Introduction
Neuroinflammation is defined as activation of the
innate immune system of the brain in response to an inflammatory
challenge and is characterized by microglial activation,
astrogliosis and neuronal loss (1).
In the central nervous system (CNS), tumor necrosis factor-α
(TNF-α), a key proinflammatory cytokine, exerts homeostatic as well
as pathophysiological effects. Under normal conditions, TNF-α
exerts regulatory functions on physiological processes such as
synaptic plasticity, synaptic transmission, learning and memory.
Under pathological conditions, microglia and a proportion of
astrocytes and oligodendrocytes secrete TNF-α, which is an
important component of neuronal apoptosis associated with
neuroinflammation (2). Neuronal
apoptosis is the most crucial event in neuronal loss associated
with neurological diseases and is regulated by pro- and
anti-apoptotic factors (3). At
present, it is urgent to seek the underlying molecular mechanisms
of neuronal apoptosis induced by neuroinflammation.
PC12 cells, which originate from a rat adrenal
medullary tumour (pheochromocytoma), have been widely used as a
neuronal model system to assess processes such as neuronal
differentiation, neurite outgrowth, neuronal toxicity and neuronal
apoptosis (4–6). Puerarin, known as Ge-gen in Chinese, is
isolated from Radix puerariae and has protective effects on
the nervous and cardiovascular system to prevent osteoporosis,
liver injury and inflammation (7).
An increasing body of evidence has indicated that puerarin has
anti-apoptotic effects and mitigated apoptosis in multiple cell
types, including neurons, microglia, osteoblasts and cardiomyocytes
(8–11). A previous study reported that
puerarin protected PC12 cells against β-amyloid-induced cell
injury, which was associated with its antioxidant effects (12). Another study confirmed that puerarin
protected differentiated PC12 cells from
H2O2-induced apoptosis via Akt
phosphorylation (13). However,
little is known regarding the effect of puerarin on PC12 cell
apoptosis induced by TNF-α and its underlying mechanisms.
The present study demonstrated that puerarin
prevented TNF-α-induced apoptosis in PC12 cells via activation of
the phosphoinositide-3 kinase (PI3K)/Akt signaling pathway to
provide potential mechanisms underlying the neuroprotective effect
of puerarin, which may be used as a novel neuroprotective agent
against neuroinflammation.
Materials and methods
Materials
Rat PC12 (adrenal gland pheochromocytoma) cells were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Rat TNF-α was obtained from R&D Systems
(Minneapolis, MN, USA). The specific PI3K/Akt inhibitor LY294002
(10 µM) was from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Puerarin (purity, >98%) was purchased from the National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China) and dissolved in dimethylsulfoxide (DMSO) at 10
mM.
PC12 cell culture
PC12 cells were cultured in Dulbecco's modified
Eagle's medium (Hyclone; GE Healthcare, Chalfont, UK) supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and antibiotics (100 units/ml penicillin and 100
units/ml streptomycin; Gibco, Thermo Fisher Scientific, Inc.) at
37°C in a humidified incubator with 5% CO2. Cells were
supplied with fresh medium three times per week, and were split at
a 1:3 ratio twice per week.
MTT assay
PC12 cells were seeded into 96-well plates at
1×104 cells/well. Puerarin was added at the desired
concentration for 2 h prior to treatment with TNF-α, after which
the cells were incubated for an additional 24 h. MTT
(Sigma-Aldrich) working solution (0.5 mg/ml) was added to each
well, followed by incubation for 4 h at 37°C. Subsequently, DMSO
(Sigma-Aldrich) was added. The optical density (OD) was measured at
a wavelength of 570 nm using a plate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The inhibitory rate of PC12 cell
proliferation was calculated as follows: Inhibitory rate (%) =
(ODcontrol group - ODtest group) ×
100%/ODcontrol group.
Flow cytometric analysis
Flow cytometric analysis was used to detect the
apoptotic rate of cells processed with a fluorescein isothiocyanate
(FITC) Apoptosis Detection kit (BD Pharmingen, San Jose, CA, USA).
