Introduction
As one of the most serious complications following
cardiac arrest (CA), global cerebral ischemia/reperfusion (I/R)
injury directly leads to serious neurological dysfunction and
mortality. It has been reported that <5% of individuals with an
out-of-hospital CA are able to survive, and only 25% of patients
survive the subsequent hospitalization following initial
resuscitation (1–4). Therefore, it is necessary to develop
novel therapeutic options in order to reduce cerebral I/R injury in
patients experiencing CA.
Ischemic postconditioning was first described by
Zhao et al in 2003 (5), and
consists of short episodes of ischemia after a prolonged cardiac
ischemia to reduce cardiac infarct size. Previous results have also
confirmed the neuroprotection effect of ischemic postconditioning
(6). However, brain ischemic
postconditioning may be difficult to apply in clinical practice due
to potential lesions of brain vessels and tissues. Compared with
ischemic postconditioning, inhaled anesthetic postconditioning is
relatively easy to administer and carries low risk. It has been
shown that postconditioning by the administration of inhaled
anesthetics, including isoflurane (Iso), sevoflurane and xenon, can
protect against cerebral I/R injury both in vitro and in
vivo (7–14). The mechanisms responsible for
anesthetic postconditioning are still unclear, but it is evident
that anesthetic postconditioning and ischemic postconditioning have
similarities, such as regulating pro-survival signaling downstream,
activating the phosphoinositide 3-kinase/Akt pathway, attenuating
mitochondrial damage and inflammation reaction, and reducing
oxidative stress and apoptosis (11,15).
However, the requirement for a vaporizer and related equipment
limits the use of anesthetic postconditioning in pre-hospital
resuscitation. A new type of anesthetic, emulsified isoflurane
(EIso), can offset these limitations. As a recently developed
formulation (a combination of emulsion and Iso), EIso is suitable
for intravenous administration. Large-animal experiments indicated
that EIso provides more rapid induction and recovery compared with
propofol, and presents a notable hemodynamic stability (16). A few animal studies have shown that
EIso possesses protective effects on ischemic heart, liver and lung
(17–20). However, studies on EIso for global
cerebral I/R injury are very limited.
Recently, a phase I clinical trial of EIso has been
completed and the results have shown that EIso possesses a strong
anesthetic potency and can be safely used in humans (21). Therefore, it was hypothesized that
EIso postconditioning, administered following the return of
spontaneous circulation (ROSC), could provide cerebral protection
(22). The present study aimed to
evaluate whether EIso postconditioning improved the survival and
neurological outcomes in a rat model of CA.
Materials and methods
Animals
A total of 73 healthy male adult Sprague-Dawley rats
(250–350 g, ~2 months old, provided by the Center of Experimental
Animals at Sichuan University, Chengdu, China) were used in the
present study, and the animal experiment protocol was approved by
the Institutional Animal Experimental Ethics Committee of West
China Hospital, Sichuan University (approval number: 20120112B).
The animals were maintained under a 12:12 h light/dark cycle in a
temperature- (20–25°C) and humidity-conditioned (60±5%)
environment, and rats were permitted free access to food and water.
All animals were handled in accordance with the National Institutes
of Health Guide for the Care and Use of Laboratory Animals, and all
efforts were made to minimize suffering during experiment under
appropriate anesthesia and practice of appropriate animal handling
skills. EIso was prepared in our laboratory according to a
well-established protocol (18). The
concentration of EIso was then determined and confirmed by gas
chromatography (Aligent 4890 D; Tegent Technology Ltd., Shanghai,
China) at the beginning of the experiments.
Animal preparation
Under general anesthesia (10% chloral hydrate, 300
mg/kg, Chengdu Kelong Chemical Co., Ltd., Chengdu, China), the rats
were orotracheally intubated with a 16-G cannula (B. Braun
Melsungen AG, Melsungen, Germany) and mechanically ventilated using
a rodent ventilator (HX-300S; Chengdu Taimeng Technology Co., Ltd.,
China) with room air at a tidal volume of 10 ml/kg. The respiratory
rate was set at a frequency of 60 breaths/min to maintain
normocapnia. Furthermore, the rectal temperature was kept at
37±0.5°C throughout the experiment with the aid of a heating lamp.
The femoral artery cannulation was established with a 24-G catheter
for blood pressure monitoring and blood sampling. Another 22-G
catheter was inserted into the left femoral vein for drug
administration. Arterial blood was collected for blood gas analysis
(ABL800; Radiometer Inc., Copenhagen, Denmark) at three time points
as follows: Baseline, and 30 and 60 min after ROSC. The mean
arterial pressure (MAP) and electrocardiogram were continuously
monitored using a physiological recorder (Biolap420E; Chengdu
Taimeng Technology Co., Ltd.).
CA and resuscitation
The anesthetized rats were paralyzed with
succinylcholine [0.5 mg/kg intravenously (i.v.); Shanghai Xudong
Haipu Pharmaceutical Co., Ltd., Shanghai, China]. Subsequently,
asphyxia was induced by stopping the ventilator and clamping the
tracheal tube at the end of exhalation. CA was defined as a
systolic blood pressure ≤25 mmHg. Resuscitation was achieved after
6 min of asphyxia. Briefly, rats were mechanically ventilated with
100% oxygen at a tidal volume of 10 ml/kg, the respiratory rate was
set at a frequency of 80 breaths/min, and the thoracic compression
was maintained at a rate of 200 compressions/min. Adrenaline (0.02
mg/kg i.v.; Grand Pharmaceutical Co., Ltd., Wuhan, China) and 5%
NaHCO3 (1 mmol/kg i.v.; Huiyinbi Group Jiangxi Dongya
Pharmaceutical Co., Ltd., Jiangxi, China) were simultaneously
administered by thoracic compression. ROSC was defined as an
increase of MAP of >50 mmHg for >10 min (23).
