Introduction
Insomnia is a subjective experience characterized by
a difficulty in falling asleep and/or staying asleep, which results
in the failure of sleep quality or quantity to meet an individual's
normal physiological needs and affects their social functions. It
is the most common sleep disorder with very high rates of morbidity
(1). According to an investigation
by the World Health Organization, ~1/3 of the world's population
suffers from sleep disorders (2).
The percentage of people with various types of sleep disorders in
China is significantly higher (35%) than the rest of the world
(27%). Thus, effective prevention and treatment of insomnia is a
main focus of research in the world (3).
In clinical treatment, hypnotics are primarily used
in modern medicine. This drug treatment may be accompanied by many
side effects. It is not an ideal therapeutic regimen due to its
poor long-term efficacy and addiction, and tolerance, in the case
of long-term administration (4).
Mongolian medicine has a unique mechanism and good efficacy in
treatment of insomnia. In Mongolian medicine, it is believed that
the imbalance among Heyi, Xila, and Badagan, predominance of Heyi
in the heart and white meridian, and Heyi blood intermingling
arising from dysfunctions are the basic etiology and pathogenesis.
Such negative emotions as tension, worry, fear, depression and
anxiety; and social environment, diet, daily life and movement
conditions are external factors. In the Mongolian medicine, the
treatment of insomnia mainly focuses on relieving Heyi and
regulating Heyi, Xila, and Badagan (5–7). The
warm needling therapy is one of most common methods for the
treatment of insomnia in Mongolian medicine as it functions in
warming and smoothing meridians, regulating qi and blood,
regulating voxels, enhancing immunity, and preventing and treating
diseases. It is widely accepted way of treatment by the patients
due to such characteristics as high efficiency, safety, no side
effects, simplicity and no drug dependence (8). Thus, the warm needling therapy has
considerable advantages in the treatment of insomnia, such as
significant efficacy, safety, simplicity, and economy.
Our study first investigates the role of the
Mongolian medical warm acupuncture in hypnotizing the PCPA-induced
insomnia models. We also discuss the levels of related microRNAs
(miRNAs or miRs) and target genes before and after warm
acupuncture. Finally, we observed the insomnia-related cytokines
and neurotransmitter content, probed into the biological foundation
of Mongolian medical warm acupuncture in treating insomnia in
combination with the clinical tests, and preliminarily explain the
multi-dimension complex mechanism of the Mongolian medical warm
acupuncture.
Materials and methods
Rat modeling and grouping
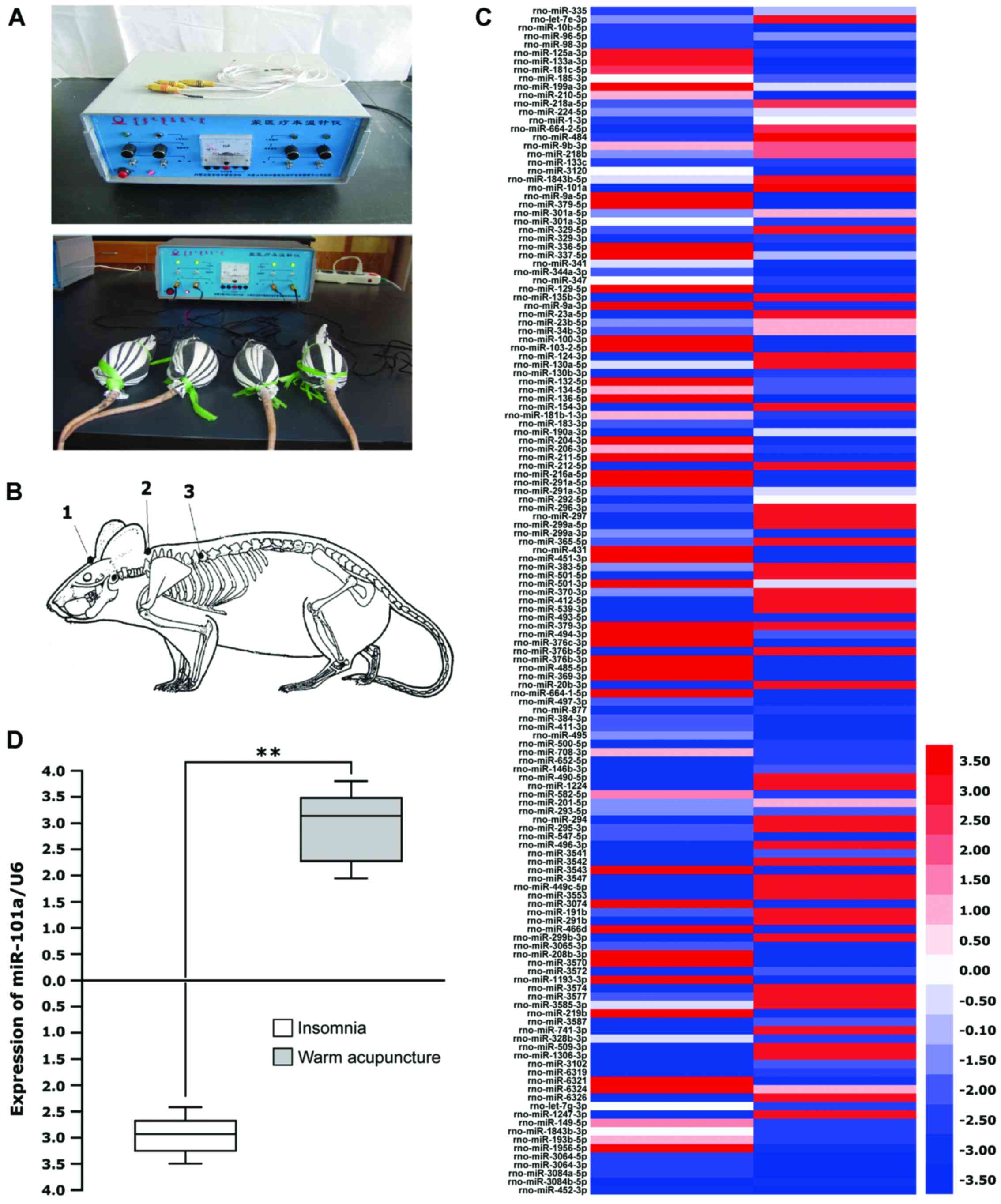
Experimental apparatus of the Mongolian medicine
Model MY-I electric heating needle warmers (patent no. ZL 2011 2
0058078.0) (Fig. 1A) and sterile
Mongolian medicine silver needles were produced by Inner Mongolia
Medical University (Hohhot, China). Experimental animals and
modeling A of a total of 60 clean Sprague-Dawley rats weighing
160–180 g (provided by the Laboratory Animal Center of Inner
Mongolia University) were raised adaptively for 7 days and then
intraperitoneally injected with PCPA (Lanbote, Beijing, China) at a
dose of 300 mg/kg between 8:30-9:00 a.m. each day for 2 consecutive
days at the 8th day for establishing models of insomnia rats. PCPA
was dissolved with normal saline (pH 7.0–8.0) and made into a
suspension as per l ml/100 g.