PC12 cells (5×105) were collected from 24-well culture
plates at the end of the drug incubation. Cells were then washed
and incubated in buffer containing 5 µl FITC-Annexin V and 5 µl
propidium iodide (PI). The mixture was then incubated for 5 min at
room temperature in the dark and immediately analyzed with a flow
cytometer (BD FACSVerse) followed by analysis with BD FASCuite
software (BD Biosciences, Franklin Lakes, NJ, USA).
Enzymatic assay for caspase-3 and
caspase-9 activity
Caspase activity was detected using the caspase-3
and caspase-9 Activity Assay kit (KeyGen Biotech Co., Ltd.,
Nanjing, China) according to standard procedures. Cells were
collected and extracted on ice in lysis buffer. The protein content
of the supernatant was subsequently measured using the
bicinchoninic acid (BCA) method with an assay kit (Thermo Fisher
Scientific, Inc.). An equal amount of protein (50 µg) was detected
using reaction buffer containing chromogenic substrates at 37°C for
2 h in the dark. The absorbance values were measured at 405 nm with
a spectrophotometer (Bio-Rad Laboratories, Inc.).
Western blot analysis
PC12 cells (1×106) were collected, washed
and lysed with radioimmunoprecipitation assay buffer. The protein
concentration was determined using a BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.). The protein samples (30 µg) were
