-forming gene p38 mitogen-activated protein kinase (MAPK). In
the models of MRSA-induced mouse acute suppurative osteomyelitis,
the application of p38 MAPK agonist could promote MBD-14 at the
infection site releasing the increased inhibition of the
osteomyelitis (3). The present study
further verified the influence of HBD-3 on the growth status and
inflammatory response of the bacteria in the infectious biofilm as
well as the cell cytokines in the infectious bone tissue of the
implant in the mouse and provide the drug effect target and new
theoretical basis for the clinical application of HBD-3.
Materials and methods
Construction of MRSA-induced implant
drug-resistant bacteria biofilm infection model in the mouse left
tibial bone marrow
Main reagents: S. aureus standard strain ATCC
25923 was purchased from the Bacterial Culture Laboratory of the
Third Military Medical University, HBD-3 and vancomycin were
purchased from Sigma-Aldrich (St. Louis, MO, USA), the nutrient
broth (pH 7.2+0.2), tryptic soy agar (TSA) culture medium and
tryptic soy broth (TSB) were purchased from Qingdao Haibo
Biotechnology Co., Ltd. (Shandong, China); the nutrient agar was
purchased from Hangzhou Microbiological Agents Co., Ltd. (Hangzhou,
China), Live/Dead BacLight™ Bacterial Viability kit was purchased
from Molecular Probes Europe BV (Leiden, The Netherlands); the
calcofluor white was purchased from R&D (Minneapolis, MN, USA).
Mouse monoclonal nuclear factor-κB (NF-κB) antibody (dilution,
1:500; cat. no. sc-56735), mouse monoclonal toll-like receptor 4
(TLR-4) antibody (dilution, 1:500; cat. no. sc-293072) and rabbit
anti-mouse biotin-labeled secondary antibody (dilution, 1:2,000;
cat. no. sc-358917) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Diaminobenzidine (DAB) color-producing
reagent kit was purchased from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. (Beijing, China) and enzyme-linked
immunosorbent assay (ELISA) kit was purchased from Invitrogen
(Carlsbad, CA, USA).
Main instruments: The sterile super clean bench was
purchased from Shanghai Boxun Industrial Co., Ltd. (Shanghai,
China); the electro-heating standing-temperature cultivator type
DHP-9162 was purchased from Beijing Liuyi Instrument Factory
(Beijing, China), the laser scanning confocal microscope was
purchased from Leica Microsystems Inc. (Buffalo Grove, IL, USA),
the titanium rods (diameter, 0.5 mm; length, 3 mm) were processed
and manufactured by Chongqing Lidakang Company (Chongqing, China),
the Mindray BS-380 automatic biochemical analyzer was purchased
from Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (Shenzhen,
China) and the ordinary optics microscope was purchased from
Olympus (Tokyo, Japan).
The titanium rod was placed in 6-well flat bottom
cell culture plate, the S. aureus was cultured in the TSA
for 20 h, after the culture, and a single colony was cultured in
TSB for 20 h constantly shaking. The bacterial liquid was placed in
each well of the 6-well plate, diluted with normal saline to 0.5
McIntosh turbidity unit, each well was provided with 1 ml TSB and
the flat cells were cultured in the six wells, and then cultured in
the incubator for 20 h at 37°C. Healthy adult male Sprague-Dawley
(SD) rats were selected under normal feeding, at the average weight
of 230 g. The mice were abdominally anesthetized and the medullary
cavity was expanded by exposing the opening on the left-side tibial
plateau, the titanium rods with biofilm were implanted in the
medullary cavity, after 24 h medullary cavity infection, the drug
was administered by intraperitoneal injection after the local
biofilm was matured.
Experimental grouping and observation
indicators
The cases in the experiment were divided into the
model group, HBD-3 group and vancomycin group (20 in each group),
the model group was injected with 10 ml saline, HBD-3 group was
injected with 10 ml of 8 µg/ml (1 MIC), the vancomycin group was
injected with 10 ml of 0.5 µg/ml (1 MIC), 5 animals in each group
were sacrificed on days 1, 7, 14, and 21, respectively, an
observation was carried out on whether there was swelling and
purulent secretion on the local wound, 1 ml venous sinus blood of
eye socket was collected for blood routine examination and blood
culture, the laser scanning confocal microscopy was used to observe
the morphology of the biofilm on the implant surface and the number
of viable bacteria, the immunohistochemical staining was adopted to
test the expression of NF-κB and TLR-4, and ELISA method was used
to test interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α),
IL-1α and interferon-γ (IFN-γ)-inducible protein-10 (IP-10)
expression levels.
