Introduction
Intervertebral disc degeneration (IDD) is associated
with lower back pain, which has a significant impact on modern
society (1,2). The causes of IDD are multifactorial,
and the underlying pathophysiology and pathogenesis are not fully
understood (3). Animal models of IDD
are crucial for clarifying its pathological mechanisms and provide
a means for evaluating new pharmacological therapies and other
treatment modalities (4). Many
animal models of disc degeneration have been established; these
include mechanical models such as compression or
instability-induced models, structural models such as injury or
chemically induced models, and models of spontaneously developing
IDD (5–7). Each of these models has advantages and
disadvantages when used for the study of IDD pathogenesis and/or to
test novel therapies. Among these IDD models, the most commonly
used is the needle puncture or stab wound-induced model (6,8). All
such injury-induced degeneration models require a surgical step to
injure the disc and create a wound sufficient to trigger disc
degeneration (9); therefore, the
pathophysiology of injury-induced disc degeneration is more similar
to a frustrated healing response than to the process of human disc
degeneration (8). Currently, no
ideal animal model for the study of IDD exists, and the
identification of novel improved animal models remains of great
importance (10).
Excessive mechanical loading can contribute to the
initiation of IDD (11). An
epidemiological study reported a positive dose-response
relationship between an individual's cumulative occupational lumbar
load and lumbar disc herniation, as well as lumbar disc narrowing
(12). Additionally, an in
vivo study investigating the effects of mechanical loading on
the transport of nutrients into normal human lumbar discs
demonstrated that sustained mechanical loading impairs the
diffusion of nutrients into the disc and triggers changes similar
to those found in disc degeneration (13). These findings suggest that a model of
mechanical load-induced disc degeneration may stimulate the
processes involved in human disc degeneration.
Intradiscal pressure (IDP) is thought to be one of
the few determinants that indirectly influenced by axial spinal
load (14). A previous investigation
found great variations in IDP in sheep during their various daily
activities. The highest IDPs (3.73–4.78 MPa) were recorded when the
sheep stood up from a lying position, and were 5- to 6-fold greater
than the IDPs recorded while the sheep were standing (0.70–0.73
MPa) (15). This indicates that an
animal's position greatly impacts its IDP, which is considered to
be a significant factor influencing disc degeneration (13,16).
In the present study, effects of noninvasive
cumulative axial loading on the rabbit lumbar discs were explored
by making rabbits maintain an upright posture and placing a
noninvasive external load on the spine. The method used in this
study partially simulated the excessive load-induced IDD process in
humans, and thus may constitute a potential model for use in
further studies.
Materials and methods
Establishment of noninvasive
cumulative axial load-induced IDD model
A total of 24 male New Zealand white rabbits (~4
months old; 2.4–2.8 kg) provided by the animal center of Navy
General Hospital (Beijing, China) were randomly assigned to one of
two study groups: Experimental or control. Each rabbit in the
experimental group (n=12) was placed into a plastic tube designed
to maintain the rabbit in an upright posture. The tubes were
transparent, 50 cm in height, 14 cm in diameter, and equipped with
steel mesh bases for ease of cleaning. While in the tubes, the
rabbits were docile and unable to escape. Following a 1-week
acclimation period during which rabbits in the experimental group
were confined in tubes for 4–6 h a day, a 600-g collar composed of
Styrofoam and plummets was placed onto the neck of each
experimental rabbit to begin the formal experiment. Rabbits in the
experimental group were put in their tubes for 6 h every day (3 h
in the morning and 3 h in the afternoon). When not confined to
their tubes, the rabbits in the experimental group were housed and
fed in standard cages. Rabbits in the control group (n=12) were
housed and fed in standard cages throughout the entire experiment.
All rabbits were housed at 20–25°C, 50–60% humidity, and a 12 h
light/dark cycle. Each rabbit was fed with 75 g rabbit feed, twice
a day. Water was provided ad libitum. Rabbits in the two
groups were weighed every 4 weeks to assess their general
condition. Experimental methods were conducted in accordance with
recommendations in the Guide for the Care and Use of Laboratory
Animals from the National Institutes of Health (Bethesda, MD, USA).
The study protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of Navy General Hospital (Beijing,
China; no. 2013–0624).
Radiographs
Prior to the experiment, lateral radiographs were
taken of each rabbit in the experimental group while the animal was
sitting in an upright position in its tube to observe its body
position within the tube. Rabbits in the experimental and control
groups were subsequently anesthetized with xylazine (3 mg/kg
intramuscularly, Shengda Pharmaceutical Co., Ltd., Jilin, China)
and ketamine (40 mg/kg intramuscularly, Jiangsu Hengrui Medicine
Co., Ltd., Jiangsu, China) and lumbar lateral radiographs were
taken with the rabbits in the lateral decubitus position. The
differences in disc height of rabbits in the experimental group
were determined by measuring the disc heights on the upright and
lateral decubitus radiographs.
At 4, 8 and 12 weeks following the start of the
study, lumbar lateral radiographs of rabbits in the experimental
and control groups were taken when rabbits were in the lateral
decubitus position to examine changes in disc height. Disc heights
were measured and expressed as the disc height index (DHI) using a
previously reported method (6). The
DHI was calculated using measurements obtained from the anterior,
middle and posterior portions of the intervertebral disc, and was
divided by the average of adjacent vertebral body heights. The
formula DHI=2x(A+M+P)/(A1+M1+P1+A2+M2+P2) was used for all
radiograph measurements, where A, M, P indicate anterior, middle
and posterior disc height, respectively; A1, M1, P1 indicate
anterior, middle and posterior upper adjacent vertebral body
height, respectively; and A2, M2, P2 indicate anterior, middle and
posterior lower adjacent vertebral body height, respectively
(Fig. 1D). Changes in DHI were
expressed as a DHI percentage, calculated as follows: DHI (%)=DHI
in upright position/DHI in lateral decubitus position ×100. To
evaluate differences between the lumbar discs at different levels,
data from three lumbar segments were analyzed. These segments were
discs L2-3, L4-5, and L6-7, representing the upper, middle and
lower lumbar discs, respectively. All measurements were recorded by
two independent observers, and the mean values were calculated.
