Introduction
In recent years, the incidence rates of diabetes
have been increasing each year (1).
Currently, diabetes is the fifth leading cause of mortality
following infectious diseases, cardiovascular diseases, cancer and
trauma (1). Diabetes is a chronic
progressive disease characterized by high blood glucose levels.
There are two types of diabetes, Type I and Type II, which are
characterized by the cause of the disease (2). Type II diabetes is associated with a
high fat diet and obesity, is characterized by insulin resistance
and accounts for ~90% of cases of adult diabetes (3). Type II diabetes is responsible for
~5.2% of the global mortality rate (4).
Diabetes may cause systemic microvascular and large
vascular lesions, diabetic retinopathy, diabetic nephropathy,
diabetic cerebrovascular disease and diabetic cardiovascular
disease (5). In addition to causing
vascular disease, diabetes may cause myocardial cell damage and
eventually lead to the development of diabetic cardiomyopathy (DCM)
(6). DCM is a disease caused by
diabetes that is independent of coronary artery disease,
hypertension and heart valve disease. The main characteristics of
DCM include oxidative stress, cardiac hypertrophy, apoptosis,
myocardial fibrosis and impaired cardiac function (7).
Nuclear factor erythroid 2 (Nrf2), one of the ‘cap
‘n’ collar’ family members, is a master regulator of cell toxicity
and redox (8). When cells are
affected by oxidative stress or electrophilic compound stimulation,
Nrf2 will translocate into the nucleus and bind to an antioxidant
response element to induce the expression of genes required for the
antioxidant defense system (9). The
antioxidant defense system is the main protective mechanism against
oxidative damage, resulting in the neutralization of oxidants and
electrophiles by antioxidants in the cells (10).
Oleanolic acid (OL) is a pentacyclic triterpenoid
[chemical name, (3P)-3-hydroxy-olean-12-en-28-oic acid] with a
natural chemical composition formed from free or combined
glycosides. OL widely exists in plants, such as white snake tongue
grass, hawthorn fruit, clove, papaya, Ligustrum lucidum and
Prunella vulgaris (11).
Experimental studies have demonstrated that OL has effective liver
and kidney protective properties, inhibits platelet aggregation and
has hypolipidemic, hypoglycemic, anti-inflammatory, anti-cancer,
anti-stress, anti-ulcer and anti-microbial properties (12,13). OL
is used to treat acute jaundice, chronic poisoning hepatitis, liver
fibrosis and cirrhosis (12). In
addition, OL is effective in the recovery of immune function caused
by chemotherapy for malignant tumors and protection against liver
damage following administration anti-tuberculosis agents (14). Therefore, the aim of the present
study was to investigate the effects of OL against DCM in a rat
model of diabetes and the possible mechanism.
Materials and methods
Animals and induction of DCM in
rats
The experimental procedures were approved by the
Animal Care and Welfare Committee of The First Affiliated Hospital
of Zhengzhou University (Zhengzhou, China) in accordance with the
guidelines for the Care and Use of Laboratory Animals. A total of
24 male Sprague-Dawley rats (220–250 g; 6–8 weeks old) were
purchased from the Experimental Animal Center of the Academy of The
First Affiliated Hospital of Zhengzhou University and maintained in
standard conditions at 22±2°C, 55±5% humidity with a 12 h
light/dark cycle and fed normal chow. All rats were randomly
assigned into three groups: Control group (n=8), DCM model group
(n=8) and OL treatment group (n=8). Diabetes was induced in the DCM
model group using an intraperitoneal injection of streptozotocin
(STZ; 60 mg/kg; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germanu). The OL treatment group were administered with an
intraperitoneal injection of 80 mg/kg OL once every 2 days for 14
days. The control group was administered equal volumes of normal
saline.
Body weight and heart rate
A CODA 8-channel tailcuff blood pressure system
(Kent Scientific, Torrington, CT, USA) was used to record heart
rate. Subsequent to sacrifice, body weights were immediately
measured. Rats were washed with phosphate-buffered saline and their
wet weight was recorded using standard scales.
Echocardiography and hemodynamic
measurements
Left ventricular internal dimension in systole
(LVIDs), left ventricular internal dimension in diastole (LVIDd),
interventricular septal wall thickness (IVSd) and fractional
shortening were measured using a manometer-tipped catheter
(SPR-320NR; Millar, Inc., Houston, TX, USA) and recorded using a
MP150 system (Biopac Systems, Goleta, CA, USA).
Determination of p-glycogen synthase
(GS), glycogen phosphorylase (GP), methane dicarboxylic aldehyde
(MDA) and superoxide dismutase (SOD) activities
The GS, GP, MDA (A003-1) and SOD (A001-3; both
Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
activities were assayed radiochemically using ELISA kits, according
to the manufacturer's instructions. The GS and GP activities were
expressed as nmol/min/mg protein, MDA activity was expressed as
nmol/mg protein and SOD activity was expressed as U/mg protein
using an ultraviolet-visible spectrophotometer (UVmini-1240;
Shimadzu Corp., Kyoto, Japan).
Determination of glycogen content
Heart tissue samples were boiled in 30% KOH
saturated with NaSO4 for 30 min. Glycogen content was
precipitated with 95% ice-cold ethanol for 30 min. Following this,
5 ml sulfuric acid was added and the mixture was incubated on ice
for 30 min. Glycogen content was measured using an
ultraviolet-visible spectrophotometer (UVmini-1240; Shimadzu Corp.)
at 490 nm.
