Introduction
Cartilage lesions are a common occurrence in
Orthopedics due to trauma, osteochondritis, osteoarthritis and
patellar softening. Studies of tissue-engineered cartilage have
opened up new strategies for the restoration of articular
cartilage. The lack of blood supply and innervation in the
articular cartilage make injuries to these areas impossible to be
spontaneously repaired (1). The
joint trauma, infection and degenerative lesions caused by
cartilage damage can lead to long-term joint pain and dysfunction,
and even joint atrophy, leading to great pain and inconvenience.
Traditional cartilage repair methods include microfracture surgery,
subchondral drilling (2) and
periosteal transplantation (3) with
a purpose to induce the proliferation and differentiation of cells
with chondrogenic potential for reconstruction. But those
treatments usually only work well for fibrous cartilage (4) and the results in the repair of hyaline
cartilage are poor and are often followed by further degradation
and secondary ossification.
Autologous chondrocytes are currently the only
tissue- engineering cells used for clinical cartilage treatments.
However, the proliferation of mature chondrocytes is difficult, the
origin of autogenous cartilage is limited, and the extraction of
autologous chondrocyte is inconvenient. In addition, in
vitro differentiation can also easily occur, leading to the
loss of cartilage formation ability. Furthermore, the activity of
autologous chondrocytes decreases with age. Because of all these
problems autologous chondrocytes usually fail to meet the
requirements for tissue reconstruction (1,2). Based
on previous studies, we evaluated the application of amniotic
membrane-derived stem cells as a seeding cell source to provide
experimental evidence that could expand the repertoire of seeding
cells available for cartilage tissue engineering.
Materials and methods
Reagents
The reagents used in the study included, fetal
bovine serum (FBS; Institute of Biotechnology, Chinese Academy of
Medical Sciences, Beijing, China); bovine serum albumin (BSA; Yuan
Heng Sheng Ma Biotechnology Research Institute, Beijing, China);
and high glucose DMEM (Gibco Life Technologies, Carlsbad, CA, USA)
for cell culture. Type I collagenase (Gibco Life Technologies);
polylysine (Sigma-Aldrich, St. Louis, MO, USA); immunohistochemical
SP kit (Binyang, Tianjin, China); transferrin (Sigma-Aldrich);
phenylmethylsulfonyl fluoride and Triton X-100 (both from Biodee
Biotechnology, Beijing, China); trypsin (Huamei Biological
Engineering Co., Ltd., Henan, China); and high glucose EDTA
(Sigma-Aldrich) for sample processing. Insulin (Sigma-Aldrich);
vitamin C (Invitrogen Life Technologies, Carlsbad, CA, USA); type
II (Ab-1) mouse mAb and goat anti-mouse IgG (H+L) (both from Merck
Millipore, Billerica, MA, USA); and DAB coloring solution (Wuhan
Boster Biological Engineering Co., Ltd., Wuhan, China) for other
experiments.
Experimental equipment
An inverted phase contrast microscope (090-135.001;
Leica, Wetzlar, Germany) was used for sample observations. The
specialized equipment used to process and prepare the samples
included a pressure steam sterilizer (Shanghai Boxun Industrial
Co., Ltd., Shanghai, China), an automatic slicing machine (RM-2135;
Leica), a constant temperature-type baking sheet machine
(TK-218III; Hubei Taiwei Medical Technology Co., Ltd., Hubei,
China), a biological tissue automatic dehydration machine (ZT-12H;
Xiaogan Yaguang Medical-Electronic Technology, Hubei, China), and a
biological tissue paraffin embedding machine (TB-718D; Hubei Taiwei
Medical Technology, Co., Ltd.).
Processing of experimental
animals
Grouping and handling
Twenty-four 3-month-old New Zealand white rabbits
were selected without restriction on sex. Our study focused on the
femoral medial malleolus from the 24 rabbits. The rabbits were
divided into 3 groups of 8 rabbits each, according to the treatment
provided to heal an experimental femoral medial malleolus lesion.
Group I received an implanted human acellular amniotic membrane
seeded with bone marrow-derived mesenchymal stem cells (HAAM-BMSCs)
into the femoral condyle, group II a simple HAAM and the control
group received no experimental lesion or treatment. The rabbits
were sacrificed at 12 and 24 weeks after the procedure (4 rabbits
in each time-point). Specimens from the knee joints were collected,
processed, and stained with hematoxylin and eosin (H&E) and
toluidine blue and finally subjected to type II collagen
immunohistochemical staining. Then the samples were subjected to
Micro-CT and observation under inverted phase contrast microscope.