Laser scanning confocal microscope
observation
After fluorescence staining, the polysaccharide
protein complex became blue, a variety of bacteria on the bacterial
biofilm was washed with phosphate-buffered saline (PBS), soaked in
2.5% glutaraldehyde solution for 1 h, washed with PBS and added
with calcofluor white, after 40 min, they were wet, and confocal
laser scanning microscopy (CLSM) was used to observe the biofilm
morphology at 485/530 nm wavelength. The stain SYT09 in L13152
Live/Dead BacLight™ Bacterial Viability kits allowed viable
bacteria to emit green fluorescence, and propidium iodide (PI)
allow the dead bacteria to emit red fluorescence, the viable
bacteria and dead bacteria was distinguished depending on the
different fluorescence colors. The SYT09:PI:distilled water was
prepared as 1.5:1.5:l in the dark; the culture solution was
removed; the deionized water was used for washing; the fluorescent
dye was used for staining, and then the solution was incubated in
the dark at 37°C for 15 min and placed under CLSM for testing;
Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA) image analysis software was used for processing; three
horizons were randomly selected, and finally the colony IOD, bulk
density, and polysaccharide matrix staining area were
calculated.
Immunohistochemical staining
The tibial tissue slices were conventionally
manufactured through dewaxing, hydration, washing with PBS, enzyme
inactivation with 3% H2O2, antigen retrieval,
5% normal goat serum closure, declining of primary antibody working
solution (β-actin; dilution, 1:500; cat. no. sc-130300) and
incubation at 37°C for 4 h, washing with PBS, using appropriate
amount of 50 µl biotin-labeled secondary antibody working solution
(dilution, 1:2,000; cat. no. sc-358917), incubation at 37°C for 30
min then washed with PBS, DAB developing, hematoxylin re-staining,
dehydration, resulting in transparency and slice closure. The
positive cells strained brown and yellow. The total cell count per
slice was counted under complete horizon under high-power field
(x400), three horizons were randomly selected, and then the mean
value was taken.
Statistical analysis
SPSS 19.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for analysis; the quantitative data are expressed
as mean ± standard deviation; the comparison among the groups was
analyzed by one-way ANOVA and the comparison among the groups was
analyzed by variance requiring repeated data measurement; P<0.05
was considered to indicate a statistically significant
difference.
Results
General observations
Three mice in the model group died with swelling and
white purulent secretion on the wound due to infection (15%); no
deaths occurred in the other two groups due to infection, and 1
case had significant wound swelling, respectively, in each group,
but there was no purulent secretion.
Blood routine examination and blood
culture
The percentage of the total white blood cells and
neutrophil granulocytes in the model group was gradually increased
with time, while that in the HBD-3 group and vancomycin group was
decreased with time, and the comparative difference among groups
was statistically significant (P<0.05); that in the HBD-3 group
and vancomycin group at each time-point was significantly decreased
compared with the model group, and the comparative difference was
statistically significant (P-value between groups <0.05); in
terms of the comparison between HBD-3 group and vancomycin group,
the difference was not statistically significant (P>0.05)
(Table I).
 | Table I.Comparison of the results of blood
routine examination. |
Table I.
Comparison of the results of blood
routine examination.
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Cells | Day | Model | HBD-3 | Vancomycin | F-value | P-value |
|---|
| Total white | 1 | 10.6±1.8 | 8.5±1.0 | 8.8±1.2 | 6.857 | 0.027 |
| blood cells
(x109/l) | 7 | 12.2±2.0 | 7.3±1.1 | 7.5±1.3 | 8.201 | 0.009 |
|
| 14 | 14.5±2.2 | 6.6±0.8 | 6.7±1.0 | 8.634 | 0.002 |
|
| 21 | 17.3±2.6 | 5.0±0.6 | 5.2±0.8 | 9.125 | <0.001 |
| F-value | 9.538 | 7.628 | 7.549 |
|
|
|
| P-value | <0.001 | 0.012 | 0.013 |
|
|
|
| Neutrophil | 1 | 82.6±5.3 | 78.9±4.6 | 80.2±4.7 | 7.322 | 0.026 |
| granulocytes (%) | 7 | 83.4±5.5 | 75.3±4.4 | 75.5±4.8 | 7.564 | 0.023 |
|
| 14 | 86.6±5.6 | 73.4±4.2 | 73.6±4.6 | 7.747 | 0.020 |
|
| 21 | 88.0±6.0 | 72.5±4.3 | 72.6±4.5 | 7.865 | 0.016 |
| F-value | 8.030 | 7.534 | 7.632 |
|
|
|
| P-value | <0.001 | 0.013 | 0.012 |
|
|
|
Through identification of the blood culture
bacteria, the bacteria in the three groups were due to MRSA.