Western blot analysis
Rat hearts (50 mg) were homogenized in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) supplemented with a protease
inhibitor cocktail. Total protein concentrations were measured
using a BCA protein assay kit (Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). A total of 50 µg protein from each sample
was separated by 8–10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Merck Millipore). Membranes were blocked with
5%-skim milk powder in TBS with Tween-20 (TBST) and incubated with
anti-heme oxygenase (HO)-1 (1:200; sc-10789), anti-Nrf2 (1:300;
sc-33569), anti-phosphorylated-GP (p-GP; 1:200; sc-66913),
anti-phosphorylated GS (p-GS; 1:400; sc-99029) and anti-β-actin
(1:500; sc-130656; all Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) primary antibodies at 4°C overnight. Following washing three
times with TBST, the membranes were incubated with a secondary
horseradish peroxidase-conjugated immunoglobulin G antibody
(1:3,000; sc-2357; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h,
visualized using an enhanced chemiluminescence reagent kit (P0018A;
Beyotime Institute of Biotechnology) and quantified using Image J
3.0 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean using InStat version 5 (GraphPad Software, Inc., La
Jolla, CA, USA). Data between two groups were compared using
unpaired t-tests. Comparisons for three or more groups were
performed using one-way or two-way analysis of variance. P<0.05
was considered to indicate a statistically significant
difference.
Results
OL protects against body weight and
heart rate alterations in rats with DCM
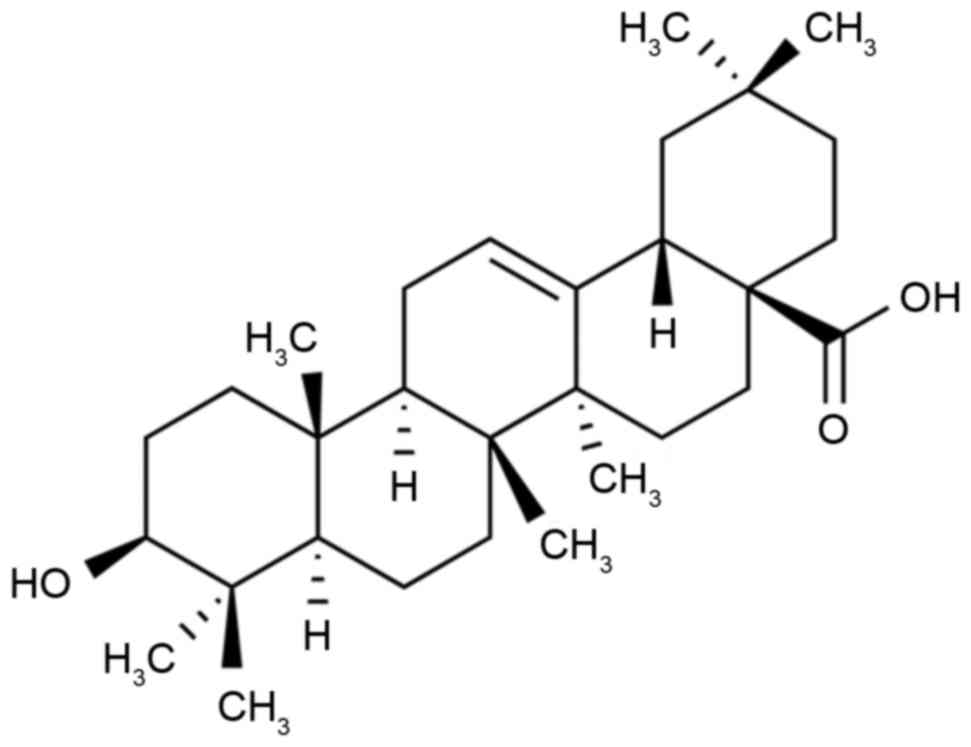
Fig. 1 presents the
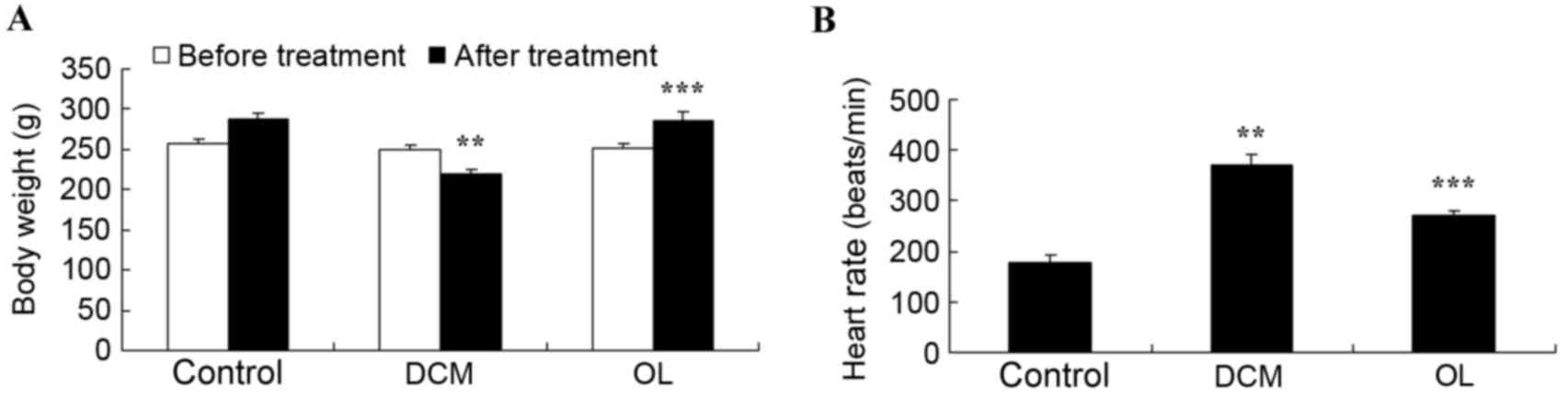
chemical structure of OL. The body weights of rats with DCM were
lower than those of the control rats following treatment (P=0.0081;
Fig. 2A) and the heart rates of rats
with DCM were higher than those of the control rats (P=0.0042;
Fig. 2B). Treatment with OL
significantly increased the DCM-induced reduction of body weight
(P=0.0096) and significantly decreased the DCM-induced increase in
heart rate (P=0.0067) in rats with DCM (Fig. 2).