The evaluation of the PCPA insomnia rats were as
follows: significantly frequent activities, abnormal sensitivity to
such stimulus as sound and light, strengthened excitability,
enhanced aggressiveness, greyish white excrement, circadian rhythm
disorders and frequent daytime activities, occurred to the rats at
28–30 h after the second intraperitoneal injection of PCPA, which
indicated that the models were successfully established (9). This study was approved by the Animal
Ethics Committee of Inner Mongolia Medical University.
The Sprague-Dawley rats were divided into 4 groups
randomly, the normal, model, warm acupuncture and medication groups
of 15 rats each. In the normal group, the rats were adaptively
raised for 7 days and intraperitoneally injected with normal saline
(0.l ml/kg) for 2 consecutive days from the 8th day. The rats were
also fed twice at a fixed time for 1 h each day without any other
stimuli. In the model group, the rats were adaptively raised for 7
days and intraperitoneally injected with PCPA (300 mg/kg) in the
morning of each day for 2 consecutive days from the 8th day in
order to establish insomnia rat models without any other stimuli.
In the warm acupuncture group, the rats were adaptively raised for
7 days and intraperitoneally injected with normal saline (0.l
ml/kg) between 8:30-9:00 a.m. each day for 2 consecutive days from
the 8th day. The Dinghui Acupoint, Heyi Acupoint, and Xin Acupoint
of each rat was stimulated with warm acupuncture for 15 min each
time (Mongolian Model MY-I electric heating needle warmers) at
~40°C (be careful not to burn the needling site) once each day for
7 consecutive days starting from the 10th day. In the medication
group, the rats in the model group were adaptively raised for 7
days and intraperitoneally injected with diazepam (batch no.
20150126; North China PharmaceuticaL Co., Ltd., Shijiazhuang,
China) once a day at a dose of 0.92 mg/kg/day for 7 consecutive
days.
Criteria for acupoint selection and
acupoints
The criteria for the normal warm acupuncture group
and model + warm acupuncture group are as follows: The effective
acupoints were selected on the basis of the classic theory of
Mongolian medicine and clinical treatment (Fig. 1B). Acupoints: i) Dinghui Acupoint
refers to the intersection between the line drawn above the middle
of the two eyebrows and the line drawn above the middle of the
superior margins of the two lobes, i.e., Baihui Acupoint in
traditional Chinese medicine. The acupoint was stimulated to
alleviate ‘Heyi hoarseness’, daftness, visual deterioration,
dizziness and headache. ii) Heyi Acupoint is located at the center
of the superior fovea of first thoracic vertebrae and stimulated to
treat such Heyi diseases as daftness, palpitation, agitation,
stammer, staying awake at night, pale coated tongue and neck
rigidity. iii) Xin Acupoint is located at the center of the
inferior fovea of seventh thoracic vertebrae and stimulated to
treat palpitation, atrial fibrillation, daftness, ‘Badagan and
Heyi’ heart diseases, insomnia, delirium, anorexia and delirium
(10).
Sampling method
The rats in the normal, model, and warm acupuncture
groups were decollated. The prefrontal cortex, hypothalamus, and
hippocampus of each rat were removed quickly on ice, weighed, and
preserved at −70°C.
Determination of the differential
expression profile of microRNA in the brain tissue of the insomnia
rats before and after Mongolian medical warm acupuncture
Analysis of the microRNA expression profile
The brain tissue of the insomnia rat model and the
rats receiving warm acupuncture was collected. The total RNA was
extracted using the TRIzol reagent. The miRNAs chip was used to
analyze the expression profiles of miRNAs in the cells of both
groups.
The principle of the miRNAs chip technology are as
follows: The miRNAs in the specimens to be tested are collected and
hybridized with the complementary probes on specific chips. The
miRNAs 3′ terminal end in the specimens to be tested are usually
marked with fluorescence groups. The fluorescence intensity could
be scanned after hybridization and non-specific elution. The miRNAs
with significant differential expression can be screened following
substantial data processing. The significance of changes in miRNA
expression was determined by screening for possible target miRNAs.
The differential expression of miRNAs was detected using the
stem-loop real-time quantitative RT-PCR method using the special
miRNAs quantitative detection kit (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China).
Prediction and verification of the biological
information of the target gene in the differentially expressed
miRNAs
The prediction of biological information of target
genes of miRNAs was done using the online budget software. It
primarily uses the following three types of software: TargetScan,
PicTar, and miRanda. The eukaryotic expression carrier was
established for the differential miRNAs. Bioinformatics was used to
predict and verify whether the target molecules of miRNAs include
PAX8. The miRNAs closely associated with target molecules that
served as the research subject.
miR-101a mimics and inhibitor
miR-101a inhibitor was the reverse complementary
sequence of the miR-101a mature individuals and performed
2′-O-methyl modification for all basic groups. The negative control
(scramble) of the miRNA inhibitor was a sequence that does not
match with any known human miRNA sequence. All of them were
synthesized by the Guangzhou RiboBio Co., Ltd. The miR-101a
sequence mimics were (5′-TCCCCCGGGCCAGAGGTTGTAACGTTGTCTAT-3′);
miR-101a inhibitor sequence
(5′-GAAACCCAGCAGACAAAGCTTTGTTGCCTAACGAAC-3′); and internal
reference U6snRNA (5′-CACCACGTTTATACGCCGGTG-3′).
Real-time fluorescent quantitative PCR
detection of the expression levels of miR-101a in the brain tissue
of rats in the warm acupuncture treatment group
Reverse transcription of miRNAs
Small molecular RNA (1 µg) was diluted to 10 µl. A
total of 2 µl of the RT primer working solution and 4.5 µl of DEPC
water were added. The mixture was denatured for 5 min at 72°C and
quickly cooled on ice. After the addition of the following reverse
transcription components (the above mixture; 5 µl of 5X buffer, 2.5
µl 10 mM dNTP, 0.5 µl RNase inhibitor and 1.0 µl ReverTra Ace). The
solution was reacted for 1 h at 42°C and for 5 min at 95°C, cooled
on ice, and then preserved.