This study was approved by the Animal Ethics Committee of Xiaoshan
Hospital of Traditional Chinese Medicine.
Animal modeling
The rabbits were anesthetized with 3% pentobarbital
sodium (30 mg/kg) injected through the ear vein. Under aseptic
conditions, the skin of the bilateral knee joints was prepared and
a medial incision was made on the medial region. Then, the joint
capsule was cut along the quadriceps tendon medial edge. The
patella was turned to reveal the femoral condyle and drill a hole
with a 4-mm diameter drill to a depth of 3 mm. According to the
experimental design, a HAAM or HAAM-BMSCs complex was sutured to
the medial femoral condyle. After the implant, the patella was
reset and the tissue was suture-closed layer by layer. Finally, the
animals were left to move freely and each of them was fed in a
single cage.
Handlings on BMSCs
Isolation and culture
The rabbits were routinely disinfected in the
operation area. A bone marrow puncture needle was used to penetrate
into the proximal femur and tibia and 10 ml syringe was connected
to extract 4–5 ml of bone marrow fluid. Next, the obtained bone
marrow fluid was transferred into a centrifuge tube containing
heparin and DMEM/F12. BMSCs were separated by whole bone marrow
adherent separation and were then used for culture. Then bone
marrow fluid was centrifuged at 1,050 × g for 5 min at 4°C, the
supernatant was discarded and appropriate amount of DMEM/F12 was
added to wash again for a second time. After centrifugation at
1,000 × g for 5 min at 4°C, the supernatant was discarded, and the
cell number was counted and adjusted to a cell concentration of
5×105/ml. FBS (15%) and DMEM/F12 complete medium were
added to make a cell suspension. The suspension was inoculated into
a culture flask, and the flask was placed into an incubator for
culture at 37°C with a 5% CO2. The medium was completely
replaced after 48 h, and then every 48 or 72 h. Seven or eight days
later, when the cell fusion reached 85% confluence, the samples
were digested with 0.25% trypsin-EDTA for no more than 30 sec or 1
min. A dilution ratio for subculturing of 1:2 or 1:3 was used. The
FBS concentration was at 10% during successive culture transfers.
The growth status of the cells was periodically observed and
recorded under an inverted phase contrast microscope. BMSCs with
uniform morphology, strong refraction and no granularity in their
cytoplasm from the third generation were reserved for HAAM
seeding.
Identification of BMSCs
Morphological observation. The morphology of
adherent cells, their proliferation rate and population growth
patterns were observed and photographed under an inverted phase
contrast microscope.
Specimen processing
Evaluation of cartilage, tissue processing
The cartilage tissue from the rabbit models was
taken for frozen sectioning. DAPI was used to stain nuclei, the
survival of BMSCs after implantation was evaluated with
fluorescence microscopy. In addition, all the samples were
subjected to general observations, H&E staining, toluidine blue
staining and type II collagen immunohistochemical staining. The
Wakitani scoring was used for histological scoring. The scoring
criteria included the semi-quantitative analysis of cell type,
medulla staining (metachromatic), surface integrity and thickness
of cartilage. Scores ranged from 0 to 12 points. Lower scores
indicate degrees of regenerative repair closer to the normal
conditions (Table I).
 | Table I.Modified Wakitani scores for cartilage
defect evaluation. |
Table I.
Modified Wakitani scores for cartilage
defect evaluation.
| Items | Criteria | Value |
|---|
| Cell type | Hyaline
cartilage | 0 |
|
| Most of them are
hyaline cartilage | 1 |
|
| Most of them are
fibrocartilage | 2 |
|
| Most of them are
non-cartilage | 3 |
|
| No cartilage | 4 |
| Medulla staining
(metachromatic) | Normal | 0 |
|
| Slight decrease | 1 |
|
| Significantly
reduced | 2 |
|
| No change | 3 |
| Surface
integritya | Smooth (>3/4) | 0 |
|
| Medium
(>1/2-3/4) | 1 |
|
| Irregular
(1/4-1/2) | 2 |
|
| Severely irregular
(<1/4) | 3 |
| Thickness of
cartilageb | >2/3 | 0 |
|
| 1/3-2/3 | 1 |
|
| <1/3 | 2 |
| Maximum total
score |
| 3 |
Immunohistochemical staining of type II
collagen
The specimens were fixed, decalcified and paraffin
fixed. After sectioning, immunohistochemical staining of type II
collagen was carried out as follows: i) tissue slides were placed
in the oven at 50°C for 2 h; ii) de-waxing was performed with 3
times washing using xylene, then hydration was performed by washing
4 times with 100, 90, 80 and 70% alcohol, respectively. Then the
samples were washed 3 times with phosphate-buffered saline (PBS);
iii) 1 volume 30% H2O2 was mixed with 10
volumes of distilled water at room temperature for 10 min to
inactivate endogenous enzymes and then the samples were washed 3
times with PBS; iv) a composite digestive solution was added and
then left standing for 10 min at room temperature before washing 3
times with PBS; v) 5% BSA blocking solution was added and the
samples slides were incubated at room temperature for 20 min, to
finally remove all excess liquid; vi) the primary antibody was
diluted (mouse anti-rabbit type II collagen antibody) to 10 µg/ml.
