Introduction
Adult cells generally cannot undergo chromatin
remodeling (i.e., cell reprogramming reaction) under normal
conditions. However, previous research groups separately used four
exogenous transcription factors (octamer-binding transcription
factor 3/4, (Oct3/4), (sex determining region Y)-box 2 (Sox2),
c-Myc, and Kruppel-like factor 4 (Klf4) by Yamanaka, and Oct3/4,
Sox2, Nanog, and Lin28 by Thomson) to induce complex reprogramming
reactions in adult cells to obtain induced pluripotent stem (iPS)
cells comparable with embryonic stem cells (ESCs) (1–3). These
iPS cells were very similar to ESCs in many respects, including
pluripotent differentiation, self-proliferation capacity, and the
ability to form teratomas in vivo (4,5). Because
these seed cells of the iPS cells were adult cells sourced from
humans or animals, the preparation of the iPS cells did not involve
ethical constraints. Additionally, iPS cells advantageously have
low immunogenicity and diverse sources. Thus, they could be ideal
materials for cell and gene therapies (4,5).
The Yes-associated protein (Yap) is a downstream
transcriptional coactivator of the Hippo-Yap pathway (6,7). During
normal growth and development, activated Yap can induce the
transcription of downstream genes and maintain organ development
and cell growth. Yap loses activity after phosphorylation by large
tumor suppressor 1/2 (Lats1/2) kinases, resulting in the inhibition
of the transcription of downstream genes and the subsequent
termination of cell proliferation and organ hyperplasia. Therefore,
Yap activation directly influences the growth and development of
tissues and organs (7–9). The gene for human Yap is localized on
chromosome 11q12. Except for peripheral white blood cells that do
not express Yap protein, other tissues and organs extensively
express Yap (6,10,11).
Additionally, many studies have indicated that Yap plays important
roles in maintaining stem cell pluripotency, promoting stem cell
proliferation, and regulating stem cell differentiation (9–11).
In this study, we combined two Yamanaka factors,
Oct4 and Sox2, and a key factor in the Hippo-Yap pathway, Yap, to
investigate whether human amniotic epithelial cells (HuAECs) could
be induced for reprogramming into iPS cells.
Materials and methods
Isolation and culture of HuAECs
According to previously reported methods (4,12),
amniotic membranes were washed with 4°C phosphate-buffered saline
(PBS; Gibco, Gaithersburg, MD, USA) three times and cut into small
pieces. The pieces were then digested in 20 ml 0.125%
Trypsin-ethylenediaminetetraacetic acid (EDTA; Gibco) at 37°C for
30 min and mixed thoroughly in 20 ml Dulbecco's modified Eagle's
medium (DMEM):F12 (1:1) cell culture medium (containing 15% fetal
bovine serum, FBS). The cell suspension was filtered through a
200-mesh filter (Millipore, Bedford, MA, USA). The cell filtrate
solution was collected and centrifuged at 1,500 rpm for 10 min. The
supernatant was discarded, and the cell pellet was resuspended in
DMEM:F12 (1:1) cell culture medium (containing 15% FBS; Gibco). The
cell density was adjusted to 1×105/ml and directly
inoculated onto 6-cm cell culture dishes. Cells were cultured in a
cell incubator set at 37°C and 5% CO2. The cell culture
medium was replaced after 48 hr.
Preparation of iPS cells
According to previously reported methods (1,3,13), HuAECs in the logarithmic growth phase
were used at a cell density of 1×106/ml. The original
culture medium was discarded, and 2 ml Opti-MEM (Gibco) culture
medium was added. pLVX-Oct3/4, pLVX-Sox2, and pLVX-Yap1
lentiviruses were added (virus concentrations were 1×108
infectious units [IFU]/plaque-forming units [PFU]; Novobio,
Shanghai, China), gently but thoroughly mixed, and reacted in a
37°C water bath for 120 min. After the reactions were finished, 4
ml mTeSR™1 medium (STEMCELL Technologies, Inc., MA, USA) was added.
The cells were cultured in a 37°C and 5% CO2 cell
incubator. The cell culture medium was replaced after 24 h.
Preparation of embryoid bodies
In accordance with previously reported methods
(1,2), the concentration of iPS cells was
adjusted to 1×105/ml using cell differentiation culture
medium (DMEM, 15% FBS, 0.1 mmol/l non-essential amino acids, 2
mmol/l glutamate, and 0.1 mmol/l β-mercaptoethanol; all from
Gibco). Cell suspensions at 2 µl were dropped onto the covers of
cell culture dishes. After the covers were fully covered with cell
suspension, they were placed onto the bottom of the dishes. Cells
were continuously cultured for 48 h.