After a successful resuscitation, mechanical
ventilation was maintained until spontaneous respiration was well
restored. At 1 h after ROSC, the catheters were withdrawn, vessels
were ligated, and the wounds were closed. Following extubation, the
rats were placed in a chamber with 50% O2 for 30 min,
and then room air was provided for 30 min. Finally, rats were
returned to their cages.
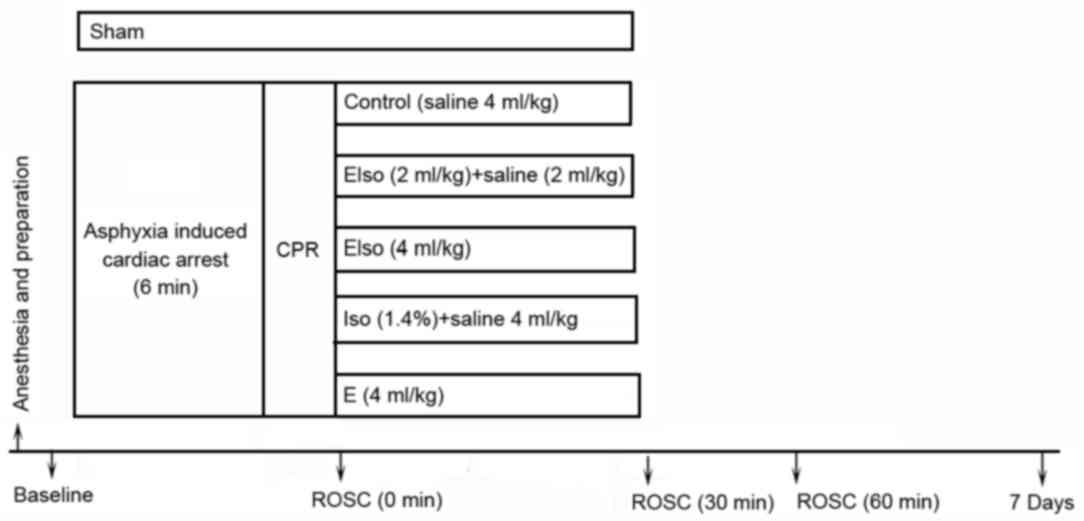
Experimental design
After ROSC, 60 rats were randomly and evenly
assigned into five groups (12 rats in each group), and a further 4
rats were included in the sham group (without CA and CPR) for brain
morphology analysis (Fig. 1). All
interventions were administered immediately among post-CA rats
after ROSC and maintained for 30 min. The control group received an
intravenous infusion of saline (4 ml/kg). The EIso-2ml group
received an intravenous infusion of EIso (2 ml/kg) and saline (2
ml/kg). The EIso-4ml group received an intravenous infusion of EIso
(4 ml/kg). The Iso group inhaled 1.4% Iso and received an
intravenous infusion of saline (4 ml/kg) and finally, the emulsion
(E) group received an intravenous infusion of emulsion (4
ml/kg).
Survival condition and neurological
function evaluations
Post-CA rats were monitored for 7 days, and the
survival rate was recorded daily. All evaluations of the
neurological functions were blindly performed by an independent
investigator 1 day prior to the operation, and 1 and 7 days after
ROSC.
Neural deficit score (NDS)
Neurological functions were assessed with an
established NDS (24), which
evaluates general behavior and cranial function, sensory and motor
function and coordination. A normal rat has an NDS of 500, and 0
represents mortality.
Novel object recognition test
This test was performed based on the spontaneous
tendency of a rat to explore a novel object, and it was conducted
as previously described by Bevins and Besheer (25). Briefly, 1 day prior to the test, each
rat was allowed to stay in the test apparatus for 20 min in order
to become familiar with the environment. At the beginning of the
test, two identical objects (sample objects) were positioned in the
back left and right corners of the apparatus. The rat was placed at
the mid-point of the wall opposite the objects, with its body
parallel to the sidewalls, and its nose pointed away from the
objects. Subsequently, the rat was given 10 min to freely explore
the objects in the apparatus, and then it was placed back to its
home cage. After 1 h, one of the sample objects and a novel object
were replaced in the apparatus, and the rat was placed in exactly
the same manner as described above. The movement of the rat was
monitored for 5 min, and the time that the rat interacted with both
objects was recorded. The recognition index (RI=novel object
interaction time/total object interaction time) was determined for
later analysis, where RI reflects the novel object discrimination
capability of rats and a higher RI demonstrates a better memory
condition.
Contexual fear conditioning
Fear conditioning represents a form of associative
learning that has been well used in numerous species, including
rats (26,27). Briefly, 1 day prior the operation,
the subject rat was placed in a chamber and given 120 sec for free
exploration. Next, a mild foot shock (0.8 mA for 1 sec) was
administered, and such a procedure was repeated five times with
intervals of 120 sec. At day 1 and 7 after ROSC, the rat was placed
into the same training chamber for 5 min, during which the presence
of freezing response of the rat (absence of movement except for
respiration) was observed and the freezing time was recorded for
later analysis.
Brain morphology evaluations
At 7 days after ROSC, the rats were sacrificed and
10-µm paraffin-embedded coronal sections were prepared at the
hippocampal level (approximately at bregma −3.0 mm). Terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) staining was adopted to detect DNA fragmentation as
previously described (28). The
hippocampal CA-1 sector was thoroughly analyzed at a magnification
of ×400 by counting all TUNEL-positive cells (CAST system, Revision
0.9.5; Olympus, Ballerup, Denmark). In order to evaluate viable
neurons of the hippocampal CA-1 sector, the same method was used on
Nissl-stained 10-µm sections at the same level of the hippocampus.
These examinations were blindly performed by a pathologist.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The homogeneity of variance was evaluated using Levene's test.