The diluted antibody was added followed by incubation at 37°C for 2
h, then slides were washed again with PBS 3 times for 3 min each;
vii) a biotinylated goat anti-mouse IgG was added followed by
incubation at 37°C for 20 min, and then washing with PBS 3 times
for 3 min; viii) a streptavidin-biotin-peroxidase (SAB) complex was
added followed by incubation at 37°C for 30 min, and then washed
with PBS 3 times for 5 min; ix) for DAB color development, DAB was
added and the samples were kept at room temperature for color
development. Microscopic observation was used to control reaction
time. Flushing with distilled water for 10 min terminated the
reactions.
Hematoxylin staining was performed by covering
slides with hematoxylin for 10 min and then flushing for 30 min
with water. The samples were dehydrated by washing 3 times with 80,
90 and 100% ethanol. Finally, the samples were made transparent by
washing 3 times with xylene, 3 min for each time. Finally the
samples were sealed with neutral gum for observation under the
microscope.
Toluidine blue staining
Tissue sections were de-waxed and hydrated with
conventional described methods above. The samples were kept in
toluidine blue solution for 30 min, and then gently washed with
water. Next, samples were kept in glacial acetic acid solution
until the nucleus and particles became clear, then gently washed
with water and dried with cold air. Finally, the samples were
treated with xylene to become transparent, and sealed with neutral
gum.
Statistical analysis
The analysis software SPSS version 11 (IBM SPSS,