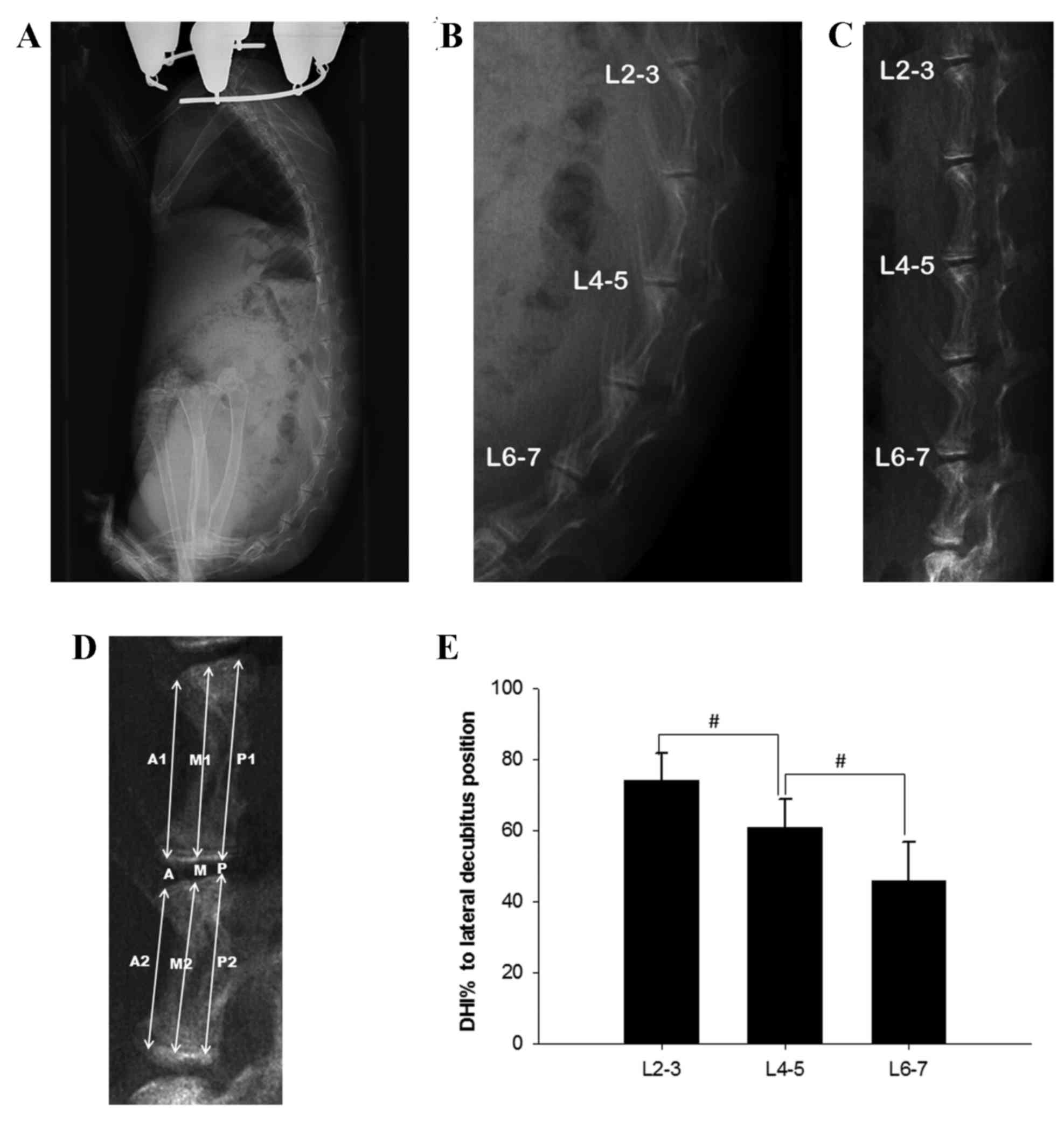 | Figure 1.Body position of rabbits in the
experimental group and disc height measurements under different
conditions. (A) Lateral radiograph of an experimental rabbit while
maintained in an upright posture by a tube, and (B) a locally
enlarged lumbar image from this lateral radiograph. (C) Lateral
radiograph of the same rabbit in the lateral decubitus position.
(D) Schematic representation of the measurements taken to calculate
the DHI. (E) Changes in the DHI, where DHI% is the DHI in the
upright position as a percentage of DHI in the lateral decubitus
position. All data are expressed as the mean ± standard deviation.
#P<0.05. DHI, disc height index. A, M, P indicate
anterior, middle and posterior disc height, respectively; A1, M1,
P1 indicate anterior, middle and posterior upper adjacent vertebral
body height, respectively; A2, M2, P2 indicate anterior, middle and
posterior lower adjacent vertebral body height, respectively. |
Magnetic resonance imaging (MRI)
examination
Rabbits in the two groups underwent MRI prior to and
4, 8, 12 and 14 weeks following commencement of the study. From
12–14 weeks, rabbits in the experimental group did not receive any
treatment, and were housed and fed in the same manner as the
control group while allowing time for disc recovery.
The hydration status of the nucleus pulposus (NP)
was graded using a modified Schneiderman's score as previously
described (17). SilverPACS-ADViewer
software version 4.8 (Zhejiang SilverPAC HEA Ltd., Zhejiang, China)
was used to determine grayscale values for the NP of discs of
interest and cerebrospinal fluid in the same image on a T2-weighted
sagittal MRI scan. The grayscale value for the NP was normalized
against that of the cerebrospinal fluid, which was arbitrarily
assigned a value of one. To evaluate differences among lumbar discs
at different levels, the data from the three lumbar segments were
analyzed. All measurements were recorded by two independent
observers and the mean values were calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of gene
expression
At 14 weeks, rabbits from the two groups were
sacrificed by injection of 100 mg/kg pentobarbital (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany). The NPs of L5-6 were obtained
and immediately frozen at −196°C in liquid nitrogen for subsequent
RT-qPCR analysis.
Total ribonucleic acids (RNA) of tissue was
extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). 2 U DNase I enzyme was mixed
with 1 µg extracted RNA and incubated at 37°C for 20 min to remove
genomic DNA using the DNA-free™ kit (Takara Bio, Inc., Otsu,
Japan), followed by inactivation of DNase using 1 µl of the
provided DNase inactivation reagent. RNA concentration and
integrity were analyzed in denaturing electrophoresis gel and
spectrophotometer (A260/A280 and A230/A280 ratios). RT-qPCR was
conducted using PrimeScript™ RT Master Mix (Takara Bio, Inc.).
