Introduction
Peripheral nerve injuries occur with a high
frequency, accounting for up to 3% of all trauma injuries (1,2). In the
majority of cases, surgical intervention is necessary due to the
self-regenerative capability of nerves; however, this is
time-consuming and incomplete, as described in (3), which may cause functional impairment.
Although autograft transplantation is the first choice of
treatment, the shortage of donor resources and the repercussions of
this invasive treatment to the donor present as major limitations
(4,5). However, the discovery of an alternative
therapy to replace autografts and treat peripheral nerve injury has
presented as a challenge.
Among the typical approaches for treating nerve
crush injury, Schwann cell (SC)-based therapy is highly recommended
(6). SCs, the principle glia in the
peripheral nervous system, have an important role in the
development, function and regeneration of peripheral nerves
(7). Following peripheral nerve
injury, SCs aid in phagocytizing the damaged end of the axon and
provide physical support to regenerate axons by forming ‘Bands of
Büngner’. Furthermore, SCs create a suitable axonal growth
environment by producing neurotrophic factors, such as
brain-derived neurotrophic factor (BDNF), glial cell-derived
neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF) and
neurotrophic factors-3, −4/5 and −6 (NT-3, NT-4/5, and NT-6,
respectively) (8–12). However, the slow growth rate of SCs
is reported to be one of the major limitations of SC application in
regenerative medicine (13). In
addition, elevating the proliferation ability of SCs is important
for constructing tissue-engineered nerves (6). As a result, researchers have been
exploring various promoting agents for SCs proliferation, such as
interleukin-1β (14) and tanshinone
IIA (15).
Utilizing plant-derived traditional Chinese
medicines to treat various types of diseases has a long history in
East Asian countries, such as China, Korea and Japan (16). Furthermore, some western medicines
are derived from major constituent of traditional Chinese medicine
(17) Scutellaria baicalensis
Georgi (Huangqin in Chinese), a traditional Chinese medicine, has
been used to treat inflammation, fever, ulcers and cancer for
hundreds of years (18–20) and a recent study has reported that
flavonoids from the stems and leaves of S. baicalensis
Georgi have neuroprotective effects (21). Baicalin, one of the major flavonoid
isolated from the root of S. baicalensis, has a variety of
biological functions, including anti-inflammatory, anti-oxidant and
anti-apoptotic activities (22–24).
Previous studies have revealed that baicalin had neuroprotective
effects on permanent brain ischemia in rats (25) and was able to promote the neuronal
differentiation of neural stem cells (26,27).
However, little is known on whether baicalin is capable of exerting
positive or negative effects on SC proliferation and
differentiation. The present study aimed to investigate the effects
of different concentrations of baicalin on the viability of RSC96
SCs. The results revealed that baicalin was able to promote the
viability of RSC96 SCs at a particular concentration.
Materials and methods
Cell culture
RSC96 SCs were purchased from China Center for Type
Culture Collection (Wuhan, China) and cultured in Dulbecco's
modified Eagle medium (DMEM)-F12 (1:1; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China) and 1% of penicillin/streptomycin in an incubator
at 37°C with 95% air and 5% CO2. Baicalin (Chengdu
Best-Reagent Chemical, Co., Ltd., Chengdu, China) was dissolved in
0.2% dimethyl sulfoxide (DMSO) and prepared as a stock solution
with a final concentration of 100 mM and stored at −20°C. The stock
solution was diluted with culture medium immediately prior to
treatment.
Cytotoxicity assay
To determine the level of cytotoxicity of baicalin
on RSC96 SCs, cell cytotoxicity was detected with a MTT assay
(Gibco; Thermo Fisher Scientific, Inc.) method. RSC96 SCs were
seeded in 96-well plates at a density of 1,000 cells/well and the
cell viability was determined by using the MTT assay on day 3.
Following treatment with various concentrations of baicalin (0 to
1,000 µM where 0 µM was used as a control) for 3 days, 20 µl MTT (5
mg/ml) was added to each well and plates were incubated in the dark
at 37°C for 4 h. Once MTT was removed, cells were treated with 200
µl DMSO (Amresco, LLC, Solon, OH, USA) for crystal solubilization.
The spectrometric absorbance at 570 nm was read using Multiskan™ GO
microplate spectrophotometer (Thermo Fisher Scientific, Inc.,
USA).
Measure of cell viability via the MTT
assay
RSC96 SCs were seeded in 96-well plates at a density
of 1,000 cells/well and the cell viability was determined by using
the MTT assay on days 2, 4, and 6. Once cells were treated with
various concentration of baicalin (5, 10 and 20 µM), MTT solution
(5 mg/ml) was added and the cells were incubated for 4 h at 37°C.
Following removal of the incubation medium, the dark blue formazan
crystals formed in the intact cells and all samples were
solubilized with 200 µl DMSO. Subsequently, the absorbance was
measured at 570 nm on a microplate spectrophotometer (Thermo Fisher
Scientific, Inc.).
Measure of cell viability via
fluorescein diacetate staining
Live RSC96 SCs were examined using fluorescein
diacetate (FDA) on days 2, 4 and 6. A stock solution of FDA
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was prepared
by dissolving 5 mg FDA in 1 ml acetone. Staining solution was
prepared by mixing 5 ml PBS with 8 µl FDA stock solution. Once the
culture medium was removed, 0.5 ml staining solution was added and
the cells were stained in the dark for 5 min. The evaluation of
viability was conducted by fluorescent microscopy (magnification,
×100, Nikon Corporation. Tokyo, Japan). ImageJ software (version
1.48v; National Institutes of Health, Bethesda, MA, USA) was used
for quantitative analysis of the fluorescein diacetate stained
cells.
Hematoxylin and eosin staining
RSC96 SCs were grown at 1×105 cells/ml in
DMEM/F12 (1:1) with 0, 5, 10 or 20 µM of baicalin for 2, 4, and 6
days on a 24-well plate with a coverslip set at the bottom.
Following fixing in 95% ethanol for 20 min, the coverslip contents
were washed in PBS twice, immersed in hematoxylin for 2 min, and
washed in water for 1 to 3 sec to remove hematoxylin. The coverslip
was washed in 1% hydrochloric acid and ethanol for 2 to 3 sec,
water for 10 sec, ammonia for 15 sec and running water for 10 sec.
Eosin staining was performed for 1 min and the stain was removed by
washing with water for 2 sec, 80% ethanol for 2 sec, 95% ethanol
for 5 min and 100% ethanol for 10 min. Subsequently, the coverslip
was air-dried and mounted with neutral gum for light microscopy
analysis. Images of five random fields of the culture were captured
(magnification, ×100).