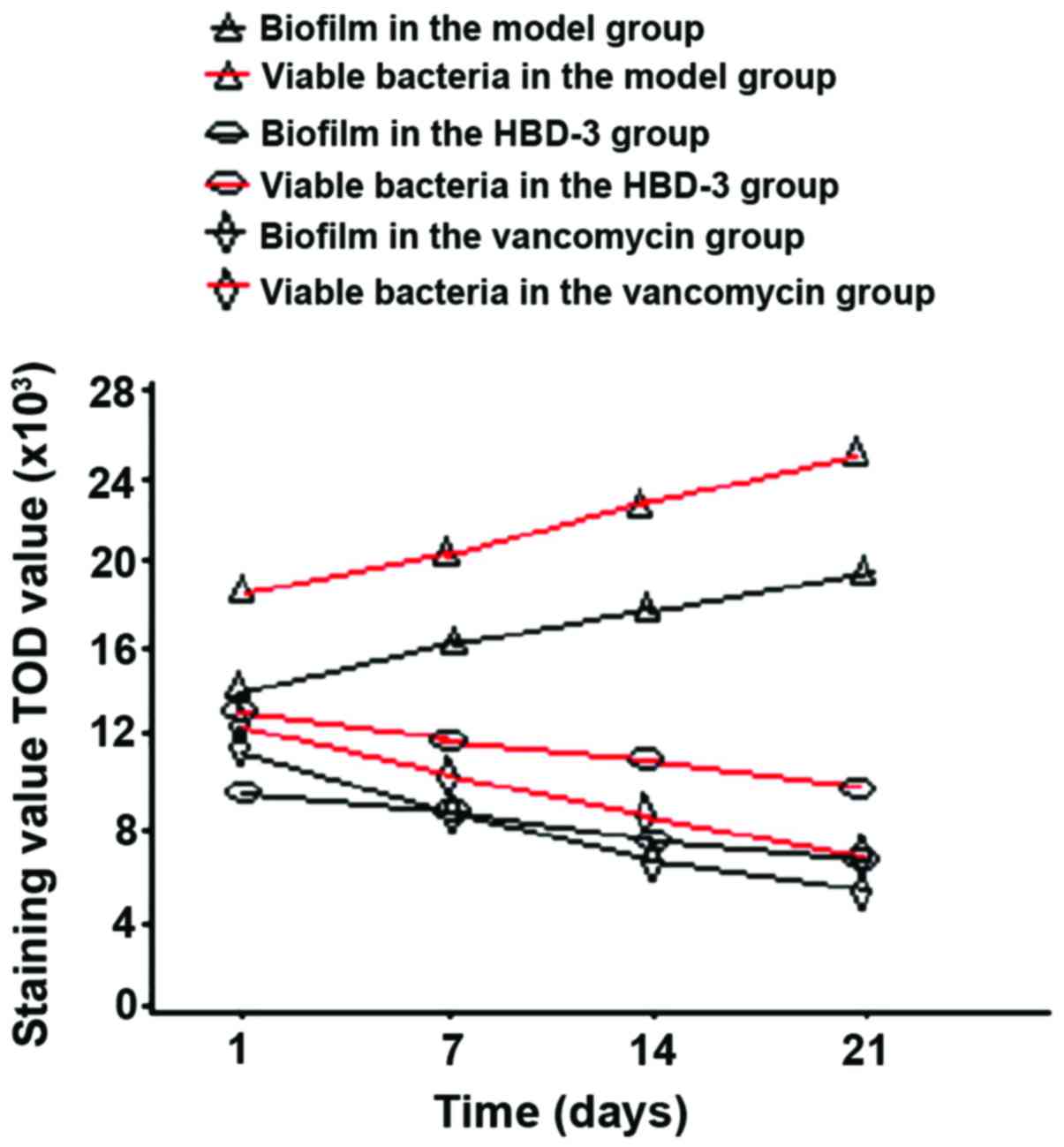
Biofilm morphology and the number of
viable bacteria
The biofilm morphology and the number of viable
bacteria in the model group were gradually increased with time,
while those in the HBD-3 group and vancomycin group were decreased
with time, the comparative difference among the groups was
statistically significant (P<0.05), whereas, those in the HBD-3
group and vancomycin group at each time-point were significantly
decreased compared with the model group, the comparative difference
among groups was statistically significant (P<0.05); in terms of
the comparison between HBD-3 group and vancomycin group, the
difference was not statistically significant (P>0.05) (Fig. 1).