Physiological variables and viable neuron counts were analyzed by
one-way analysis of variance with a Bonferroni post hoc test
between multiple experimental groups. Furthermore, the survival
rate was compared using Fisher's exact test, and the 7-day survival
condition was compared using Kaplan-Meier analysis and log-rank
test. Statistical analysis was performed using SPSS software
version 18.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Physiological variables
The results shown in Table I reveal that there were no
significant differences among the five groups in terms of
physiological variables at baseline, 30 and 60 min after ROSC. In
addition, as shown in Table II, no
significant differences were observed in resuscitation-associated
variables with regard to the asphyxia time (time from ventilation
termination to CA), CA time [time from CA to initiation of
cardiopulmonary resuscitation (CPR)] and ROSC time (time from
initiation of CPR to ROSC).
 | Table I.Physiological variables before and
after restoration of spontaneous circulation. |
Table I.
Physiological variables before and
after restoration of spontaneous circulation.
| Variable | Control | EIso-2ml | EIso-4ml | Iso | E |
|---|
| Baseline |
|
|
|
|
|
| Body
weight (g) | 272±31 | 280±14 | 272±23 | 289±20 | 289±26 |
| MAP
(mmHg) | 97±17 | 93±18 | 98±13 | 90±19 | 98±15 |
| Body
temperature (°C) | 36.9±0.5 | 37.2±0.5 | 37.0±0.4 | 36.8±0.3 | 37.0±0.5 |
| pH | 7.35±0.03 | 7.34±0.02 | 7.34±0.03 | 7.36±0.04 | 7.36±0.02 |
|
PaCO2 (mmHg) | 35±5 | 35±4 | 34±6 | 32±4 | 36±4 |
|
PaO2 (mmHg) | 192±55 | 239±80 | 241±98 | 195±68 | 196±89 |
|
SaO2 (%) | 96.4±2.1 | 96.6±1.5 | 97.0±0.7 | 96.4±1.7 | 96.2±1.4 |
|
HCO3−
(mmol/l) | 19.7±1.5 | 19.2±.4 | 19.0±2.0 | 19.2±2.2 | 20.5±1.2 |
| Base
excess (mmol/l) | −6.0±2.1 | −6.6±1.9 | −6.9±2.8 | −6.8±2.5 | −5.0±1.8 |
|
Hemoglobin (g/dl) | 14.1±1.2 | 13.4±1.6 | 12.8±2.4 | 13.2±2.0 | 14.3±0.9 |
|
Potassium (mmo/l) | 3.8±0.4 | 3.9±0.5 | 3.5±0.7 | 3.6±0.6 | 3.8±0.6 |
| Lactic
acid (mmol/l) | 1.1±0.4 | 1.1±0.4 | 0.9±0.3 | 1.3±0.8 | 1.1±0.3 |
| At 30 min after
ROSC |
|
|
|
|
|
| MAP
(mmHg) | 70±14 | 66±11 | 71±17 | 65±14 | 79±10 |
| pH | 7.29±0.09 | 7.22±0.07 | 7.27±0.07 | 7.27±0.10 | 7.28±0.06 |
|
PaCO2 (mmHg) | 34±11 | 36±6 | 35±7 | 34±14 | 38±11 |
|
PaO2 (mmHg) |
136±24 | 120±24 | 110±15 | 120±19 | 124±27 |
|
SaO2 (%) | 93.2±3.0 | 90.6±4.2 | 90.7±2.8 | 91.3±3.9 | 91.2±3.0 |
|
HCO3−
(mmol/l) | 16.3±2.0 | 15.8±2.5 | 16.2±2.1 | 15.8±2.1 | 17.2±1.2 |
| Base
excess (mmol/l) | −10.3±2.6 | −10.7±3.2 | −10.2±2.9 | −10.9±3.1 | −8.7±2.1 |
|
Hemoglobin (g/dl) | 15.1±2.0 | 13.2±1.0 | 12.7±1.9 | 13.0±2.2 | 14.1±2.7 |
|
Potassium (mmol/l) | 4.7±1 | 4.0±0.9 | 4.2±0.8 | 4.5±1 | 4.6±0.9 |
| Lactic
acid (mmol/l) | 3.0±0.9 | 2.7±1.7 | 2.5±0.9 | 3.0±0.4 | 2.4±0.8 |
| At 60 min after
ROSC |
|
|
|
|
|
| MAP
(mmHg) | 71±16 | 71±15 | 76±21 | 78±20 | 81±8 |
| pH | 7.29±0.06 | 7.26±0.06 | 7.24±0.07 | 7.28±0.09 | 7.29±0.04 |
|
PaCO2 (mmHg) |
42±7 | 43±8 | 46±7 | 40±10 | 42±9 |
|
PaO2 (mmHg) | 160±54 | 142±36 | 139±28 | 149±50 | 157±50 |
|
SaO2 (%) | 94.8±2.5 | 92.7±2.6 | 92.3±3.1 | 92.8±3.8 | 94.1±2.2 |
|
HCO3−
(mmol/l) | 19.2±2.1 | 17.8±1.8 | 18.8±2.5 | 18.0±2.2 | 18.9±1.6 |
| Base
excess (mmol/l) | −6.0±2.7 | −7.6±2.5 | −5.8±3.5 | −7.6±2.7 | −6.4±2.6 |
|
Hemoglobin (g/dl) | 15.7±1.4 | 13.7±1.6 | 14.6±2.0 | 14.3±1.7 | 14.9±1.3 |
|
Potassium (mmol/l) | 5.0±0.7 | 4.8±1.0 | 5.1±1.0 | 4.7±1.0 | 4.3±0.9 |
| Lactic
acid (mmol/l) | 2.0±0.8 | 2.3±1.2 | 2.0±0.6 | 2.7±1.2 | 1.9±0. 4 |
 | Table II.Times of different procedures during
cardiac arrest and cardiopulmonary resuscitation. |
Table II.