OL protects against alterations to
echocardiography in rats with DCM
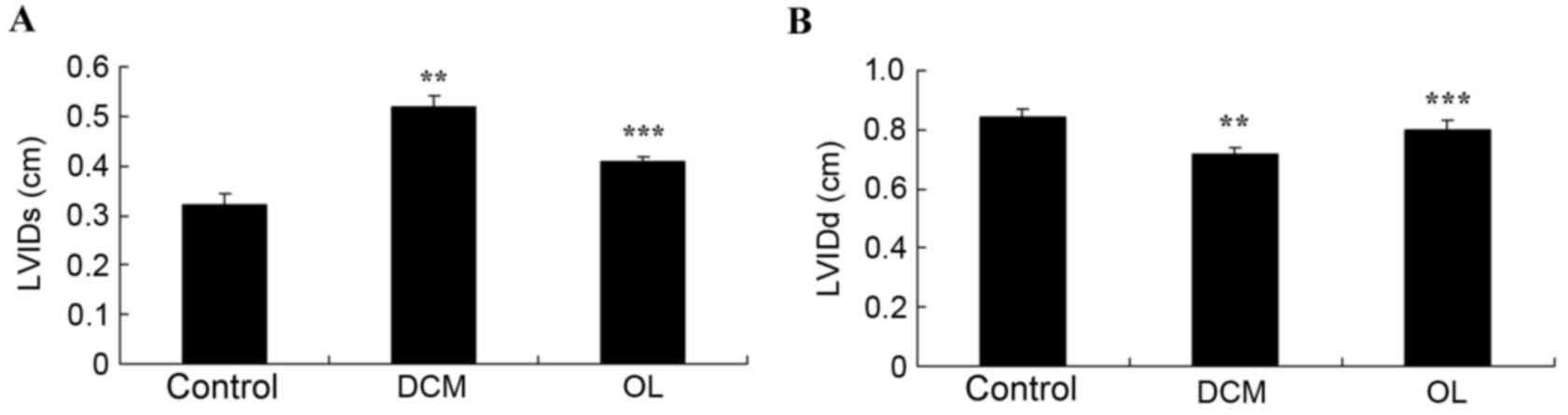
To investigate whether OL protects against
echocardiographic alterations in rats with DCM, LVIDs and LVIDd
were measured. LVIDs and LVIDd were significantly higher (P=0.0063)
and lower (P=0.0086), respectively, in rats with DCM compared with
normal controls (Fig. 3). Treatment
with OL significantly inhibited decreased (P=0.0077) and increased
(P=0.0096) LVIDs and LVIDd measurements, respectively, as compared
with rats with DCM (Fig. 3).
OL protects against alterations in
hemodynamics in rats with DCM
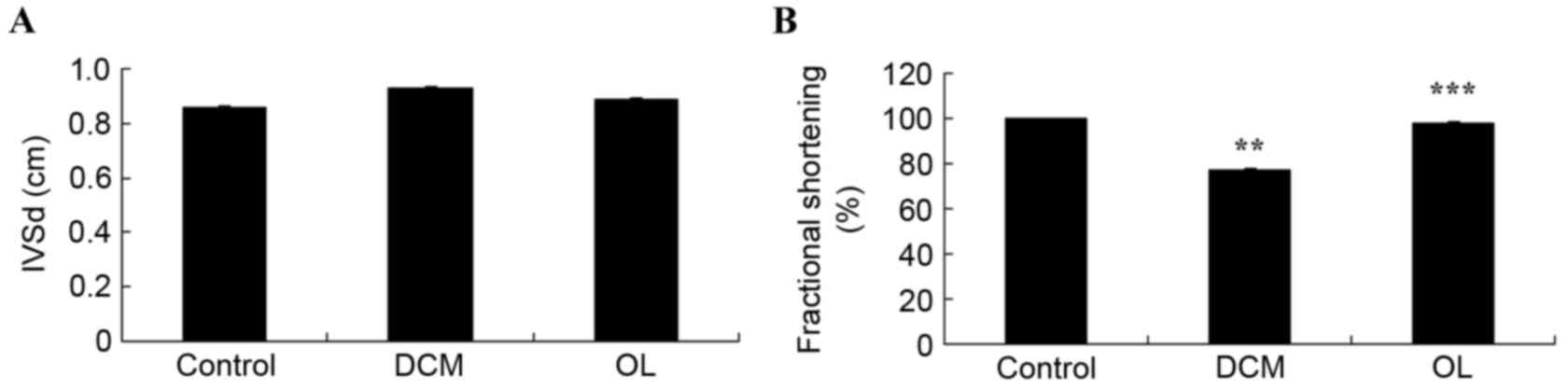
Fig. 4 demonstrates
the effects of OL on the hemodynamics of rats with DCM. No
significant differences were observed in IVSd between the three
groups; however, rats with DCM exhibited a significant decrease in
fractional shortening (P=0.0067) compared with the control rats.
Treatment with OL decreased the DCM-induced reduction of fractional
shortening, resulting in a significant increase (P=0.0073) in
fractional shortening in the OL treatment group compared with the
DCM model group (Fig. 4).
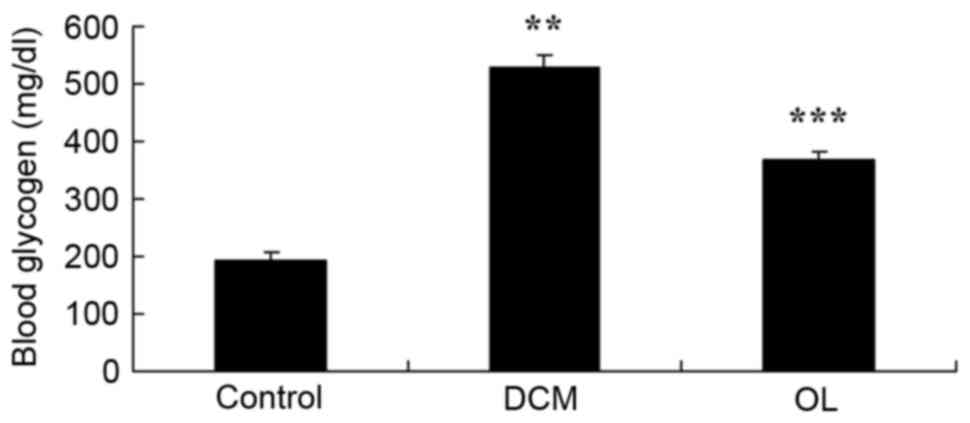
OL protects against increased glycogen
levels in rats with DCM
The present study demonstrated that DCM
significantly increased blood glycogen levels in rats with DCM
(P=0.0031) compared with the control group; however, treatment with
OL significantly reduced (P=0.0068) the blood glycogen levels of
the rats in the treatment group compared with the glycogen levels
of the untreated rats with DCM (Fig.