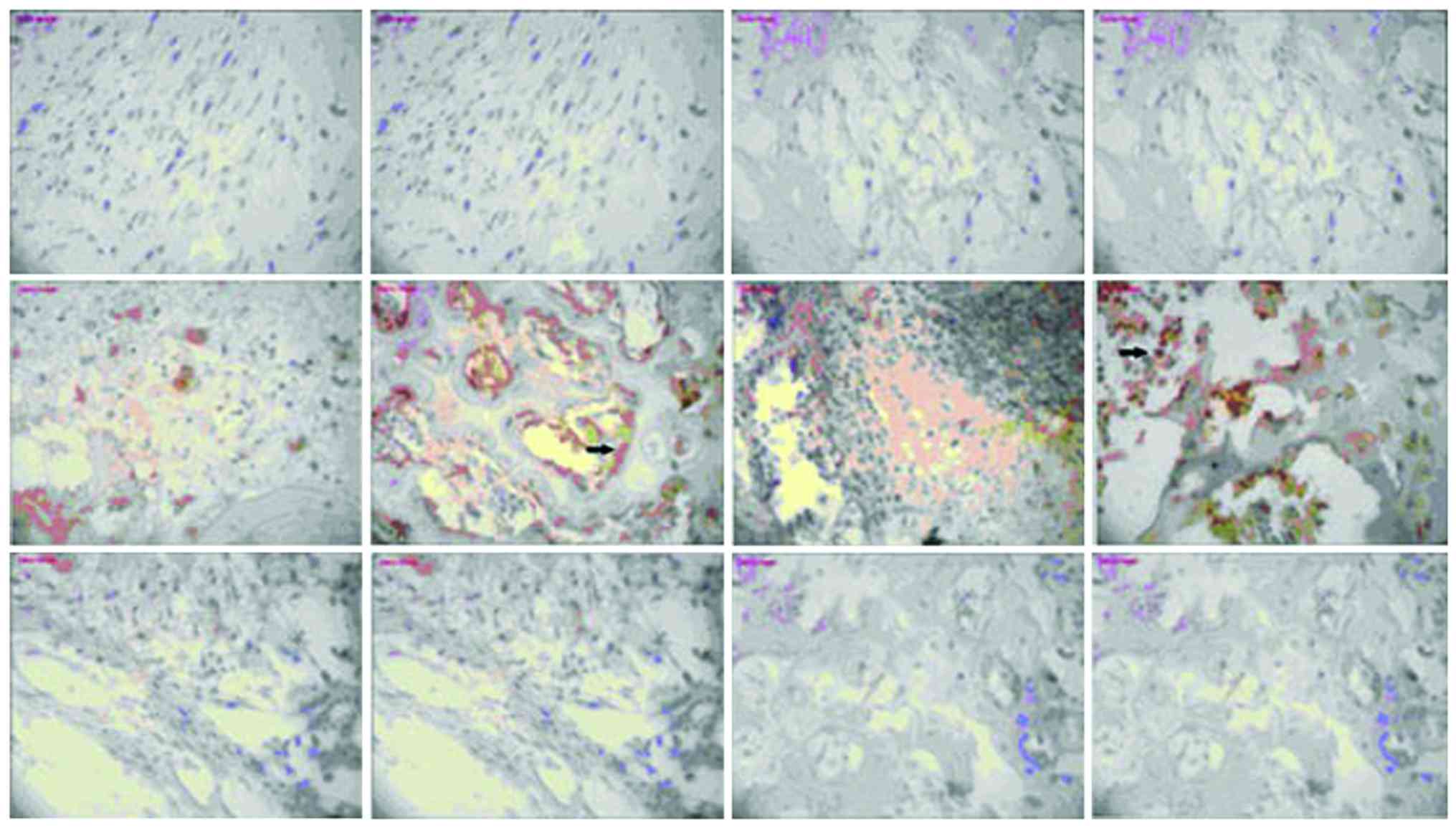
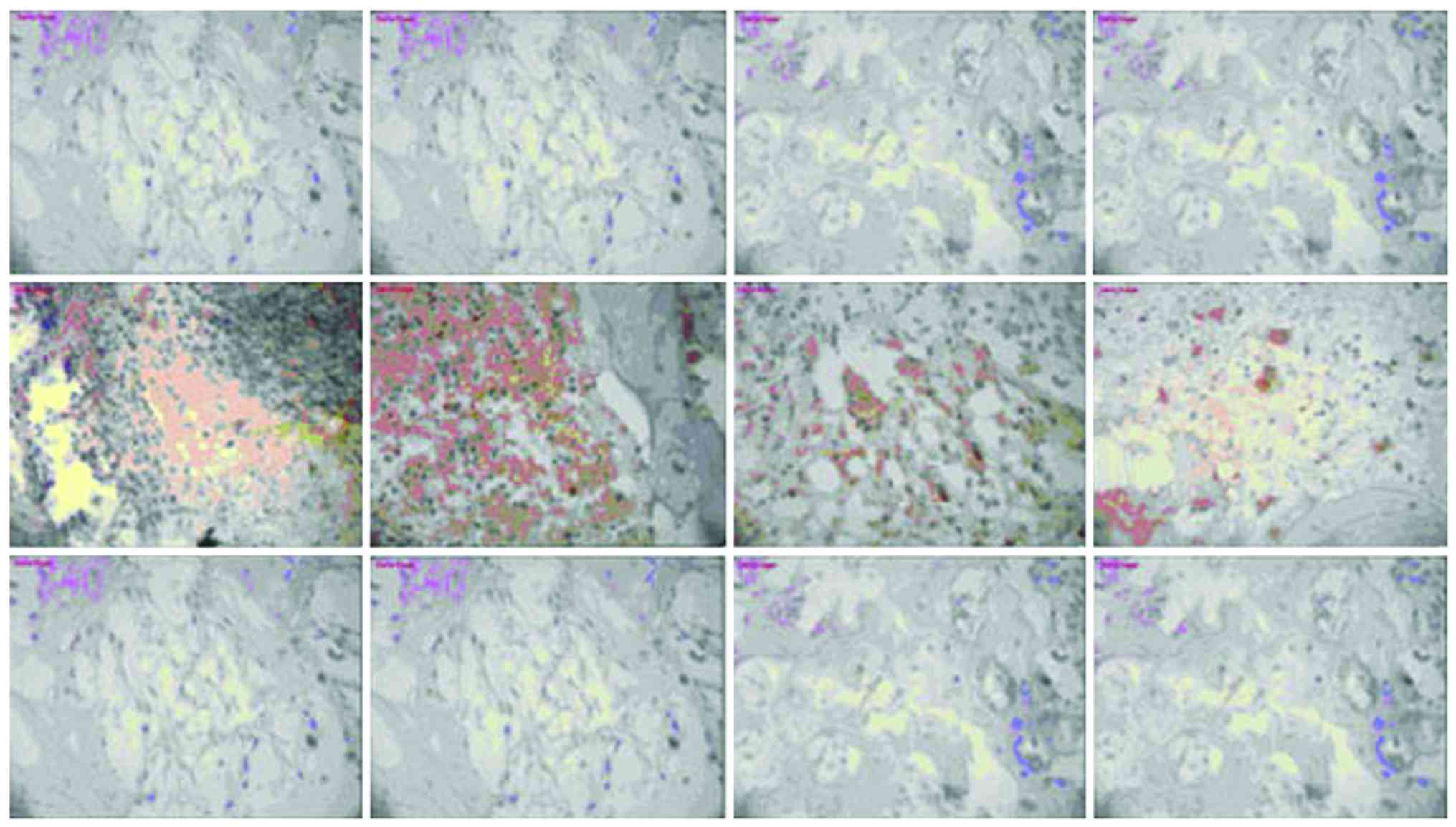
Expression of NF-κB and TLR-4
There was no significant change in NF-κB or TLR-4
expression in the model group or vancomycin group at each
time-point, those in the HBD-3 group began to increase on the 1st
day, reached the peak on the 7th day and began to decrease on the
14th day, the comparative difference at each time-point was
statistically significant (P<0.05), whereas, those in the HBD-3
group at each time-point were significantly higher than the model
group and vancomycin group, and the difference was statistically
significant (P<0.05) (Figs. 2 and
3).