Times of different procedures during
cardiac arrest and cardiopulmonary resuscitation.
| Procedure | Control | EIso-2ml | EIso-4ml | Iso | E |
|---|
| Asphyxia (sec) | 193±35 | 197±20 | 196±38 | 197±20 | 192±14 |
| CA (sec) | 167±35 | 163±20 | 164±38 | 163±20 | 168±14 |
| ROSC (sec) | 68±23 | 65±21 | 69±21 | 68±21 | 66±20 |
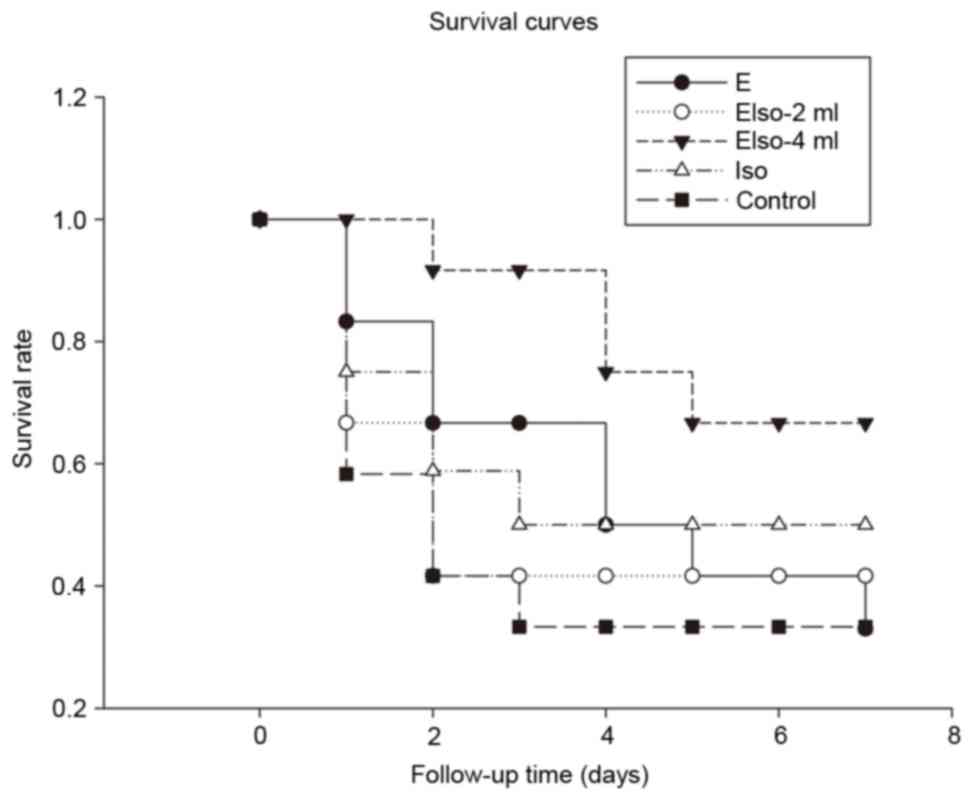
Survival conditions
A total of 73 rats were included in the present
study, among which 4 rats were included in the sham group for brain
morphology analysis, 7 rats failed to achieve ROSC and 2 rats died
due to blood loss caused by femoral artery cannulation failure. The
survival rate 1 day after ROSC in the EIso-4ml group was
significantly higher than in the control group (100 vs. 58.3%,
P<0.05). Furthermore, the survival rate 7 days after ROSC was
33.3% in the control and E groups, and 41.7, 66.7 and 50% in the
EIso-2ml, EIso-4ml (P<0.05 vs. control group) and Iso groups,
respectively (Fig. 2).
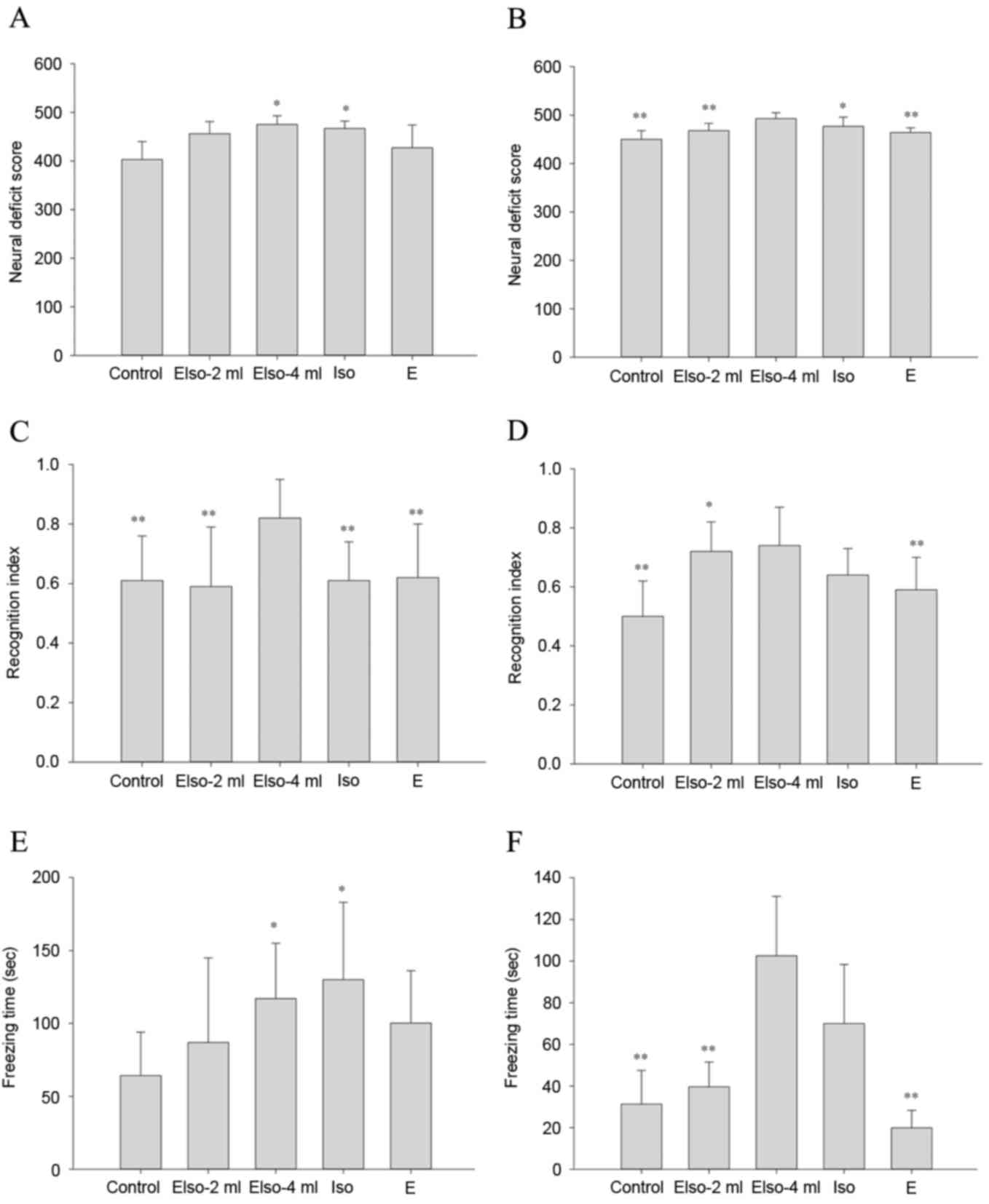
NDS
At 1 day after ROSC, the EIso-4ml and Iso groups
showed higher NDS values compared with the control group. At 7 days
after ROSC, NDS values were still higher in the EIso-4ml and Iso
groups compared with the control group, and the EIso-4ml group also
exhibited a higher NDS than the EIso-2ml and E groups (Fig. 3A and B).