RNA extraction and qPCR
According to previously reported methods (4,12), the
total RNA from cells in all groups were extracted based on the
manufacturer instructions for the Trizol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). The total RNA was treated with
DNase I (Sigma-Aldrich, St. Louis, USA), quantified, and reverse
transcribed into cDNA using a ReverTra Ace-α First Strand cDNA
Synthesis kit (Toyobo, Shanghai, China; Biotech Co., Ltd.,
Shanghai, China). Quantitative polymerase chain reaction (qPCR) was
performed using a RealPlex4 real-time PCR detection system
(Eppendorf Co., Ltd., Hamburg, Germany). A SYBR-Green Real-Time PCR
Master Mix (Toyobo) was used as the fluorescence dye for nucleic
acid amplification. qRT-PCR was performed for 40 amplification
cycles of the following steps: 95°C denaturation for 15 sec, 58°C
annealing for 30 sec, and 72°C extension for 42 sec. The relative
gene expression levels were calculated and determined using the
2−ΔΔCt method as follows: ΔCt = Ct_genes - Ct_18sRNA and
ΔΔCt = ΔCt_all_groups - ΔCt_blank control_group. The mRNA
expression levels were calibrated based on the expression level of
18 s rRNA. The primers used are shown in Table I.
 | Table I.qRT-PCR primers. |
Table I.
qRT-PCR primers.
| Gene product | Forward (F) and
reverse (R) primers (5′→3′) | Size (bp) |
|---|
| Oct4 | F:
GTGGAGGAAGCTGACAACAA | 118 |
|
| R:
TCTCCAGGTTGCCTCTCACT |
|
| Sox2 | F:
AGAAAAACGAGGGAAATGGG | 120 |
|
| R:
GTCATTTGCTGTGGGTGATG |
|
| Rex1 | F:
GGTGGCATTGGAAATAGCAG | 148 |
|
| R:
TGCCTAGTGTGCTGGTGGT |
|
| Nanog | F:
GATTTGTGGGCCTGAAGAAA | 119 |
|
| R:
CAGGGCTGTCCTGAATAAGC |
|
| Ssea3/4 | F:
CTTTGAGGCTCTGCAGCTTA | 150 |
|
| R:
CTGGTTCGCTTTCTCTTTCG |
|
| Mst | F:
AGAAGGATGGGGTGGCTC | 117 |
|
| R:
CAGGTGCTGTAGCTCTGTGC |
|
| Lats1 | F:
TTTCTTGGCACAAACACCAT | 130 |
|
| R:
GGGTCCTCGGCAAAGTTTA |
|
| Mob1 | F:
TGACTTGGGTTCAAGATCAGC | 128 |
|
| R:
ATGGGCATAAACCCTGAACA |
|
| Yap | F:
TTGGGAGATGGCAAAGACAT | 113 |
|
| R:
CTGTGACGTTCATCTGGGAC |
|
| 18S
rRNA | F:
CAGCCACCCGAGATTGAGCA | 223 |
|
| R:
TAGTAGCGACGGGCGGTGTG |
|
Semi-quantitative RT-PCR
According to a previously reported method (1), the total RNA from cells in all groups
were extracted based on the manufacturer instructions for the
TRIzol reagent (Invitrogen). The total RNA was treated with DNase I
(Sigma-Aldrich, St. Louis, USA), quantified, and reverse
transcribed into cDNA using a ReverTra Ace-α First Strand cDNA
Synthesis kit (Toyobo). Semi-quantitative RT-PCR was performed in a
PTC-200 PCR machine (MJ Research Inc., Waltham, MA, USA). Each
sample used 100 ng cDNA template. Additionally, 5 pmoles PCR
forward and reverse primers, 200 µM dNTP, 1 unit RED-Taq
Polymerase, and 1X RED-Taq polymerase buffer were added (all
reagents were purchased from Sigma-Aldrich). The reaction volume
was adjusted to 20 µl using nuclease-free deionized water. The
qRT-PCR was performed for 32 amplification cycles of the following
steps: 95°C denaturation for 15 sec, 58°C annealing for 30 sec, and
72°C extension for 42 sec. The PCR product was subjected to 1.5%
agarose gel electrophoresis (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The mRNA expression levels were calibrated based on the
expression level of 18s rRNA. The primers used are shown in
Table I.
Detection of alkaline phosphatase
According to the manufacturer instructions of the
BCIP/NBT Alkaline Phosphatase Color Development kit (Beyotime
Biotechnology Co., Ltd., Zhejiang, China), cell samples were fixed
in 1 ml 4% paraformaldehyde (Sigma-Aldrich) at room temperature for
30 min. The fixative solution was discarded, and the
5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium
(BCIP/NBT) staining working solution was added and incubated at
room temperature for 30 min. The BCIP/NBT staining working solution
was subsequently discarded, and the cells were washed with
distilled water twice to terminate the coloring reaction.
Western blot analysis
According to previously reported methods (4,12), the
total protein samples of all groups were subjected to 12% SDS-PAGE
denaturing gel electrophoresis (Bio-Rad) and transferred onto
polyvinylidene difluoride PVDF membranes (Millipore, Bedford, MA,
USA). After the membranes were blocked and washed, primary
antibodies were added and incubated at 37°C for 45 min (Table II). Then, after the membranes were
fully washed, secondary antibodies were added and incubated at 37°C
for 45 min (Table II). The
membranes were washed with Tris-buffered saline containing Tween-20
(TBST, Bio-Rad) at room temperature four times for 14 min per wash.
The results were developed using the enhanced chemiluminescence
(ECL) method (Bio-Rad). The membranes were exposed using Kodak
XAR-5 films (Sigma-Aldrich).
 | Table II.Primary antibodies, their source and
dilutions. |
Table II.