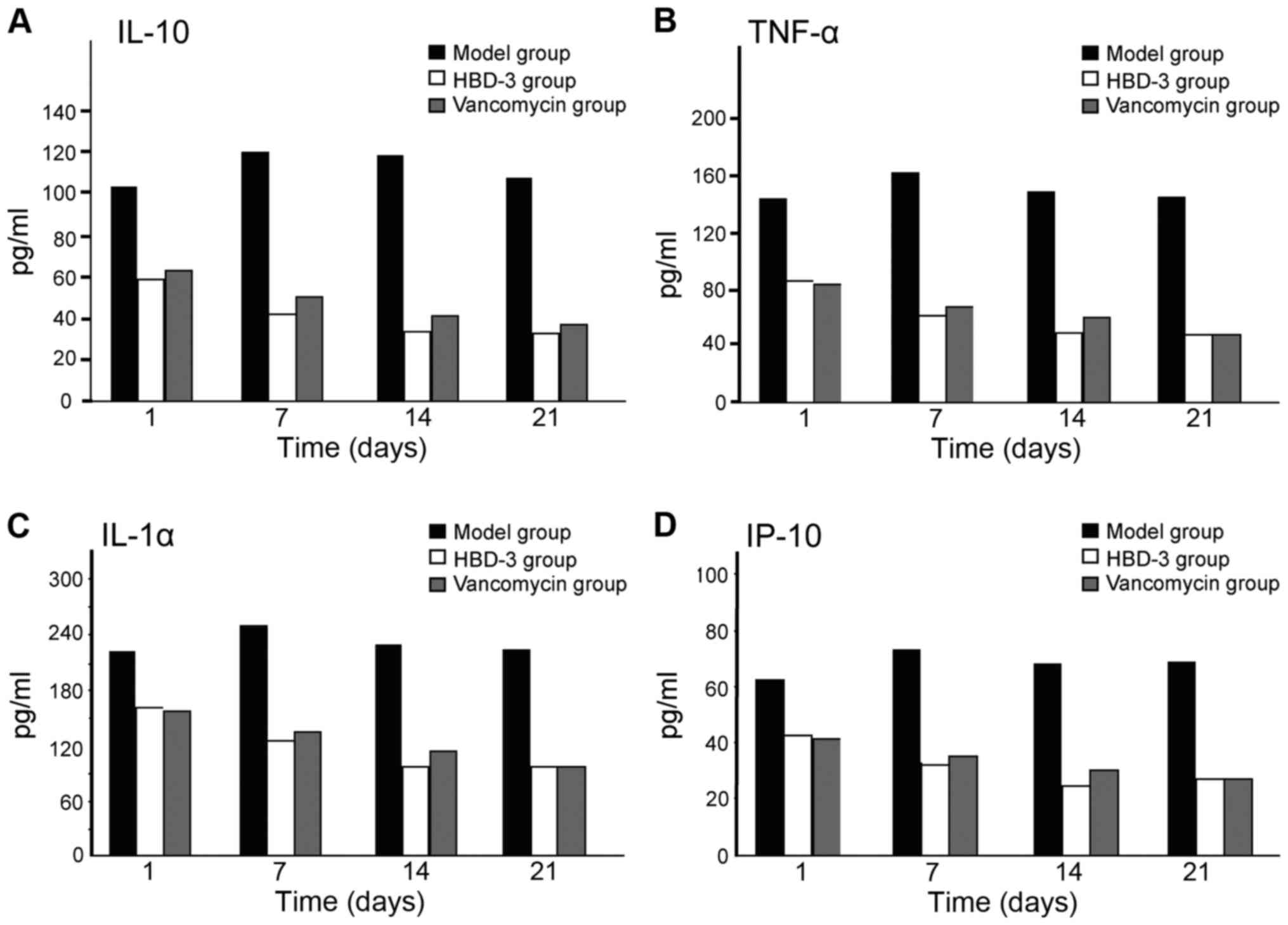
The expression levels of IL-10, TNF-α,
IL-1α and IP-10
The expression levels of IL-10, TNF-α, IL-1α and
IP-10 at each time-point in the model group were significantly
higher than the other two groups, and the difference was
statistically significant (P<0.05); in terms of the comparison
between the HBD-3 group and vancomycin group, the difference was
not statistically significant (P>0.05) (Fig. 4).