Briefly, 1 ml RNA was mixed with 2 ml 5X PrimeScript RT MasterMix
and 10 ml RNase free dH2O was added. Mixed solutions
were incubated for 15 min at 37°C and then at 85°C for 5 sec, and
finally stored at −80°C prior to qPCR. qPCR assays were performed
with a SYBR Premix Ex Taq™ PCR kit (Takara Bio, Inc.) and a Mini
Opticon Detector system (Bio Med Sciences, Inc., Allentown, PA,
USA). Briefly, 10 ul SYBR-Green (containing DNA polymerase), 10
pmol forward and reverse primer, and 100 ng cDNA were used in a
final volume of 20 µl. All primers were designed using Premier 5.0
software (Premier Biosoft International, Palo Alto, CA, USA) and
are presented in Table I. GAPDH was
used as a housekeeping gene due to its stable expression in all
tissue. The qPCR thermal cycling conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing at 60°C for 30 sec and
extension at 72°C for 30 sec. Melting curve analysis was performed
following the final amplification period via a temperature gradient
of 95°C for 15 sec, 60°C for 15 sec and 95°C for 15 sec. The
log2 (Δ-Δ-Cq) was performed by comparing the
mean experimental Δ-Cq to the mean control animal
Δ-Cq (18). The mean
value for each sample was used in the final analysis. All the
experiments were performed in triplicate.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequence | GeneBank accession
no. |
|---|
| GAPDH | Forward:
5′-GGAGAAAGCTGCTAA-3′ | L23961 |
|
| Reverse:
5′-ACGACCTGGTCCTCGGTGTA-3′ |
|
| Collagen Iα | Forward:
5′-ATGGATGAGGAAACTGGCAACT-3′ | D49399 |
|
| Reverse:
5′-GCCATCGACAAGAACAGTGTAAGT-3′ |
|
| Collagen IIα | Forward:
5′-CCTGTGCGACGACATAATCTGT-3′ | AF027122 |
|
| Reverse:
5′-GGTCCTTTAGGTCCTACGATATCCT-3′ |
|
| Aggrecan | Forward:
5′-GCTACGGAGACAAGGATGAGTTC-3′ | L38480 |
|
| Reverse:
5′-CGTAAAAGACCTCACCCTCCAT-3′ |
|
Histological analysis
Following sacrifice, disc segment L6-7, which
exhibited the most obvious degenerative changes on MRI examination,
was removed using a miniature electronic saw and prepared for
histological analysis. The specimens were immediately fixed in 10%
formaldehyde at 25°C for 1 week, decalcified using Perenyi's fluid
(4% nitric acid, 0.15% chromic acid and 30% ethanol) for 6 days and
washed with running water for 12 h to remove excess acid. Tissue
samples were subsequently cut mid-sagittally, embedded in paraffin,
cut into 5-µm slices, stained with hematoxylin and eosin (H&E,
10 min hematoxylin staining and 1 min eosin staining) at 25°C and
examined via light microscopy. Additional sections were stained
with 0.05% picrosirius red for 30 min at 25°C and examined via
polarized light microscopy to observe any changes in collagen
fibers that may have occurred during disc degeneration.
Statistical analysis
All statistical analysis was performed using SPSS
version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Differences between means of data from radiographic and MRI
measurements were analyzed using repeated measurement analysis of
variance and Fisher's least significant differences post hoc test.
The homogeneity of variances was initially assessed using Levene's
test. If the variances were homogeneous, the least-significant
difference test was performed; otherwise, Tamhane's T2 was used.
All data are expressed as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
General conditions
During weeks 3 and 7 of the study, two rabbits in
the experimental group became agitated and died; their data were
not included in the final results. The remaining 10 rabbits in the
experimental group tolerated the treatment well. Throughout the
14-week experiment, the mean body weight of rabbits in the
experimental and control groups increased from initial values of
2.56 and 2.62 kg, respectively, to final values of 3.63 and 3.81
kg, respectively. No significant differences in weight were
observed between the experimental and control groups at any time
point.
Radiography
A representative lateral radiograph of an
experimental group rabbit being maintained in an upright position
in its tube is displayed in Fig. 1A and
B. In this position, there was a marked kyphotic curvature of
the lower lumbar spine, and the observed disc height is markedly
less than that in the image of the same rabbit in a lateral
decubitus position (Fig. 1C). A
schematic representation of the technique used to measure DHI is
displayed in Fig. 1D. Changes in the
DHI when rabbits were in an upright posture were expressed as
percentage of DHI and normalized to measurements obtained while the
rabbits were in the lateral decubitus position. Significant
differences between the disc heights in the upright and lateral
decubitus positions were observed for all of the three measured
sections (all P<0.05). For L2-3, the mean disc height in the
upright position was 74.4% of its height in the lateral decubitus
position. This ratio dropped to 60.7% for L4-5 and 46.5% for L6-7.
A significant difference in disc height reduction was also observed
among different segments (P<0.05; Fig. 1E).