SYBR ExTaq Mix quantitative PCR amplification was
performed for the expression of miR-101a in brain tissue of the
warm acupuncture rats, insomnia model rats, and normal control
rats. U6 was amplified for the internal reference control. The
reaction system was as follows: 12.5 µl of SYBR ExTaq Mix II
(Guangzhou RiboBio Co., Ltd.), 1 µl of upstream primer, 1 µl of
downstream primer, 2 µl of cDNA template, and 8.5 µl of
dH2O. The reaction conditions were as follows: 95°C for
10 sec, 95°C for 5 sec; 58°C for 30 sec, microplate reading at 72°C
for 30 sec, microplate reading for 30 cycles at 72°C for 10 min,
55°C for 5 min, the solubility curve 55–95°C, and 0.3°C/microplate
reading for 1 sec. U6 served as the internal reference gene. The
relative expression of the target gene was calculated using formula
2−ΔΔCq (11).
Detection of the expression levels of PAX8 with
western blotting
The brain tissue of rats in the blank control,
insomnia model, and warm acupuncture groups were collected,
respectively. The histocytes were lysed and the cytoplasm and
nuclear protein supernatant were extracted for a SDS-PAGE gel. The
wet transfer method was used after it was electrophoresed for 2 h
at a constant voltage of 120 V. It was electrically transferred
onto the PVDF membrane, immersed in TBST blocking buffer containing
5% skim milk for 1 h at room temperature, and rinsed with TBST. The
primary antibody was added (Beyotime Biotech, Nanjing, China) and
vibrated overnight at 4°C. It was then rinsed several times with
TBST solution. After addition of the rabbit anti-human related
polyclonal antibody (Beyotime Biotech), it was vibrated for 1 h and
rinsed 3 times. The PAX8 level was detected after ECL
development.
Culture of neurocytes of adult rats
After the rats were euthanized, the hippocampus was
separated under the microscope to remove the meninges and blood
vessels. They were rinsed 3 times with the D-Hank's solution
(Beyotime Biotech), cut into milky fluid-type pieces and digested
in the CD-Hank'S-dispase-DNase-papin (DDDP) (Beyotime Biotech) at
37°C. The DMEM/F12 containing 10% fetal bovine serum (FBS) was
added to terminate the digestion. The solution was centrifuged for
5 min at 600 × g; the supernatant was discarded. Cells were
re-suspended with DMEM/F12 containing 10% FBS and inoculated into a
culture flask containing poty-omithine (10 µg/m1) and laminin (5
µg/m1). The solution was replaced by serum-free DMEM/F12 cell
culture medium at 24 h. In addition, 1% N2, 2% B27, and 20 ng/ml
EGF and bFGF (mesenchymal stem cell medium, SCM) were added. When
the cells grew to 80–90% confluency, they were digested with
Acctuase (Beyotime Biotech) for passaging and inoculated into a new
culture flask in the ratio of 1:3. HPCs were digested into a
single-cell suspension and inoculated in the culture plate or
culture dish containing poly-omithine (50 µg/m1) and laminin (10
µg/m1). In order to improve cell survival, the media was changed to
differential medium (DMEM/F12, 1 ng/ml bFGF, 1% FBS, 100 nmol/l
retinoic acid (Beyotime Biotech) at 24 h after cell adherence; the
solution was changed every 2 days. The cells were differentiated
for 14 days and then turned into neuronal cells (12).
Dual-luciferase reporter gene experiments
Results indicated that miR-101a might have a
targeted-regulating relationship with the PAX8 gene after the
bioinformatic prediction of the miRNA target gene using the on-line
budgeting software TargetScan, PicTar, and miRanda. The luciferase
reporter gene plasmid containing PAX8 3′UTR (Applied Biosystems,
Foster City, CA, USA) was established. The eukaryotic expression
plasmid of the miR-101a gene and the luciferase reporter gene
plasmid containing 3′UTR was used to co-transfect the HEK293 cells.
A luciferase dual reporter gene experiment was conducted to
identify the target relationship between miR-101a and PAX8. The
luciferase reporter vector pMIR-Report™ was purchased from the
Applied Biosystems.
Luciferase activity experiment
The 3 groups of neuronal cells were inoculated into
a 24-well microplate with 5×104 cells/well and
transfected with calcium phosphate at 24 h. All types of required
DNA were transfected. The receptor expression vector and the
scramble, miR-101a mimics/inhibitor expression plasmids containing
the luciferase reporter gene were transfected into the cells. The
media was discarded. Cells were washed once with PBS and 120 µl of
lysis buffer was added to each well. It was centrifuged for 5 min
at 9,500 × g. The supernatant was collected. A total of 20 µl of
cell lysis buffer was well mixed with 10 µl of luciferase detection
reagent. Spectrafluo plus was used to detect the fluorescence
signal and the ratio between the fluorescence value and the
corresponding OD value of the B-gal galactosidase was the relative
activity of the calibrated luciferase.
Detection of related cytokines,
neurotransmitters, and receptors
The enzyme-linked immunosorbent assay (ELISA)
(BioSino Bio-Technology and Science Inc., Beijing, China) was used
to detect the levels of the interleukins of IL-1, IL-2, and IL-6
and the tumor necrosis factor-α (TNF-α) at the above sites.
Detection of monoamine neurotransmitters in the brain tissue was
conducted using the high performance liquid
chromatography-electrochemical detection method to determine the
levels of noradrenaline (NE), dopamine (DA), 5-hydroxytryptamine
(5-HT), glutamic (Glu) acid, and γ-aminobutyric acid (GABA). The
high performance post-column derivatization-electrochemical
detector method was used to determine the level of acetylcholine
(Ach) (BioSino Bio-Technology and Science Inc.).
Statistical analysis
SAM and TIGR Multiple Array Viewer software package
(TMeV version 4.0) (J. Craig Venter Institute, La Jolla, CA, USA)
were used to conduct an unsupervised cluster analysis for the
miRNAs chip expression profile. The fluorescent real-time
quantitative PCR detection used the Sequence Detection system (SDS)
2.3 software for data analysis. The expression level of miRNAs were
expressed with the ΔCq value (Cq miRNA-Cq U6). The biological
experimental data were expressed with mean ± standard deviation.
The intergroup difference between the groups was subject to
Student's t-test. The comparison of intergroup enumeration data
used the Chi-square test or the Fisher's exact probability method.