Primary antibodies, their source and
dilutions.
| Antibodies | Companies | Applications |
|---|
| Rabbit anti-human
Oct4 (no. 2890) | Cell Signaling
Technology, Danvers, MA, USA | IF (1:100) |
|
|
| WB (1:1,000) |
| Rabbit anti-human
Sox2 (no. 3579) | Cell Signaling
Technology, Danvers, MA, USA | WB (1:1,000) |
| Rabbit anti-mouse
SSEA3/4 (no. 4755) | Cell Signaling
Technology, Danvers, MA, USA | IF (1:100) |
| Rabbit anti-human
MST (no. 14946) | Cell Signaling
Technology, Danvers, MA, USA | WB (1:1,000) |
| Rabbit anti-human
p-MST (no. 3681) | Cell Signaling
Technology, Danvers, MA, USA | WB (1:1,000) |
| Rabbit anti-human
LATS1 (no. 3477) | Cell Signaling
Technology, Danvers, MA, USA | WB (1:1,000) |
| Rabbit anti-human
MOB1 (no. 13730) | Cell Signaling
Technology, Danvers, MA, USA | WB (1:1,000) |
| Rabbit anti-human
Yap (no. 14074) | Cell Signaling
Technology, Danvers, MA, USA | WB (1:1,000) |
| Rabbit anti-human
p-Yap (no. 13008) | Cell Signaling
Technology, Danvers, MA, USA | WB (1:1,000) |
| Rabbit anti-human
GAPDH (no. 5174) | Cell Signaling
Technology, Danvers, MA, USA | WB (1:1,000) |
Chromatin immunoprecipitation
(ChIP)-PCR
In accordance with previously reported methods
(4,12), the manufacturer instructions of the
EZ-ChIP kit (Millipore, Bedford, MA, USA) were followed. Briefly,
cells were fixed in 1% paraformaldehyde at 37°C for 30 min and
incubated in 125 mM glycine at room temperature for 10 min to
terminate cross-linking. Cells were sonicated on ice until the DNA
was broken into chromatin fragments of 200–1000 bp. The primary
antibody was added, and the samples were incubated at 4°C
overnight. Protein A/G and agarose were added for adsorption, and a
final immune precipitate was obtained. PCR amplification was then
performed for 33 amplification cycles of the following steps: 95°C
denaturation for 30 sec, 55°C annealing for 30 sec, and 72°C
extension for 30 sec. The 2-ΔCt calculation method was performed to
determine the relative expression levels of the PCR products as
follows: ΔCt = Ct_all_groups - Ct_Input_group. The primers used are
listed in reference (2).
Analysis of chromosome karyotype
According to a previously reported method (4), iPS cells with excellent growth status
were incubated with 0.1 µg/ml colchicine (Sigma-Aldrich) for 30
min. The supernatant was discarded, and the cells were collected
and mixed thoroughly in 9 ml 0.075% KCl (Sigma-Aldrich) by
pipetting. Hypotonic treatment was performed at 37°C for 30 min.
The recovered cell pellet was fixed in a fixative solution
(Beyotime Biotechnology) at room temperature three times for 15 min
per fix. The cell suspension was dropped onto slides and baked at
80°C for 2 h. Cells were digested with 0.25% Trypsin-EDTA (Gibco)
for 3 min, washed with deionized water three times, and stained
with Giemsa staining solution (Beyotime Biotechnology) at room
temperature for 10 min. After washing again with deionized water
three times, the slides were mounted in neutral balsam (Beyotime
Biotechnology).
Preparation of the teratoma
In accordance with previously reported methods
(4,12), iPS cells with excellent growth status
were inoculated into back subcutaneous tissues of nude mice in a
sterile environment. Each nude mouse was inoculated at one point
with approximately 1×108/ml iPS cells. Nude mice were
fed under normal conditions until tumor formation.
H&E staining
According to a previously reported method (4), tissues were fixed in 4%
paraformaldehyde at room temperature for 12 h. Frozen tissue
sections were prepared at thicknesses of approximately 5 µm.
Sections were fixed in 95% anhydrous ethanol for 2 min, stained in
hematoxylin for 5 min, and differentiated in differentiation
solution for 2 min. Sections were immersed in weak ammonia solution
for 3 min, washed with deionized water for 5 min, stained with
eosin for 5 min, and washed with deionized water for 5 min. Tissue
sections were immersed in 70, 80, and 90% alcohol solution once for
1 min, washed with anhydrous ethanol twice for 1 min each wash,
cleared in xylene twice for 1 min each wash, and mounted using
neutral balsam. These reagents and materials were all purchased
from Beyotime Biotechnology Co., Ltd., Zhejiang, China.
Immunofluorescence staining
According to previously reported methods (4,12), cell
samples were fixed in 1 ml 4% paraformaldehyde (Sigma-Aldrich) at
room temperature for 30 min and blocked in blocking solution
(Beyotime Biotechnology) at 37°C for 60 min. The blocking solution
was discarded, and the cells were washed with an
immunohistochemistry washing solution (Beyotime Biotechnology) at
room temperature three times for 5 min each wash. Primary
antibodies (Table II) were added
and incubated at 37°C for 45 min. The antibodies were discarded,
and the cells were washed with the immunohistochemistry washing
solution (Beyotime Biotechnology) at room temperature three times
for 5 min each wash. Secondary antibodies (Table II) were added and incubated at 37°C
for 45 min. The antibodies were discarded, and the cells were
washed with the immunohistochemistry washing solution (Beyotime
Biotechnology) at room temperature three times for 5 min each.
Finally, the cells were mounted in immunofluorescence mounting
fluid (Sigma-Aldrich).
Results
Overexpression of Yap, Oct4, and Sox2
induced HuAECs to express high levels of ESC markers
HuAECs were isolated from fetal amniotic membrane
and infected with lentiviruses carrying Oct4, Sox4, and Yap (OSY)
coding sequences. These cells were cultured using iPS cell culture
methods to investigate whether the OSY factors could induce HuAEC
reprogramming into iPS cells (Fig.