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). Total RNA was extracted from RSC96 SCs using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions. cDNA was synthesized
from reverse transcribed total RNA using a PrimeScript RT reagent
kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian,
China). Briefly, residual DNA was removed as follows: 10 µl total
volume of 2 µl 5x gDNA eraser buffer, 1 µg total RNA, 1 µl gDNA
eraser and RNase-free dH2O at 42°C for 2 min. For
reverse transcription, 20 µl total volume was used with 10 µl of
the reaction solution as described, 4 µl 5x PrimeScript buffer 2, 1
µl PrimeScript RT enzyme mix I, 1 µl RT primer mix and 4 µl
RNase-free dH2O. This reaction was performed at 37°C for
15 min, followed by incubation in an 85°C water bath for 5 sec. The
synthesized cDNA was cooled at 4°C for 5 min and then stored at
−20°C until real-time quantitative PCR reactions. PCR was performed
on Mastercycler® ep realplex 4 system (Eppendorf,
Hamburg, Germany) using FastStart Universal SYBR Green Master
(Roche Diagnostics, Indianapolis, IN, USA) according to the
manufacturer's protocol. Briefly, a total reaction volume of 20 µl
was used containing 10 µl SYBR Master Mix, 0.4 µl each primer (0.4
µmol/l), 2 µl cDNA, and 7.6 µl RNase-free dH2O. The
cycling conditions were as follows, for 35 cycles: Denaturing, at
94°C for 30 sec, annealing at 54°C for 30 sec and extension at 72°C
for 30 sec. A final melting curve analysis was performed utilizing
conditions of 95°C for 15 sec, 60°C for 60 sec, followed by 95°C
for 15 sec. The PCR products for glial cell-derived neurotrophic
factor (GDNF), BDNF and ciliary neurotrophic factor (CNTF) were
129, 182 and 191 bp, respectively. The primer sequences are
indicated in Table I. All reactions
were performed in triplicate. The relative expression levels of
mRNA were calculated using the comparative 2−ΔΔCq method
(28) and normalized against
GAPDH.
 | Table I.Genes and oligonucleotide primers
used in PCR analysis. |
Table I.
Genes and oligonucleotide primers
used in PCR analysis.
| Gene | Primer sequence (5′
to 3′) | Length (bp) | Amplicon size
(bp) |
|---|
| GDNF | F:
AGACCGGATCCGAGGTGC | 18 | 129 |
|
| R:
TCGAGAAGCCTCTTACCGGC | 20 |
|
| BDNF | F:
TACCTGGATGCCGCAAACAT | 20 | 182 |
|
| R:
TGGCCTTTTGATACCGGGAC | 20 |
|
| CNTF | F:
ATGGCTTTCGCAGAGCAAAC | 20 | 191 |
|
| R:
CAACGATCAGTGCTTGCCAC | 20 |
|
| GAPDH | F:
GTCATCATCTCAGCCCCCTC | 20 | 99 |
|
| R:
GGATGCGTTGCTGACAATCT | 20 |
|
Immunohistochemistry
RSC96 SCs were fixed in 95% ethanol for 20 min and
washed in PBS twice. Cells were incubated in
H2O2 (3%) for 10 min to block peroxidase and
rinsed using distilled water. Sections were subsequently washed
with PBS three times for 2 min. Rabbit anti-rat S100B antibody
(1:200; catalogue no. BA0120; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) was added and incubated at room temperature for
2 h and subsequently rinsed with PBS, containing 0.05% Tween-20,
three times for 2 min. Slides were incubated with
peroxidase-conjugated goat anti-rabbit IgG (1:100; catalogue no.
SP-9001; Zhongshan Jin Qiao Biotechnology Co., Beijing, China) for
30 min at 37°C. Following incubation, sections were washed with
PBS, containing 0.05% Tween-20, three times for 2 min.
Diaminobenzidine was added to visualize primary antibody staining
and samples were washed in distilled water. Subsequently, slides
were counterstained with hematoxylin for 20 sec, washed once in
water, mounted, dried and dehydrated by immersing in 70% ethanol
for 10 min, 95% ethanol for 10 min and 100% ethanol for 10 min.
Following dehydration, the mounted slides were observed by using a
Nikon light microscope at a magnification, ×100).
Statistical analysis
Data were statistically analyzed using the SPSS
software package, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Statistical analysis among multiple samples was performed by
one-way analysis of variance followed by post hoc least significant
difference (LSD) tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
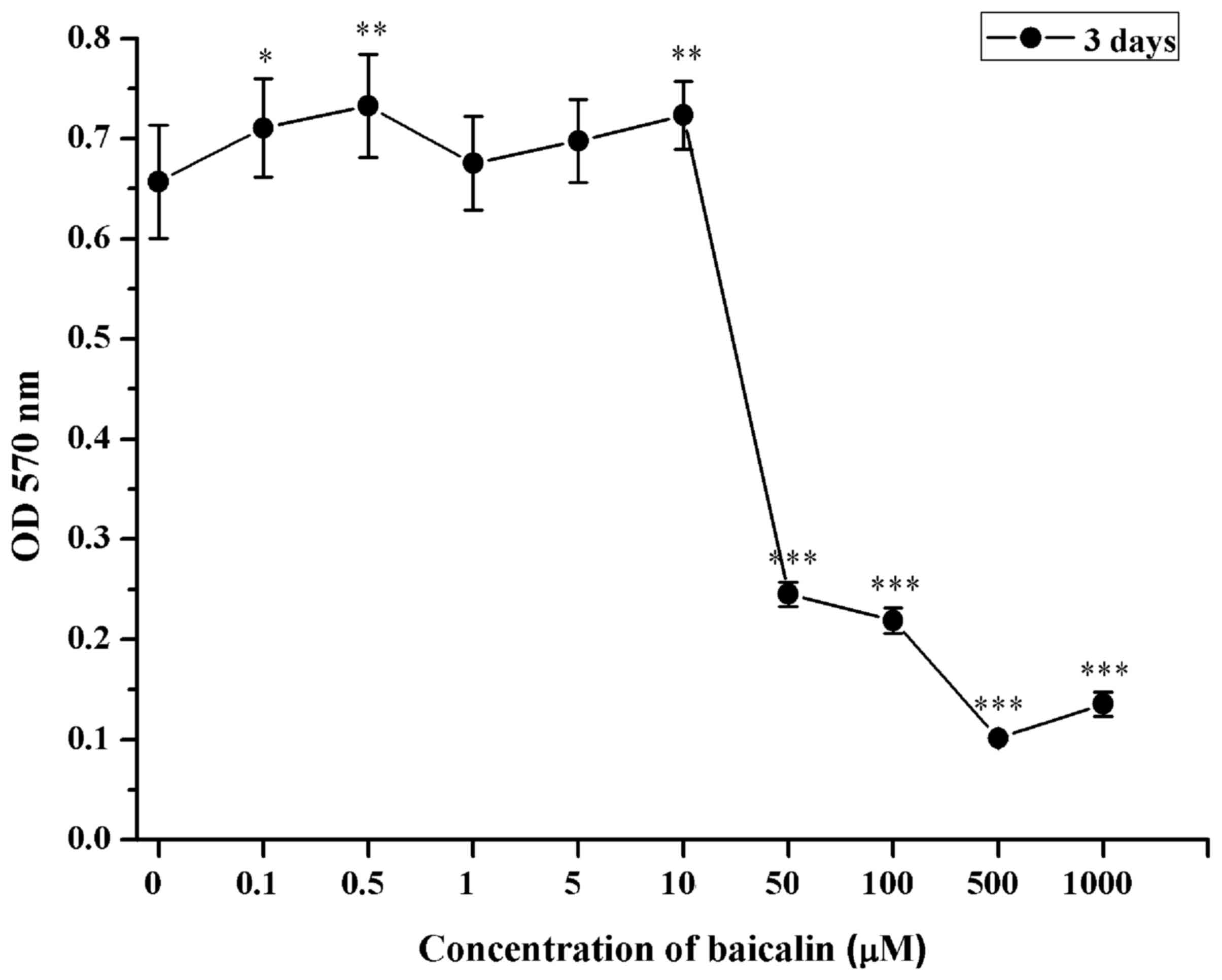
Cytotoxicity of baicalin
The cytotoxicity of baicalin on RSC96 SCs was
examined by MTT assay. RSC96 SCs were treated with baicalin at
increasing concentrations (0.1 to 1,000 µM). Minimal cytotoxic
effects were observed when RSC96 SCs were treated with baicalin for
3 days at doses 0.1, 0.5, 1, 5 or 10 µM (Fig. 1). However, significant cytotoxic
effects were observed in cells treated with >50 µM, indicated by
the significantly reduce viability exhibited by the SCs (P<0.001
vs. 0 µM; Fig. 1). Therefore,
concentrations of 5, 10 or 20 µM of baicalin were selected for
subsequent investigations.