Discussion
A previous study (4)
has shown that after bacterial infection, the osteoblasts could be
stimulated to secret cytokines, chemokines, and endogenous
antibiotic peptide β-defensin, suggesting that in addition to the
inherent bone metabolism regulation function, the osteoblast could
also help active immune and defense response and acquired immune
regulation of the host. While under the bacterial inflammation
condition, the β-defensins secreted by the osteoblasts, as a kind
of inhibitor for bacterial infection newly discovered in recent
years, are attracting increasing attention in clinical and basic
research because of its broad antibacterial spectrum, greater
action speed, the relative difficulty of bacterial mutation
resistance to the drug, inherent immunological compatibility and
special role in bone infection immune regulation and control
(5).
β-defensin, as a kind of cationic protein
polypeptide with the molecular weight of 2–6 kDa, has potent
antimicrobial activity (6) if the
concentration is from µmol to nmol. Compared with other
antimicrobial peptides, HBD-3 has a broad antibacterial spectrum,
stable physical and chemical properties as well as a strong
bactericidal effect on the Gram-positive, negative and fungi. It
can pass through the mediation of TLR and connect with congenital
and acquired immune responses (7).
The amino acid sequence of the MBD-14 is highly congenetic
(8) to that of HBD-3. Furthermore,
the in vitro antibacterial experiments and immune chemotaxis
experiments have also showed (9)
that the recombinant MBD-14 has a similar broad-spectrum
antibacterial and chemotactic cytokine activity with HBD-3.
β-defensin-class antibacterial mechanism may be due to the peptide
molecules with cationic charges that can be combined first on the
bacterial plasma membrane with the cationic charge, then the
hydrophobic segments and amphipathic α-helixes in the molecules
will be inserted into the plasma membrane, eventually the ion
channel can be formed by mutual displacement of the membrane
molecules, thereby resulting in bacterial membrane structural
damage and causing bacteria death (10) due to loss of membrane potential.
Since the β-defensin-class antibacterial mechanism is mainly a
physical effect, it is significantly different from the mechanism
of traditional antibiotics. Therefore, it is difficult for
microorganism to produce resistance mutation against it, this is
the reason that β-defensins can still be provided with a
bactericidal effect on many drug-resistant bacteria even including
resistance to the vancomycin Enterococcus faecalis, and it
will not damage its own cells and visceral organs (11).
Our study results show that there was no death due
to infection in the HBD-3 group and vancomycin group, there was
only one case with significant wound swelling in the two groups,
but there were no purulent secretions, suggesting that HBD-3 and
vancomycin had a strong antibacterial activity. The percentage of
the total white blood cells and the neutrophil granulocytes in the
HBD-3 group and vancomycin groups as well as the biofilm morphology
and the number of viable bacteria were decreased with time, those
at each time-point were significantly decreased compared with the
model group, the comparative differences among the groups and in
the group were statistically significant; in terms of the
comparison between the HBD-3 group and vancomycin group, the
difference was not statistically significant. It was suggested that
HBD-3 and vancomycin had a strong bacteriostatic effect on the
bacterial growth and biofilm formation and the bacteriostatic
effects were equivalent (12). The
expression of NF-κB and TLR-4 in the model group and vancomycin
group was not significantly changed at each time-point, those in
the HBD-3 group began to increase on the 1st day, reached the peak
on the 7th day and began to decline on the 14th day, those in the
HBD-3 group at each time-point were significantly higher than the
model group and the vancomycin group and the difference was
statistically significant. It was suggested that the bacteriostatic
effect of HBD-3 may be related to the upregulation of the
expressions of NF-κB and TLR-4 (13), while the bacteriostatic effect of
vancomycin may be accomplished by other means. The expression
levels of IL-10, TNF-α, IL-1α and IP-10 in the model group at each
time-point were significantly higher than the other two groups, and
the difference was statistically significant; in terms of the
comparison between the HBD-3 group and vancomycin group, the
difference was not statistically significant. It was suggested that
HBD-3 and vancomycin could significantly reduce the IL-10, TNF-α,
IL-1α and IP-10 mediated inflammatory response (14). In conclusion, β-defensin 3 can
inhibit the bacterial growth by regulating inflammation and immune
response in MRSA-induced implant drug-resistant bacteria biofilm
infection in mouse tibial bone marrow.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81401815), the China
Postdoctoral Science Foundation (grant no. 2015M582900) and the
Jiangsu Postdoctoral Science Foundation (grant no. 1501146C).
