Introduction
Chronic prostatitis (CP) is a frequently occurring
disease of the male urogenital system. It has a complicated
pathogenesis and its morbidities differ depending on the region it
is reported in. The incidences of prostatitis reported in different
studies vary due to the application of different epidemiological
investigation methods and population structures. In the USA, the
incidence of prostatitis among males aged 20 to 79 years old was
reported as 2.2–16% in 1996 (1,2). In
Europe, the incidence among males aged 20 to 59 years old was
reported as 14.2% in 1990 (2), and
in Asia, the incidence among males aged from 20 to 79 years old was
reported to be 2.7–8.7% in 2000 (3,4).
According to the dynamic medicine investigation database of
America, CP accounts for >8% of all patients visiting the
Urologic Surgery Clinic (5), but
reaches up to 33% in China (6).
According to the classification of prostatitis of the National
Institutes of Health (NIH) of America, type III prostatitis
(chronic prostatitis/chronic pelvic pain syndrome, CP/CPPS), also
known as the chronic non-bacterial prostatitis/chronic pelvic pain
syndrome, accounts for >90% of CP cases (7). However, the pathogenesis of CP/CPPS
remains unknown as its etiology is complicated and may be
associated with the systemic inflammatory and autoimmune mechanism
(8). Some reports suggest that
CP/CPPS is caused by multiple etiological factors; some have
reported that it is attributable to some undistinguishable disease
with the same or similar clinical presentation, even though they
have been cured (9). Currently, many
scholars believe that the combined action of pathogenic infection,
inflammation, abnormal pelvic floor neuromuscular activity and
immunologic mechanism is the major cause of CP/CPPS (10,11).
According to the number of white blood cells in the expressed
prostatic secretion of patients, the syndrome may be further
divided into two subtypes: IIIA (inflammatory CPPS) and IIIB
(non-inflammatory CPPS) (12).
Considering the prevalence of CPPS can reach 15% in males,
understanding and investigating its pathogenesis is crucial
(13). Experimental autoimmune
prostatitis (EAP) refers to a non-infection autoimmunity-driven rat
model of CPPS; following the subcutaneous injection of prostate
antigen (PAG) and the induction of adjuvant, pain occurs in rats
allowing the pain sense test to be completed.
Interleukin-17 (IL-17) is a novel pro-inflammatory
cytokine, a glycoprotein that contains terminal signal peptide and
is secreted by a group of independent cluster of differentiation
(CD)4 + T cell subsets (14,15). It has been demonstrated that IL-l7 is
associated with the occurrence and development of various types of
disease; its expression increases in a number of autoimmune
diseases including rheumatoid arthritis, systemic lupus
erythematosus and autoimmune thyroid disease (16). It has been suggested that IL-17 may
serve a vital role in autoimmune diseases and animal experiments
verify that a number of different types of autoimmune disease in
mice may be treated by inhibiting IL-17 expression (17). Previously, type III prostatitis was
thought to be a type of autoimmune disease (11), thus it is of great importance to
investigate IL-17 expression in the prostate.
It has previously been demonstrated that chemotactic
factors serve a vital role in the development of CPPS (18). Chemokine ligand 2 (CCL2) has a
chemotaxis and activation effect on inflammation-related natural
killer cells, mononuclear macrophages, immunoreaction related T
cells and dendritic cells, which may stimulate the accumulation
inflammatory cells in tissue lesions (19), thus causing inflammation in cells and
tissues.
The experiments in the current study were performed
to analyze the expression of IL-17 and CCL2 in the prostate of EAP
rats in addition to assessing the association between IL-17 and
CCL2 expression. Pelvic pain in EAP rats was assessed and rats were
subjected to the pain behavioral test. Following intervention using
tacrolimus and celecoxib, the pain behavioral test was performed
again and changes in the expression of IL-11 and CCL2 in the
prostate were detected. The results of the current study may
improve understanding regarding CPPS pathogenesis. The effect of
cytokines and chemotactic factors that mediate the prostate and
thus, derive the pelvic pain immunologic adaptive response should
be further studied.
Materials and methods
Animals
A total of 44 two-month old male specific pathogen
free (SPF) Sprague Dawley (SD) rats weighing 220±18 g and 20
four-month-old SPF SD rats weighing 350±20 g were purchased from
the animal experimental center of Kunming Medical University in
China (Kunming, China). All rats were raised in individually
ventilated cages in the animal house of Kunming Institute of
Zoology in the Chinese Academy of Sciences (Kunming, China). The
room temperature was 18–22°C, the humidity was kept at 50%, rats
experienced a 12-h light/dark cycle, and all rats had ad libitum
access to food and water. All of the experimental protocols in the
present study were approved by the Animal Care and Use Committee at
Kunming Institute of Sciences (Yunnan, China).
Modeling
The 20 two-month-old SD rats were sacrificed by
cervical dislocation following intraperitoneal injection of 10%
chloral hydrate (1011268; Shanghai Rongbai Biological Technology
Co., Ltd., Shanghai, China). The complete prostate tissue was
harvested from the rats, weighed, and cut into sections. The tissue
was subsequently transferred to a glass homogenizer in an ice bath
at 0°C for 15 min. The obtained homogenate was centrifuged at 5,738
× g at 4°C for 30 min, followed by the extraction of PAG as per the
method proposed by Donadio and Depiante-Depaoli (20). Briefly, the protein concentration of
PAG was detected using a bicinchoninic acid (BCA) protein assay kit
(PC0020-50; Beijing Solarbio Science and Technology Co., Ltd.,
Beijing, China) and subsequently diluted to 60 mg/ml solution with
PBS. To induce EAP, 34 SD rats (two-month old and weighing 220±18
g) were administered with 1 ml mixed emulsion PAG and complete
Freund's adjuvant (F5881; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany; 1:1) by subcutaneous injection at multiple points (under
the skin of pelvis area of the lower abdomen and the bilateral
shoulders) on days 0, 7, 14 and 28. A total of 10 SD rats in the
normal group (NG; two-months old and weighing 220±18 g) were
administered 1 ml normal saline by subcutaneous injection on the
same days.