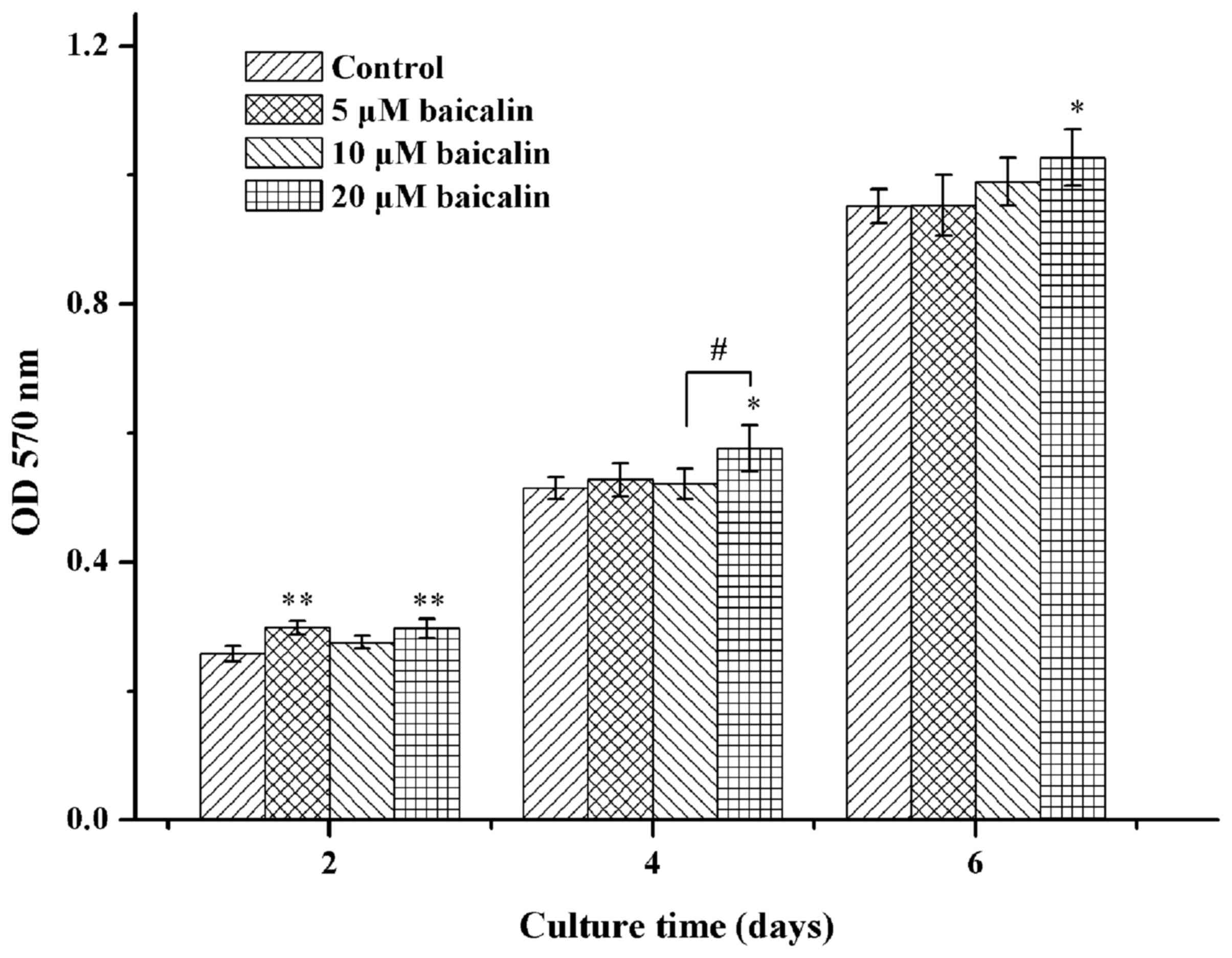
Cell viability
The cell viability of RSC96 SCs was explored using
the MTT assay in the present study. The viability of SCs was
indicated to be time- and dose-dependent (Fig. 2). Furthermore, SCs were more viable
when incubated with various concentrations of baicalin (0, 5, 10 or
20 µM) when compared with the control at different time points.
Cell viability following treatment of baicalin (20 µM)
significantly increased up to ~15% when compared with the control
on day 2 (P<0.05; Fig. 2). In all
groups of SCs treated with baicalin, 20 µM of baicalin was the
optimal concentration that promoted the highest cell viability of
RSC96 SCs.