1A). Microscopy showed that HuAECs showed typical epithelial
cell characteristics and had cobblestone morphologies (Fig. 1B). After transducing with OSY
factors, the cells were cultured for 14 days. Microscopy showed the
gradual development of clone-like cell masses (Fig. 1C). These clones were identified as
OSY-iPS. The alkaline phosphatase assays suggested these clone-like
cells exhibited dark-purple, positive reactions (Fig. 1D). The semi-quantitative PCR results
indicated the mRNA levels of ESC markers (Oct4, Sox2, Nanog, Rex1,
and Ssea3/4) and Yap in OSY-iPS cells were higher than those in the
HuAECs (Fig. 1E). Additionally,
immunofluorescence staining results suggested that the expression
of Oct4 and SSEA3/5 proteins in OSY-iPS cells was positive
(Fig. 1F). Finally, Western blot
results showed that the expression levels of Oct4, Sox2, and YAP
proteins in the OSY-iPS cells were significantly higher than those
in the HuAECs (Fig. 1G). Therefore,
the three OSY factors induced HuAECs to express high levels of ESC
markers.
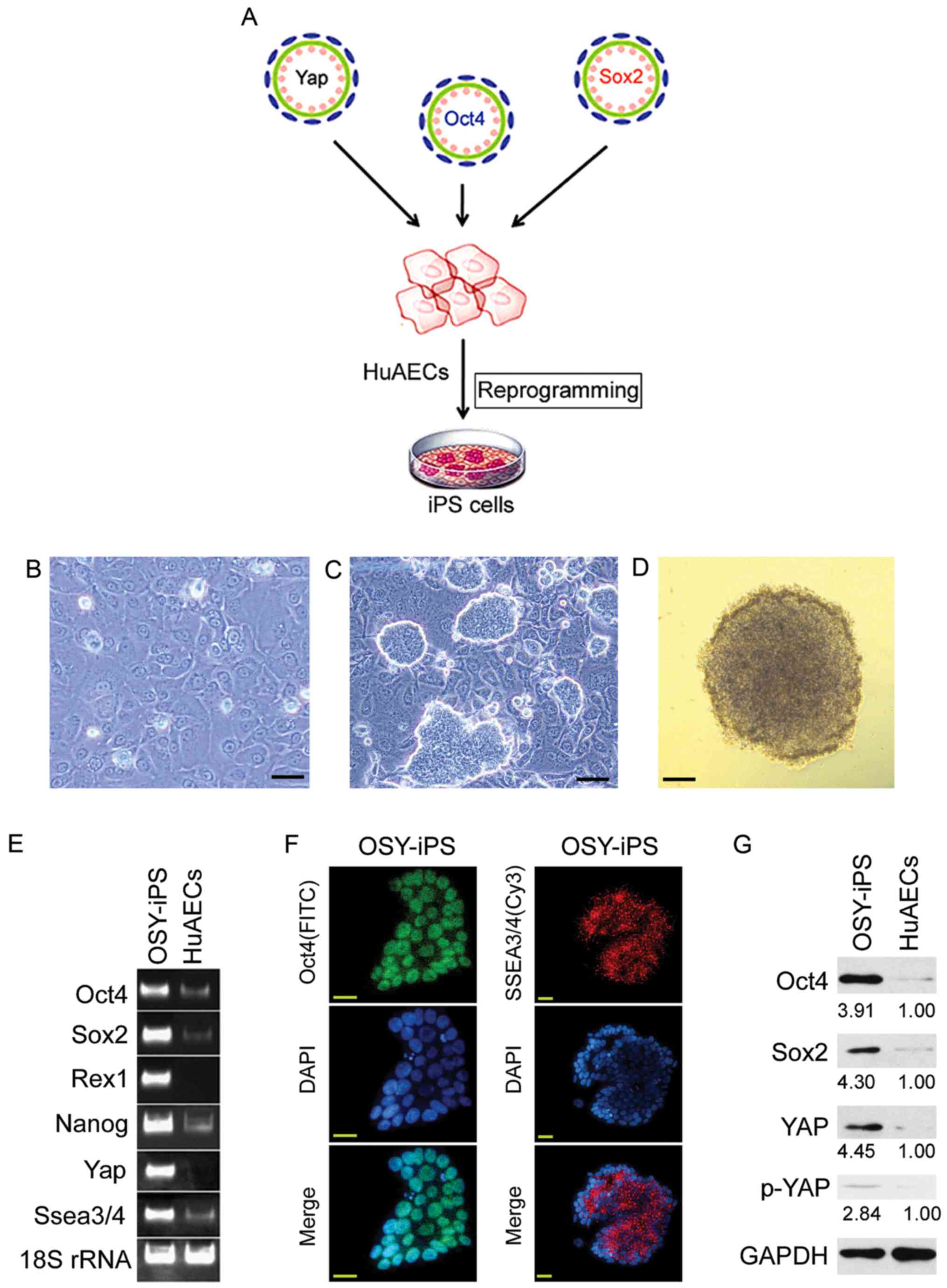 | Figure 1.Overexpression the three factors, OSY,
induced HuAECs to express high levels of ESC markers. (A) The
process of induction of HuAEC reprogramming into iPS cells by OSY.