P<0.05 was considered to indicate a statistically significant
difference. The difference was considered remarkably statistically
significant when P<0.01. The statistical analysis was conducting
using the SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Differences in expression of miR-101a
in the brain tissue before and after treating the rats for insomnia
with Mongolian medical warm acupuncture
Based on analysis of the chip data, it was found
that differences were present in the expression of 141 miRNAs after
the Mongolian medical warm acupuncture compared to the normal rats
(Fig. 1C). Changes in miR-101a were
the most significant and increased 98 times compared to that of the
model group. Then, we conducted a fluorescent real-time
quantitative PCR study on the levels of miR-101a in 15 insomnia
rats receiving warm acupuncture. It was found that the median
increased to 3.2 times from the −2.9 times in the model group
(P<0.001; Fig. 1D).
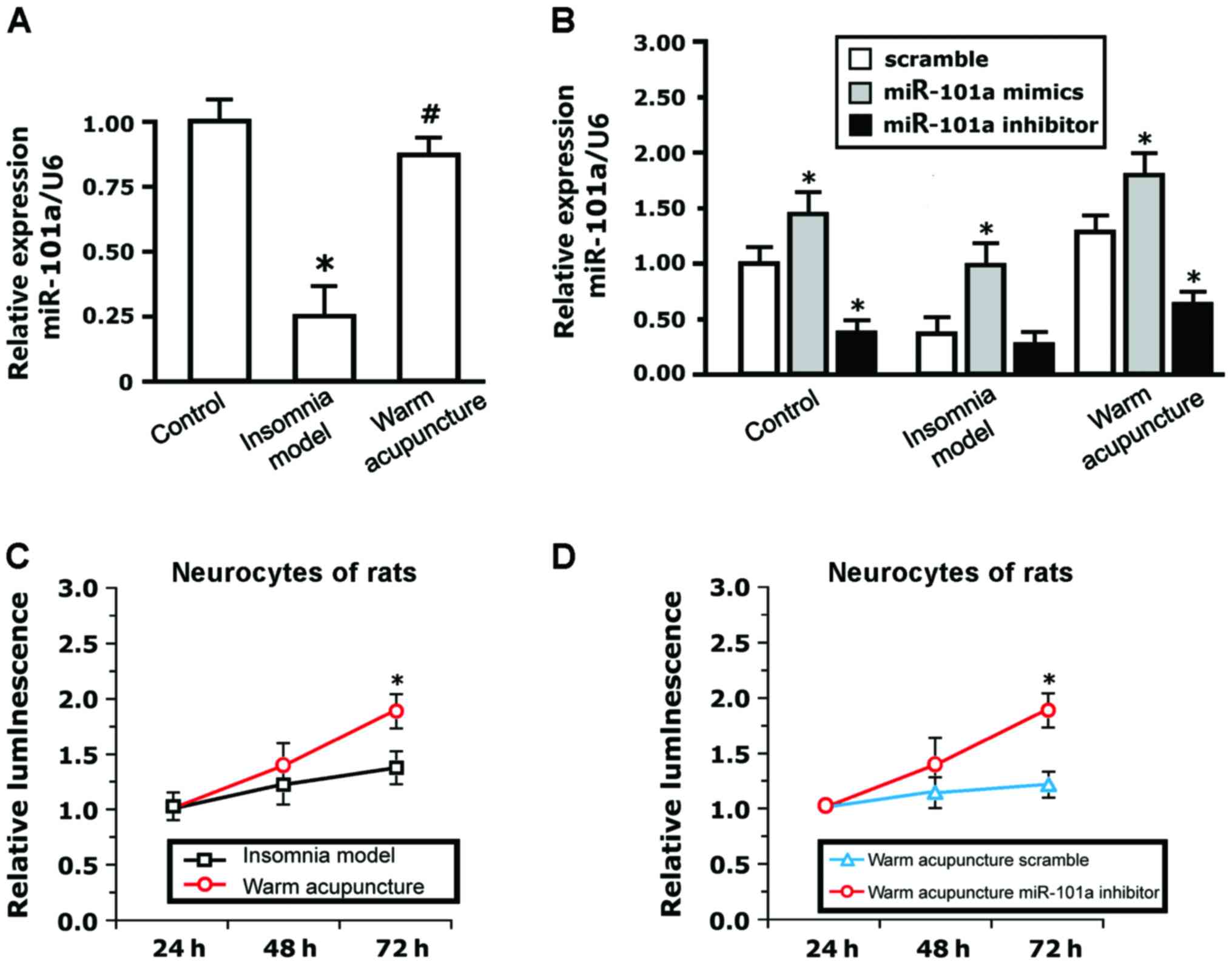
Expression of miR-101a in the
hippocampus before and after treating the insomnia rats with
Mongolian medical warm acupuncture
To study the biological functions of miR-101a,
fluorescent real-time quantitative PCR was used to detect the
expression of miR-101a in the hippocampus before and after
treatment of the insomnia rats with Mongolian medical warm
acupuncture. The results indicated that the expression levels of
miR-101a in the brain tissue of rats in the insomnia model group
decreased significantly compared to that in the brain histiocytes
of normal rats (P<0.05). The expression levels of miR-101a
increased significantly and approximated the normal value following
warm acupuncture (P<0.05; Fig.
2A). The expression of miR-101a in the blank neurocytes
transfected with miR-101a mimics increased significantly at 72 h
(P<0.05) compared to that in the negative control group. The
expression of miR-101a in the blank neurocytes transfected with
miR-101a inhibitor decreased significantly at 72 h (P<0.05;
Fig. 2B). The expression of miR-101a
in cells transfected with miR-101a mimics at 72 h in the insomnia
model group increased significantly (P<0.05) compared to that in
the negative control group. The expression of miR-101a in cells
transfected with miR-101a inhibitor at 72 h in the insomnia model
group had no significant changes (Fig.
2B). The expression of miR-101a in cells transfected with
miR-101a mimics increased significantly at 72 h (P<0.05)
following Mongolian medical warm acupuncture compared to that in
the negative control group. The expression levels of miR-101a in
cells transfected with miR-101a inhibitor decreased significantly
(P<0.05; Fig. 2B).
There were no differences in the luciferase activity
of neuronal cells cultured in vitro between the model group
and the Mongolian medical warm acupuncture group within the initial
24 h. The cell activity in the Mongolian medical warm acupuncture
group increased compared to that in the model group at 48 h. The
cell activity in the Mongolian medical warm acupuncture group
increased significantly compared to that in the model group at 72 h
(P<0.05; Fig. 2C). In order to
further verify the effects of miR-101a on the activity of the
neurocytes, the neuronal cells of the rats receiving Mongolian
medical warm acupuncture were transfected with miR-101a inhibitor
and scramble control. As seen in Fig.