Armonk, NY, USA) was used for statistical analyses. The gross and
histological scores of the experimental and control groups at
different time-points using the t-test were determined. A P<0.05
was considered to be statistically significant.
Results
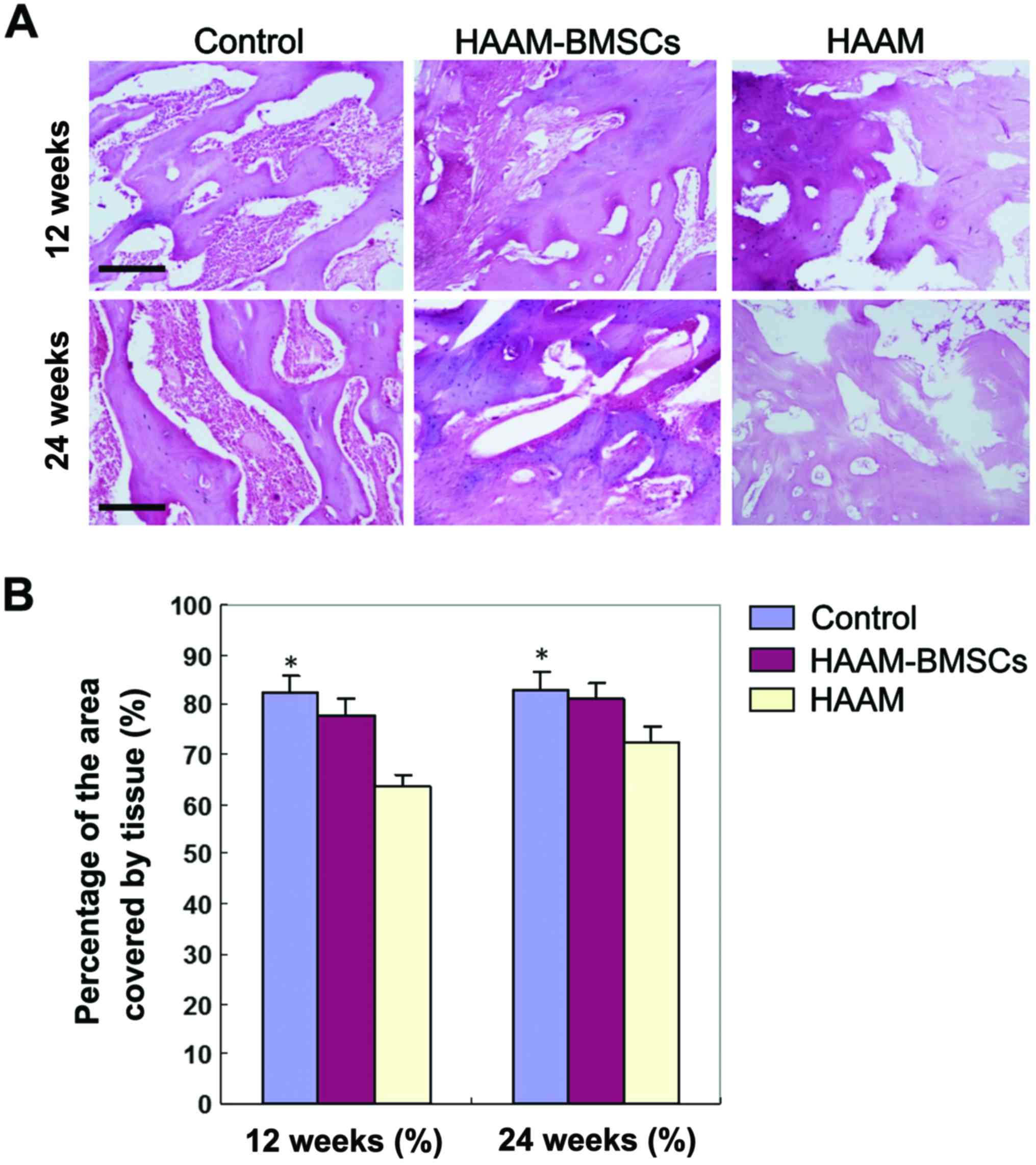
H&E staining of rabbit cartilage
at 12 and 24 weeks
H&E staining of rabbit cartilage tissue in each
group showed that tissue growth of HAAM-BMSCs group was better than
that of HAAM group at both 12 and 24 weeks. The tissue coverage in
the HAAM-BMSCs group was significantly higher than that of the HAAM
group (p<0.05), and there was no significant difference between
the HAAM-BMSCs and the control groups (p>0.05). H&E staining
images are shown in Fig. 1A.
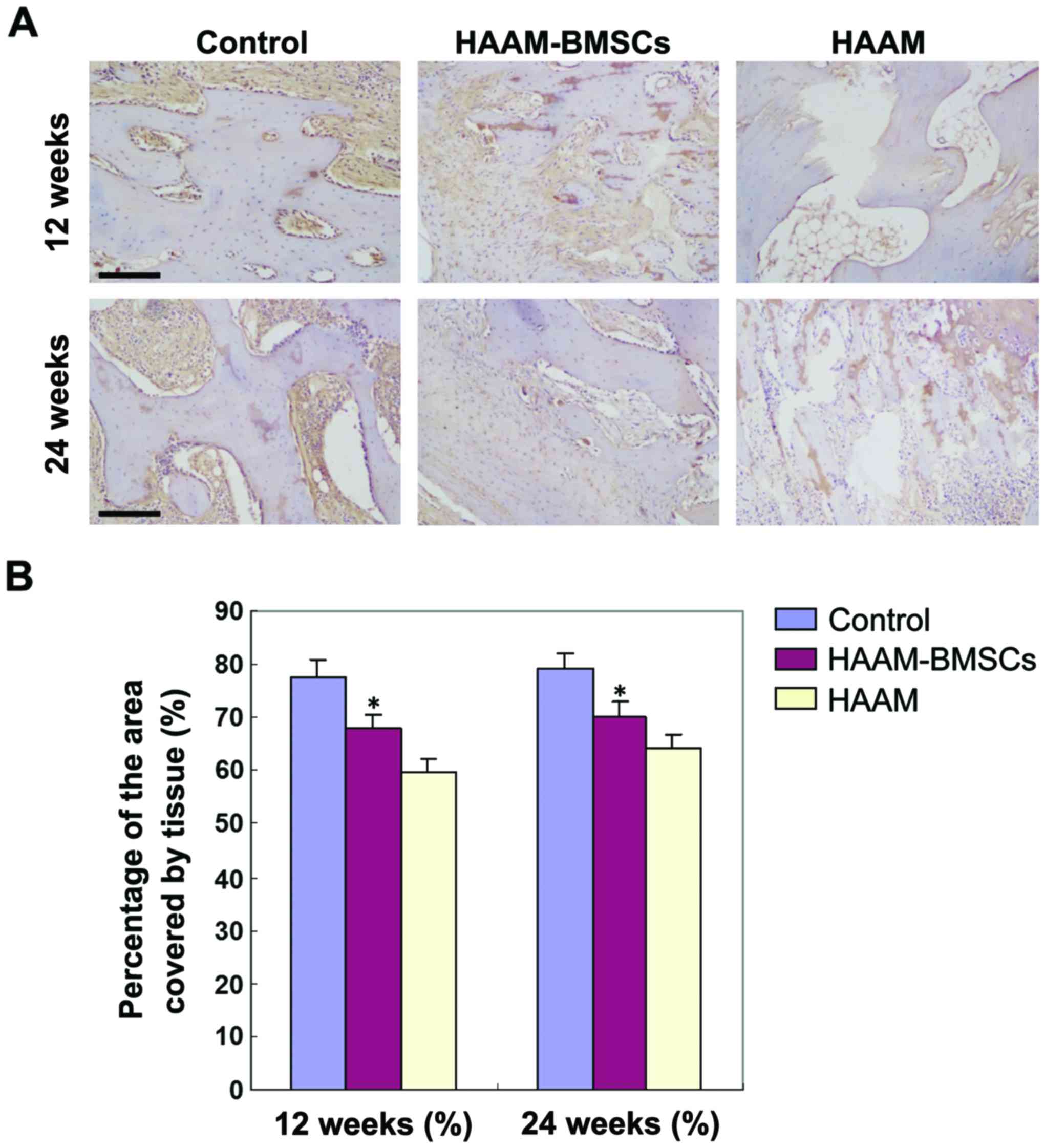
Collagen II expression detected by
immunohistochemical staining
Immunohistochemical staining was used to detect the
expression levels of collagen II in each group. The percentage of
collagen II-positive area in HAAM-BMSCs group was significantly
higher than that in HAAM group (p<0.05), and there was no
significant difference between the HAAM-BMSCs and the control
groups (p>0.05) (Fig. 2).
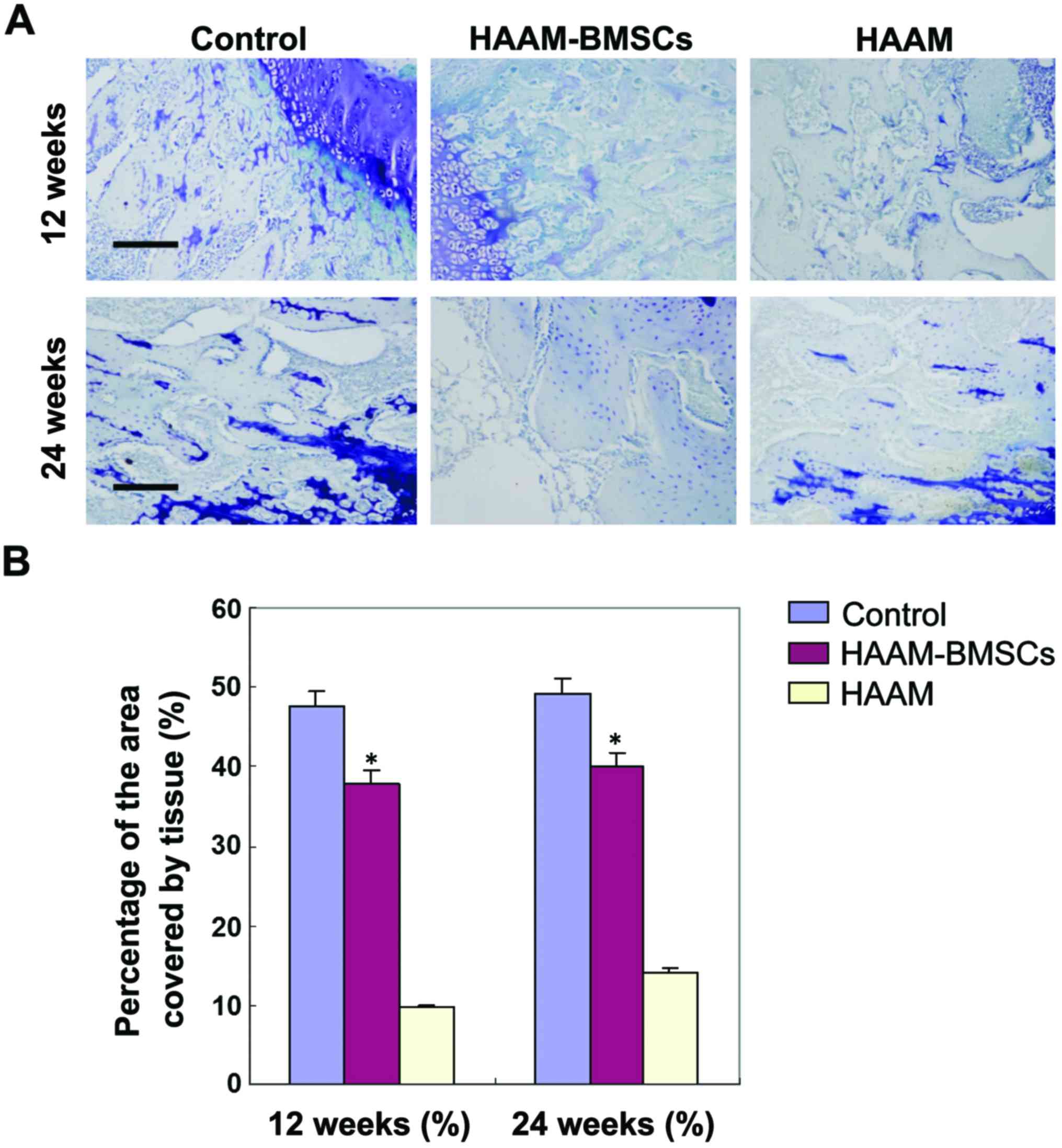
Toluidine blue staining at 12 and 24
weeks
In order to detect the expression of DNA in rabbit
cartilage tissue, we used toluidine blue staining to detect the
number of stained chondrocytes in the HAAM-BMSC group. The number
of condrocytes in HAAM-BMSC group was significantly higher than
that in HAAM group (p<0.05), and there was no statistically
significant difference between the number in the HAAM-BMSCs and