5).
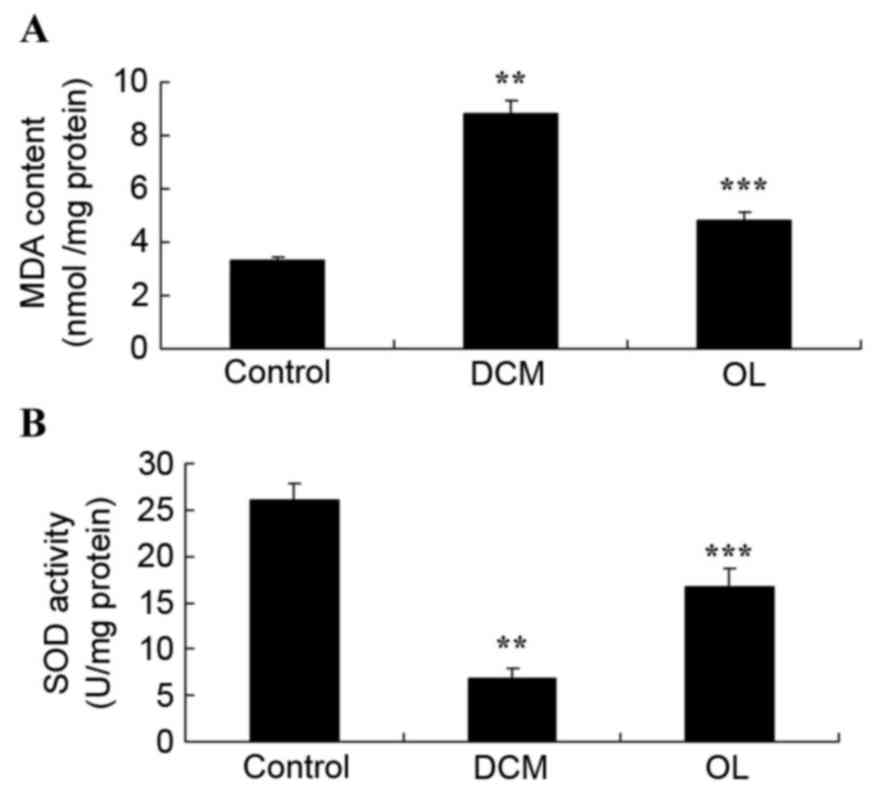
OL protects against oxidative stress
in rats with DCM
In the present study, rats with DCM exhibited a
significant increase (P=0.0022) in MDA activity and a significant
decrease (P=0.0013) in SOD activity compared with control group
rats (Fig. 6). Rats treated with OL
exhibited decreased oxidative stress through the significant
suppression (P=0.0052) of MDA activity and the significantly
increased (P=0.0038) levels of SOD activity compared with untreated
rats with DCM (Fig. 6).
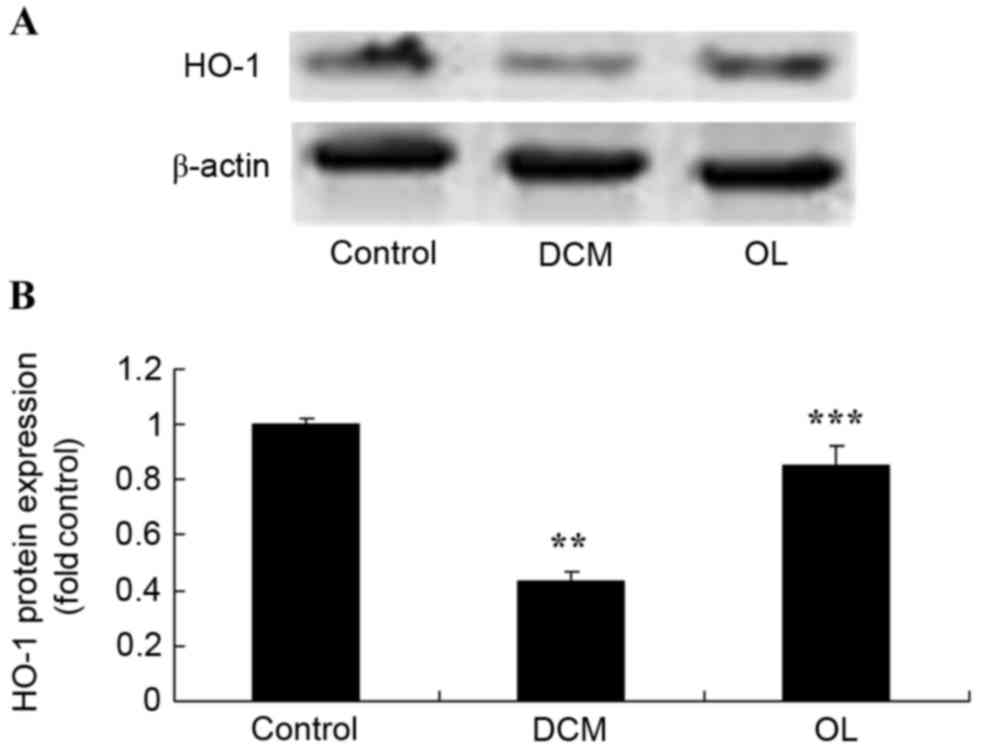
OL protects against decreased HO-1
protein expression levels in rats with DCM
In order to elucidate the possible roles of the HO-1
protein, the present study determined HO-1 protein expression
levels in vivo. Results demonstrated that there was a
significant decrease (P=0.0031) in the HO-1 protein expression
level in rats with DCM when compared with the control group;
however, OL-treated rats exhibited significantly increased
(P=0.0067) HO-1 protein expression levels compared with untreated
rats with DCM (Fig. 7).