2D, the activity of the cells transfected with miR-101a
inhibitor was higher at 48 h compared to the activity of the cells
transfected with scramble. The activity of cells transfected with
miR-101a inhibitor increased significantly at 72 h (P<0.05).
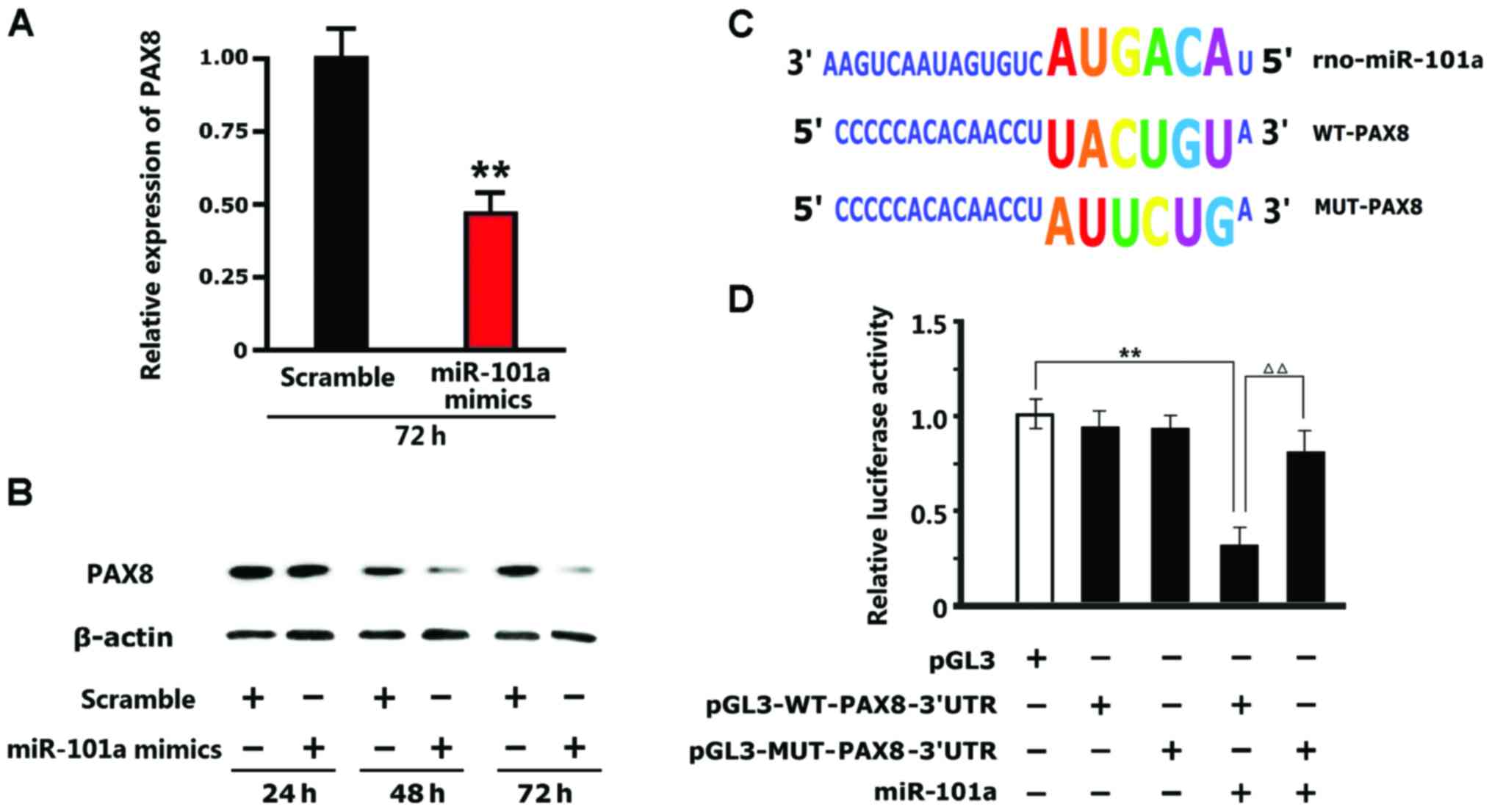
miR-101a inhibits the expression of
the PAX8 protein
The results of western blotting indicated that the
expression of the PAX8 protein in the neuronal cells of the
insomnia model rats cultured in vitro with miR-101a mimics
added was inhibited and downregulated significantly (P<0.01)
compared to the expression of the PAX8 protein in the neuronal
cells of the insomnia model rats cultured in vitro with
scramble control (Fig. 3A and B).
The results of luciferase in the 293T cells indicated that
pGL3M-MUT-PAX8-3′UTR and pGL3M-WT-PAX8-3′UTR in the negative
control group had no significant changes compared with pGL3M in the
blank plasmid group. After addition of miR-101a mimics, no
significant changes occurred to the activity in the MUT group while
the fluorescence intensity in the WT group decreased significantly.
This indicated that miR-101a can bind to the specific sequence in
the WT-PAX8-3′UTR promoter and change the specific sequence of the
promoter whereas miR-101a does not function (Fig. 3C and D).