that in the control group (p>0.05) (Fig. 3).
Wakitani scoring of histological
cartilage defect
The Wakitani scoring for histological cartilage
defects was used to further evaluate tissue repair in each group.
The Wakitani scores of the HAAM and HAAM-BMSC groups were both
significantly higher than those of the control group (p<0.05).
However, compared with the HAAM group, the Wakitani score of the
HAAM-BMSCs was significantly lower (p<0.05) but still higher
than that of the normal control group (p<0.05). At 24 weeks, the
Wakitani score in group I was significantly lower compared with the
score at 12 weeks (p<0.05). There was no significant difference
in Wakitani score in the HAAM-BMSCs group between the 2 time-points
(Table II).
 | Table II.Wakitani scoring of histological
cartilage defects. |
Table II.
Wakitani scoring of histological
cartilage defects.
| Groups | 12 weeks (%) | 24 weeks (%) | T-value | P-value |
|---|
| Control group | 0.00±0.01 | 0.00±0.01 | 0.43 | 0.52 |
| HAAM (group I) | 10.38±0.21 | 9.47±1.11 | 0.44 | 0.57 |
| HAAM-BMSCs (group
II) |
6.33±0.38a |
3.05±1.28a | 1.28 | 0.03 |
Discussion
Articular cartilage injuries do not get well
repaired spontaneously, and are often the harbingers of
degenerative arthritis, eventually leading to joint dysfunction.
Therefore, interventions to repair damaged cartilage in order to
avoid or delay the occurrence of secondary osteoarthritis are
actively sought by researchers. In recent years, developments in
tissue engineering for cartilage repair have widened the prospects.