separated by 10% SDS-PAGE and transferred to a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). After
blocking with 5% nonfat dry milk, the membrane was incubated
overnight at 4°C with primary antibodies [1:1,000 dilution; rabbit
antibodies to cleaved-caspase-3 (9664), caspase-3 (9665),
phosphorylated (p)-Akt (Ser473) (4058), Akt (4685) and GAPDH
(5174); all from Cell Signaling Technology, Inc., Beverly, MA, USA;
rabbit antibodies to Bax (ab32503) and Bcl-2 (ab59348); both from
Abcam, Cambridge, MA, USA]. The blotted membranes were then washed
and probed with horseradish peroxidase-conjugated secondary
antibodies (1:2,000 dilution; anti-rabbit) (7074; Cell Signaling
Technology, Inc.). Immunoreactive bands were visualized using an
enhanced chemiluminescence kit (32106; Thermo Fisher Scientific,
Inc.). Images were captured using a scanner (Amersham Life Science,
Arlington Heights, IL, USA). Intensities of protein bands were
quantified using Image J software version 1.44 (National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Differences between two groups were evaluated using
Student's t-test. One-way analysis of variance was used for
multi-group comparison. P<0.05 was considered to indicate a
statistically significant difference. Results were analyzed using
SPSS 13.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Results
Puerarin attenuates TNF-α-induced
cytotoxicity in PC12 cells
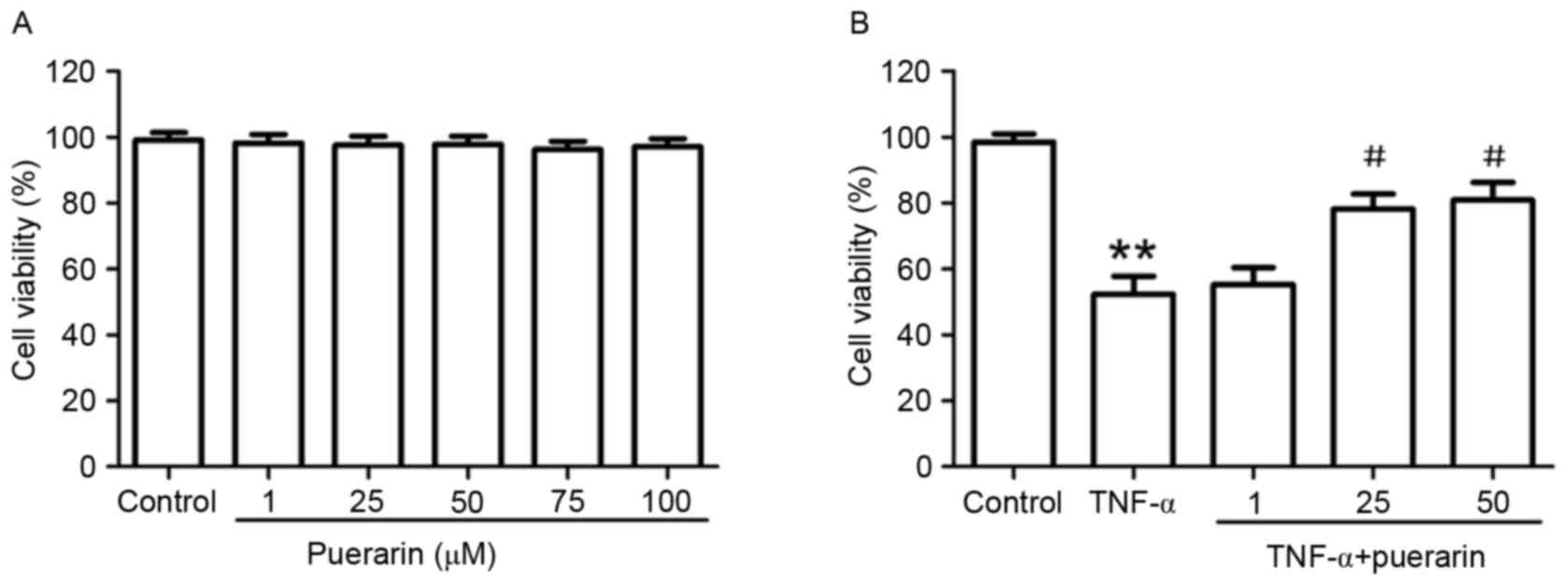