Pain sense test
On days 5, 10, 20, 30, 35, 40 and 45 following the
PAG injection, the Von Frey filament behavioral test was performed
to detect the pain sense in the pelvic area of rats in the model
group and control group. The Von Frey filament behavioral test was
developed in the late 19 century; sets of filaments with different
hardness were designed to evaluate sensitivity to pain when touched
(21,22). The filament is a piece of
non-invasive experimental equipment used to assess the mechanical
pain sense in addition to the touch threshold (21). As animal models are unable to express
their discomfort directly, the results of this experiment are
assessed through a series of behaviors including sudden contraction
of the abdomen, immediate licking or scratching of simulated sites
and jumping, all of which were defined as positive reactions. The
abnormal pain sense test (22) was
performed on days 0, 10, 20 and 30 following intervention.
Intervention
A total of 24 EAP rats were divided into three
groups (n=8) and each group was administered either 1 ml tacrolimus
(Astellas Pharma Inc., Tokyo, Japan; 0.8 mg/kg), 1 ml celecoxib
(Pfizer Inc., New York, NY, USA; 40 mg/kg) or 1 ml normal saline as
a control by intragastric administration once a day for thirty
consecutive days.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used for the detection of IL-17 and
CCL2. TRIzol was used for the extraction of mRNA; tissues specimens
were incubated with TRIzol for 5–10 min at room temperature and
subsequently centrifuged at 8,263 × g at 4°C for 10 min. cDNA was
obtained following reverse transcription; the primer was designed
according to sequences; target sequences were amplified using a
TIANScript RT kit (cat. no. KR104-02; Tiangen Biotech Co., Ltd.,
Beijing, China) according to the manufacturer's protocol and the
reaction system was constructed for PCR. The housekeeping gene
β-actin was used as the internal control in sample results. The
procedures for RT-qPCR were as follows: Firstly, 1 µg of total RNA,
2 µl of Oligo (dT) and 2 µl of Super Pure dNTP were added into a
reaction tube and mixed. The reaction mixture was diluted to 14.5
µl through the addition of RNase-Free ddH2O and heated
at 70°C for 5 min. The mixture was immediately cooled on ice for 2
min, following which the reaction liquid was centrifuged at 51 × g
at room temperature for 10 sec to collect the reaction liquid as
some liquid was adhered to the wall following heating. A total of
14.5 µl of mixture, 0.5 µl of 5X First-Strand Buffer, 0.5 µl of
RNasin and 1 µl of TIANScrip M-MLV were added. The product was
agitated prior to incubation for 10 min at 25°C, for 50 min at 42°C
and 5 min at 95°C. For PCR, the reaction system (20 µl) comprised
10 µl of SuperReal PreMix Plus, 0.6 µl of upstream primer (10 µM),
0.6 µl of downstream primer (10 µM), 100 ng of cDNA and 0.4 µl of
ROX Reference Dye and RNase-free ddH2O to volume. The
primer sequences were as follows: IL-17, forward CAC TGA GGC CAA
GGA CTT and reverse CGT GGA ACG GTT GAG GTA; MCP1, forward CAT GCT
TCT GGG CCT GCT GT and reverse AGG TGA GTG GGG CGT TAA CT. The
reaction procedures included pre-degeneration at 95°C for 15 min,
and 40 cycles of degeneration at 95°C for 10 sec, annealing at 58°C
for 30 sec and extension at 72°C for 30 sec. The quantification of
each specimen was repeated three times. Relative quantitative
analysis was performed using a fluorescent quantitative PCR
amplifier, SuperReal PreMix Plus (SYBR Green; cat. no. FP205-02;
Tiangen Biotech Co., Ltd.) and the 2−∆∆Cq method
(23).
Western blot analysis
Western blot analysis was used to detect the gray
values of IL-17 and CCL2. Radioimmunoprecipitation assay (RIPA)
lysis solution (R0020; Beijing Solarbio Science and Technology Co.,
Ltd.) was used for cell lysis; the prostate tissues were ground
with liquid nitrogen and centrifuged with RIPA lysis solution at
8,263 × g at 4°C for 15 min. The supernatant was collected and
protein concentrations were measured using a BCA protein
concentration assay kit (PC0020-500; Beijing Solarbio Science and
Technology Co., Ltd.) according to the manufacturer's protocol.
Proteins were separated by 12% SDS-PAGE. The amount of protein
loaded per lane was: 80 µg of IL-17, 160 µg of MCP and 20 µg of
β-actin (different sample loading standards were formulated for
different target proteins). Proteins were transferred onto a
polyvinylidene fluoride membrane and blocked with electrophoretic
buffer solution for 1.5 h in an ice bath. Each liter of solution
contained the following: 3 g Tris base (25 Mm; cat. no. 04816100),
14.4 g of glycine (cat. no. 04808822; 192 Mm), 1 g of SDS (0.1%;
cat. no. 04811030), all MP Biomedicals (Santa Ana, CA, USA). The
membrane was subsequently incubated with primary antibodies at 4°C
overnight against the following: IL-17 (1:500; A0688; ABclonal
Biotech Co., Ltd., Lake Bluff, IL, USA), MCP1 (1:600; ab7202;
Abcam, Cambridge, UK) and β-actin (1:10,000; TA-09; Origene
Technologies, Inc., Rockville MD, USA). Membranes were washed with
PBST (PBST was prepared by adding TWEEN-20 into PBS at a ratio of
1:5,000) three times (10 min each time) and incubated with
rabbit-derived antibody (1:3,000; ZB-2301) and mouse-derived
antibody (1:3,000; ZB-2305; both Origene Technologies, Inc.) for
1.5 h at room temperature. Supersignal West Dura Extended Duration
Substrate (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used for the detection of enhanced chemiluminescence;
exposure, developing and photographic fixing of X-ray film
indicated positive bands. The housekeeping gene β-actin was used as
the internal control in sample results. The software used for
quantification was GIS 1D analysis software version 4.2 (Tanon,
Shanghai, China) (24).