The selection of seeding cells is a prerequisite and key to the
success of tissue engineering. In most cases, BMSCs were used as
seeding cells (2). However, due to
the limited amounts of bone marrow for cell extraction, and the
fact that the number of stem cells decreases gradually with age,
the use of BMSCs is not without problems (3).
Amniotic MSCs, with their strong in vitro
proliferation and differentiation capabilities are easy to obtain
in large quantities with only slight damage to the donor site.
Therefore, adipose-derived MSCs have become a new source of tissue
engineering seeding cells and gene therapy target cells. Besides
the nature of seeding cells, the local presence of cytokines is
another important factor that can promote the formation of
cartilage locally (4). In our study,
bone marrow-derived stem cells were harvested by mechanical
isolation, enzymatic digestion and centrifugation. We confirmed the
identity of the stem cells, and proved they could differentiate
into adipocytes and chondrocytes.
The space between cartilage cells is filled with
cartilage matrix, which is the scaffold of cartilage tissue. The
cartilage matrix is indispensable to maintain the biomechanical
characteristics of articular cartilage. It reduces the friction
coefficient, buffers mechanical shock and mediates the signal
transmission between cells (5,6). For
tissue engineering of cartilage, a cartilage matrix has the ideal
three-dimensional structure, physical and chemical properties, and
good biocompatibility with bone marrow-derived stem cells (7), making it a reliable stem cell carrier.