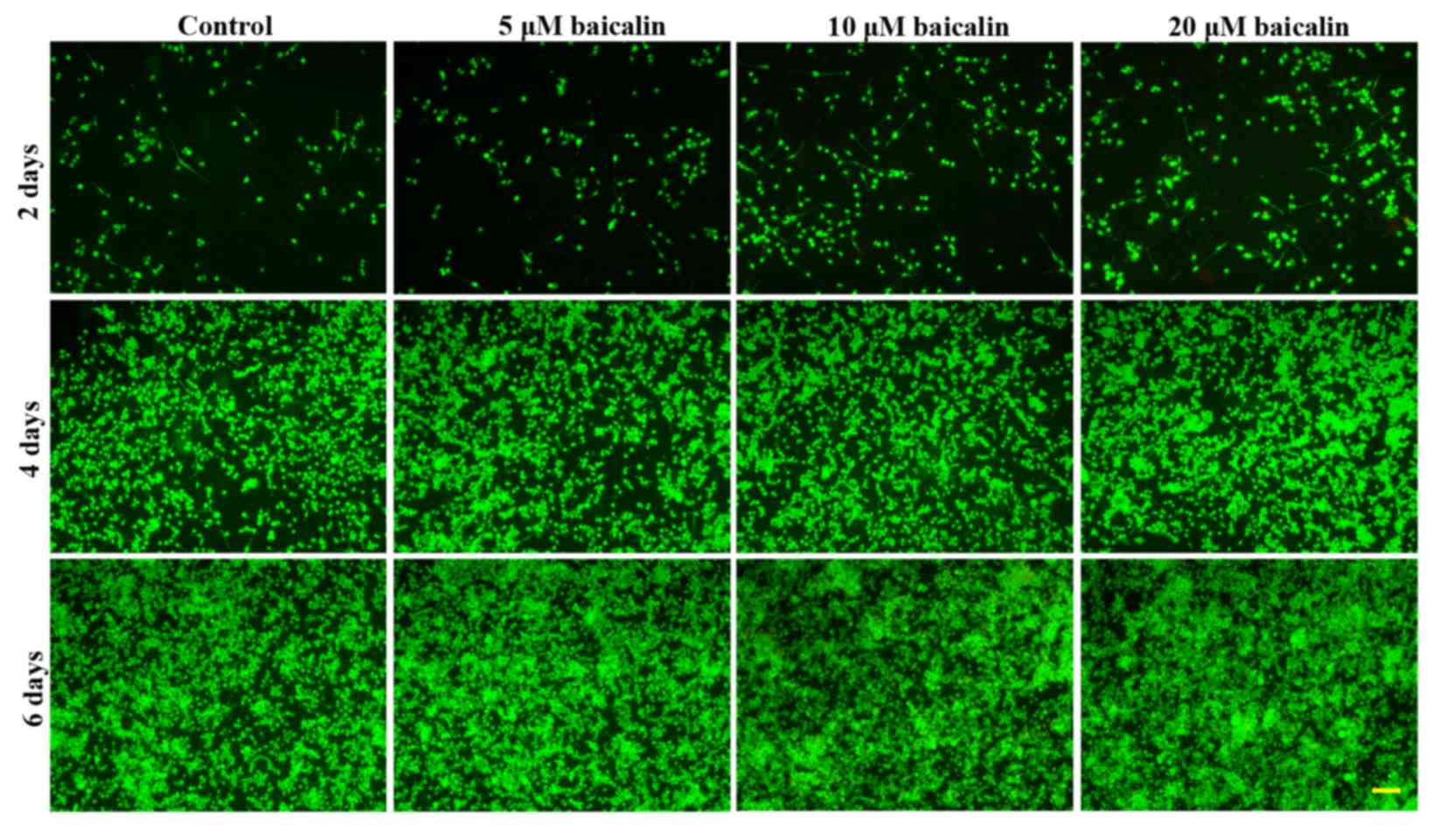
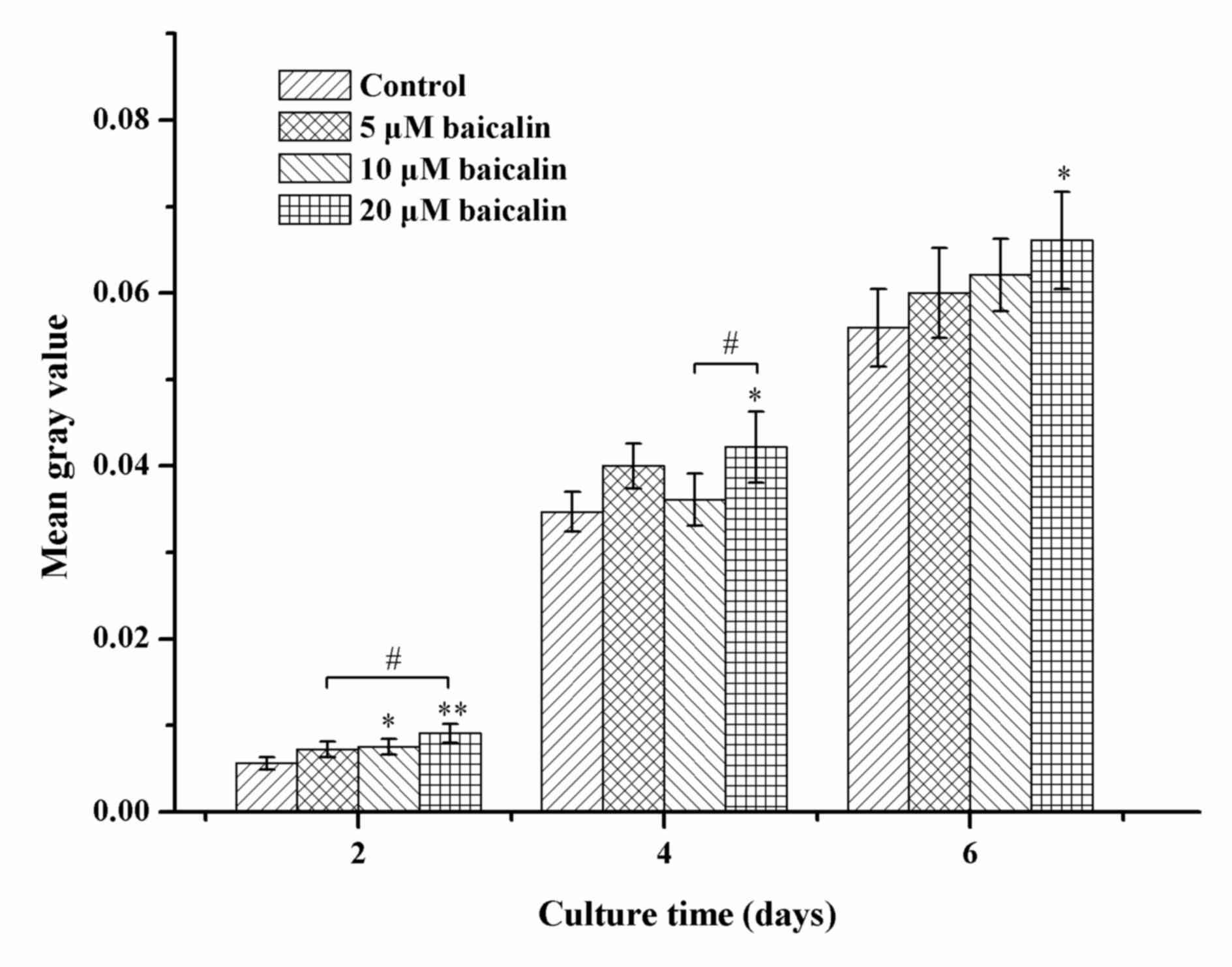
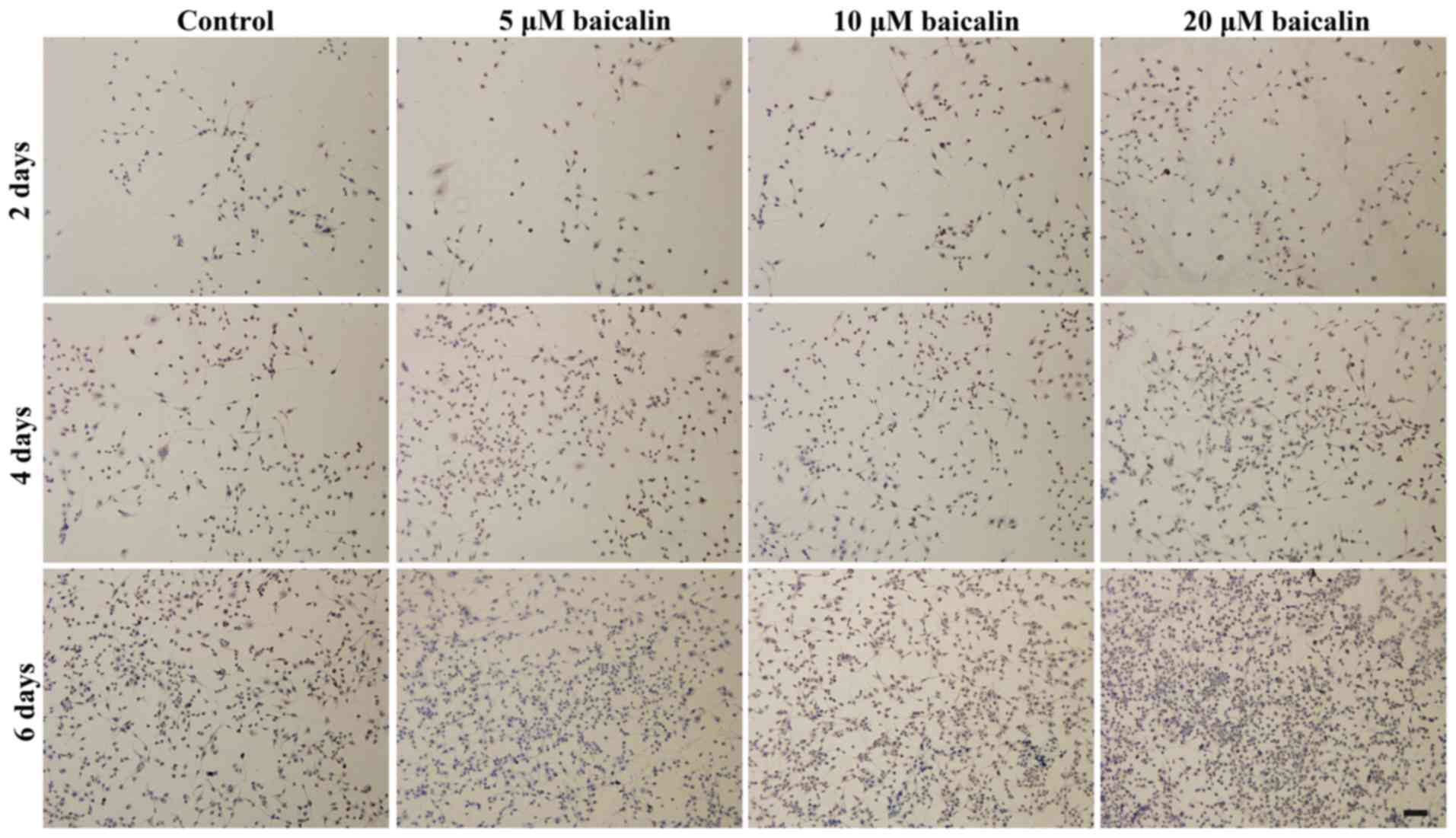
To further investigate the effects of baicalin on
RSC96 SC viability, the live viability of RSC96 SCs was analyzed by
FDA staining. As shown in Fig. 3,
the number of viable cells, which were green in color, increased
with time in all groups. In agreement with the MTT analysis, a
greater number of viable cells were presented in baicalin-treated
groups when compared with the control at different corresponding
culture times. These data support the beneficial effect of baicalin
on SC survival. In all baicalin groups, the number of viable cells