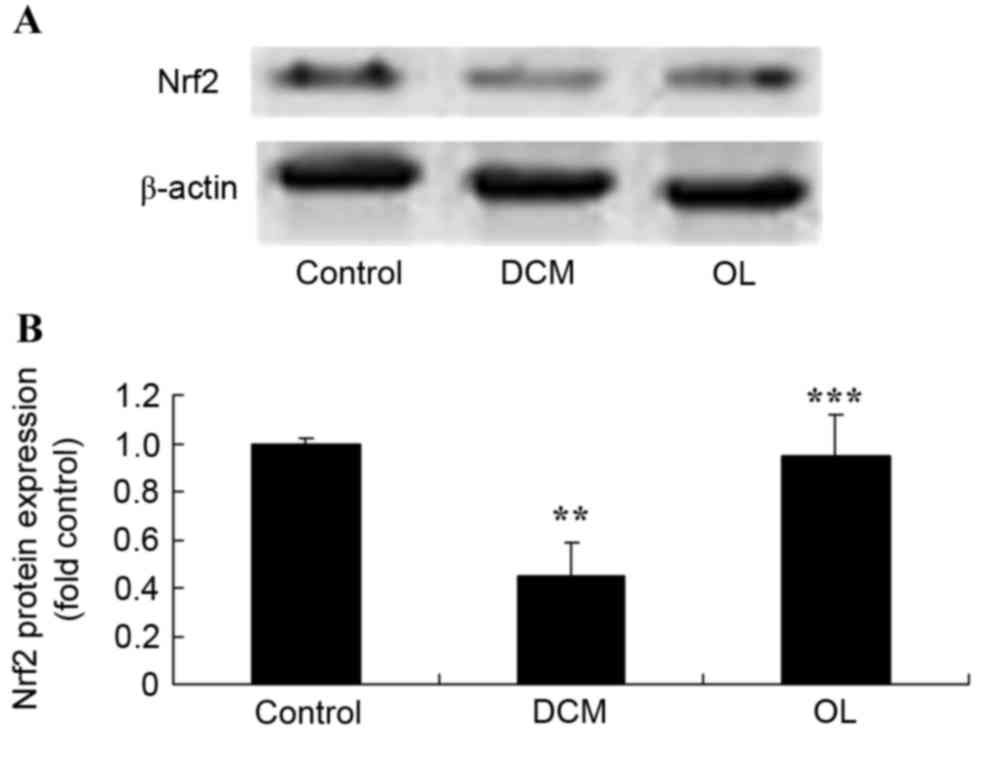
OL protects against decreased Nrf2
protein expression levels in rats with DCM
In order to elucidate the role of Nrf2 and the
effects of OL against DCM, the present study measured Nrf2 protein
expression levels using western blot analysis. As shown in Fig. 8, DCM significantly inhibited
(P=0.0046) the protein expression levels of Nrf2 compared with
control rats; however, treatment with OL significantly increased
(P=0.0072) the Nrf2 protein expression levels in DCM rats compared
with the untreated DCM model group.
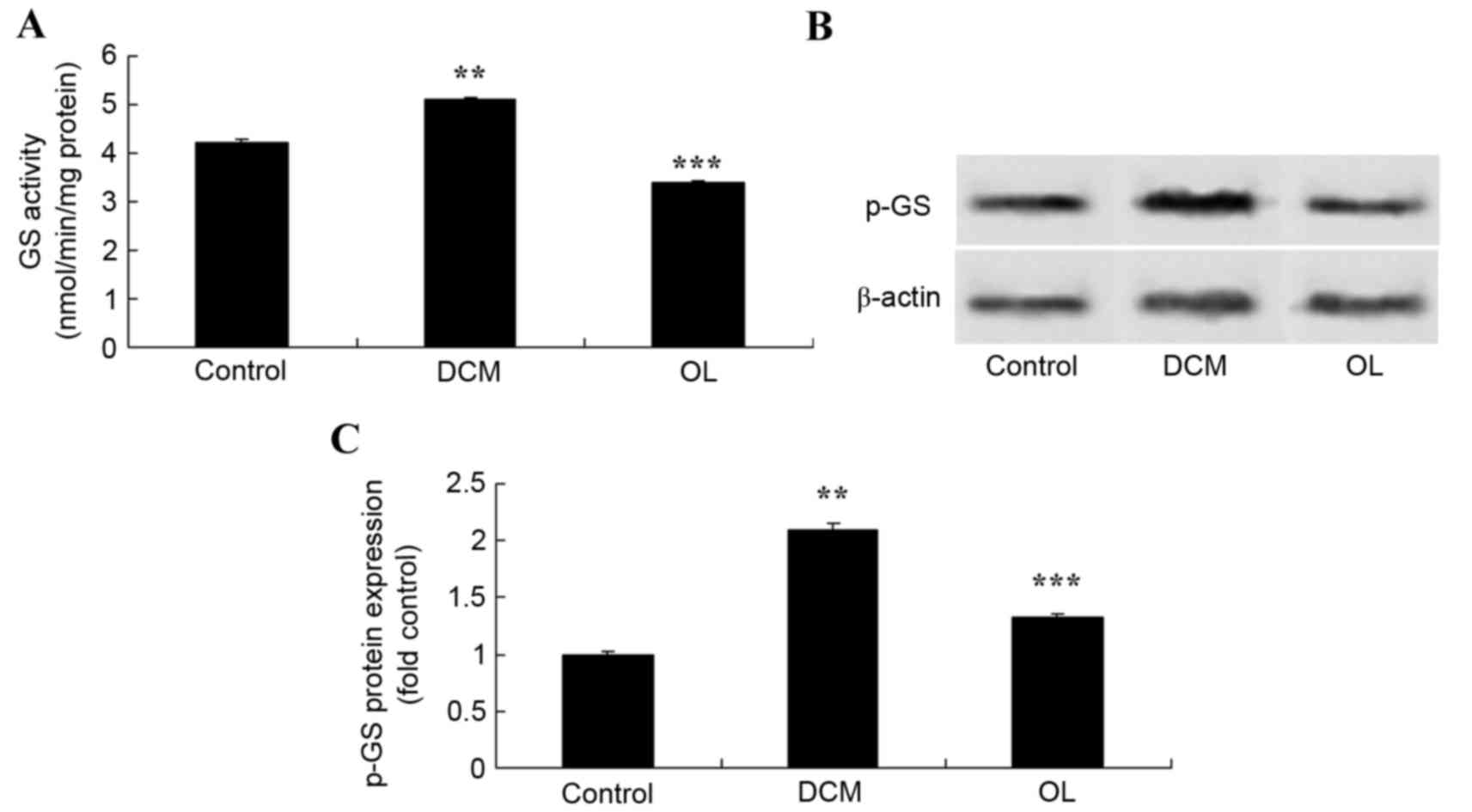
OL protects against increased GS
activity and protein expression levels in rats with DCM
In order to determine the effects of OL on the GS
signaling pathway, the activity and protein expression levels of GS
were measured using an ELISA kit and western blot analysis. Results
demonstrated that the activity and protein expression levels of GS