Statistical analysis
Prism version 5.01 (GraphPad Software, Inc., La
Jolla, CA, USA) was used for statistical analysis. Two-way analysis
of variance was used for data analysis of abnormal tactile sense.
P<0.05 was determined to represent a statistically significant
difference.
Results
High expression of IL-17 and CCL2 in
prostate tissues of EAP rats
CPPS rat models are currently widely used to study
the mechanism between pain and inflammation in human prostatitis
(25). EAP rat modeling refers to
subcutaneously injecting homologous rat prostate antigens into SD
rats to induce prostatitis and paraesthesia of pelvis. The Von Frey
filament was adopted for behavioral tests in the present study.
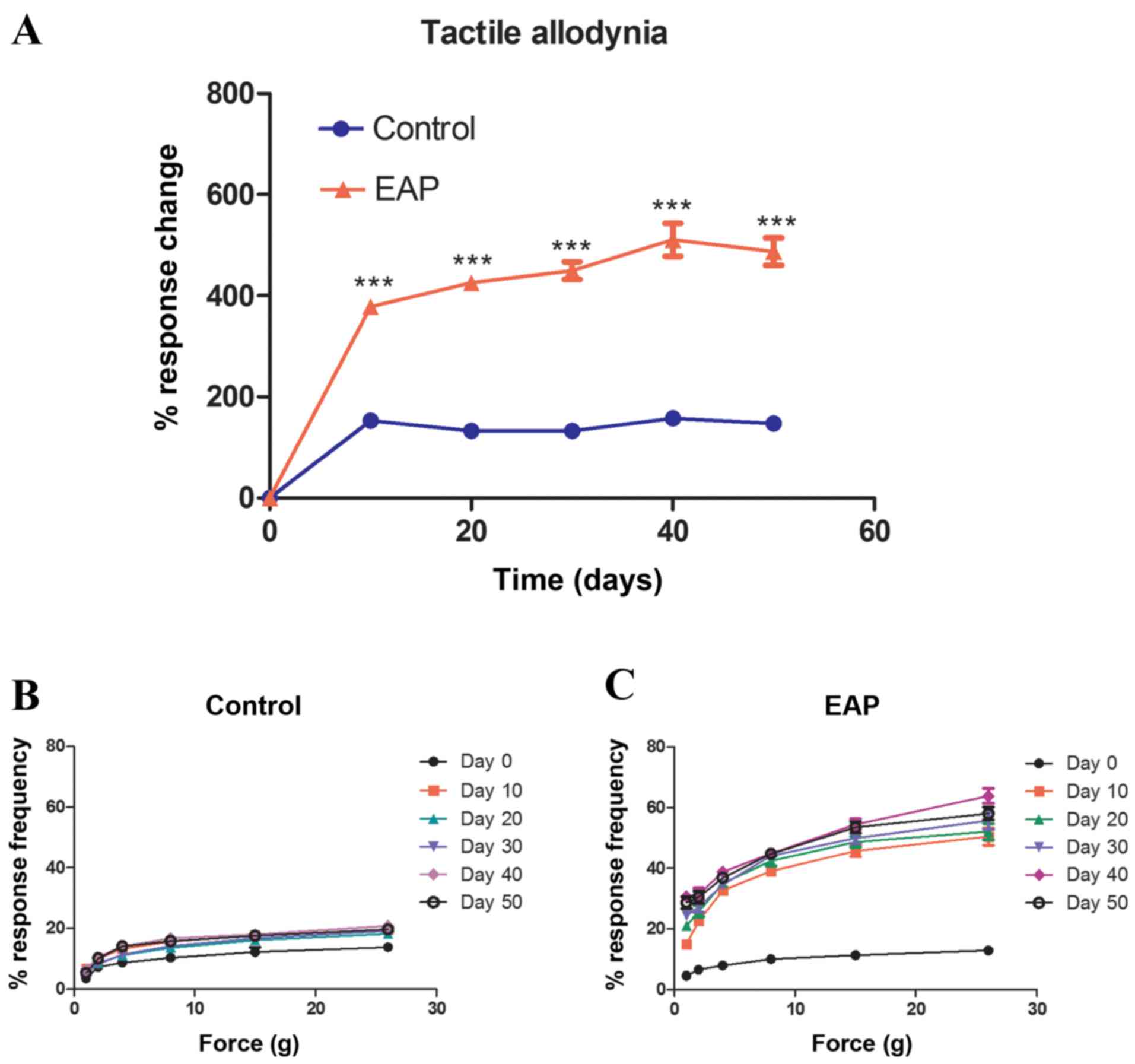
Results indicate that the peak value of allodynia in EAP rats
occurred 40 days following antigen injection and that the response
to tactile alloydnia in EAP rats was significantly higher than that
of control rats at all time points (P<0.001; Fig. 1A). Fig.
1 indicates the increase of frequency in response to an
abnormal pain stimulus that was exhibited by rats in the EAP group,
following the Von Frey test (performed every 10 days) from day 0 to
50, compared with the control group. As the filament strengthened,
the response of the control and EAP groups differed significantly
at every time point (P<0.05; Fig. 1B
and C).