was highest when incubated in medium with 20 µM baicalin (Fig. 4).
Cell morphology
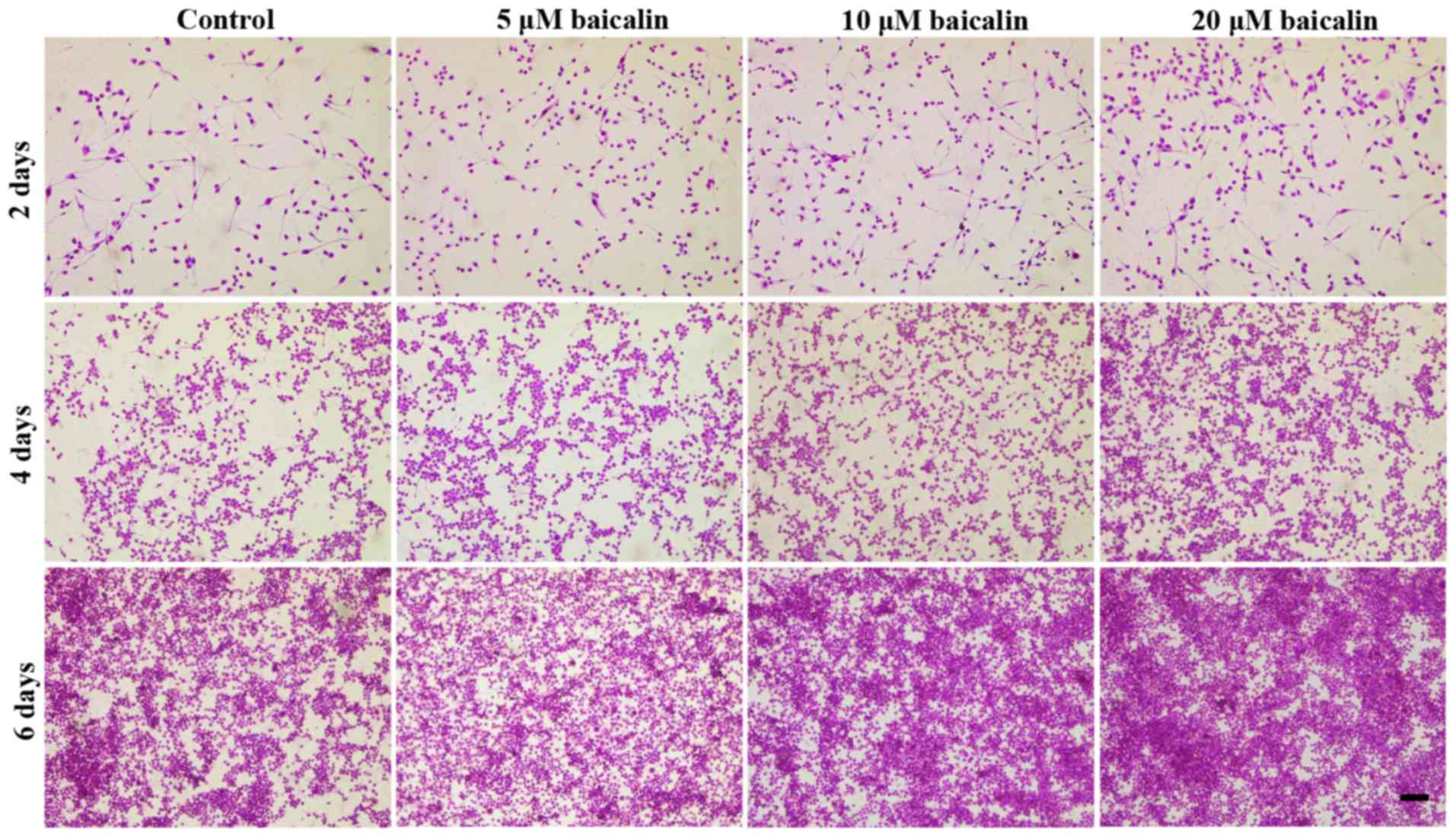
Hematoxylin and eosin staining was used to observe
RSC96 SC morphology. Dendrites, the typical component of nerve
cells, were clearly observed under the microscope following 2 days
of culture; however, over time, the number of cells with dendrites
decreased whereas the number of rounded cells increased. As showed
in Fig. 5, the SCs grew slower in
control when compared with the groups treated with baicalin at 2, 4
and 6 days. Furthermore, among the three concentrations, the
present data suggests that 20 µM of baicalin stimulated cell
proliferation the most prominently.