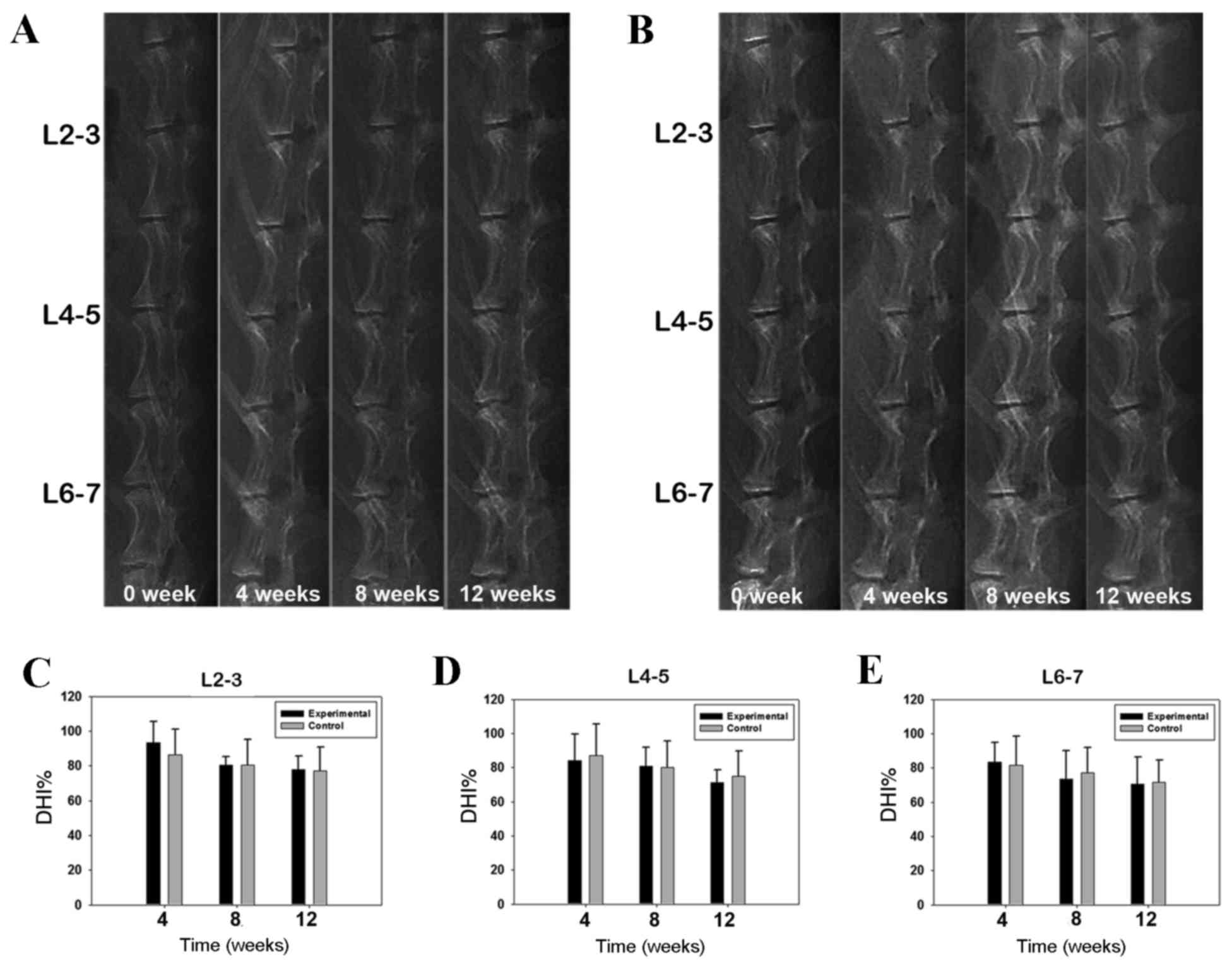
Fig. 2A and B present
representative lateral radiographs of rabbits from the control and
experimental groups, respectively; these were obtained prior to
treatment and again at 4, 8 and 12 weeks post-treatment. A steady
reduction in DHI values was observed over time for the control and
experimental groups; however, no significant difference was
observed between the two groups at any lumbar level or at any time
point (Fig. 2C-E).
MRI
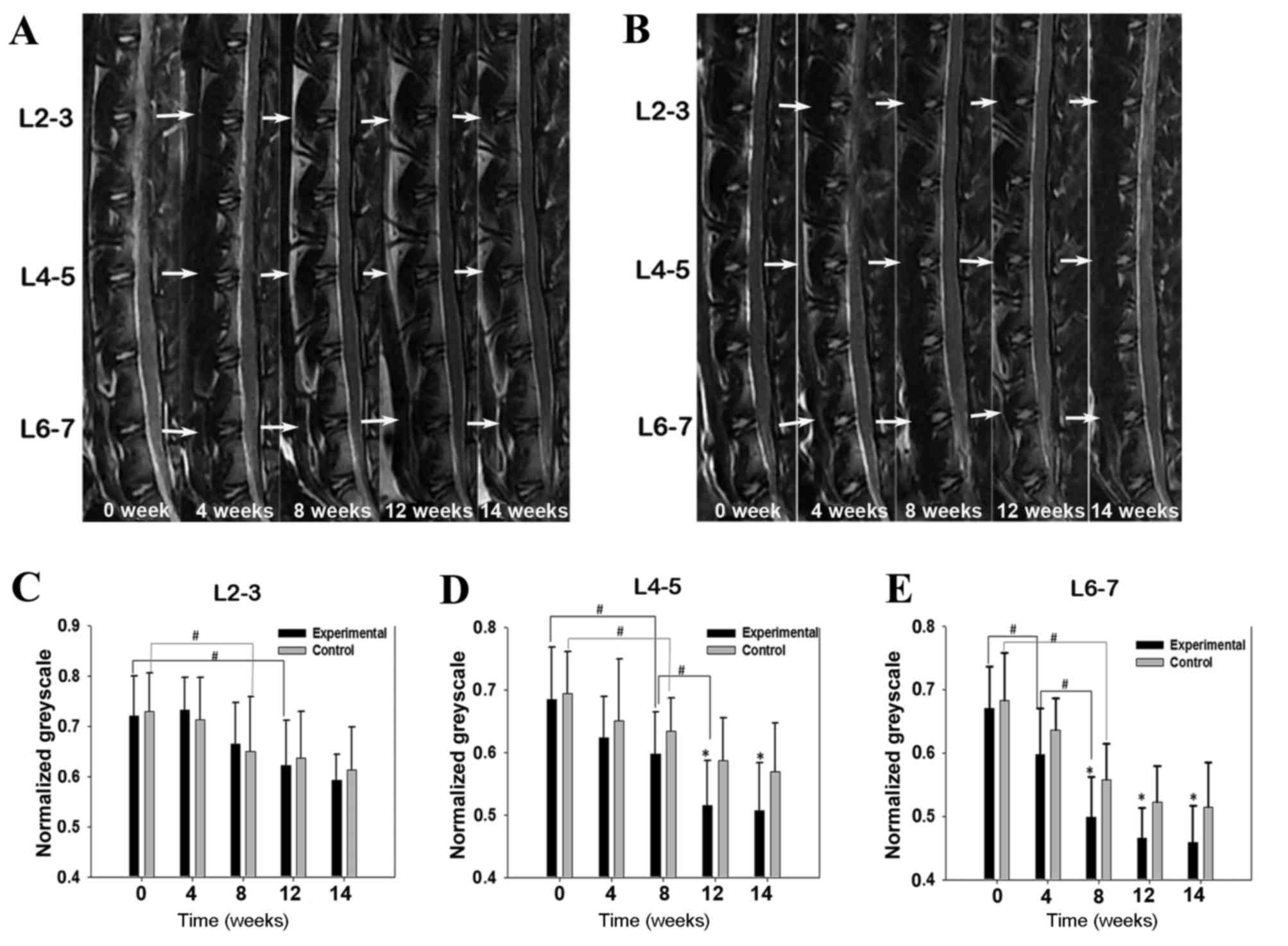
Serial MRI scans of the lumbar spinal areas of
animals in the experimental and control groups were obtained prior
to and at 4, 8, 12 and 14 weeks following treatment. For each
group, representative, T2-weighted, mid-sagittal plane images from
the same rabbit at different time points are displayed in Fig. 3. Signal intensities of lumbar discs
from rabbits in the experimental group decreased progressively over
the 14-week experimental period, particularly in the lower lumbar
discs (Fig. 3A). By contrast, NP
signal intensities in the control group decreased more slowly
during the same period (Fig. 3B).