were significantly increased (P=0.0061) in rats with DCM compared
with the control group (Fig. 9).
Treatment with OL significantly inhibited (P=0.0078) GS activity
and protein expression levels in rats with DCM compared with the
untreated DCM model group (Fig.
9).
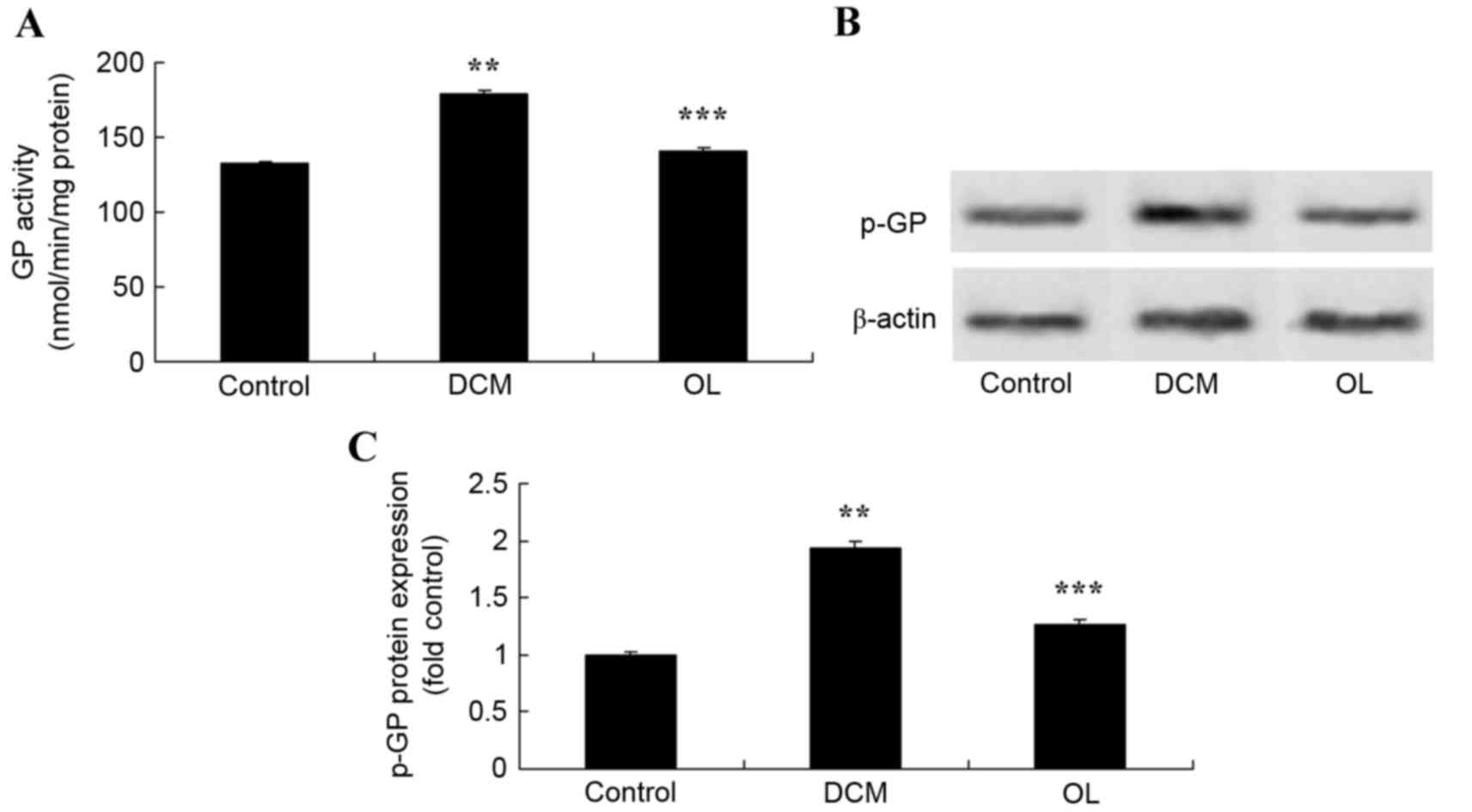
OL protects against increased GP
activity and protein expression levels in rats with DCM
In order to determine whether OL affects the GP
signaling pathway in order to protect against DCM in rats, GP
activity and protein expression levels were measured using an ELISA
kit and western blot analysis. Results demonstrated that the
activity and protein expression levels of GP were significantly
increased (P=0.0066) in rats with DCM compared with the control
group (Fig. 10). Treatment with OL
significantly inhibited (P=0.0081) GP activity and protein
expression levels in rats with DCM compared with the untreated DCM
model group (Fig. 10).