(B) Cell morphology of HuAECs; scale, 30 µm. (C) OSY-iPS cells had
clone-like morphology; scale, 30 µm. (D) Alkaline phosphatase
staining identification of OSY-iPS cells was positive; scale, 30
µm. (E) Semi-quantitative PCR results indicated that OSY-iPS cells
expressed high levels of ESC markers (Oct4, Sox2, Nanog, Rex1, and
Ssea3/4) and Yap. (F) Immunofluorescence staining results suggested
that OSY-iPS cells expressed high levels of Oct4 and SSEA3/4
proteins; scale, 30 µm. (G) Western blot results showed that
OSY-iPS cells expressed high levels of Oct4, Sox2, and Yap
proteins. |
Overexpression of Yap, Oct4, and Sox2
induced HuAECs to undergo chromatin reprogramming
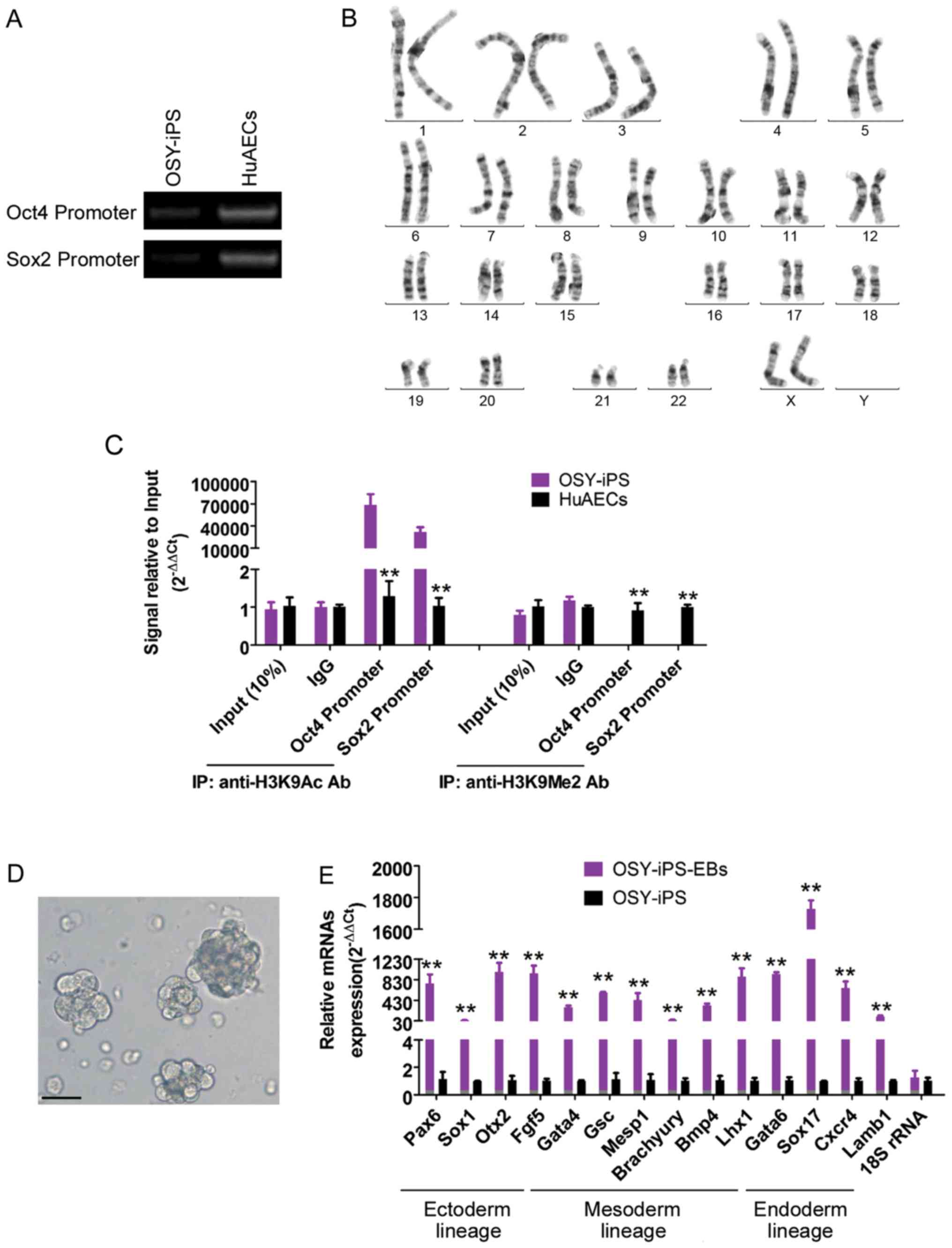
The methylation PCR results showed that the
methylation levels of endogenous Oct4 and Sox2 gene promoter
regions were significantly higher than those in HuAECs (Fig. 2A). Additionally, the ChIP results
showed that the Oct4 and Sox2 gene promoter regions in the OSY-iPS
cells primarily interacted with H3K9 acetylation sites, whereas the
Oct4 and Sox2 gene promoter regions in the HuAECs primarily
interacted with H3K9 dimethylation sites (Fig. 2B). H3K9 acetylation can activate gene
transcription, and H3K9 methylation can inhibit the transcription
activities of genes. Additionally, chromosome karyotype analyses
showed that these iPS cells had a normal female chromosome core
(46XX), indicating that chromatin reprogramming did not cause
chromosome abnormalities in the cells (Fig. 2C). Furthermore, the natural
differentiation of the OSY-iPS cells was induced in vitro
using the embryoid body culture method. The expression of the
makers of the three germ layers was identified using qPCR. These
results indicated that the OSY-iPS cells expressed high levels of
markers associated with the three germ layers after six days of
natural, induced differentiation. These results indicated that the
OSY factors induced chromatin reprogramming in HuAECs.