References
|
1
|
Montanaro L, Speziale P, Campoccia D,
Ravaioli S, Cangini I, Pietrocola G, Giannini S and Arciola CR:
Scenery of Staphylococcus implant infections in orthopedics. Future
Microbiol. 6:1329–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Batoni G, Maisetta G, Esin S and Campa M:
Human beta-defensin-3: a promising antimicrobial peptide. Mini Rev
Med Chem. 6:1063–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi Y, Song W, Feng ZH, Zhao YT, Li F,
Tian Y and Zhao YM: Disinfection of maxillofacial silicone
elastomer using a novel antimicrobial agent: recombinant human
beta-defensin-3. Eur J Clin Microbiol Infect Dis. 28:415–420. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu C, Tan H, Cheng T, Shen H, Shao J, Guo
Y, Shi S and Zhang X: Human β-defensin 3 inhibits
antibiotic-resistant Staphylococcus biofilm formation. J Surg Res.
183:204–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu C, He N, Cheng T, Tan H, Guo Y, Chen
D, Cheng M, Yang Z and Zhang X: Ultrasound-targeted microbubble
destruction enhances human β-defensin 3 activity against
antibiotic-resistant Staphylococcus biofilms. Inflammation.
36:983–996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh PK, Shiha MJ and Kumar A:
Antibacterial responses of retinal Müller glia: production of
antimicrobial peptides, oxidative burst and phagocytosis. J
Neuroinflammation. 11:332014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colavita I, Nigro E, Sarnataro D, Scudiero
O, Granata V, Daniele A, Zagari A, Pessi A and Salvatore F:
Membrane protein 4F2/CD98 is a cell surface receptor involved in
the internalization and trafficking of human β-Defensin 3 in
epithelial cells. Chem Biol. 22:217–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hinrichsen K, Podschun R, Schubert S,
Schröder JM, Harder J and Proksch E: Mouse beta-defensin-14, an
antimicrobial ortholog of human beta-defensin-3. Antimicrob Agents
Chemother. 52:1876–1879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Röhrl J, Yang D, Oppenheim JJ and Hehlgans
T: Identification and biological characterization of mouse
beta-defensin 14, the orthologue of human beta-defensin 3. J Biol
Chem. 283:5414–5419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bedran TB, Mayer MP, Spolidorio DP and
Grenier D: Synergistic anti-inflammatory activity of the
antimicrobial peptides human beta-defensin-3 (hBD-3) and
cathelicidin (LL-37) in a three-dimensional co-culture model of
gingival epithelial cells and fibroblasts. PLoS One. 9:e1067662014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Semple F, MacPherson H, Webb S, Cox SL,
Mallin LJ, Tyrrell C, Grimes GR, Semple CA, Nix MA, Millhauser GL,
et al: Human β-defensin 3 affects the activity of pro-inflammatory
pathways associated with MyD88 and TRIF. Eur J Immunol.
41:3291–3300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Funderburg N, Lederman MM, Feng Z, Drage
MG, Jadlowsky J, Harding CV, Weinberg A and Sieg SF:
Human-defensin-3 activates professional antigen-presenting cells
via toll-like receptors 1 and 2. Proc Natl Acad Sci USA.
104:18631–18635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ning R, Zhang X, Guo X and Li Q:
Staphylococcus aureus regulates secretion of interleukin-6 and
monocyte chemo-attractant protein-1 through activation of nuclear
factor kappaB signaling pathway in human osteoblasts. Braz J Infect
Dis. 15:189–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu C, Wang J, Cheng T, Li Q, Shen H, Qin
H, Cheng M and Zhang X: The potential role of increasing the
release of mouse β-defensin-14 in the treatment of osteomyelitis in
mice: a primary study. PLoS One. 9:e868742014. View Article : Google Scholar : PubMed/NCBI
|