Novel object recognition test
At 1 day after ROSC, the EIso-4ml group showed a
greater RI than the other four groups. At 7 days after ROSC, the
RIs in the EIso-2ml and EIso-4ml groups were both higher compared
with that of the control group (Fig. 3C
and D).
Contextual fear conditioning
At 1 day after ROSC, the freezing times in the
EIso-4ml and Iso groups were greater than that in the control
group. At 7 days after ROSC, the freezing time in the EIso-4ml
group was greater than that in the control, EIso-2ml and E groups
(Fig. 3E and F).
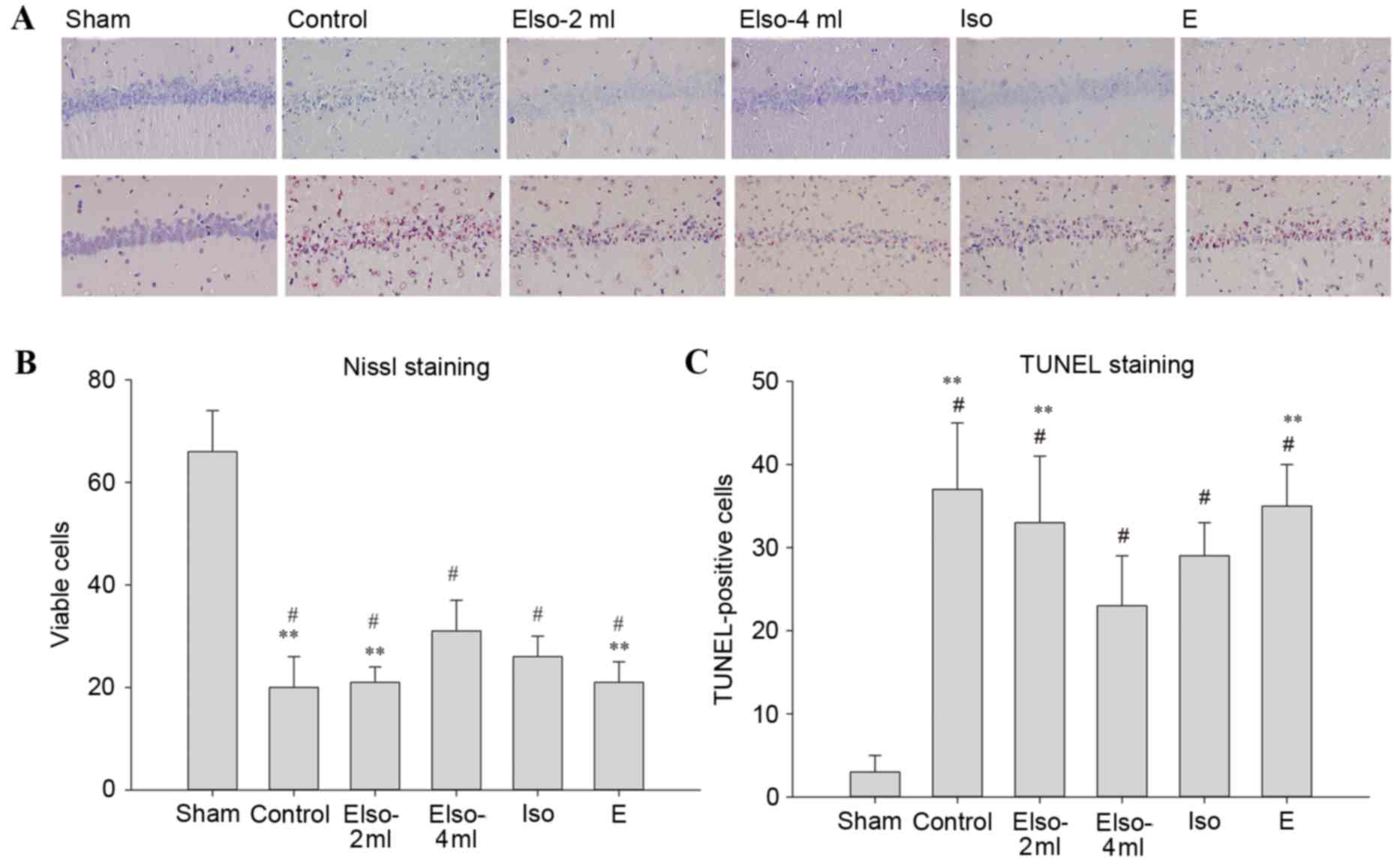
Brain morphology evaluations
Compared with the sham group (n=4), the viable
neurons in the other five groups were significantly decreased 7
days after ROSC. The number of viable neurons in the hippocampal
CA-1 region was significantly preserved in the EIso-4ml group
compared with the control, EIso-2ml and E groups. The number of
TUNEL-positive cells in the EIso-4ml group was less than those in
the control, EIso-2ml and E groups (Fig.