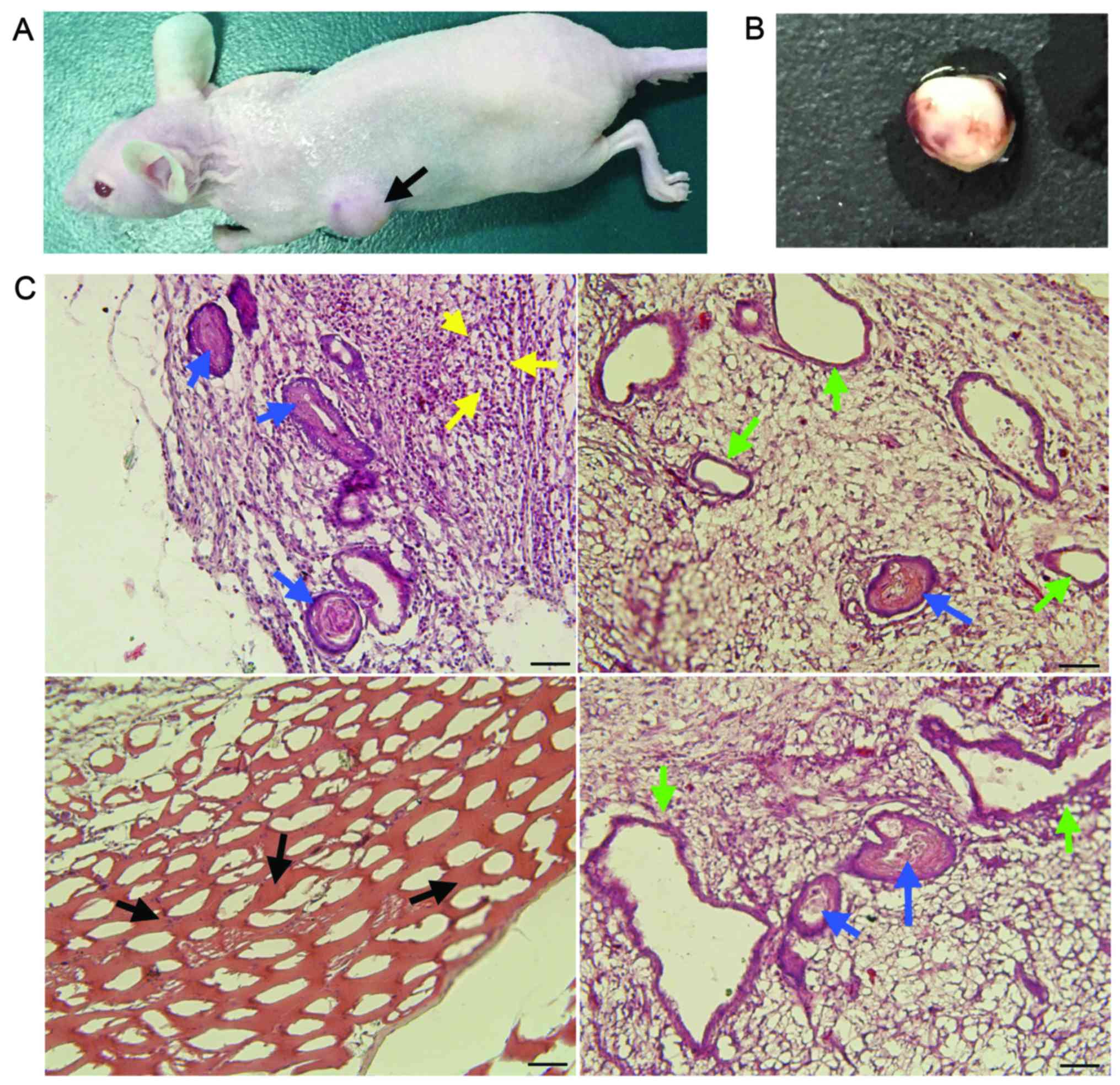
OSY-iPS cells have pluripotency
To confirm that the OSY-iPS cells had ESC-like
pluripotent differentiation capacity, the OSY-iPS cells were
injected into nude mice. After a certain time, the left back sides
of the nude mice developed tumor bodies (Fig. 3A). The surfaces of these tumor bodies
were smooth and had a soft texture. Obvious blood vessel
distribution could be observed on the surfaces (Fig. 3B). Pathological identification showed
that these tumor bodies contained many types of tissues and cells,
including glands and intestinal epithelia of the endoderm, striated
muscles of the mesoderm, and neural tubes and naïve neurons of the
ectoderm (Fig. 3C). Therefore, these
tumor bodies exemplified typical teratoma. The results of in
vivo experiments showed that the OSY-iPS cells had pluripotent
differentiation capacity and could form teratomas containing cells
from three germ layers in nude mice.