In the process of inducing stem cell differentiation into
chondrocytes, the cartilage matrix plays 3 different roles, it is a
single-cell factor (8); a combined
cytokine (9); and a continuous
cytokine (10).
Amniotic membranes contain immature undifferentiated
cells because they form in the early stages of embryogenesis.
Amniotic membrane cells are amniotic epithelial cells, fibroblasts
and undifferentiated mesenchymal cells. Fibroblasts can produce
collagen fibers, elastic fibers, reticular fibers, a variety of
glycoproteins, proteoglycans, collagen, hyaluronic acid and
fibronectin (11,12). Mesenchymal cells have the potential
to differentiate during inflammation and can do so into dendritic
cells and fibroblasts. Amniotic epithelial cells in vitro
can express glial fibrillary acidic protein and liver parenchymal
cell differentiation markers, albumin and α-fetoprotein, which
indicates that they can be seeding cells for nerve or liver tissue
engineering (13). Amniotic
epithelial cells rarely express human leukocyte antigens A, B and C
or DR or other antigens and therefore their transplantation does
not usually lead to immune rejection. Studies have been carried out
to explore the use of amniotic epithelial cells to treat nerve
damage, amniotic membrane premature rupture, and corneal epithelial
defects (14–16). Thus, the use of amniotic epithelial
cells for tissue engineering may lead to successful outcomes.
In a study, human embryonic periosteum-derived
osteoblasts and human amniotic cells were combined in vitro
to construct a tissue engineered periosteum with the ability to
promote differentiation of new osteoblasts, and increase expression
levels of Cbfa1 (17). As a scaffold
material, human amniotic membrane can induce and promote osteoblast
differentiation. Tissue engineered periosteum constructed by the
combination of osteoblasts and human amniotic membrane has been
used for production of bone tissue at a molecular level, where the
proliferation of condrocytes was evidenced (18). The amniotic membrane has showed good
ability for water absorption, cell adsorption and biocompatibility
in vitro. Condylar cells can adhere and proliferate on the
amniotic membrane. The amniotic membrane has been proposed as a
scaffold for condylar tissue engineering (19–22).
In our study, we evaluated the use of
amniotic-loaded stem cells to repair a skeletal cartilage lesion,
by implanting a BMSC-HAAM complex into an injured medial condyle of
rabbits. At 12 and 24 weeks after implantation, the number of
chondrocytes, the collagen II expression level and the Wakitani
scores in the medial condylar cartilage of the BMSC-HAAM group were
all significantly better than those in the HAAM group. The results
suggest that amniotic membrane-loaded stem cells have a good
reparation effect on the damaged medial condyle of rabbits, which
is consistent with the results of previous studies. We believe that
the application of amniotic membrane-loaded stem cells, as a tissue
engineering repair material, has good clinical prospects.
Based on our findings, the amniotic membrane-derived
loaded stem cell repair strategy used had a good therapeutic effect
on the osteochondral defect, in the weight-bearing area of the
femoral medial malleolus. The number of chondrocytes in the injured
area increased significantly with the HAAM-BMSC treatment, which
accelerated the repair of damaged tissue in rabbits. For clinical
experiments in humans further experiments and ethical aspects still
need to be considered.