4).
Discussion
The present study showed that EIso postconditioning
had a beneficial effect on cerebral I/R injury. Additionally, EIso
also showed a dose-dependent response for its cerebroprotective
effects. Intravenous administration of volatile anesthetics may
have some advantages compared with the inhaled mode of delivery,
such as more rapid induction and recovery as well as ease of
pre-hospital administration (16).
Organ protection has previously been achieved by EIso
preconditioning in different animal models, with protection of the
heart, liver, kidney and lungs being observed (19,29,30).
Anesthetic postconditioning has been shown to be as effective as
preconditioning; Hu et al (31) have demonstrated that postconditioning
with EIso at the start of reperfusion is capable of producing
myocardial protection against I/R injury. A previous study of EIso
has shown that a lower dose of Iso (~80% less for anesthetic
induction and 20% less for maintenance) is required to obtain
comparable anesthetic and organ-protective effects (32). In the present study, EIso
postconditioning profoundly improved the survival of rats, and
neurological outcomes were also enhanced at 1 and 7 days after
ROSC. The results confirmed and extended previous findings. To the
best of our knowledge, the present study for the first time
indicated that administration of EIso following ROSC improved the
survival and neurological outcomes in a rat model of CA.
Although the EIso-2ml and Iso groups showed improved
NDS values, memory function and survival conditions, no significant
protective effect on brain morphology was detected. Previous
studies demonstrated that EIso (2 ml/kg) is useful for preventing
heart injury in rats, and apoptotic cardiomyocytes are
significantly reduced in EIso-treated rats compared with the
control group (18,31). Fang et al (14) reported that cerebral infarct ratios
were significantly decreased in rats post-conditioned with 1.4% Iso
for 30 min using a middle cerebral artery occlusion (MCAO) model.
Another study demonstrated that postconditioning of myocardial
infarction with EIso (2 ml/kg) is as effective as 1 minimal
alveolar concentration (MAC) of Iso in rats (32). Since the present study is our first
of EIso in a rat model of CA, EIso (2 ml/kg) and 1.4% Iso
(corresponding to 1 MAC of Iso in rats) were selected as
interventions based on the previous studies. The results of the
present study showed that EIso (2 ml/kg) and 1.4% Iso did not
manifest significant protective effects on cerebral I/R injury
following CA. Possible explanations for these results were the
differences in the target organ and cerebral ischemic model. The
greatest proportion of the post-CA mortality and morbidity is
caused by global ischemic brain damage, and brain neurons are more
sensitive to ischemic insult compared with cardiomyocytes (33). Furthermore, EIso (2 ml/kg) may have
useful effects on ischemic cardiomyocytes, but did not show
significant benefits on an ischemic brain. In addition, ischemic
injury in the CA model was more serious and diffused compared with
the occlusion of left anterior descending coronary artery and MCAO
models. Therefore, the discordance between a previous MCAO model
and the present study is possible, and further studies should be
performed to elaborate on this observation.
Due to the lack of knowledge regarding the
appropriate intravenous dosage of EIso for cerebral I/R injury
protection, the references used for guidance were based on EIso in
myocardial protection. Chiari et al (30) demonstrated that intravenous infusion
of 6.9% EIso (3.5 ml/kg/h) for 30 min has a protective effect on
rabbit models of myocardial infarction. In addition, Rao et
al (17) revealed that
continuous infusion of 8% EIso (2–3 ml/kg) exerts infarct-limiting
effects on rabbits. Furthermore, EIso (2 and 4 ml/kg) have better
effects on an ischemic heart in rats compared with EIso (1 ml/kg)
(34). Therefore, the
dose-dependence of EIso indicates that a higher dose of EIso may
have better protective effects. Besides EIso (2 ml/kg) and 1.4%
Iso, EIso (4 ml/kg) was additionally selected to evaluate the
dose-effect on cerebral ischemia following CA. The results revealed
that EIso (2 ml/kg) and 1.4% Iso indeed exhibited a tendency to
improve survival conditions. However, EIso (4 ml/kg) had a better
effect in terms of both the survival rate and neurological outcomes
compared with EIso (2 ml/kg) and Iso.
Although it is necessary to further explore the
dose-effect relationship between EIso and cerebral protection, it
may be concluded that EIso (4 ml/kg) had a better protective effect
than EIso (2 ml/kg) and 1.4% Iso on cerebral I/R injury following
CA in rats. Furthermore, previous studies have demonstrated that
ischemic brain injury is a process characterized by progressive
neuronal loss for at least 7–14 days after ischemic insults in
rodents (35,36). The results of the present study
highly support this, demonstrating that EIso (4 ml/kg)
significantly reduced apoptotic cells and protected living neurons
7 days after ROSC.
More importantly, emulsion is critical in this
protective process. There is evidence that intravenous emulsion
therapy has a positive effect on hemodynamics (37,38), and
it protects the heart by decreasing apoptosis (39,40). In
the experiments of the present study, MAP at 30 and 60 min after
ROSC in the E group was the highest among five groups, and memory
function and survival rate at 1 day after ROSC in the E group were
better compared with the control group, although these differences
were not statistically significant. Therefore, it was possible that
emulsion reduced hemodynamic changes and improved the blood supply
and oxygen of the brain following CPR, which consequently improved
the survival rate and neurological outcomes. As a combination of
emulsion and Iso, the protective effect of EIso may be associated
with the emulsion. However, this speculation cannot be confirmed
until further mechanistic studies are conducted.