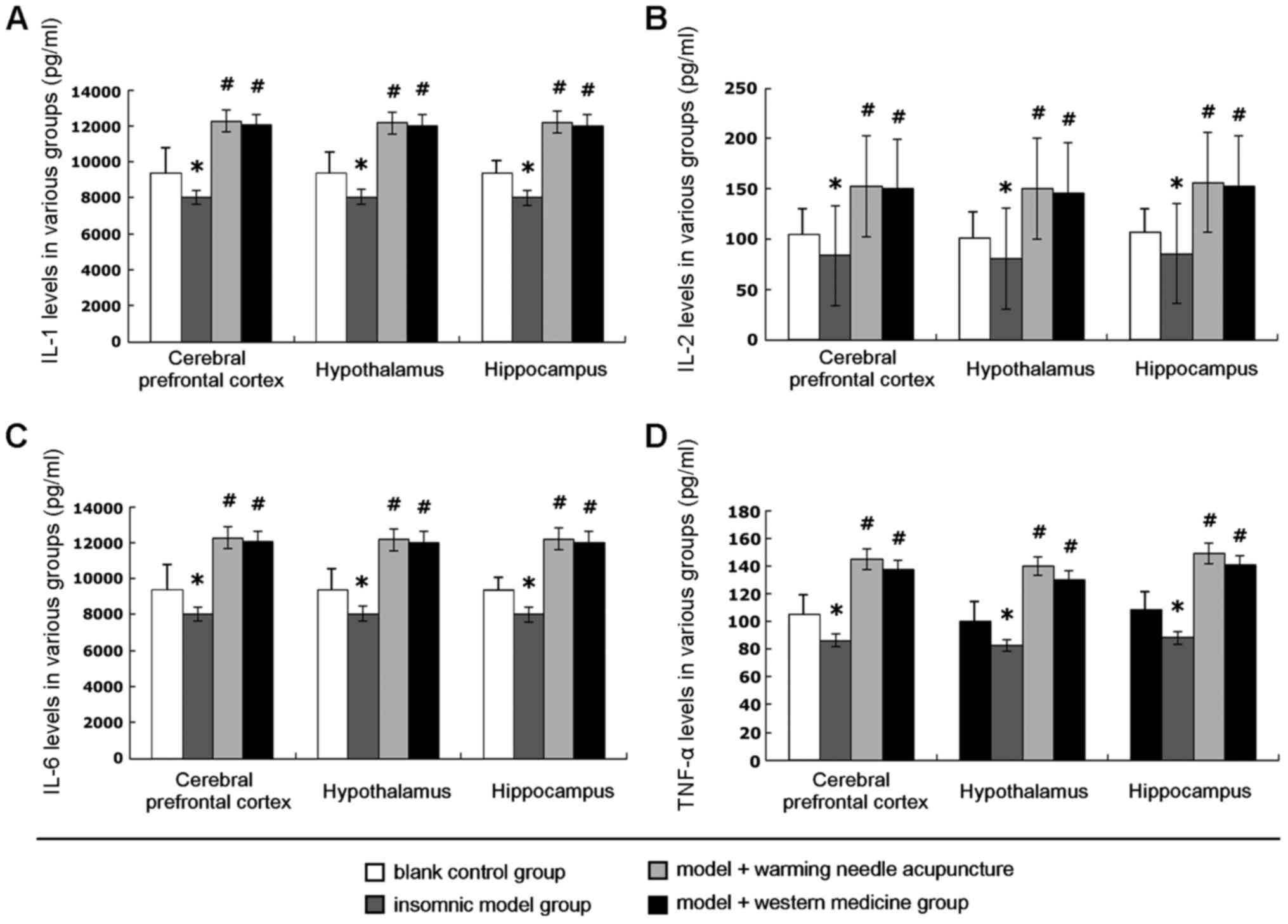
Detection of the levels of IL-1, IL-2,
IL-6, and TNF-α
The results of detection of levels of interleukin
IL-1, IL-2, and IL-6, and TNF-α are shown in Fig. 4. The levels of interleukins IL-1,
IL-2, and IL-6 and TNF-α in the hypothalamus, hippocampus, and
prefrontal cortex tissue of the insomnia rats decreased
significantly compared with those in the blank control group;
differences were significant (P<0.05). After treatment with warm
acupuncture or western medicine, the levels of the interleukins
IL-1, IL-2, IL-6, and TNF-α at various sites increased
significantly compared to those in the model group; there were
statistical differences (P<0.05). Results indicate that warm
acupuncture had significant efficacy in treatment of insomnia rats.
Its basic efficacy was equivalent to that of the drug
treatment.
Detection of the levels of 5-HT, NE,
Ach, DA, Glu, and GABA
The results of the levels in the hypothalamus,
hippocampus, and prefrontal cortex tissue of the rats are shown in
Fig. 5. The detection results
indicated that the levels of NE, DA, and Glu of the rats in the
insomnia model group were significantly higher than those in the
blank control group (P<0.05). After warm acupuncture or
treatment with western medicine, the levels of NE, DA, and Glu
decreased significantly compared to those in the insomnia model
group; differences were statistically significant (P<0.05). The
difference in efficacy between warm acupuncture treatment and
western medicine treatment was not significant, indicating that the
treatment outcomes were not different. The levels of 5-HT, GABA,
and Ach of the rats in the insomnia model group were significantly
lower than those in the blank control group; there was a
statistically significant difference (P<0.05). The levels of
5-HT, GABA, and Ach of the insomnia rats increased significantly
after warm acupuncture or western drug treatment compared to those
in the insomnia model group. There was a statistically significant
difference (P<0.05). No significant difference was observed
between the warm acupuncture and western drug treatment,
demonstrating that the treatment outcome of Mongolian medical warm
acupuncture was similar to the outcome of western drug
treatment.
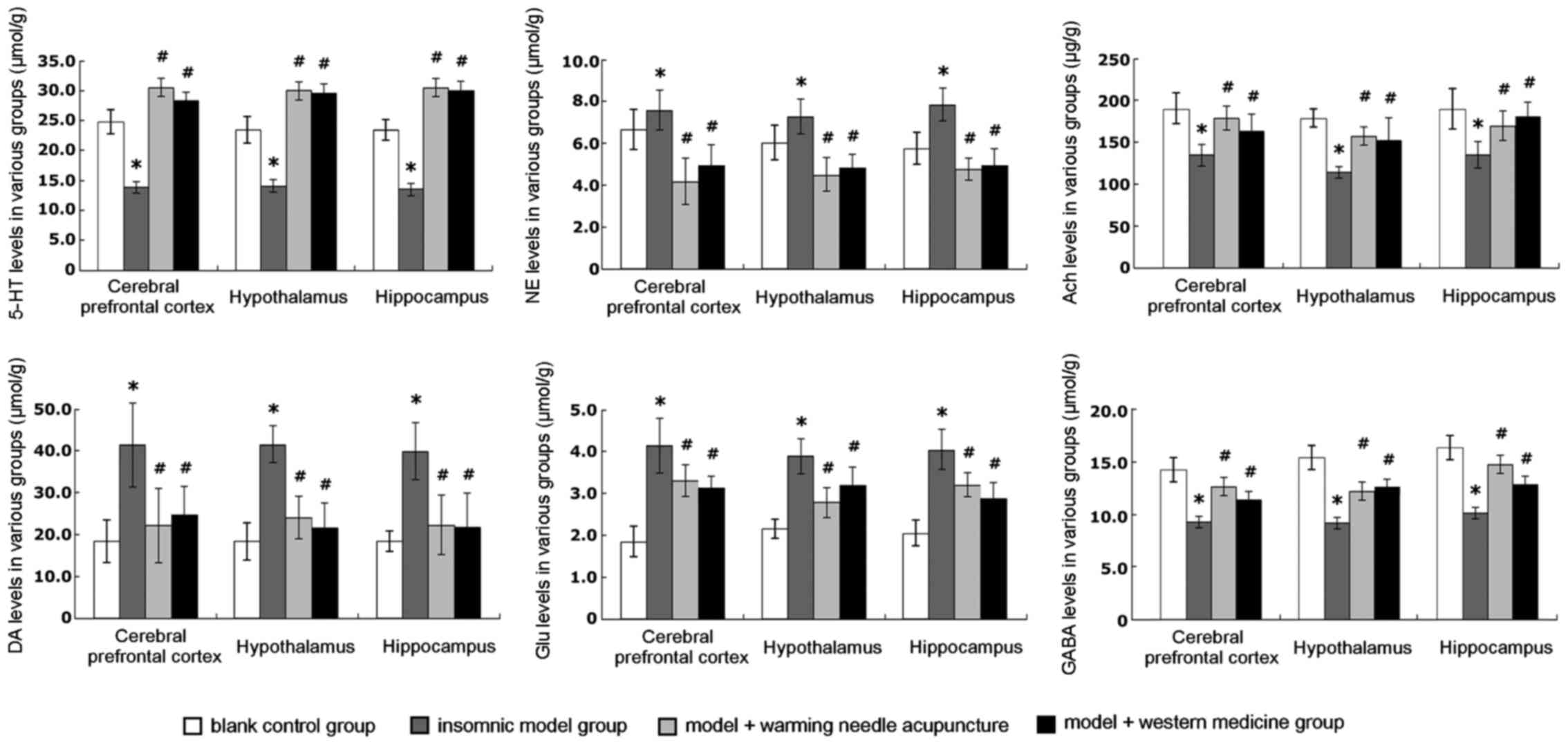 | Figure 5.Levels of 5-hydroxytryptamine (5-HT),
noradrenaline (NE), acetylcholine (Ach), dopamine (DA), glutamic
(Glu), and γ-aminobutyric acid (GABA in the hypothalamus,
hippocampus, and prefrontal cortex of the rats. There is a
significant difference between the insomnia model group and blank
control group, *P<0.05; #there is a significant
difference between the warm acupuncture group, drug treatment
group, and the insomniamodel group, *P<0.05. |
Discussion
In the Mongolian medical warm acupuncture therapy,
patients are subject to the insertion of special silver needles
into fixed acupoints and warming moxibustion for the prevention and
treatment of diseases and recovery of patients. The acupuncture
effect, hyperthermia effect, and specific stimulation of acupoints
are combined to produce some biological effects for treatment of
diseases (13). A great deal of
clinical research indicates that Mongolian medical warm acupuncture
is simple, efficient, and safe in the treatment of insomnia
(14).
Mongolian medicine can improve sleep and treat
insomnia by alleviating Heyi, circulating Heyi blood, and balancing
Heyi, Xila, and Badagan (15). In
the Mongolian medicine, it is believed that the warm needling
therapy can dredge the meridians, regulate qi and blood,
voxel, and strengthen the immunity. Modern research demonstrates
that acupuncture plays a role in regulating neurohumor,
strengthening and activating the anti-disease ability, and
improving the immunity of the organism. It is a good choice for
insomnia patients. There are many clinical reports on the treatment