Grayscale values for the NP areas decreased gradually in both
groups over time, in particular during the first 8 weeks. In the
L2-3 segment, no significant difference was observed between the
experimental and control groups at any time point (Fig. 3C). In segment L4-5, a significant
reduction was observed in the experimental group compared with the
control group at week 12, and was maintained over the following
2-week recovery period (P<0.05; Fig.
3D). In segment L6-7, a significant reduction was observed in
the experimental group compared with the control group at week 8,
which persisted until the end of the experiment (P<0.05;
Fig. 3E).
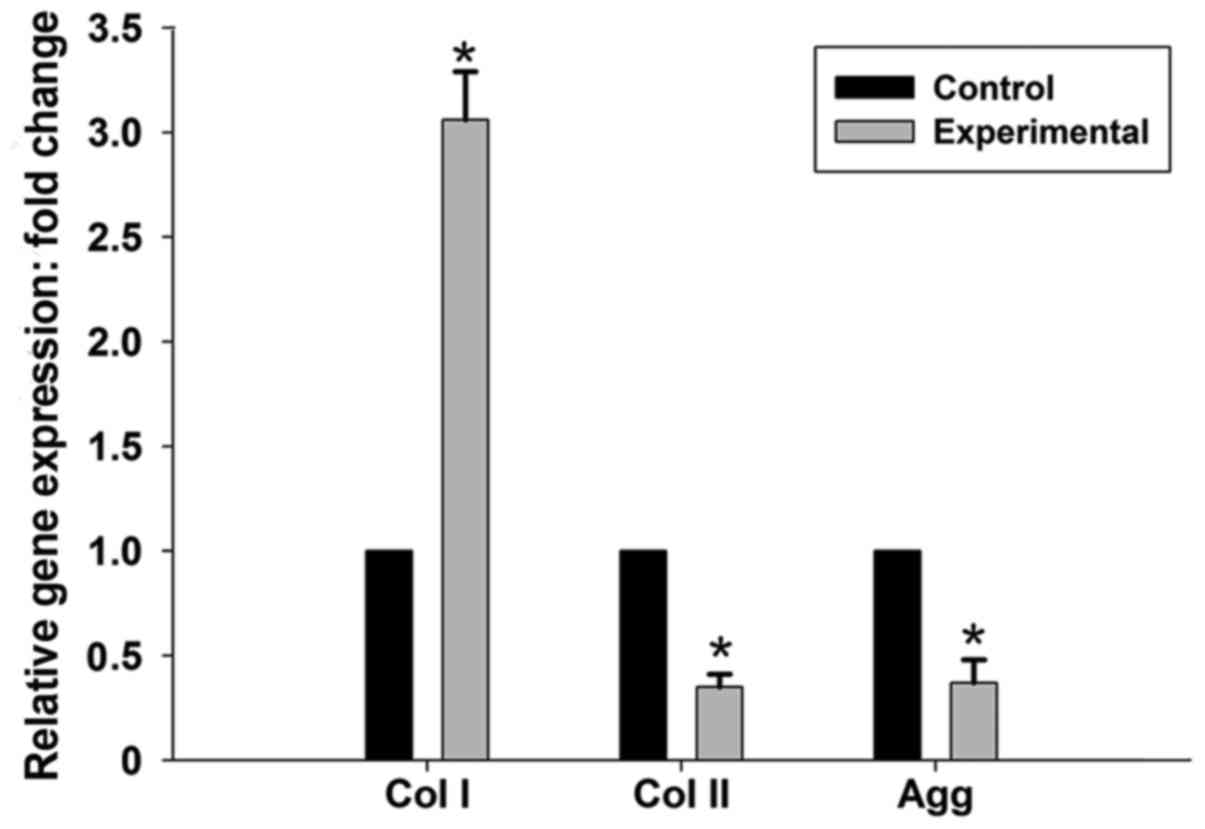
RT-qPCR
The relative expression levels of matrix genes in
the experimental and control groups were assessed by comparing the
mean experimental animal Δ-Cq with the mean control
animal Δ-Cq. The expression of collagen type Iα mRNA was
significantly higher in the experimental group compared with the
control group (3.06-fold; P<0.05), whereas collagen type IIα and
aggrecan mRNA expression levels were significantly downregulated
(0.35- and 0.37-fold, respectively; P<0.05; Fig. 4).