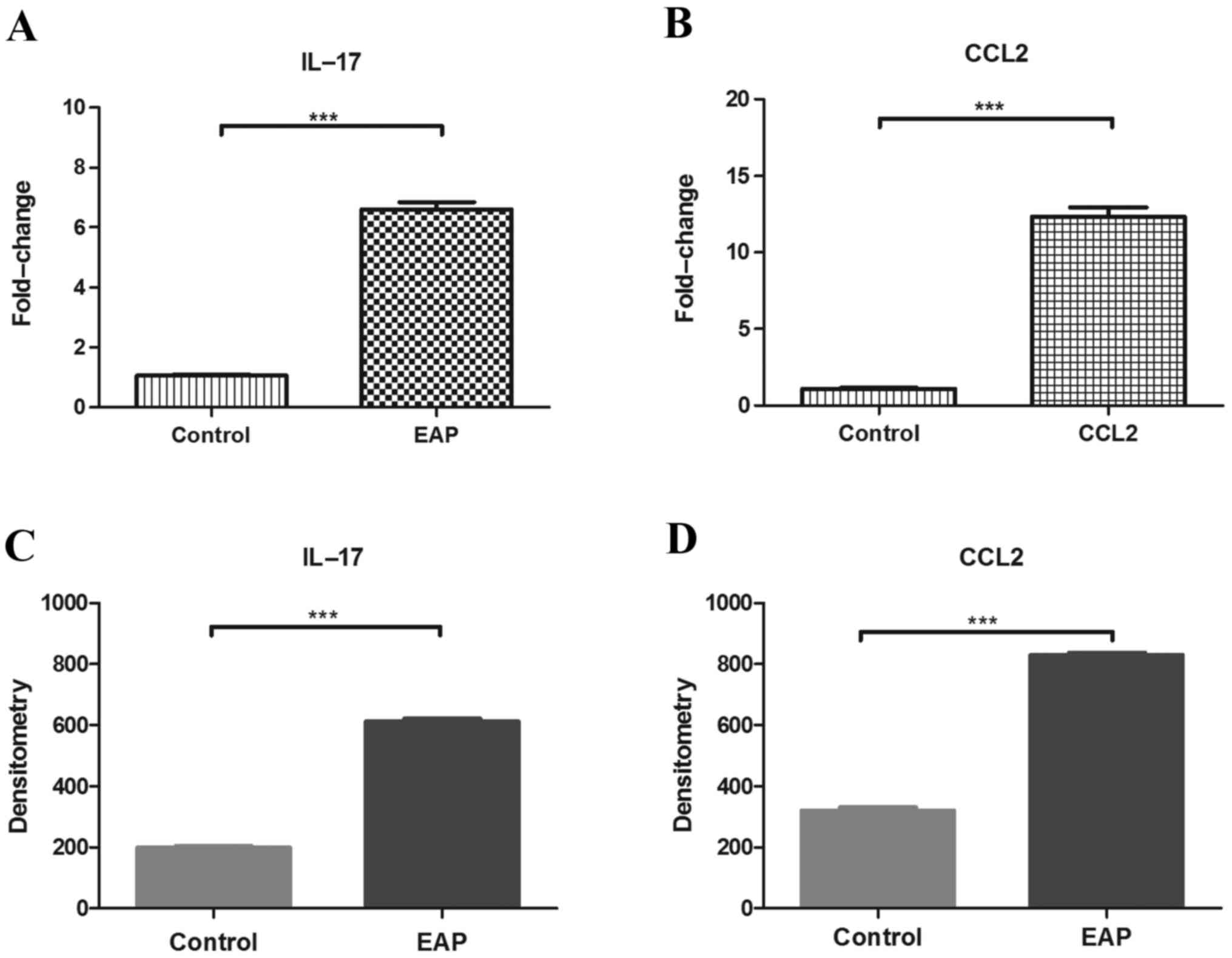
Fifty days following the injection of antigens,
prostate tissues of rats in the control group and the EAP group
were subjected to RT-qPCR and the expression of IL-17 and CCL2 were
compared. Western blot analysis was completed to assess and compare
the protein level of IL-17 and CCL2 in prostate tissues of rats.
Fig. 2A and B indicate that prostate
tissues of rats in the control group and the EAP group exhibited
significantly higher levels of IL-17 and CCL2 mRNA, compared with
the control (P<0.001). Fig. 2C and
D demonstrate that prostate tissues of rats in the EAP group
exhibited significantly higher levels of IL-17 and CCL2 protein,
compared with rats in the control group (P<0.001). These results
indicate that EAP rats experienced pelvic pain. Furthermore, the
high expression of IL-17 and CCL2 indicate that both may
participate in the occurrence and development of inflammation.
Mediation of IL-17 and CCL2 in pelvic
pain of EAP rats
IL-17 and CCL2 exhibited high expression in the
prostate tissues of EAP rats, indicating that IL-17 and CCL2 serve
an important role in the occurrence and development of
inflammation. In order to assess the effect of IL-17 and CCL2 in
the mediation of pelvic pain, tacrolimus, celecoxib or normal
saline was administered to EAP rats for 30 days. During the
intervention treatment, the frequency of abnormal pain reaction
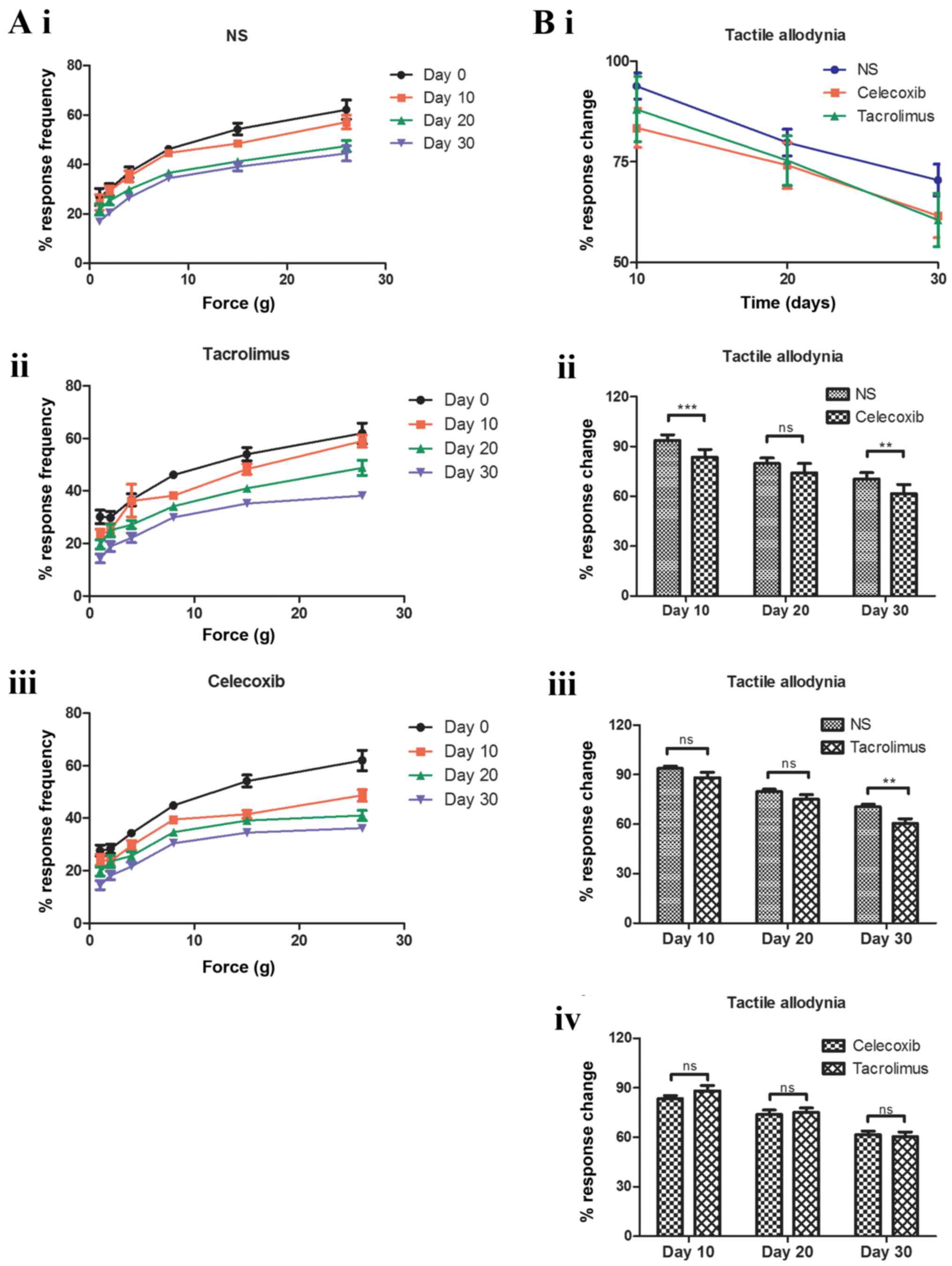
demonstrated different degrees of decrease. Fig. 3A (i-iii) demonstrates the differences
between each group when each filament was strengthened constantly.
Fig. 3A (i) describes the abnormal
pain reaction frequency of the mice on days 0 (one day prior to
treatment), 10, 20 and 30 after being gavaged with normal saline;
the reaction frequency decreased as time went on. Fig. 3A (ii) shows the abnormal pain
reaction frequency of mice on days 0, 10, 20 and 30 after being
gavaged with tacrolimus; the reaction frequency decreased as time
went on. Fig. 3A (iii) demonstrated
the abnormal pain reaction frequency of the mice on days 0, 10, 20
and 30 after being gavaged with tacrolimus; the reaction frequency
decreased as time went on. Fig. 3B
(i) describes results from the Von Frey test. Tests were
performed every 10 days following intervention until day 30, and
results are presented in Fig. 3B
(i). Compared with the NS control group, the frequency of
abnormal pain reaction of tacrolimus and celecoxib groups
decreased, although this decrease was not significant.
Comparison of the therapeutic effect
of tacrolimus and celecoxib on inflammation and pelvic pain of EAP
rats
Fig. 3B (ii-iv)
presents the results of abnormal pain tests at different time
points following intervention, indicating that tacrolimus and
celecoxib had lessened pelvic pain in EAP rats. Tacrolimus and