of insomnia with Mongolian medical warm acupuncture (16). The Mongolian medical warm acupuncture
therapy is used in all of these clinical reports. The Dinghui
Acupoint, Heyi Acupoint, and Qianding Acupoint are largely selected
for the treatment of insomnia with acupuncture and satisfactory
efficacy is achieved. It is thus clear that Mongolian medical warm
acupuncture is very efficacious in treating insomnia (17).
There is no uniform criteria for the quantitative
and qualitative assessment of the acupuncture treatment indexes in
traditional Mongolian medicine. The investigation of the indexes of
several neurotransmitters does not completely represent the
physiological indexes of the whole organism and conform to the
holistic concept in the Mongolian medicine theory. As the
acupuncture mechanism is very complex, it is difficult to analyze
and master the mechanism from a physiological point-of-view.
However, the changes in miRNA levels in an organism can be
determined in a standard and objective manner. Thus, studying
Mongolian medical warm acupuncture with miRNA levels as the medium
can embody the macroscopic and integral characteristics and conform
to the microcosmic and targeted characteristics of the modern
molecular biological techniques (18).
The expression levels of miR-101a in the Mongolian
medical warm acupuncture treatment group is increased compared to
the insomnia rat model group. Transfection of miR-101a inhibitor
can successfully decrease the expression of endogenous miR-101a in
cells. It can be inferred that the target gene of miR-101a may be
the paired box gene 8, PAX8 (19)
through anylsis by the TargetScan, PicTar and miRanda software. In
2014, researchers investigated >47,000 Europeans and ~5,000
African-Americans. Researchers compared their genetic information
and the time for falling asleep at night and results have shown
that the sleep mode is influenced by the genetic difference. A
certain gene region may influence the sleep mode by regulating the
thyroid hormone levels. This DNA region is close to the PAX8 gene,
which is associated with the development and functions of the
thyroid gland (20). Patients with
hypothyroidism are prone to drowsiness and patients with thyroid
hyperfunction may suffer from insomnia. We discovered that the
principle of Mongolian medical warm acupuncture in improving
insomnia is directly associated with the role of miR-101a in the
regulation of the PAX8 gene.
In modern medicine, research on insomnia has shown
that the sleep-wake up cycle is a physiological process involving
coordination and integration of multiple systems and centers with a
complex regulatory mechanism. It is primarily associated with such
structures as sleep activated cells of the preoptic region of the
reticular system of the brain stem, histaminergic neurons in the
papilla nodule region and cerebral cortex. The neurotransmitters
closely associated with the wakeup-sleep cycle such as GABA, Ach,
5-HT, DA, NE, histamine and orexin; IL-1 and TNFs have sleep
regulation roles, and non-peptide substances aid in regulating
sleep (21–23). Furthermore, we also demonstrated that
treating insomnia with Mongolian medical warm acupuncture is also
associated with the above neurotransmitters.
Mongolian medical warm acupuncture integrates
acupuncture effect, hyperthermia, and acupoint specific stimulation
and achieves organism regulation and disease treatment via the
complex multi-system, multi-channel, and multi-path mechanism
relevant to blood circulation, nervous system and immune function
(24). Our study demonstrates that
the upregulation of expression of miR-101a is directly associated
with PAX8 regulation in treating rats with warm acupuncture. Our
study verifies multiple neurotransmitter regulation networks which
provide biological research demonstration for modern treatment with
Mongolian medical warm acupuncture and scientific support for the
modernization development of the traditional national medicine.