Histology
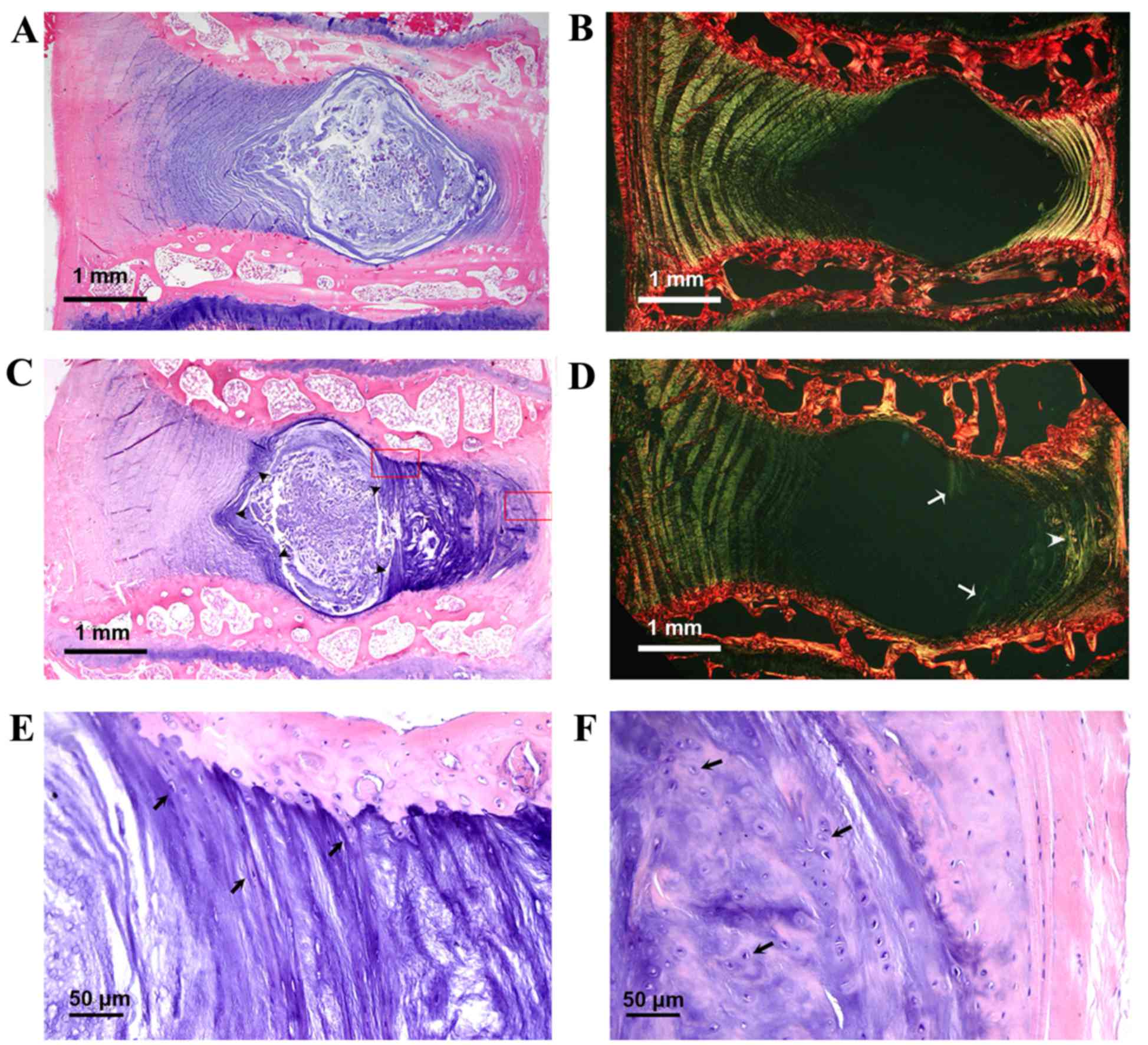
Representative histological sections of disc segment
L6-7 in the midsagittal plane are displayed in Fig. 5. These include light microscopy
images following H&E staining and polarized microscopy images
following picrosirius red staining. In the control group, H&E
staining revealed a clear demarcation between the NP and annulus
fibrosus (AF), and scattered clusters of NP cells within an
abundant gelatinous matrix (Fig.
5A). Picrosirius red staining revealed a nearly intact AF, with
a normal pattern of fibrocartilage lamellae and a well-defined
border between the AF and NP (Fig.
5B). In the experimental group, H&E staining revealed a
marked increase in fibrocartilage lamellae at the inner border of
the posterior AF, resulting in a markedly decreased gelatinous NP
area (Fig. 5C). Locally enlarged
images (indicated by red boxes in Fig.
5C) demonstrated that the increased inner lamellae had the
appearance typical of fibrocartilage, and originated from cartilage
endplates (Fig. 5E), whereas the
outer border of the posterior AF lost its fibrocartilage lamellar
structure and resembled hyaline cartilage (Fig. 5F). Picrosirius red staining revealed
newly formed birefringent collagen fibers infiltrating the NP from
the margins of the cartilage endplates (Fig. 5D). The thickened posterior AF lacked
the normal structure of fibrocartilage lamellae, and exhibited an
uneven staining pattern (Fig.
5D).
Discussion
The present study explored the effects of
noninvasive cumulative axial loading on the intervertebral discs in
a rabbit model. The animals used in this study were young rabbits
(~4 months old), which are readily obtainable by any institution.
The total death rate of 16.7% appears comparable to that seen in
other disc degeneration models (19). Compared with previously established
disc degeneration models, the model used in the present study was
implemented without producing disc injury and thus avoided surgical
intervention. The loading pattern used in the present study
partially mimics the cumulative effects of occupational lumbar
loading in humans, which has been reported to have positive
dose-response associations with lumbar disc herniation and
spondylosis (12,20).
To increase the IDP in lumbar discs, rabbits were
placed into frictionless tubes designed to maintain them in an
upright posture, and the ‘body weight’ above the lumbar disc was
increased using a heavy collar. A pilot study revealed that rabbits
were able to withstand this treatment as long as the external
loading weight was ≤1/4 of its body weight (600 g), and the working
time was ≤6 h per day; otherwise, rabbits may experience adverse
effects including irritability or anorexia. To ensure that rabbits
in the experimental group were properly fed, they were allowed to
eat and rest at noontime. Apart from the time spent in the tubes,
the rabbits in the experimental group were housed under the same
conditions as the control group.
Radiographs captured when rabbits were in the
upright position revealed that the lower lumbar disc space was
significantly narrowed relative to its height in the lateral
decubitus position (>50% in disc segment L6-7), indicating
increased IDP in the lumbar discs when rabbits were upright. The
disc narrowing was most severe in the lower lumbar spine. This may
be due in part to greater compression forces being exerted on the
lower lumbar discs; however, the primary cause may be the kyphotic
curvature of the lower lumbar spine producing concentrated areas of
stress and the muscle force required to stabilize the spine, which
may contribute to increasing the IDP of experimental rabbits
(15,21,22).
With classic needle puncture-induced models of IDD a
significant difference in DHI reduction between groups may be
observed as early as 2 weeks post-treatment (6); however, in the present study no
significant differences were observed in the DHI measurements at
any disc level throughout the 12-week observation period. While a
disc showing obvious signs of narrowing is considered to be
severely degenerated, such a disc may not be an ideal model for
investigating novel biological therapies for early stage disc
degeneration (8). Therefore, the
upright rabbit model using in the present study may represent an
ideal research tool for assaying the effectiveness of such
treatments for early and mild degeneration. It was notable that the
DHI reduced obviously over time in both the experimental and the
control group. This may be a result of the young age of the animals
and their skeletal immaturity. The speed of intervertebral disc
growth may not be as great as that of the adjacent vertebral body,
resulting in a reduction in DHI during this period. However when
the rabbits became skeletally mature at week 8, the drop in DHI was
not so precipitous.