Discussion
DCM is a cardiac disease that is distinct from
hypertension and coronary artery atherosclerotic heart disease
(1). Research has confirmed the
existence of DCM and it is believed that a particularly significant
symptom of the disease is left ventricular diastolic dysfunction.
As the disease progresses, symptom develop into left ventricular
hypertrophy and systolic dysfunction occurs and, ultimately, this
may result in the development of heart failure (7). In the present study, it was
demonstrated that OL protects against DCM-induced body weight and
heart rate alterations and improves echocardiographic and
hemodynamic measurements in DCM rats.
Currently, the specific pathogenesis of DCM is
unclear and, due to the lack of clinical research on this disease,
correct diagnosis and treatment of DCM is often difficult, with the
disease often being misdiagnosed as coronary heart disease. In the
majority of cases, the diagnosis of DCM is confirmed by autopsy
(15). Previous studies have
demonstrated that the occurrence and development of DCM is related
to a variety of factors (16,17). Due
to disruption of the cellular antioxidant defense balance in DCM,
increased numbers of free radicals are generated, resulting in
oxidative stress and cell damage. Possible mechanisms for this
damage include metabolic disorders, changes in tissue structure and
function, small vessel disease, autonomic dysfunction, insulin
resistance and cell factor abnormalities (9). In the present study, OL treatment
inhibited the increased glycogen content in the blood of rats with
DCM. A study by Mukundwa et al (14) demonstrated that OL affects the
insulin signaling pathway of diabetic male Sprague-Dawley rats.
A previous study has demonstrated that oxidative
stress is the most important factor in DCM development (18). Oxidative stress results in the
increase of intracellular oxidative metabolites and, when cell
antioxidant mechanisms are lacking, it promotes the accumulation of
reactive oxygen species, resulting in cell toxicity (19). Oxidative stress may result from
various causes, including myocardial cell damage, exercise,
psychological stress, ischemia and hypoxia (17,20).
Recent studies have demonstrated that, when using different blood
glucose gradients to develop myocardial cells, cell apoptosis is
proportional to concentration of blood glucose, with high blood
glucose levels inducing oxidative stress through the generation of
reactive oxygen species (21,22). The
increased number of reactive oxygen species results in gene
expression abnormalities, alterations to signal transduction and
activation of the programmed cell death pathway (23,24).
Inhibition of myocardial cell death can significantly prevent heart
toxicity in diabetes (23,24). In the present study, it was observed
that OL reduced oxidative stress in rats with DCM. A study by
Sarkar et al (23)
demonstrated that OL prevents fluoride-induced metabolic and
oxidative stress in rat brain through the suppression of oxidative
stress.
HO-l is a microsomal oxidase with various functions
in the process of heme metabolism (10). HO-1 is a myocardial protection factor
and has anti-inflammatory, anti-oxidant, anti-apoptotic and
anti-arrhythmia properties. In addition, HO-1 has an important role
in cardiovascular disease and research has demonstrated that HO-1
is able to inhibit cardiac hypertrophy; however, oxidative stress
leads to DCM and results in the reduction of HO-1 expression
(24,25). HO-1 expression has become a universal
marker of protection against oxidative stress. In the present
study, treatment of the STZ-induced rat model of DCM with OL
significantly activated HO-1 protein expression in rats with DCM. A
study by Hong et al (26)
suggested that OL attenuates tubulointerstitial fibrosis through
Nrf2/HO-1 signaling in chronic cyclosporine nephropathy.
Studies have demonstrated that Nrf2 protection
against heart damage due to oxidative stress may involve various
mechanisms, including: Regulation of the sensitivity of apoptosis
by Nrf2; and, Nrf2 inhibiting the Fas-induced apoptosis pathway,
thus, Nrf2 overexpression may protect cells against Fas-induced
apoptosis (8,27). Nrf2 has anti-inflammatory effects,
inhibiting foam cell formation and pathogenesis formation of
atherosclerosis (28). In the
present study, treatment with OL significantly increased Nrf2
protein expression levels and significantly suppressed the protein
expression levels and activity of GS and GP in rats with DCM. A
study by Liu et al (11)
reported that OL alleviates ethanol-induced hepatic injury through
Nrf2 in rats.
In conclusion, the present study identified novel
protective effects of OL against DCM through the inhibition of
DCM-induced body weight and heart rate alterations, improved
echocardiographic and hemodynamic measurements, and reduced blood
glycogen levels in DCM rats. These findings demonstrate that the
HO-1/Nrf2 signaling pathway has an important role in OL protection
against cardiac damage through insulin modulation of the GS/GP
signaling pathway in rats with DCM. These findings also indicate
that OL may have the potential to be used as a novel therapeutic
treatment for DCM.
References
|
1
|
Saengtipbovorn S and Taneepanichskul S:
Lifestyle Change Plus Dental Care (LCDC) program improves
knowledge, attitude, and practice (KAP) toward oral health and
diabetes mellitus among the elderly with type 2 diabetes. J Med
Assoc Thai. 98:279–290. 2015.PubMed/NCBI
|
|
2
|
Barrett HL, Nitert M Dekker, Jones L,
O'Rourke P, Lust K, Gatford KL, De Blasio MJ, Coat S, Owens JA,
Hague WM, et al: Determinants of maternal triglycerides in women
with gestational diabetes mellitus in the Metformin in Gestational
Diabetes (MiG) study. Diabetes Care. 36:1941–1946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jorgensen CH, Gislason GH, Ahlehoff O,
Andersson C, Torp-Pedersen C and Hansen PR: Use of secondary
prevention pharmacotherapy after first myocardial infarction in
patients with diabetes mellitus. BMC Cardiovasc Disord. 14:42014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harashima SI, Ogura M, Tanaka D, Fukushima
T, Wang Y, Koizumi T, Aono M, Murata Y, Seike M and Inagaki N:
Sitagliptin add-on to low dosage sulphonylureas: Efficacy and
safety of combination therapy on glycaemic control and insulin
secretion capacity in type 2 diabetes. Int J Clin Pract.