References
|
1
|
Takao M, Uchio Y, Kakimaru H, Kumahashi N
and Ochi M: Arthroscopic drilling with debridement of remaining
cartilage for osteochondral lesions of the talar dome in unstable
ankles. Am J Sports Med. 32:332–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mithoefer K, Williams RJ 3rd, Warren RF,
Potter HG, Spock CR, Jones EC, Wickiewicz TL and Marx RG: The
microfracture technique for the treatment of articular cartilage
lesions in the knee. A prospective cohort study. J Bone Joint Surg
Am. 87:1911–1920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grande DA, Breitbart AS, Mason J, Paulino
C, Laser J and Schwartz RE: Cartilage tissue engineering: current
limitations and solutions. Clin Orthop Relat Res. 367
Suppl:S176–S185. 1999. View Article : Google Scholar
|
|
4
|
Enosawa S, Sakuragawa N and Suzuki S:
Possible use of amniotic cells for regenerative medicine. Nihon
Rinsho. 61:396–400. 2003.(In Japanese). PubMed/NCBI
|
|
5
|
Okawa H, Okuda O, Arai H, Sakuragawa N and
Sato K: Amniotic epithelial cells transform into neuron-like cells
in the ischemic brain. Neuroreport. 12:4003–4007. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bilic G, Ochsenbein-Kölble N, Hall H, Huch
R and Zimmermann R: In vitro lesion repair by human amnion
epithelial and mesenchymal cells. Am J Obstet Gynecol. 190:87–92.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parmar DN, Alizadeh H, Awwad ST, Li H,
Neelam S, Bowman RW, Cavanagh HD and McCulley JP: Ocular surface
restoration using non-surgical transplantation of tissue-cultured
human amniotic epithelial cells. Am J Ophthalmol. 141:299–307.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pollok JM and Vacanti JP: Tissue
engineering. Semin Pediatr Surg. 5:191–196. 1996.PubMed/NCBI
|
|
9
|
Eyrich D, Wiese H, Maier G, Skodacek D,
Appel B, Sarhan H, Tessmar J, Staudenmaier R, Wenzel MM, Goepferich
A, et al: In vitro and in vivo cartilage engineering using a
combination of chondrocyte-seeded long-term stable fibrin gels and
polycaprolactone-based polyurethane scaffolds. Tissue Eng.
13:2207–2218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Shu XZ and Prestwich GD:
Osteochondral defect repair with autologous bone marrow-derived
mesenchymal stem cells in an injectable, in situ, cross-linked
synthetic extracellular matrix. Tissue Eng. 12:3405–3416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Freed LE, Vunjak-Novakovic G, Biron RJ,
Eagles DB, Lesnoy DC, Barlow SK and Langer R: Biodegradable polymer
scaffolds for tissue engineering. Biotechnology. 12:689–693.
1994.PubMed/NCBI
|
|
12
|
Mahgoub MA, Ammar A, Fayez M, Edris A,
Hazem A, Akl M and Hammam O: Neovascularization of the amniotic
membrane as a biological immune barrier. Transplant Proc. 36:pp.
1194–1198. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim BS and Mooney DJ: Development of
biocompatible synthetic extracellular matrices for tissue
engineering. Trends Biotechnol. 16:224–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burg KJ, Porter S and Kellam JF:
Biomaterial developments for bone tissue engineering. Biomaterials.
21:2347–2359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura T and Kinoshita S: Ocular surface
reconstruction using cultivated mucosal epithelial stem cells.
Cornea. 22 Suppl 7:S75–S80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura T, Endo K, Cooper LJ, Fullwood
NJ, Tanifuji N, Tsuzuki M, Koizumi N, Inatomi T, Sano Y and
Kinoshita S: The successful culture and autologous transplantation
of rabbit oral mucosal epithelial cells on amniotic membrane.
Invest Ophthalmol Vis Sci. 44:106–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ducy P, Starbuck M, Priemel M, Shen J,
Pinero G, Geoffroy V, Amling M and Karsenty G: A Cbfa1-dependent
genetic pathway controls bone formation beyond embryonic
development. Genes Dev. 13:1025–1036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leyva-Leyva M, Barrera L, López-Camarillo
C, Arriaga-Pizano L, Orozco-Hoyuela G, Carrillo-Casas EM,
Calderón-Pérez J, López-Díaz A, Hernandez-Aguilar F,
González-Ramírez R, et al: Characterization of mesenchymal stem
cell subpopulations from human amniotic membrane with dissimilar
osteoblastic potential. Stem Cells Dev. 22:1275–1287. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inatomi T, Nakamura T, Koizumi N, Sotozono
C and Kinoshita S: Current concepts and challenges in ocular
surface reconstruction using cultivated mucosal epithelial
transplantation. Cornea. 24 Suppl 8:S32–S38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura T, Inatomi T, Sotozono C, Amemiya
T, Kanamura N and Kinoshita S: Transplantation of cultivated
autologous oral mucosal epithelial cells in patients with severe
ocular surface disorders. Br J Ophthalmol. 88:1280–1284. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inatomi T, Nakamura T, Koizumi N, Sotozono
C, Yokoi N and Kinoshita S: Midterm results on ocular surface
reconstruction using cultivated autologous oral mucosal epithelial
transplantation. Am J Ophthalmol. 141:267–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ti SE, Grueterich M, Espana EM, Touhami A,
Anderson DF and Tseng SC: Correlation of long term phenotypic and
clinical outcomes following limbal epithelial transplantation
cultivated on amniotic membrane in rabbits. Br J Ophthalmol.
88:422–427. 2004. View Article : Google Scholar : PubMed/NCBI
|