celecoxib were able to relieve pelvic pain and control the
expression of IL-17 and CCL2 in inflammation. As presented in
Fig. 3B (ii-iii), the effectual time
of celecoxib group was earlier than that of the tacrolimus group;
10 days following the intervention treatment, the difference
between the celecoxib group and the NS control group was
significant [P<0.001; Fig 3B
(ii)]. However, the difference between the tacrolimus group and
the NS control group was significant 30 days following intervention
[P<0.01; Fig. 3B (iii)]. In
Fig. 3B (iv), the comparison between
the celecoxib and tacrolimus groups demonstrated no significant
difference between the two treatments at the three time points.
Therefore, although the effectual time of celecoxib group was
earlier than that of the tacrolimus group, there was no significant
difference in therapeutic effect between the two treatments at any
time point.
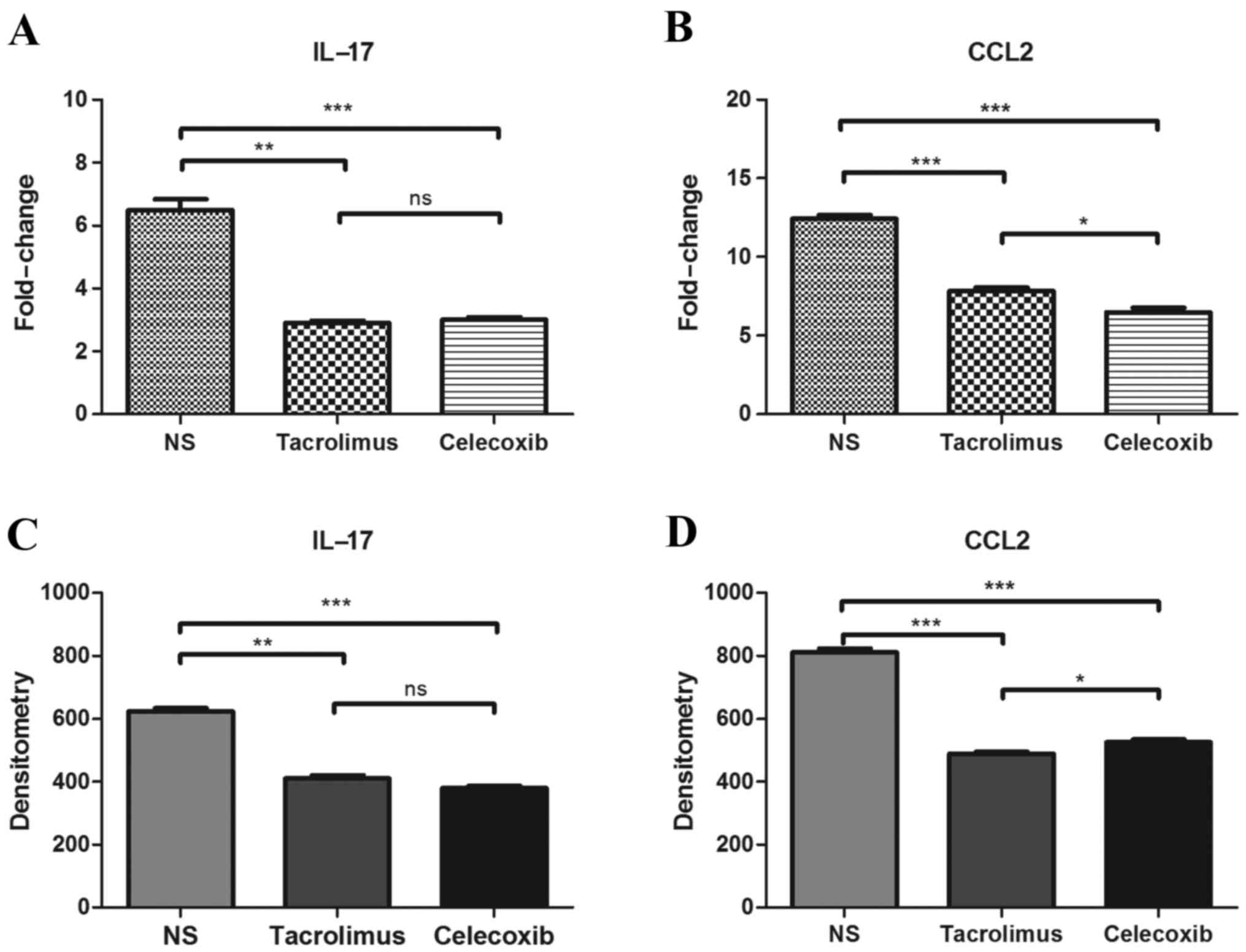
In addition, in prostate tissues, the expression of
IL-17 and CCL2 decreased, compared with that of the NS group. As
presented in Fig. 4A and B, 30 days
following intervention of EAP rats, prostate tissues of rats in
each group were removed for RT-qPCR analysis. Results demonstrated
that, compared with the NS group, the expression of IL-17 and CCL2
significantly decreased in the tacrolimus and celecoxib groups
(P<0.01). Prostate tissues of rats in each group were obtained
30 days following intervention and subjected to western blot
analysis. Results indicated that, compared with the NS group, both
tacrolimus and celecoxib decreased the gray value contrast
(Fig. 4C and D). This indicates that
IL-17 and CCL2 not only serve an important role in occurrence and
development of inflammation, but also in the mediation of pelvic
pain.
Discussion
Chronic prostatitis is a disease caused by a number
of factors (25) that may lead to
discomfort in the groin area, pelvic pain, irritable urination and
sexual dysfunction (26). Its
histological characterization is the infiltration of multinucleate
and mononuclear cells into interstitial connective tissues
(27). However, the pathogenesis and
diagnostic criteria for chronic prostatitis remains unclear, thus
research in this area is hindered (28).
Good animal models may not only help with the
analysis of the pathogenesis of chronic prostatitis and chronic
pelvic pain syndrome, but may also provide evidence for the
efficiency of different treatment methods. Therefore, appropriate
animal models are of great value to understanding and treating this
disease (29,30). With the development of immunology and
molecular biology, the effect of autoimmune factors on morbidity of
chronic prostatitis is receiving greater attention (31). A number of studies (18,32,33) have
assessed the association between the immune reaction and
pathogenesis of chronic prostatitis to investigate the pathogenesis