Nevertheless, the present study has certain
limitations. Firstly, although two different Elso dosages were
investigated, it is not possible to conclude an appropriate dosage
nor whether an increased infusion duration was associated with
better protection, based on our experimental design. Secondly, the
protective effects of EIso (4 ml/kg) and equivalent Iso were not
compared on the rat model of CA due to a lack of studies comparing
MAC between EIso and Iso in rats. In addition, the blood and
end-tidal concentrations of Iso in the Iso and EIso groups were not
measured; therefore, unequal doses of Iso may have been
administered to rats. Thirdly, transient global ischemia induces
neuronal damage, particularly in the CA-1 region of a rat's
hippocampus. Therefore, the brain morphology analysis of the
present study was only restricted to the CA-1 region (41). Moreover, the cortex, thalamus and
striatum were not evaluated in detail due to the difficulty of
defining an exact area in these structures. Finally, the EIso
postconditioning effect was evaluated using a 6-min asphyxia.
Although a previous study has shown that CA induced by 6-min of
asphyxia is enough to exhibit ischemic neuronal injury in rats
(42), further exploration is
required to clarify the protective effect of EIso after a longer
period of asphyxia. In addition, an asphyxia-induced CA model was
investigated, and therefore nonasphyxial causes, such as
ventricular fibrillation, cannot be precluded.
In conclusion, the present study demonstrated that
EIso postconditioning improved survival and neurological outcomes
on a rat model of CA. Furthermore, the observations of the present
study suggested a potentially novel and easily applicable method to
treat post-CA syndrome. Therefore, the present study may pave the
way for a successful translation of EIso postconditioning into
clinical practice.
Acknowledgements
The authors would like to thank Liang Zhao for
statistical review of the article, and the support from the
National Natural Science Foundation of China (grant no.
81101403).
References
|
1
|
Stiell IG, Wells GA, Field B, Spaite DW,
Nesbitt LP, De Maio VJ, Nichol G, Cousineau D, Blackburn J, Munkley
D, et al: Advanced cardiac life support in out-of-hospital cardiac
arrest. N Engl J Med. 351:647–656. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Langelle A, Tyvold SS, Lexow K, Hapnes SA,
Sunde K and Steen PA: In-hospital factors associated with improved
outcome after out-of-hospital cardiac arrest: A comparison between
four regions in Norway. Resuscitation. 56:247–263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fishman GI, Chugh SS, Dimarco JP, Albert
CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X,
et al: Sudden cardiac death prediction and prevention: Report from
a National heart, lung, and blood institute and heart rhythm
society workshop. Circulation. 122:2335–2348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nichol G, Thomas E, Callaway CW, Hedges J,
Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, et al:
Regional variation in out-of-hospital cardiac arrest incidence and
outcome. JAMA. 300:1423–1431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: Comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao H, Sapolsky RM and Steinberg GK:
Interrupting reperfusion as a stroke therapy: Ischemic
postconditioning reduces infarct size after focal ischemia in rats.
J Cereb Blood Flow Metab. 26:1114–1121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meybohm P, Gruenewald M, Albrecht M,
Müller C, Zitta K, Foesel N, Maracke M, Tacke S, Schrezenmeir J,
Scholz J and Bein B: Pharmacological postconditioning with
sevoflurane after cardiopulmonary resuscitation reduces myocardia
dysfunction. Crit Care. 15:R2412011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riess ML, Matsuura TR, Bartos JA,
Bienengraeber M, Aldakkak M, McKnite SH, Rees JN, Aufderheide TP,
Sarraf M, Neumar RW and Yannopoulos D: Anaesthetic postconditioning
at the initiation of CPR improves myocardial and mitochondrial
function in a pig model of prolonged untreated ventricular
fibrillation. Resuscitation. 85:1745–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fries M, Nolte KW, Coburn M, Rex S, Timper
A, Kottmann K, Siepmann K, Häusler M, Weis J and Rossaint R: Xenon
reduces neurohistopathological damage and improves the early
neurological deficit after cardiac arrest in pigs. Crit Care Med.
36:2420–2426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Derwall M, Timper A, Kottmann K, Rossaint
R and Fries M: Neuroprotective effects of the inhalational
anesthetics isoflurane and xenon after cardiac arrest in pigs. Crit
Care Med. 36 11 Suppl:S492–S495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y, Lekic T, Fathali N, Ostrowski RP,
Martin RD, Tang J and Zhang JH: Isoflurane posttreatment reduces
neonatal hypoxic-ischemic brain injury in rats by the
sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway.
Stroke. 41:1521–1527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McMurtrey RJ and Zuo Z: Isoflurane
preconditioning and postconditioning in rat hippocampal neurons.
Brain Res. 1358:184–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L and Zuo Z: Isoflurane
postconditioning induces neuroprotection via Akt activation and
attenuation of increased mitochondrial membrane permeability.
Neuroscience. 199:44–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Q Fang, Xu H, Sun Y, Hu R and Jiang H:
Induction of inducible nitric oxide synthase by isoflurane
post-conditioning via hypoxia inducible factor-1α during tolerance
against ischemic neuronal injury. Brain Res. 1451:1–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan YY, Hu WW, Nan F and Chen Z:
Postconditioning-induced neuroprotection, mechanisms and
applications in cerebral ischemia. Neurochem Int pii. S0197–0186.
Jan 11–2017.Epub ahead of print.
|
|
16
|
Lucchinetti E, Schaub MC and Zaugg M:
Emulsified intravenous versus evaporated inhaled isoflurane for
heart protection: Old wine in a new bottle or true innovation?
Anesth Analg. 106:1346–1349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rao Y, Wang YL, Zhang WS and Liu J:
Emulsified isoflurane produces cardiac protection after
ischemia-reperfusion injury in rabbits. Anesth Analg.