On observation of the MRI images, the degenerative
changes in the lower lumbar discs were more marked than those in
the upper lumbar discs. This degenerative pattern is similar to
that found in humans; disc degeneration in individuals typically
occurs in the lower lumbar region as a result of increased
mechanical loading and the structural properties of the motion
segment (23). Following 2 weeks of
recovery time, NPs in the experimental group did not recover their
normal hydration status, which indicates that irreversible
pathological changes occurred.
Matrix gene expression may vary in different IDD
models. In a rabbit annular laceration model, the expression of
collagen type Iα and type IIα were upregulated 2- to 8-fold while
the decorin gene was downregulated ~6-fold compared with its
expression in the control group (24). However, in another annular stab
model, aggrecan and collagen type IIα mRNA levels decreased
markedly, whereas collagen type Iα mRNA gradually increased
throughout the course of the degeneration (25). Hee et al (26) demonstrated that the collagen and
glycosaminoglycan content in inner AF and NP cells cultured under
0.2 MPa compressive stress was significantly higher than that in
control cells, whereas it was significantly lower than that in
control cells grown under 0.4 MPa compressive stress. This
indicates that the biosynthetic characteristics of human inner AF
and NP cells may vary with the degree of compressive stress that
they experience. The results for relative matrix gene expression in
the present study are similar to those of many other load-induced
degeneration models (27–29); in the experimental group the
expression of collagen type Iα mRNA was increased, whereas the mRNA
expression of collagen type IIα and aggrecan were significantly
decreased compared with their respective levels in the control
group. However, the examination of matrix gene expression was
confined to NP tissue in the present study, and the lack of data
regarding gene expression in the AF is a limitation.
It is generally believed that an increase in
collagen production in the AF of discs subjected to compression may
be a desirable anabolic remodeling response working to maintain the
strength of the AF, whereas an increase in collagen fiber
production in the nucleus may be more characteristic of an
undesirable degenerative change (16). In accordance with the results of MRI
and RT-qPCR, the histological examination in the present study
revealed visible structural changes for the cumulative axial
load-induced degeneration. Abundant fibrocartilage-like tissue grew
into the NP from vertebrae endplates, dividing the NP tissue into
small pieces. The NPs of the experimental group appear to have
undergone accelerated fibrotic changes, which may be responsible
for the dehydration changes in the MRI results. Although the widths
of the posterior AFs in the experimental group appeared to be
significantly increased, those AFs had lost their normal
fibrocartilaginous lamellar structure, and appeared similar to
hyaline cartilage (Fig. 5C and F).
These structural changes may undermine the tensile strength of the
posterior AFs, and may therefore be the pathological basis of disc
herniation.
Polarized light microscopy was used to identify
newly formed fibrocartilage containing birefringent collagen fibers
(30). Picrosirius red staining
followed by polarizing microscopy is able to selectively reveal
collagen and allows for the differentiation of procollagens and
pathological collagen fibers, which are not as tightly packed as
normal fibers (31). In the present
study, picrosirius red staining demonstrated that the distinction
between the NP and AF was less marked in the experimental group
compared with the control group, and that newly formed fibrous
bands were included in the border of the inner AF in the
experimental group. There was evidence of NPs in the experimental
group having undergone accelerated ‘fibrotic’ changes. Such changes
are considered a typical sign of disc degeneration, and are
difficult to reverse (23).
The present study had some limitations that should
be mentioned. The animals used were skeletally immature 4-month old
rabbits and mechanical loading of their lumbar discs may not be
directly comparable to the mechanical loading of skeletally mature
animals or adult humans. However, degenerative changes may occur in
humans prior to skeletal maturity (32); therefore, assessment of young rabbits
may be relevant to early-onset degeneration. Another major
limitation is that the exact in vivo IDP of rabbits in the
experimental group was not measured while they were in the tubes.
Additionally, it may have been possible to reduce the death rate by
optimizing the loading pattern in the experiment.
In conclusion, the present study may provide a novel
and feasible method for establishing a noninvasive cumulative
load-induced lumbar disc degeneration model that is able to
simulate the load-induced disc degeneration that occurs in
humans.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301579). The
authors would like to thank Dr Minhua Huang and Dr Yongjun Zuo for
their assistance with radiographs and MRI examinations.
References
|
1
|
Kandel R, Roberts S and Urban JP: Tissue
engineering and the intervertebral disc: The challenges. Eur Spine
J. 17 Suppl 4:S480–S491. 2008. View Article : Google Scholar
|
|
2
|
Shi L, Teng H, Zhu M, Li C, Huang K, Chen
BI, Dai Y and Wang J: Paeoniflorin inhibits nucleus pulposus cell
apoptosis by regulating the expression of Bcl-2 family proteins and
caspase-9 in a rabbit model of intervertebral disc degeneration.
Exp Ther Med. 10:257–262. 2015.PubMed/NCBI
|
|
3
|
Issy AC, Castania V, Castania M, Salmon
CE, Nogueira-Barbosa MH, Bel ED and Defino HL: Experimental model
of intervertebral disc degeneration by needle puncture in Wistar
rats. Braz J Med Biol Res. 46:235–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan DD, Khan SN, Ye X, Curtiss SB, Gupta
MC, Klineberg EO and Neu CP: Mechanical deformation and
glycosaminoglycan content changes in a rabbit annular puncture disc
degeneration model. Spine (Phila Pa 1976). 36:1438–1445. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lotz JC: Animal models of intervertebral
disc degeneration: Lessons learned. Spine (Phila Pa 1976).
29:2742–2750. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masuda K, Aota Y, Muehleman C, Imai Y,
Okuma M, Thonar EJ, Andersson GB and An HS: A novel rabbit model of
mild, reproducible disc degeneration by an annulus needle puncture:
Correlation between the degree of disc injury and radiological and
histological appearances of disc degeneration. Spine (Phila Pa
1976). 30:5–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun F, Qu JN and Zhang YG: Animal models
of disc degeneration and major genetic strategies. Pain Physician.