and pathophysiological process of the disease, meaning that the
current model is improving (34). In
the antigen-induced animal models of chronic prostatitis,
Pacheco-Rupil et al (35)
proposed an autoimmune model of antigen-induced inflammation of the
prostate of rodents. A mixture of homogenate of the accessory
glands of male Wistar rats and the complete Freund's adjuvant was
used as an antigen substance and subcutaneously injected into the
rats. The results demonstrated that only 3 out of 8 rats (38%)
underwent a prostatitis reaction 21 days following the injection.
In a later trial (35), 9 out of 20
rats (45%) exhibited symptoms of prostatitis 30 days following the
injection. A previous study (21)
used a model with purified prostate protein (antigen induction) of
rats or mice and the complete Freund's adjuvant to construct an
autoimmune prostatitis animal model. The method of the current
study was simple and highly effective; the mortality rate of
animals was low and the model was stable, reliable and had good
pathology specificity; the pathological changes were similar to the
clinical manifestation and the pathogenesis was similar. Therefore,
the model may be beneficial in the study of pathogenesis and
efficiency for novel treatments for human chronic prostatitis.
Physiologist Maximilien Von Frey was a pioneer of
pain research in the late 20th century. He designed the Von Frey
filaments, which have differing levels of pressure to assess
human's sensitivity to pain when their skin is touched (21). The machine was then named Von Frey
filaments or Semmes-Weinstein monofilaments, as a type of
non-invasive experimental equipment used to test the mechanical
pain sense (point) in addition to the touch threshold. Von Frey
filaments are composed of 20 filaments, providing 0.008–300 g
tactile stimulus power. During experiments, the size of Von Frey
filaments should be selected according to practical situations and
the extension length should be adjusted appropriately; filaments
are used to stimulate the skin vertically and the curve of
filaments indicates complete stress. In short, Von Frey filaments
may provide a non-invasive evaluation procedure for cutaneous
sensation and the obtained results are objective and are
repeatable. During the test of Von Frey filaments, humans may
express a direct response. However, in animal model experiments,
animals are unable to express discomfort directly. Thus, rats or
animals may express a response to stimulation through a series of
behaviors. In 2000, Ishigooka et al (36) hypothesized that CP/CPPS pain was a
type of neuropathic pain and the research focused primarily on
mechanical pain sense. The mechanical pain sense measurement of
prostatitis animal models was not only used for the research on
pathogenesis, but was also extended to pain assessment and
intervention treatment.