PC12 cells were treated with different
concentrations of puerarin (1–100 µM) for 24 h. As shown in
Fig. 1A, puerarin had no
cytotoxicity on PC12 cells. Based on the inhibitory rate of TNF-α
on PC12 cells (IC50=2.856×105 U/l; Table I), 3×105 U/l TNF-α was
used to induce apoptosis in PC12 cells as an in vitro model
of neuroinflammation-associated neuronal apoptosis. Of note,
pre-treatment with puerarin (25 and 50 µM) significantly attenuated
TNF-α-induced cytotoxicity (P<0.05; Fig. 1B).
 | Table I.Inhibitory rate of TNF-α on the
proliferation of PC12 cells. |
Table I.
Inhibitory rate of TNF-α on the
proliferation of PC12 cells.
| TNF-α (U/l) | OD570 | Inhibitory rate
(%) |
|---|
| 0 | 0.996±0.005 | 0 |
| 1×104 | 0.968±0.016 | 9.716±0.563 |
| 5×104 | 0.782±0.023 | 10.038±0.862 |
| 1×105 | 0.604±0.018 | 13.718±1.056 |
| 5×105 | 0.427±0.026 | 53.582±1.256 |
| 1×106 | 0.295±0.012 | 84.535±0.682 |
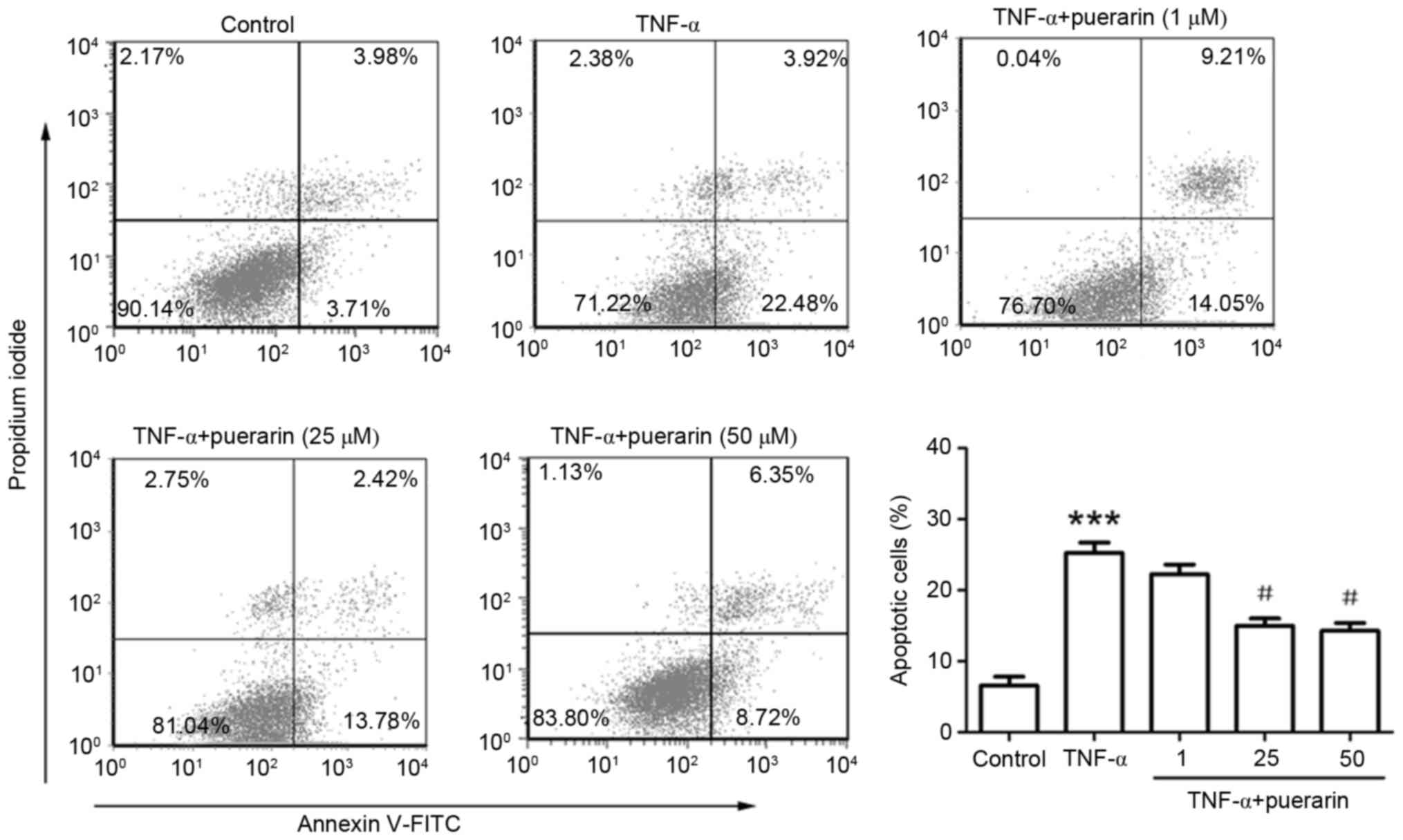
Puerarin prevents TNF-α-induced
apoptosis in PC12 cells
As shown in Fig. 2,
Annexin V/PI staining and flow cytometric analysis revealed that
TNF-α obviously increased the apoptotic rate of PC12 cells
(25.25±1.46 vs. 6.56±1.18% in the control group; P<0.001).
Pre-treatment with puerarin at 25 or 50 µM significantly prevented
TNF-α-induced apoptosis (apoptotic rate, 14.98±1.05 or 14.26±1.12
vs. 25.25±1.46% in TNF-α group; P<0.05; Fig. 2).