106:1353–1359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu ZY, Luo NF and Liu J: The protective
effects of emulsifled isoflurane on myocardial ischemia and
reperfusion injury in rats. Can J Anaesth. 56:115–125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Luo N, Liu J, Duan Z, Du G, Cheng
J, Lin H and Li Z: Emulsified isoflurane preconditioning protects
against liver and lung injury in rat model of hemorrhagic shock. J
Surg Res. 171:783–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YL, Wang ZP and Zhu W: Effect of
preconditioning with different doses of emulsifled isoflurane on
focal cerebral ischemia-reperfusion injury in rats. Chin J
Anesthesiol. 30:1243–1246. 2010.(in Chinese).
|
|
21
|
Huang H, Li R, Liu J, Zhang W, Liao T and
Yi X: A phase I, dose-escalation trial evaluating the safety and
efficacy of emulsified isoflurane in healthy human volunteers.
Anesthesiology. 120:614–625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YJ, Wu MJ, Li Y and Yu H:
Cardiocerebral protection by emulsified isoflurane during
cardiopulmonary resuscitation. Med Hypotheses. 84:20–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Idris AH, Becker LB, Ornato JP, Hedges JR,
Bircher NG, Chandra NC, Cummins RO, Dick W, Ebmeyer U, Halperin HR,
et al: Utstein-style guidelines for uniform reporting of laboratory
CPR research. A statement for healthcare professionals from a task
force of the American Heart Association, the American College of
Emergency Physicians, the American College of Cardiology, the
European Resuscitation Council, the Heart and Stroke Foundation of
Canada, the Institute of Critical Care Medicine, the Safar Center
for Resuscitation Research, and the Society for Academic Emergency
Medicine. Writing Group. Circulation. 94:2324–2336. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Che D, Li L, Kopil CM, Liu Z, Guo W and
Neumar RW: Impact of therapeutic hypothermia onset and duration on
survival, neurologic function, and neurodegeneration after cardiac
arrest. Crit Care Med. 39:1423–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bevins RA and Besheer J: Object
recognition in rats and mice: A one-trial non-matching-to-sample
learning task to study ‘recognition memory’. Nat Protoc.
1:1306–1311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JJ and Jung MW: Neural circuits and
mechanisms involved in Pavlovian fear conditioning: A critical
review. Neurosci Biobehav Rev. 30:188–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Curzon P, Rustay NR and Browman KE: Cued
and contextual fear conditioning for rodentsMethods of Behavior
Analysis in Neuroscience. Buccafusco JJ: CRC Press; Boca Raton:
2009
|
|
28
|
Penna C, Pasqua T, Amelio D, Perrelli MG,
Angotti C, Tullio F, Mahata SK, Tota B, Pagliaro P, Cerra MC and
Angelone T: Catestatin increases the expression of anti-apoptotic
and pro-angiogenetic factors in the post-ischemic hypertrophied
heart of SHR. PLoS One. 9:e1025362014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin Z, Lv E, Zhan L, Xing X, Jiang J and
Zhang M: Intravenous pretreatment with emulsified isoflurane
preconditioning protects kidneys against ischemia/reperfusion
injury in rats. BMC Anesthesiol. 14:282014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiari PC, Pagel PS, Tanaka K, Krolikowski
JG, Ludwig LM, Trillo RA Jr, Puri N, Kersten JR and Warltier DC:
Intravaneous emulsied halogenated anesthetics produce acute and
delayed preconditioning against myocardial infarction in rabbits.
Anesthesiology. 101:1160–1166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu ZY, Abbott GW, Fang YD, Huang YS and
Liu J: Emulsified isoflurane postconditioning produces
cardioprotection against myocardial ischemia-reperfusion injury in
rats. J Physiol Sci. 63:251–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang XL, Ma HX, Yang ZB, Liu AJ, Luo NF,
Zhang WS, Wang L, Jiang XH, Li J and Liu J: Comparison of minimum
alveolar concentration between intravenous isoflurane lipid
emulsion and inhaled isoflurane in dogs. Anesthesiology.
104:482–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Laver S, Farrow C, Turner D and Nolan J:
Mode of death after admission to an intensive care unit following
cardiac arrest. Intensive Care Med. 30:2126–2128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu ZY and Liu J: Effects of emulsified
isoflurane on haemodynamic and cardiomyocyte apoptosis in rats with
myocardial ischemia. Clin Exp Pharmacol Physiol. 36:776–783. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Chopp M, Jiang N, Yao F and Zolaga
C: Temporal profile of in situ DNA fragmentation after transient
middle cerebral artery occlusion in the rat. J Cereb Blood Flow
Metab. 15:389–397. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du C, Hu R, Csernansky CA, Hsu CY and Choi
DW: Very delayed infarction after mild focal cerebral ischemia: A
role for apoptosis? J Cereb Blood Flow Metab. 16:195–201. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Young AC, Velez LI and Kleinschmidt KC:
Intravenous fat emulsion therapy for intentional sustained-release
verapamil overdose. Resuscitation. 80:591–593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harvey MG and Grant RC: Intralipid
infusion ameliorates propanolol-induced hypotension in rabbits. J
Med Toxicol. 4:71–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu SL and Liu J: Effects of isoflurane
and intralipid on ischemia-reperfusion injury in isolated rat
heart. West China J Pharm Sci. 22:525–527. 2007.(in Chinese).
|
|
40
|
Huang H, Zhang W, Liu S, Yanfang C, Li T
and Liu J: Cardioprotection afforded by St Thomas solution is
enhanced by emulsified isoflurane in an isolated heart ischemia
reperfusion injury model in rats. J Cardiothorac Vasc Anesth.
24:99–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pulsinelli WA, Brierley JB and Plum F:
Temporal profile of neuronal damage in a model of transient
forebrain ischemia. Ann Neurol. 11:491–498. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin HW, Defazio RA, Della-Morte D,
Thompson JW, Narayanan SV, Raval AP, Dave KR and Perez-Pinzon MA:
Derangements of post-ischemic cerebral blood flow by protein kinase
C delta. Neuroscience. 171:566–576. 2010. View Article : Google Scholar : PubMed/NCBI
|