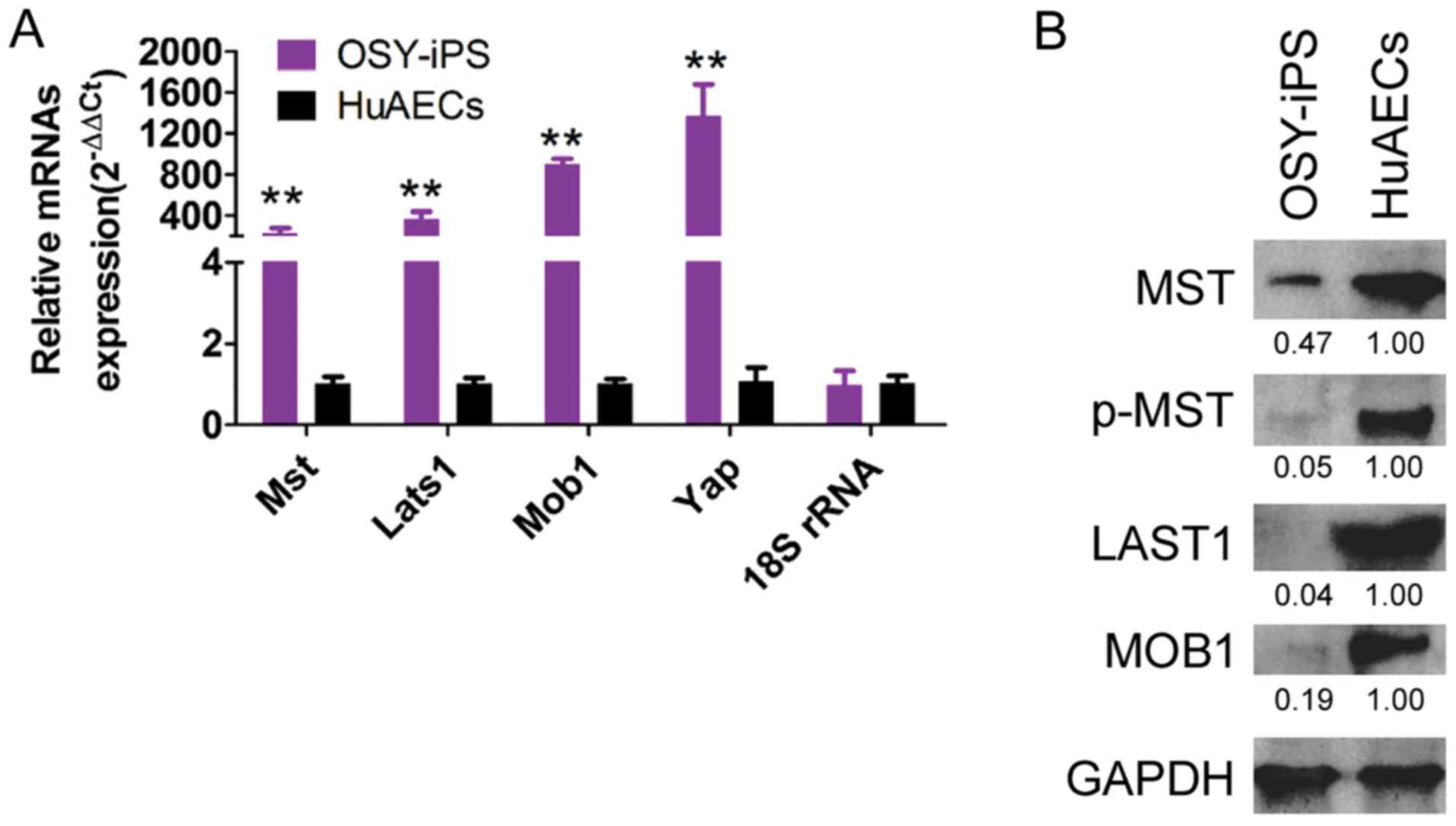
Overexpression of Yap, Oct4, and Sox2
activated the Hippo-Yap pathway in OSY-iPS cells
qPCR results indicated that the mRNA expression
levels of the Mst, Lats1, Mob1, and Yap genes in OSY-iPS cells were
significantly higher than those in the HuAECs (Fig. 4A). Additionally, the western blot
results indicated that the expression levels of the important
proteins in the Hippo-Yap pathway, Mst, Lats1, and Mob1, in the
OSY-iPS cells were significantly higher than those in the HuAECs.
Furthermore, the level of phosphorylation of Mst protein (p-Mst) in
the OSY-iPS cells was also significantly higher than that in the
HuAECs (Fig. 4B). These experimental
results indicated that the OSY factors could activate the Hippo-Yap
pathway.
Discussion
Since Yamanaka, Takahashi, and others prepared the
first strain of iPS cells in 2006, this methodology rapidly
developed (1–3). More methods have been reported for
establishing iPS cells from different sources (4,5). We used
HuAECs as seed cells and transduced two Yamanaka factors, Oct4 and
Sox2, and a key protein in the Hippo-Yap pathway, Yap, into these
cells to investigate whether iPS cells could be prepared.