Acknowledgements
This study was funded by the following projects:
National Natural Science Foundation of China (no. 81560801);
Science and Technology Innovation Fund of Provincial Department of
Finance, Inner Mongolia Autonomous Region; Collaborative Innovation
Project of Mogolian Medicine, Inner Mongolia Autonomous Region;
Technology Reserve Project of Provincial Department of Science and
Technology, Inner Mongolia Autonomous Region. Inner Mongolia
Autonomous Region Mongolian Medicine Cooperative Innovation
Project. Inner Mongolia Autonomous Region ‘Prairie excellence’
Project.
References
|
1
|
Vgontzas AN, Fernandez-Mendoza J, Liao D
and Bixler EO: Insomnia with objective short sleep duration: The
most biologically severe phenotype of the disorder. Sleep Med Rev.
17:241–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
e Silva JA Costa, Chase M, Sartorius N and
Roth T: Special report from a symposium held by the World Health
Organization and the World Federation of Sleep Research Societies:
an overview of insomnias and related disorders - recognition,
epidemiology, and rational management. Sleep. 19:412–416. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang YM, Chen HG, Song M, Xu SJ, Yu LL,
Wang L, Wang R, Shi L, He J, Huang YQ, et al: Prevalence of
insomnia and its risk factors in older individuals: A
community-based study in four cities of Hebei Province, China.
Sleep Med. 19:116–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han KH, Kim SY and Chung SY: Effect of
acupuncture on patients with insomnia: Study protocol for a
randomized controlled trial. Trials. 15:4032014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YS, Lu J, Su-Chao LM, Wu QZ, Tu Y and
Bo A: Effects of electro-warmed needle of inner-Mongolian medicine
on serum TNF-alpha, ACTH and corticosterone contents in fatigue
rats. Zhen Ci Yan Jiu. 33:258–261. 2008.(In Chinese). PubMed/NCBI
|
|
6
|
Lu J, Chen YS, A GL and Tu Y: Effects of
electric mildly-warmed needle of inner mongolian medicine on liver
MDA and GSH content, GSH-Px and SOD activity in fatigue rats. Zhen
Ci Yan Jiu. 32:167–169. 2007.(In Chinese). PubMed/NCBI
|
|
7
|
Kim TH and Jung SY: Mongolian
traditional-style blood-letting therapy. J Altern Complement Med.
19:921–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao HS: Research of Zhanbra Dorje's
contribution on the development of Mongolian medicine. Zhonghua Yi
Shi Za Zhi. 40:29–32. 2010.(In Chinese). PubMed/NCBI
|
|
9
|
Murray NM, Buchanan GF and Richerson GB:
Insomnia Caused by Serotonin Depletion is Due to Hypothermia.
Sleep. 38:1985–1993. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Si LG, Wang YH, Wuyun G, Bao LD and Bo A:
The effect of Mongolian medical acupuncture on cytokines and
neurotransmitters in the brain tissue of insomniac rats. Eur J
Integr Med. 7:492–498. 2015. View Article : Google Scholar
|
|
11
|
Sun X, Luo S, He Y, Shao Y, Liu C, Chen Q,
Cui S and Liu H: Screening of the miRNAs related to breast cancer
and identification of its target genes. Eur J Gynaecol Oncol.
35:696–700. 2014.PubMed/NCBI
|
|
12
|
Zhu X, Zhao H, Lin Z and Zhang G:
Functional studies of miR-130a on the inhibitory pathways of
apoptosis in patients with chronic myeloid leukemia. Cancer Gene
Ther. 22:573–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marrelli M, Paduano F and Tatullo M: Human
periapical cyst-mesenchymal stem cells differentiate into neuronal
cells. J Dent Res. 94:843–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu E and Amri H: China's Other Medical
Systems: Recognizing Uyghur, Tibetan, and Mongolian Traditional
Medicines. Glob Adv Health Med. 5:79–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Safavi-Abbasi S, Brasiliense LB, Workman
RK, Talley MC, Feiz-Erfan I, Theodore N, Spetzler RF and Preul MC:
The fate of medical knowledge and the neurosciences during the time
of Genghis Khan and the Mongolian Empire. Neurosurg Focus.
23:E132007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bo A, Si L, Wang Y, Xiu L, Wu R, Li Y, Mu
R, Ga L, Miao M, Shuang F, et al: Clinical trial research on
Mongolian medical warm acupuncture in treating insomnia. Evid Based
Complement Alternat Med. 2016:61902852016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yundeng: Exploration of Mongolian
translation of The Four medical tantras (rGyud-bzhi). Zhonghua Yi
Shi Za Zhi. 1989:185–188. 1989.(In Chinese).
|
|
18
|
Erihenbatu: The Mongolian medical schools
and its exponents. Zhonghua Yi Shi Za Zhi. 18:108–112. 1988.(In
Chinese). PubMed/NCBI
|
|
19
|
Burenbatu BM, Borjigin M, Eerdunduleng,
Huo W, Gong C, Zhang G Hasengaowa, Longmei, Li M, Zhang X, et al:
Profiling of miRNA expression in immune thrombocytopenia patients
before and after Qishunbaolier (QSBLE) treatment. Biomed
Pharmacother. 75:196–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riesco-Eizaguirre G, Wert-Lamas L,
Perales-Patón J, Sastre-Perona A, Fernández LP and Santisteban P:
The miR-146b-3p/PAX8/NIS regulatory circuit modulates the
differentiation phenotype and function of thyroid cells during
carcinogenesis. Cancer Res. 75:4119–4130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gottlieb DJ, Hek K, Chen TH, Watson NF,
Eiriksdottir G, Byrne EM, Cornelis M, Warby SC, Bandinelli S,
Cherkas L, et al: Novel loci associated with usual sleep duration:
The CHARGE Consortium Genome-Wide Association Study. Mol
Psychiatry. 20:1232–1239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Srichomkwun P, Admoni O, Refetoff S and de
Vries L: A Novel Mutation (S54C) of the PAX8 Gene in a Family with
Congenital Hypothyroidism and a High Proportion of Affected
Individuals. Horm Res Paediatr. 86:137–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arrigoni E and Saper CB: What optogenetic
stimulation is telling us (and failing to tell us) about fast
neurotransmitters and neuromodulators in brain circuits for
wake-sleep regulation. Curr Opin Neurobiol. 29:165–171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paulson KL and Shay BL: Sympathetic
nervous system responses to acupuncture and non-penetrating sham
acupuncture in experimental forearm pain: A single-blind randomised
descriptive study. Acupunct Med. 31:178–184. 2013. View Article : Google Scholar : PubMed/NCBI
|