16:E267–E275. 2013.PubMed/NCBI
|
|
8
|
Xi Y, Kong J, Liu Y, Wang Z, Ren S, Diao Z
and Hu Y: Minimally invasive induction of an early lumbar disc
degeneration model in rhesus monkeys. Spine (Phila Pa 1976).
38:E579–E586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elliott DM, Yerramalli CS, Beckstein JC,
Boxberger JI, Johannessen W and Vresilovic EJ: The effect of
relative needle diameter in puncture and sham injection animal
models of degeneration. Spine (Phila Pa 1976). 33:588–596. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakai D: Future perspectives of cell-based
therapy for intervertebral disc disease. Eur Spine J. 17 Suppl
4:S452–S458. 2008. View Article : Google Scholar
|
|
11
|
Stefanakis M, Luo J, Pollintine P, Dolan P
and Adams MA: ISSLS Prize winner: Mechanical influences in
progressive intervertebral disc degeneration. Spine (Phila Pa
1976). 39:1365–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seidler A, Bergmann A, Jäger M, Ellegast
R, Ditchen D, Elsner G, Grifka J, Haerting J, Hofmann F, Linhardt
O, et al: Cumulative occupational lumbar load and lumbar disc
disease-results of a German multi-center case-control study
(EPILIFT). BMC Musculoskelet Disord. 10:482009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arun R, Freeman BJ, Scammell BE, McNally
DS, Cox E and Gowland P: ISSLS Prize Winner: What influence does
sustained mechanical load have on diffusion in the human
intervertebral disc?: An in vivo study using serial postcontrast
magnetic resonance imaging. Spine (Phila Pa 1976). 34:2324–2337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guehring T, Unglaub F, Lorenz H, Omlor G,
Wilke HJ and Kroeber MW: Intradiscal pressure measurements in
normal discs, compressed discs and compressed discs treated with
axial posterior disc distraction: An experimental study on the
rabbit lumbar spine model. Eur Spine J. 15:597–604. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reitmaier S, Schmidt H, Ihler R, Kocak T,
Graf N, Ignatius A and Wilke HJ: Preliminary investigations on
intradiscal pressures during daily activities: An in vivo study
using the merino sheep. PLoS One. 8:e696102013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iatridis JC, MacLean JJ, Roughley PJ and
Alini M: Effects of mechanical loading on intervertebral disc
metabolism in vivo. J Bone Joint Surg Am. 88 Suppl 2:S41–S46. 2006.
View Article : Google Scholar
|
|
17
|
Ruan D, He Q, Ding Y, Hou L, Li J and Luk
KD: Intervertebral disc transplantation in the treatment of
degenerative spine disease: A preliminary study. Lancet.
369:993–999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sobajima S, Kompel JF, Kim JS, Wallach CJ,
Robertson DD, Vogt MT, Kang JD and Gilbertson LG: A slowly
progressive and reproducible animal model of intervertebral disc
degeneration characterized by MRI, X-ray, and histology. Spine
(Phila Pa 1976). 30:15–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seidler A, Bolm-Audorff U, Heiskel H,
Henkel N, Roth-Küver B, Kaiser U, Bickeböller R, Willingstorfer WJ,
Beck W and Elsner G: The role of cumulative physical work load in
lumbar spine disease: Risk factors for lumbar osteochondrosis and
spondylosis associated with chronic complaints. Occup Environ Med.
58:735–746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beckstein JC, Sen S, Schaer TP, Vresilovic
EJ and Elliott DM: Comparison of animal discs used in disc research
to human lumbar disc: Axial compression mechanics and
glycosaminoglycan content. Spine (Phila Pa 1976). 33:E166–E173.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue N and Orías AA Espinoza:
Biomechanics of intervertebral disk degeneration. Orthop Clin North
Am. 42:487–499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adams MA, Mcnally DS and Dolan P: ‘Stress’
distributions inside intervertebral discs. The effects of age and
degeneration. J Bone Joint Surg Br. 78:965–972. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderson DG, Izzo MW, Hall DJ, Vaccaro AR,
Hilibrand A, Arnold W, Tuan RS and Albert TJ: Comparative gene
expression profiling of normal and degenerative discs: Analysis of
a rabbit annular laceration model. Spine (Phila Pa 1976).
27:1291–1296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobajima S, Shimer AL, Chadderdon RC,
Kompel JF, Kim JS, Gilbertson LG and Kang JD: Quantitative analysis
of gene expression in a rabbit model of intervertebral disc
degeneration by real-time polymerase chain reaction. Spine J.
5:14–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hee HT, Zhang J and Wong HK: An in vitro
study of dynamic cyclic compressive stress on human inner annulus
fibrosus and nucleus pulposus cells. Spine J. 10:795–801. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paul CP, Schoorl T, Zuiderbaan HA, Doulabi
B Zandieh, van der Veen AJ, van de Ven PM, Smit TH, van Royen BJ,
Helder MN and Mullender MG: Dynamic and static overloading induce
early degenerative processes in caprine lumbar intervertebral
discs. PLoS One. 8:e624112013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang QQ, Cui XJ, Xi ZJ, Bian Q, Hou W,
Zhao YJ, Shi Q and Wang YJ: Prolonged upright posture induces
degenerative changes in intervertebral discs of rat cervical spine.
Spine (Phila Pa 1976). 36:E14–E19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yurube T, Takada T, Suzuki T, Kakutani K,
Maeno K, Doita M, Kurosaka M and Nishida K: Rat tail static
compression model mimics extracellular matrix metabolic imbalances
of matrix metalloproteinases, aggrecanases, and tissue inhibitors
of metalloproteinases in intervertebral disc degeneration.
Arthritis Res Ther. 14:R512012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K
and An HS: The origin of chondrocytes in the nucleus pulposus and
histologic findings associated with the transition of a notochordal
nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact
rabbit intervertebral discs. Spine (Phila Pa 1976). 28:982–990.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirshberg A, Sherman S, Buchner A and
Dayan D: Collagen fibres in the wall of odontogenickeratocysts: A
study with picrosirius red and polarizing microscopy. J Oral Pathol
Med. 28:410–412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sowa G, Vadalà G, Studer R, Kompel J, Iucu
C, Georgescu H, Gilbertson L and Kang J: Characterization of
intervertebral disc aging: Longitudinal analysis of a rabbit model
by magnetic resonance imaging, histology, and gene expression.
Spine (Phila Pa 1976). 33:1821–1828. 2008. View Article : Google Scholar : PubMed/NCBI
|