The emphasis of the present study was to use SD rat
models to analyze the immunological mechanism behind CPPS, in
addition to assessing the association between pain symptoms of CPPS
and the autoimmune effect. Results from a previous study indicated
that IL-17 serves a vital role in autoimmune diseases (37). In the current study, IL-17 was highly
expressed in the prostate tissues of EAP mice, which was consistent
with the above results.
Furthermore, IL-17 may also have an effect on
nervous lesions and the development of pain caused by spinal cord
injury. A previous animal model study indicated that IL-17 was
primarily responsible for the development of pain and a specific
antibody for IL-17 may markedly relieve pain (38). The results of the current study
demonstrated that the expression of IL-17 increased in prostate
tissues of EAP rats, indicating that IL-17 serves a relevant role
in autoimmune prostatitis of EAP rats. Following intervention using
tacrolimus, the expression of IL-17 in prostate tissues of EAP rats
decreased significantly (P<0.05) and pain in EAP rats was also
relieved. In a previous study completed by Murphy et al
(39), an anti-IL-17 blocking
experiment was able to treat the prostatitis and pain of EAP rats;
it was also suggested that the CD25+Forkhead box P3+CD4 T cell axis
may be a novel method of treating CPPS and chronic pain.
The results of the current study revealed that the
expression of CCL2 increased in the prostate tissues of EAP rats,
indicating that CLL2 also participates in the pathogenic process of
CPPS. Following intervention using tacrolimus, expression of CLL2
in prostate tissues of EAP rats decreased and the pain of EAP rats
was significantly relieved (P<0.05). A previous study (40) constructed EAP mice models and the
expression of CCL2 in prostate tissues of mice was increased;
changes in the CPPS pain of mice were also observed and anti-CCL2
blocking was further applied (40).
Furthermore, symptoms of pain in CCR2 deficient mice were observed
to be significantly relieved, indicating that CLL2 participated in
the occurrence of CPPS and the development of pain (40). These results were similar to the
conclusions of the present study.
Different factors have a complicated mutual
induction or inhibition, interaction effects and participate in the
immune adjustment together. The aforementioned research results
demonstrate a positive correlation between the expression of IL-17
and CCL2. The current study infers that, although IL-17 may induce
CCL2, the increase of CCL2 may also lead to the increase of IL-17.
Further studies investigating the correlation and associated
mechanisms among different factors may enable the development of
novel therapeutic interventions to treat CPPS pain.
In conclusion, the current study investigating the
intervention treatment of tacrolimus and celecoxib indicates that
both tacrolimus and celecoxib may be used to treat CPPS pain and
there is no significant difference in the therapeutic effect of
these two treatments. However, considering the high cost of
tacrolimus, celecoxib is often selected to treat CPPS. As the
conclusions of the current study are based on an experiment
involving EAP rats, more reliable data from studies in humans are
required. Non-obese diabetic (NOD) mice could be used as an
alternative animal model preparation in the future (41). Though SD mice have low risks of death
during modeling and their prostate tissues are easy to obtain,
there is a difference in the success rate of modeling between SD
mice and NOD mice.
Acknowledgements
The authors of the present study would like to thank
Professor Shen Jihong, Dr Zhao Hui and Professor Zhang from the
Institute of Zoology of Chinese Academy of Sciences.
References
|
1
|
Collins MM, Stafford RS, O'Leary MP and
Barry MJ: Distinguishing chronic prostatitis and benign prostatic
hyperplasia symptoms: Results of a national survey of physician
visits. Urology. 53:921–925. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts RO, Lieber MM, Rhodes T, Girman
CJ, Bostwick DG and Jacobsen SJ: Prevalence of a physician-assigned
diagnosis of prostatitis: The Olmsted County study of urinary
symptoms and health status Among Men. Urology. 51:578–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan JK, Png DJ, Liew LC, Li MK and Wong
ML: Prevalence of prostatitis-like symptoms in Singapore: A
population-based study. Singap Med J. 43:189–193. 2002.
|
|
4
|
Ku JH, Kim ME, Lee NK and Park YH:
Influence of environmental factors on chronic prostatitis-like
symptoms in young men: Results of a community-based survey.
Urology. 58:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krieger JN, Riley DE, Cheah PY, Liong ML
and Yuen KH: Epidemiology of prostatitis: New evidence for a
world-wide problem. World J Urol. 21:70–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo YH and Li HJ: ProstatitisPeople's
Military Medical Press; Beijing; pp. 682007
|
|
7
|
Krieger JN, Nyberg LJ Jr and Nickel JC:
NIH Consensus definition and classification of prostatitis. JAMA.
282:236–237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quick ML, Wong L, Mukherjee S, Done JD,
Schaeffer AJ and Thumbikat P: Th1-Th17 cells contribute to the
development of uropathogenic Escherichia coli-induced chronic
pelvic pain. PLoS One. 8:e609872013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang K: Guide for the Diagnosis and
Treatment of Prostatitis. People's Medical Publishing House;
Beijing: 2009
|
|
10
|
Rowe E, Smith C, Laverick L, Elkabir J,
Witherow RO and Patel A: A prospective, randomized, placebo
controlled, double-blind study of pelvic electromagnetic therapy
for the treatment of chronic pelvic pain syndrome with 1 year of
followup. J Urol. 173:2044–2047. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seethalakshmi L, Bala RS, Malhotra RK,
Austin-Ritchie T, Miller-Graziano C, Menon M and Luber-Narod J: 17
beta-estradiol induced prostatitis in the rat is an autoimmune
disease. J Urol. 156:1838–1842. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahal BA, Cohen JM, Allsop SA, Moore JB,
Bhai SF, Inverso G and Dimitrakoff JD: The role of phenotyping in
chronic prostatitis/chronic pelvic pain syndrome. Curr Urol Rep.
12:297–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wagenlehner FM, Pilatz A, Bschleipfer T,
Diemer T, Linn T, Meinhardt A, Schagdarsurengin U, Dansranjavin T,
Schuppe HC and Weidner W: Bacterial prostatitis. World J Urol.