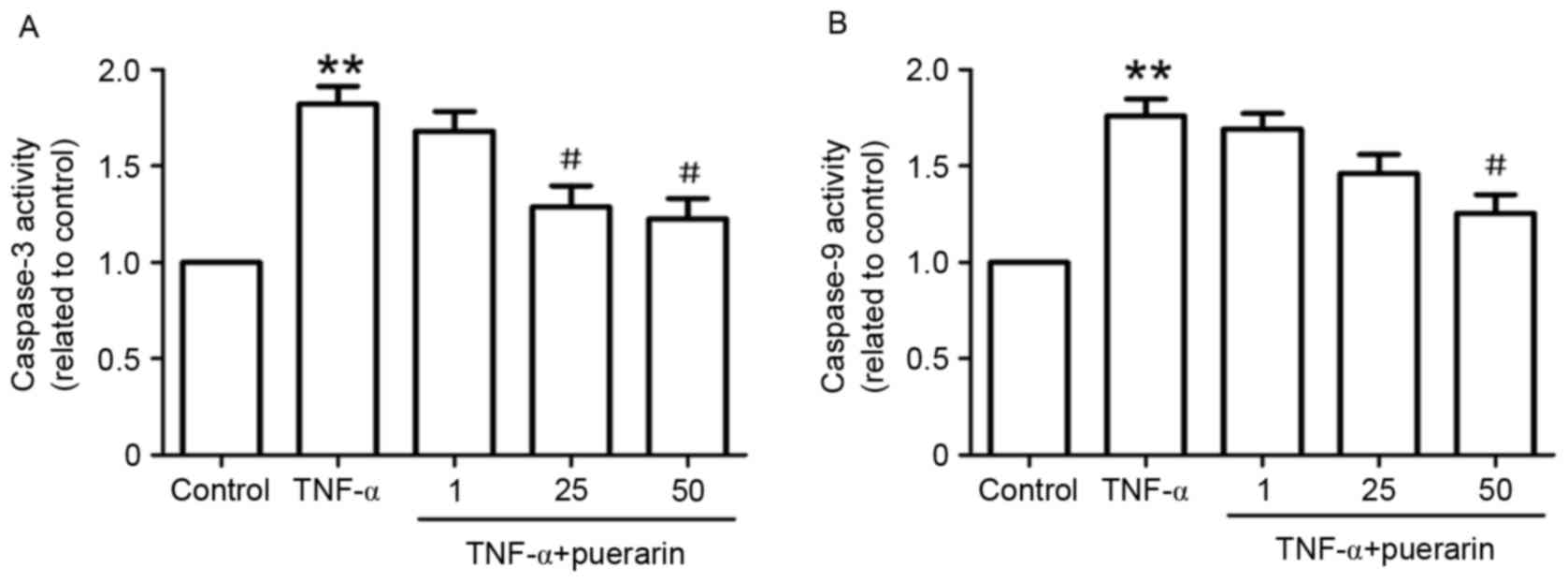
Puerarin suppresses enzymatic activity
of caspase-3 and −9
In line with the results on the apoptotic rate, the
enzymatic activity of caspase-3, a marker of apoptosis, was found
to be markedly increased in TNF-α-treated cells. Pre-treatment with
puerarin (25 and 50 µM) significantly suppressed TNF-α-induced
enzymatic activity of caspase-3 (Fig.
3A). Furthermore, the enzymatic activity of caspase-9 in
TNF-α-treated PC12 cells was investigated. As shown in Fig. 3B, puerarin (50 µM) distinctly
restrained TNF-α-induced activation of caspase-9, suggesting that
the mitochondrial apoptotic pathway may be involved in the
suppressive effect of puerarin.