66:465–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wongchareon W, Phrommintikul A,
Kanjanavanit R, Kuanprasert S and Sukonthasarn A: A predictive
model for distinguishing ischemic from non-ischemic cardiomyopathy.
J Med Assoc Thai. 88:1689–1696. 2005.PubMed/NCBI
|
|
6
|
Barsotti A, Giannoni A, Di Napoli P and
Emdin M: Energy metabolism in the normal and in the diabetic heart.
Curr Pharm Des. 15:836–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abu-Sulaiman RM and Subaih B: Congenital
heart disease in infants of diabetic mothers: Echocardiographic
study. Pediatr Cardiol. 25:137–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mann GE: Nrf2-mediated redox signalling in
vascular health and disease. Free Radic Biol Med. 75 Suppl
1:S12014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Hu X, Xie J, Xu W and Jiang H:
Beta-1-adrenergic receptors mediate Nrf2-HO-1-HMGB1 axis regulation
to attenuate hypoxia/reoxygenation-induced cardiomyocytes injury in
vitro. Cell Physiol Biochem. 35:767–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H
and Hai C: Oleanolic acid co-administration alleviates
ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing
modulating in rats. Chem Biol Interact. 221:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu K, Chu F, Li G, Xu X, Wang P, Song J,
Zhou S and Lei H: Oleanolic acid synthetic oligoglycosides: A
review on recent progress in biological activities. Pharmazie.
69:483–495. 2014.PubMed/NCBI
|
|
13
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Oleanolic acid and its synthetic
derivatives for the prevention and therapy of cancer: Preclinical
and clinical evidence. Cancer Lett. 346:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mukundwa A, Mukaratirwa S and Masola B:
Effects of oleanolic acid on the insulin signaling pathway in
skeletal muscle of streptozotocin-induced diabetic male
Sprague-Dawley rats. J Diabetes. 8:98–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Falcao-Pires I and Leite-Moreira AF:
Diabetic cardiomyopathy: Understanding the molecular and cellular
basis to progress in diagnosis and treatment. Heart Fail Rev.
17:325–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Z, He C and Zou MH: AMP-activated
protein kinase modulates cardiac autophagy in diabetic
cardiomyopathy. Autophagy. 7:1254–1255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai L, Wang Y, Zhou G, Chen T, Song Y, Li
X and Kang YJ: Attenuation by metallothionein of early cardiac cell
death via suppression of mitochondrial oxidative stress results in
a prevention of diabetic cardiomyopathy. J Am Coll Cardiol.
48:1688–1697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kajstura J, Fiordaliso F, Andreoli AM, Li
B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A and
Anversa P: IGF-1 overexpression inhibits the development of
diabetic cardiomyopathy and angiotensin II-mediated oxidative
stress. Diabetes. 50:1414–1424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Zhang L, Chen S, Feng B, Lu X,
Bai Y, Liang G, Tan Y, Shao M, Skibba M, et al: The prevention of
diabetic cardiomyopathy by non-mitogenic acidic fibroblast growth
factor is probably mediated by the suppression of oxidative stress
and damage. PLoS One. 8:e822872013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian C, Ouyang X, Lv Q, Zhang Y and Xie W:
Cross-talks between microRNAs and mRNAs in pancreatic tissues of
streptozotocin-induced type 1 diabetic mice. Biomed Rep. 3:333–342.
2015.PubMed/NCBI
|
|
21
|
Badole SL, Jangam GB, Chaudhari SM, Ghule
AE and Zanwar AA: L-glutamine supplementation prevents the
development of experimental diabetic cardiomyopathy in
streptozotocin-nicotinamide induced diabetic rats. PLoS One.
9:e926972014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Selim ME, Abd-Elhakim YM and Al-Ayadhi LY:
Pancreatic response to gold nanoparticles includes decrease of
oxidative stress and inflammation in autistic diabetic model. Cell
Physiol Biochem. 35:586–600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarkar C, Pal S, Das N and Dinda B:
Ameliorative effects of oleanolic acid on fluoride induced
metabolic and oxidative dysfunctions in rat brain: Experimental and
biochemical studies. Food Chem Toxicol. 66:224–236. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng X, Li J and Li Z: Ginsenoside Rd
mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1
signaling pathway. Int J Clin Exp Med. 8:14497–14504.
2015.PubMed/NCBI
|
|
25
|
Lappalainen J, Lappalainen Z, Oksala NK,
Laaksonen DE, Khanna S, Sen CK and Atalay M: Alpha-lipoic acid does
not alter stress protein response to acute exercise in diabetic
brain. Cell Biochem Funct. 28:644–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong YA, Lim JH, Kim MY, Kim EN, Koh ES,
Shin SJ, Choi BS, Park CW, Chang YS and Chung S: Delayed treatment
with oleanolic acid attenuates tubulointerstitial fibrosis in
chronic cyclosporine nephropathy through Nrf2/HO-1 signaling. J
Transl Med. 12:502014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Zhang J, Liu H and Zhang L: Change
of Nrf2 expression in rat hippocampus in a model of chronic
cerebral hypoperfusion. Int J Neurosci. 124:577–584. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soares MP and Ribeiro AM: Nrf2 as a master
regulator of tissue damage control and disease tolerance to
infection. Biochem Soc Trans. 43:663–668. 2015. View Article : Google Scholar : PubMed/NCBI
|