31:711–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cua DJ, Sherlock J, Chen Y, Murphy CA,
Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al:
Interleukin-23 rather than interleukin-12 is the critical cytokine
for autoimmune inflammation of the brain. Nature. 421:744–748.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Langrish CL, Chen Y, Blumenschein WM,
Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA and
Cua DJ: IL-23 drives a pathogenic T cell population that induces
autoimmune inflammation. J Exp Med. 201:233–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lubberts E, Koenders MI, Oppers-Walgreen
B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA and van den
Berg WB: Treatment with a neutralizing anti-murine interleukin-17
antibody after the onset of collagen induced arthritis reduces
joint inflammation, cartilage destruction, and bone erosion.
Arthritis Rheum. 50:650–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller LJ, Fischer KA, Goralnick SJ, Litt
M, Burleson JA, Albertsen P and Kreutzer DL: Nerve growth factor
and chronic prostatitis/chronic pelvic pain syndrome. Urology.
59:603–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arms L, Girard BM, Malley SE and Vizzard
MA: Expression and function of CCL2/CCR2 in rat micturition
reflexes and somatic sensitivity with urinary bladder inflammation.
Am J Physiol Renal Physiol. 305:F111–F122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Donadio AC and Depiante-Depaoli M:
Inflammatory cells and MHC class II antigens expression in prostate
during time-course experimental autoimmune prostatitis development.
Clin Immunol Immunopathol. 85:158–165. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmidt R, Schmelz M, Ringkamp M,
Handwerker HO and Torebjörk HE: Innervation territories of
mechanically activated C nociceptor units in human skin. J
Neurophysiol. 78:2641–2648. 1997.PubMed/NCBI
|
|
22
|
Rudick CN, Schaeffer AJ and Thumbikat P:
Experimental autoimmune prostatitis induces chronic pelvic pain. Am
J Physiol Regul Integr Comp Physiol. 294:R1268–R1275. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fen ZG, Yan WJ, Zhang J and Xu Y: Effects
of Calcium Concentration on the Soluble Gq Protein α Subunit in the
Photoreceptor Cell of Macrobrachium rosenbergi on Light Adaptation
and Dark Adaptation. Zoolog Res. 24:373–376. 2003.
|
|
25
|
Depiante-Depaoli M and Pacheco-Rupil B:
Experimental autoimmunity to rat male accessory glands (MAG):
Circulating antibodies, immunoglobulins bound to target glands, and
immunoglobulins-secreting cells. Am J Reprod Immunol Microbiol.
7:32–38. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schaeffer AJ: Clinical practice. Chronic
prostatitis and the chronic pelvic pain syndrome. N Engl J Med.
355:1690–1698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
John H, Barghorn A, Funke G, Sulser T,
Hailemariam S, Hauri D and Joller-Jemelka H: Noninflammatory
chronic pelvic pain syndrome: Immunological study in blood,
ejaculate and prostate tissue. Eur Urol. 39:72–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pontari MA: Chronic prostatitis/chronic
pelvic pain syndrome in elderly men: Toward better understanding
and treatment. Drugs Aging. 20:1111–1125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Orhan I, Onur R, Ilhan N and Ardiçoglu A:
Seminal plasma cytokine levels in the diagnosis of chronic pelvic
pain syndrome. Int J Urol. 8:495–499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alexander RB, Ponniah S, Hasday J and
Hebel JR: Elevated levels of proinflammatory cytokines in the semen
of patients with chronic prostatitis/chronic pelvic pain syndrome.
Urology. 52:744–749. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang ZY and Schluesener HJ: HDAC
inhibitor MS-275 attenuates the inflammatory reaction in rat
experimental autoimmune prostatitis. Prostate. 72:90–99. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shahed AR and Shoskes DA: Correlation of
beta-endorphin and prostaglandin E2 levels in prostatic fluid of
patients with chronic prostatitis with diagnosis and treatment
response. J Urol. 166:1738–1741. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taguchi O and Nishizuka Y: Self tolerance
and localized autoimmunity. Mouse models of autoimmune disease that
suggest tissue-specific suppressor T cells are involved in self
tolerance. J Exp Med. 165:146–156. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Motrich RD, Maccioni M, Riera CM and
Rivero VE: Autoimmune prostatitis: State of the art. Scand J
Innunol. 66:217–227. 2007. View Article : Google Scholar
|
|
35
|
Pacheco-Rupil B, Depiante-Depaoli M and
Casadio B: Experimental autoimmune damage to rat male accessory
glands. II. T cell requirement in adoptive transfer of specific
tissue damage. Am J Reprod Immunol. 5:15–19. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ishigooka M, Zermann DH, Doggweiler R and
Schmidt RA: Similarity of distributions of spinal c-fos and plasma
extravasation after acute chemical irritation of the bladder and
the prostate. J Urol. 164:1751–1756. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Quick ML, Wong L, Mukherjee S, Done JD,
Schaeffer AJ and Thumbikat P: Th 1-Th 17 cells contribute to the
development of uropathogenic Escherichia coli-induced chronic
pelvic pain. PLoS One. 8:e609872013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Day YJ, Liou JT, Lee CM, Lin YC, Mao CC,
Chou AH, Liao CC and Lee HC: Lack of interleukin-17 leads to a
modulated micro-environment and amelioration of mechanical
hypersensitivity after peripheral nerve injury in mice. Pain.
155:1293–1302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Murphy SF, Schaeffer AJ, Done J, Wong L,
Bell-Cohn A, Roman K, Cashy J, Ohlhausen M and Thumbikat P: IL17
Mediates Pelvic Pain in Experimental Autoimmune Prostatitis (EAP).
PLoS One. 10:e01256232015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Quick ML, Mukherjee S, Rudick CN, Done JD,
Schaeffer AJ and Thumbikat P: CCL2 and CCL3 are essential mediators
of pelvic pain in experimental autoimmune prostatitis. Am J Physiol
Regul Integr Comp Physiol. 303:R580–R589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rivero VE, Cailleau C, Depiante-Depaoli M,
Riera CM and Carnaud C: Non-obese diabetic (NOD) mice are
genetically susceptible to experimental autoimmune prostatitis
(EAP). J Autoimmun. 11:603–610. 1998. View Article : Google Scholar : PubMed/NCBI
|