Surprisingly, the production of typical human ESC-like clones was
observed under a microscope at approximately 2 weeks after
transduction. Further studies showed that OSY-iPS cells expressed
high levels of pluripotent markers of ESCs and could be
differentiated into cells of the three germ layers in vivo
and in vitro. iPS cells have been applied in transplantation
therapy studies in many clinical disease models (5,14,15). If
iPS cells could be readily applied in clinical therapy, this
technology would guarantee high efficiency and safety (4,12). In
this study, the preparation of iPS cells using Oct4, Sox2, and Yap
had the advantage of safety than other methods. In the past, the
four Yamanaka factors (Oct4, Sox2, Klf4, and c-Myc) have been
typically used to induce the reprogramming of adult cells into iPS
cells (1,2). However, c-Myc and Klf4 are both
proto-oncogenes. iPS cells carrying c-Myc gene have been reported
to develop malignant tumors in vivo, whereas Klf4 transmits
tumors to offspring (13,16). However, as major regulators, the
transcription factors Oct4 and Sox2 maintain the pluripotency and
self-renewal of ESCs (4,12). The use of the Yap factor could
control the balance of cells between self-proliferation and
differentiation. This combination is relatively reasonable and
efficient. Experimental results have confirmed that the combination
of the three factors, Oct4, Sox2, and Yap, could also induce the
reprogramming of general epithelial cells into iPS cells without
the involvement of c-Myc or Klf4. However, we considered that the
activation of the Hippo-Yap pathway also promoted iPS
reprogramming. Lian et al showed that during the preparation
of iPS cells using the four Yamanaka factors, the overexpression of
Yap increased the iPS cell production efficiency by two-fold
compared with the control group (10). Qin et al found that the use of
Lats2 knockout cells to prepare iPS cells shortened the
reprogramming time by approximately 5 days (11). We referenced their study results and
only used two Yamanaka factors (Oct4 and Sox2) combined with
overexpression of the key factor in the Hippo-Yap pathway, Yap, to
achieve HuAEC reprogramming. Additionally, the preparation of iPS
reprogramming using the four Yamanaka factors usually requires
approximately 1 month (1,2). However, our iPS reprogramming only
required 2 weeks. Experimental results indicated that the
activation of the endogenous Hippo-Yap pathway in cells by
overexpressing Yap could greatly shorten the iPS reprogramming
time. Overall, the combination of the three factors, Oct4, Sox2,
and Yap, could efficiently induce the reprogramming of HuAECs into
iPS cells.
In conclusion, we established a new method for iPS
induction. Through the introduction of Oct4, Sox2 and Yap to
activate the Hippo-Yap pathway, HuAECs were successfully induced to
reprogram iPS cells. And, using this method, it is possible to
shorten the time required for iPS cells reprogramming.
Acknowledgements
This study was supported by grant from the Shanghai
Natural Science Foundation (no. 16ZR1434000), and project funded by
the China Postdoctoral Science Foundation (nos. 2014M550250 and
2015T80455).
References
|
1
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okita K, Ichisaka T and Yamanaka S:
Generation of germline-competent induced pluripotent stem cells.
Nature. 448:313–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Zou G, Gao Y, Zhao X, Wang H, Huang
Q, Jiang L, Guo L and Cheng W: High efficiency of reprogramming
CD34+ cells derived from human amniotic fluid into induced
pluripotent stem cells with Oct4. Stem Cells Dev. 21:2322–2332.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu J, Ocampo A and Belmonte JC Izpisua:
Cellular metabolism and induced pluripotency. Cell. 166:1371–1385.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Pasolli HA and Fuchs E:
Yes-associated protein (YAP) transcriptional coactivator functions
in balancing growth and differentiation in skin. Proc Natl Acad Sci
USA. 108:pp. 2270–2275. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu FX, Zhao B, Panupinthu N, Jewell JL,
Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al:
Regulation of the Hippo-YAP pathway by G-protein-coupled receptor
signaling. Cell. 150:780–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoa L, Kulaberoglu Y, Gundogdu R, Cook D,
Mavis M, Gomez M, Gomez V and Hergovich A: The characterisation of
LATS2 kinase regulation in Hippo-YAP signalling. Cell Signal.
28:488–497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi YF, Yu J, Han W, Fan X, Qian H, Wei H,
Tsai YH, Zhao J, Zhang W, Liu Q, et al: A splicing isoform of TEAD4
attenuates the Hippo-YAP signalling to inhibit tumour
proliferation. Nat Commun. 7:ncomms118402016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lian I, Kim J, Okazawa H, Zhao J, Zhao B,
Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, et al:
The role of YAP transcription coactivator in regulating stem cell
self-renewal and differentiation. Gene Dev. 24:1106–1118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin H, Blaschke K, Wei G, Ohi Y, Blouin L,
Qi Z, Yu J, Yeh RF, Hebrok M and Ramalho-Santos M: Transcriptional
analysis of pluripotency reveals the Hippo pathway as a barrier to
reprogramming. Hum Mol Genet. 21:2054–2067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu T, Cheng W, Huang Y, Huang Q, Jiang L
and Guo L: Human amniotic epithelial cell feeder layers maintain
human iPS cell pluripotency via inhibited endogenous microRNA-145
and increased Sox2 expression. Exp Cell Res. 318:424–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamanaka S, Yamaguchi T, Kobayashi T,
Kato-Itoh M, Yamazaki S, Sato H, Umino A, Wakiyama Y, Arai M, Sanbo
M, et al: Generation of germline-competent rat induced pluripotent
stem cells. Plos One. 6:e220082011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lengner CJ: iPS cell technology in
regenerative medicine. Ann NY Acad Sci. 1192:38–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rowntree RK and McNeish JD: Induced
pluripotent stem cells: Opportunities as research and development
tools in 21st century drug discovery. Regen Med. 5:557–568. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Zhao Y, Xiao Z, Chen B, Wei Z,
Wang B, Zhang J, Han J, Gao Y, Li L, et al: Alternative translation
of OCT4 by an internal ribosome entry site and its novel function
in stress response. Stem cells. 27:1265–1275. 2009. View Article : Google Scholar : PubMed/NCBI
|