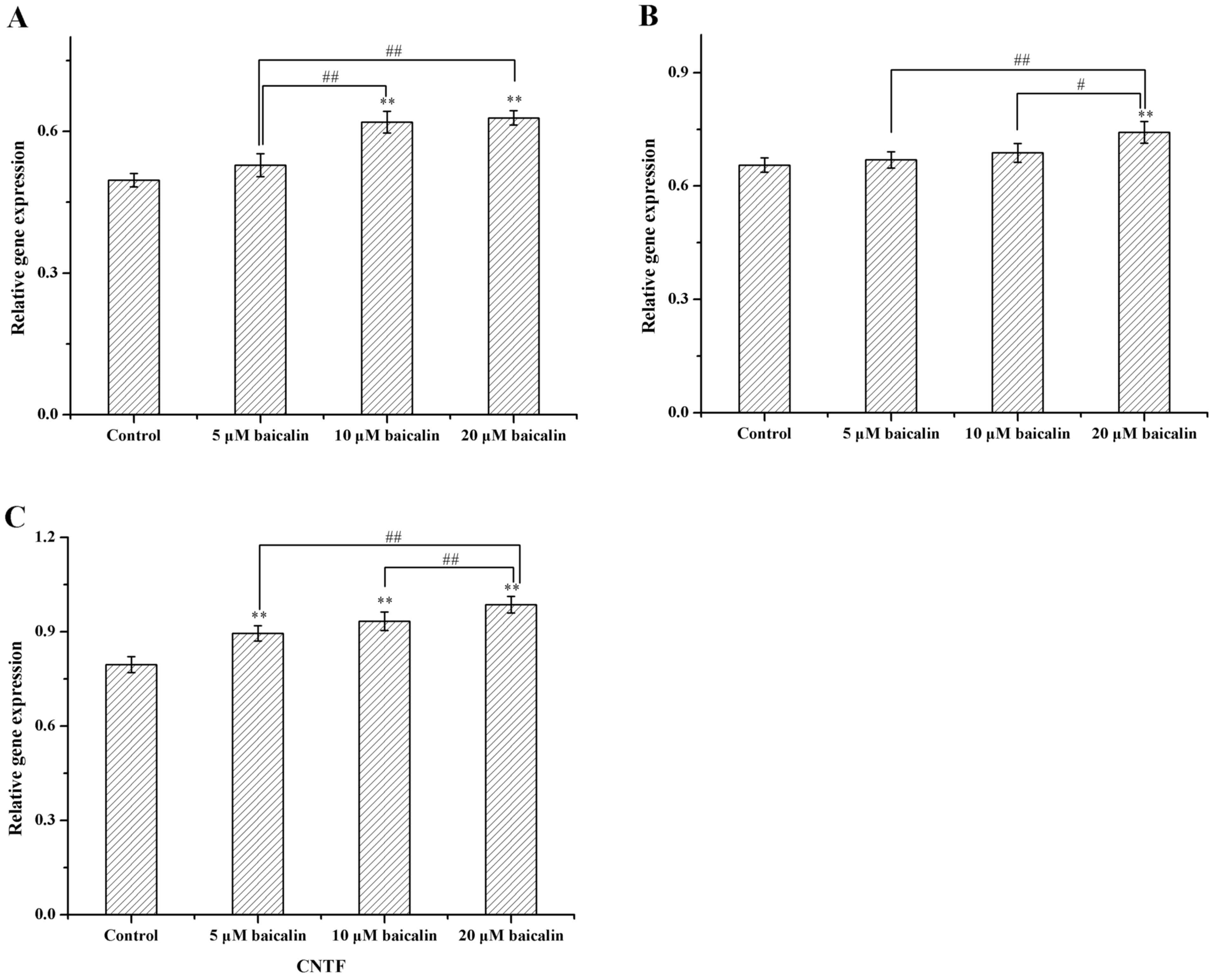
Gene expression
The effect of 0, 5, 10 or 20 µM of baicalin on RSC96
SCs was further investigated by detecting the gene expression of
the important neurotrophic factors, GDNF, BDNF and CNTF. The
expression levels of these genes were examined at 2, 4 and 6 days.
Gene expression levels of GDNF, BDNF and CNTF were markedly
increased in all baicalin-treated RSC96 SCs and significantly
increased in RSC96 SCs treated with 20 µM baicalin when compared
with the control (P<0.01), which indicated that baicalin may
stimulate the transcription of GDNF, BDNF and CNTF genes (Fig. 6). In addition, the present data
suggested that SCs treated with 20 µM baicalin exhibited the
highest gene expression levels of GDNF, BDNF and CNTF genes.
Expression of S100β
Expression of S100β was detected by
immunohistochemical staining. RSC96 SCs were treated with 0, 5, 10
or 20 µM of baicalin at different time points. As indicated in
Fig. 7, the expression of S100β was
upregulated when the concentration of baicalin increased and
treatment with 20 µM of baicalin resulted in the highest expression
of S100β in SCs.
Discussion
The present study focused on the effect of baicalin
on RSC96 SCs in vitro. The present findings indicated that
baicalin significantly enhanced the viability of SCs. In addition,
the expression of GDNF, BDNF and CNTF was significantly upregulated
in the presence of 20 µM baicalin. These findings revealed that
baicalin is capable of enhancing SCs survival and function in
vitro. This may corroborate that baicalin is a key component
that is able to contribute to nerve repair by S. baicalensis
(29). Moreover, the present study
highlights the possibility of promoting nerve regeneration in
cellular nerve grafts through baicalin-induced neurotrophin
secretion in SCs.
Acceleration of the proliferation of nerve cells is
important due to the slow axonal growth that is the cause of poor
functional recovery, which may lead to prolonged denervation of end
organs, raising the specter of permanent paralysis (30). In the present study, baicalin
exhibited an effect in a dose-dependent manner on the viability of
RSC96 SCs, whereby at the concentration of 20 µM, SCs exhibited the
highest viability, as evidenced by cell viability assay and
histological evaluation. S100, which is a SC marker (31), was elevated when SCs received
baicalin treatment, as demonstrated by the increased protein
expression levels of S100 in baicalin-treated cells when compared
with the control, via immunohistochemical examination. Natural
substrates, such as traditional medicinal herbs, are well-known for
their relatively minor adverse effects (32). Extracts from S. baicalensis
are considered to exhibit low cytotoxicity (33) and have neuroprotective properties
(34). As one of the active
components, baicalin has been reported to promote neuroprotective
effects in rats (25,35), which is in agreement with the
findings of the present study.
Nerve growth factor and several neurotrophic factors
have been reported to elicit stimulatory effects on specific
neuronal populations (36,37). They affect several vital aspects of
regeneration, including axon growth, SC function and myelination
(38). GDNF, BDNF and CNTF are
several important neurotrophic factors that are important in the
process of nerve cell regeneration (39). A previous study indicated that CNTF
is able to enhance myelin formation and myelinate regenerating
axons in the course of regrowth (40,41).
Furthermore, it has been suggested that BDNF is a necessary
component for axon regeneration (42) and a small peptide mimetic of BDNF was
demonstrated to promote peripheral myelination (43). Moreover, GDNF has been indicated to
be beneficial to peripheral nerve regeneration and functional
recovery in multiple experimental nerve injury models (44,45). In
addition, a recent study on autograft-based repair revealed that
BDNF, GDNF and nerve growth factor showed considerable promise as
these factors enhanced modality-specific axon regeneration in
autografts (46). In the present
study, when RSC96 SCs were incubated with 20 µM baicalin, the gene
expression levels of BDNF, CNTF and GDNF were significantly
elevated, as determined by RT-qPCR. These findings suggest that
baicalin likely promotes SCs viability and proliferation by
stimulating neurotrophic factors, such as CNTF and GDNF.
S100 is associated with cell proliferation and
differentiation (47). In the S100
protein family, S100B has been reported to be a potentially
important factor contributing to neuronal development (48) and differentiation. A previous study
has indicated that S100A4 is capable of stimulating neuronal
differentiation in cultures of rat hippocampal neurons (49). In the present study, S100 protein
expression levels were elevated by baicalin-treatment, as
demonstrated by immunohistochemical examination. These findings
suggest that baicalin may stimulate SC viability and
differentiation via upregulation of S100.
The present results showed that the different
concentrations of baicalin (5 to 20 µM) affected the viability of
RSC96 SCs, with 20 µM having a significant effect. Among the chosen
concentrations, treatment with 20 µM of baicalin indicated the
optimal cell viability and stimulated the most secretion of S100 in
RSC96 SCs.
In conclusion, the present study corroborated that
baicalin has a regulative effect on the viability of RSC96 SCs.
Furthermore, the present findings suggest baicalin likely affects
SC metabolism by modulating the expression of several neurotrophic
factors, such as BDNF, GDNF and CDNF. To conclude, the present
study suggests that baicalin may be a promising therapeutic agent
for peripheral nerve regeneration.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (grant no. 81160221).
This study was also supported by Research Center for Regenerative
Medicine and Collaborative Innovation Center of Guangxi Biological
Medicine.
References
|
1
|
Robinson LR: Traumatic injury to
peripheral nerves. Muscle Nerve. 23:863–873. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans GR: Peripheral nerve injury: A
review and approach to tissue engineered constructs. Anat Rec.
263:396–404. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Griffin MF, Malahias M, Hindocha S and
Khan WS: Peripheral nerve injury: Principles for repair and
regeneration. Open Orthop J. 8:199–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmidt CE and Leach JB: Neural tissue
engineering: Strategies for repair and regeneration. Annu Rev
Biomed Eng. 5:293–347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moore AM, Kasukurthi R, Magill CK, Farhadi
HF, Borschel GH and Mackinnon SE: Limitations of conduits in
peripheral nerve repairs. Hand (N Y). 4:180–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pfister BJ, Gordon T, Loverde JR, Kochar
AS, Mackinnon SE and Cullen DK: Biomedical engineering strategies
for peripheral nerve repair: Surgical applications, state of the
art, and future challenges. Crit Rev Biomed Eng. 39:81–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ide C, Tohyama K, Yokota R, Nitatori T and
Onodera S: Schwann cell basal lamina and nerve regeneration. Brain
Res. 288:61–75. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mudo G, Persson H, Timmusk T, Funakoshi H,
Bindoni M and Belluardo N: Increased expression of trkB and trkC
messenger RNAs in the rat forebrain after focal mechanical injury.
Neuroscience. 57:901–912. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toews AD, Barrett C and Morell P: Monocyte
chemoattractant protein 1 is responsible for macrophage recruitment
following injury to sciatic nerve. J Neurosci Res. 53:260–267.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tofaris GK, Patterson PH, Jessen KR and
Mirsky R: Denervated Schwann cells attract macrophages by secretion
of leukemia inhibitory factor (LIF) and monocyte chemoattractant
protein-1 in a process regulated by interleukin-6 and LIF. J
Neurosci. 22:6696–6703. 2002.PubMed/NCBI
|
|
11
|
Keilhoff G, Fansa H, Schneider W and Wolf
G: In vivo predegeneration of peripheral nerves: An effective
technique to obtain activated Schwann cells for nerve conduits. J
Neurosci Methods. 89:17–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terenghi G: Peripheral nerve regeneration
and neurotrophic factors. J Anat. 194:1–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faroni A, Rothwell SW, Grolla AA, Terenghi
G, Magnaghi V and Verkhratsky A: Differentiation of adipose-derived
stem cells into Schwann cell phenotype induces expression of P2X
receptors that control cell death. Cell Death Dis. 4:e7432013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Temporin K, Tanaka H, Kuroda Y, Okada K,
Yachi K, Moritomo H, Murase T and Yoshikawa H: Interleukin-1 beta
promotes sensory nerve regeneration after sciatic nerve injury.
Neurosci Lett. 440:130–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen JL, Chen YS, Lin JY, Tien YC, Peng
WH, Kuo CH, Tzang BS, Wang HL, Tsai FJ, Chou MC, et al: Neuron
regeneration and proliferation effects of danshen and tanshinone
IIA. Evid Based Complement Alternat Med. 2011:3789072011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park HL, Lee HS, Shin BC, Liu JP, Shang Q,
Yamashita H and Lim B: Traditional medicine in china, Korea, and
Japan: A brief introduction and comparison. Evid Based Complement
Alternat Med. 2012:4291032012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Normile D: Asian medicine. The new face of
traditional Chinese medicine. Science. 299:188–190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haranaka R, Hasegawa R, Nakagawa S,
Sakurai A, Satomi N and Haranaka K: Antitumor activity of
combination therapy with traditional Chinese medicine and OK432 or
MMC. J Biol Response Mod. 7:77–90. 1988.PubMed/NCBI
|
|
19
|
Du Z, Wang K, Tao Y, Chen L and Qiu F:
Purification of baicalin and wogonoside from Scutellaria
baicalensis extracts by macroporous resin adsorption
chromatography. J Chromatogr B Analyt Technol Biomed Life Sci.
908:143–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim AR, Kim SN, Jung IK, Kim HH, Park YH
and Park WS: The inhibitory effect of Scutellaria baicalensis
extract and its active compound, baicalin, on the translocation of
the androgen receptor with implications for preventing androgenetic
alopecia. Planta Med. 80:153–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miao G, Zhao H, Guo K, Cheng J, Zhang S,
Zhang X, Cai Z, Miao H and Shang Y: Mechanisms underlying
attenuation of apoptosis of cortical neurons in the hypoxic brain
by flavonoids from the stems and leaves of Scutellaria baicalensis
Georgi. Neural Regen Res. 9:1592–1598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li BQ, Fu T, Gong WH, Dunlop N, Kung H,
Yan Y, Kang J and Wang JM: The flavonoid baicalin exhibits
anti-inflammatory activity by binding to chemokines.
Immunopharmacology. 49:295–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang JM, Wang CJ, Chou FP, Tseng TH,
Hsieh YS, Hsu JD and Chu CY: Protective effect of baicalin on
tert-butyl hydroperoxide-induced rat hepatotoxicity. Arch Toxicol.
79:102–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung SH, Kang KD, Ji D, Fawcett RJ, Safa
R, Kamalden TA and Osborne NN: The flavonoid baicalin counteracts
ischemic and oxidative insults to retinal cells and lipid
peroxidation to brain membranes. Neurochem Int. 53:325–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tu XK, Yang WZ, Shi SS, Wang CH and Chen
CM: Neuroprotective effect of baicalin in a rat model of permanent
focal cerebral ischemia. Neurochem Res. 34:1626–1634. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Zhuang P, Shen B, Zhang Y and Shen
J: Baicalin promotes neuronal differentiation of neural
stem/progenitor cells through modulating p-stat3 and bHLH family
protein expression. Brain Res. 1429:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li M, Tsang KS, Choi ST, Li K, Shaw PC and
Lau KF: Neuronal differentiation of C17.2 neural stem cells induced
by a natural flavonoid, baicalin. Chembiochem. 12:449–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heo HJ, Kim DO, Choi SJ, Shin DH and Lee
CY: Potent Inhibitory effect of flavonoids in Scutellaria
baicalensis on amyloid beta protein-induced neurotoxicity. J Agric
Food Chem. 52:4128–4132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang YM, Kuo WH, Lai TY, Shih YT, Tsai
FJ, Tsai CH, Shu WT, Chen YY, Chen YS, Kuo WW and Huang CY: RSC96
Schwann cell proliferation and survival induced by dilong through
PI3K/Akt signaling mediated by IGF-I. Evid Based Complement
Alternat Med. 2011:2161482011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Z, Jin YQ, Chen L, Wang Y, Yang X,
Cheng J, Wu W, Qi Z and Shen Z: Specific marker expression and cell
state of Schwann cells during culture in vitro. PLoS One.
10:e01232782015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barbisan LF, Miyamoto M, Scolastici C,
Salvadori DM, Ribeiro LR, Eira AF and de Camargo JL: Influence of
aqueous extract of Agaricus blazei on rat liver toxicity induced by
different doses of diethylnitrosamine. J Ethnopharmacol. 83:25–32.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Burnett BP, Silva S, Mesches MH, Wilson S
and Jia Q: Safety evaluation of a combination, defined extract of
Scutellaria baicalensisAcacia catechu. J Food Biochem. 31:797–825.
2007. View Article : Google Scholar
|
|
34
|
Kim YO, Leem K, Park J, Lee P, Ahn DK, Lee
BC, Park HK, Suk K, Kim SY and Kim H: Cytoprotective effect of
Scutellaria baicalensis in CA1 hippocampal neurons of rats after
global cerebral ischemia. J Ethnopharmacol. 77:183–188. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao Y, Li G, Wang YF, Fan ZK, Yu DS, Wang
ZD and Bi YL: Neuroprotective effect of baicalin on compression
spinal cord injury in rats. Brain Res. 1357:115–123. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aloe L, Rocco ML, Bianchi P and Manni L:
Nerve growth factor: From the early discoveries to the potential
clinical use. J Transl Med. 10:2392012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bothwell M: NGF, BDNF, NT3 and NT4. Handb
Exp Pharmacol. 220:3–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klimaschewski L, Hausott B and Angelov DN:
The pros and cons of growth factors and cytokines in peripheral
axon regeneration. Int Rev Neurobiol. 108:137–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gordon T: The role of neurotrophic factors
in nerve regeneration. Neurosurg Focus. 26:E32009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stankoff B, Aigrot MS, Noël F, Wattilliaux
A, Zalc B and Lubetzki C: Ciliary neurotrophic factor (CNTF)
enhances myelin formation: A novel role for CNTF and CNTF-related
molecules. J Neurosci. 22:9221–9227. 2002.PubMed/NCBI
|
|
41
|
Vernerey J, Macchi M, Magalon K, Cayre M
and Durbec P: Ciliary neurotrophic factor controls progenitor
migration during remyelination in the adult rodent brain. J
Neurosci. 33:3240–3250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wilhelm JC, Xu M, Cucoranu D, Chmielewski
S, Holmes T, Lau KS, Bassell GJ and English AW: Cooperative roles
of BDNF expression in neurons and Schwann cells are modulated by
exercise to facilitate nerve regeneration. J Neurosci.
32:5002–5009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiao J, Hughes RA, Lim JY, Wong AW,
Ivanusic JJ, Ferner AH, Kilpatrick TJ and Murray SS: A small
peptide mimetic of brain-derived neurotrophic factor promotes
peripheral myelination. J Neurochem. 125:386–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tannemaat MR, Eggers R, Hendriks WT, De
Ruiter GC, van Heerikhuize JJ, Pool CW, Malessy MJ, Boer GJ and
Verhaagen J: Differential effects of lentiviral vector-mediated
overexpression of nerve growth factor and glial cell line-derived
neurotrophic factor on regenerating sensory and motor axons in the
transected peripheral nerve. Eur J Neurosci. 28:1467–1479. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Anitha M, Gondha C, Sutliff R, Parsadanian
A, Mwangi S, Sitaraman SV and Srinivasan S: GDNF rescues
hyperglycemia-induced diabetic enteric neuropathy through
activation of the PI3K/Akt pathway. J Clin Invest. 116:344–356.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hoyng SA, De Winter F, Gnavi S, De Boer R,
Boon LI, Korvers LM, Tannemaat MR, Malessy MJ and Verhaagen J: A
comparative morphological, electrophysiological and functional
analysis of axon regeneration through peripheral nerve autografts
genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or
VEGF. Exp Neurol. 261:578–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Donato R, Cannon BR, Sorci G, Riuzzi F,
Hsu K, Weber DJ and Geczy CL: Functions of S100 proteins. Curr Mol
Med. 13:24–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Donato R: Intracellular and extracellular
roles of S100 proteins. Microsc Res Tech. 60:540–551. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Novitskaya V, Grigorian M, Kriajevska M,
Tarabykina S, Bronstein I, Berezin V, Bock E and Lukanidin E:
Oligomeric forms of the metastasis-related Mts1 (S100A4) protein
stimulate neuronal differentiation in cultures of rat hippocampal
neurons. J Biol Chem. 275:41278–41286. 2000. View Article : Google Scholar : PubMed/NCBI
|