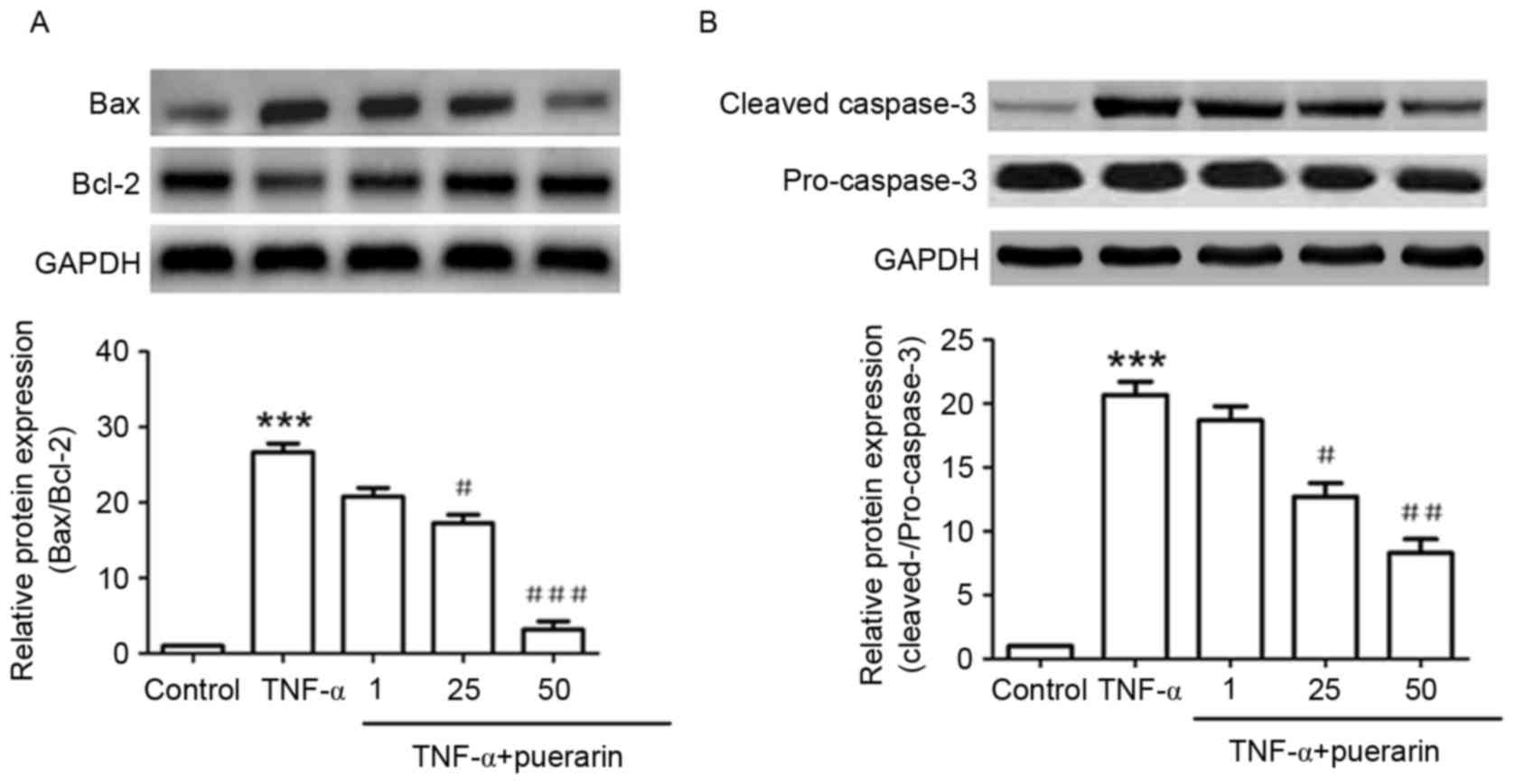
Puerarin inhibits TNF-α-induced
increases in Bax/Bcl-2 ratio and caspase-3 activation
As shown in Fig. 4A,
TNF-α significantly induced the expression of Bax and inhibited
that of Bcl-2, which was consistent with the fact that TNF-α
induced apoptosis in PC12 cells. The Bax/Bcl-2 ratio increased by
~25-fold upon treatment with TNF-α, while in cells that had been
pre-treated with 25 or 50 µM puerarin, the Bax/Bcl-2 ratio was
significantly decreased to ~16- and 3-fold of that in the control
group, respectively (P<0.05). In addition, TNF-α evidently
induced the activation of cleaved caspase-3 protein. Puerarin (25
and 50 µM) significantly decreased the levels of cleaved caspase-3,
demonstrating its inhibitive function on TNF-α-induced apoptosis
(P<0.05; Fig. 4B).
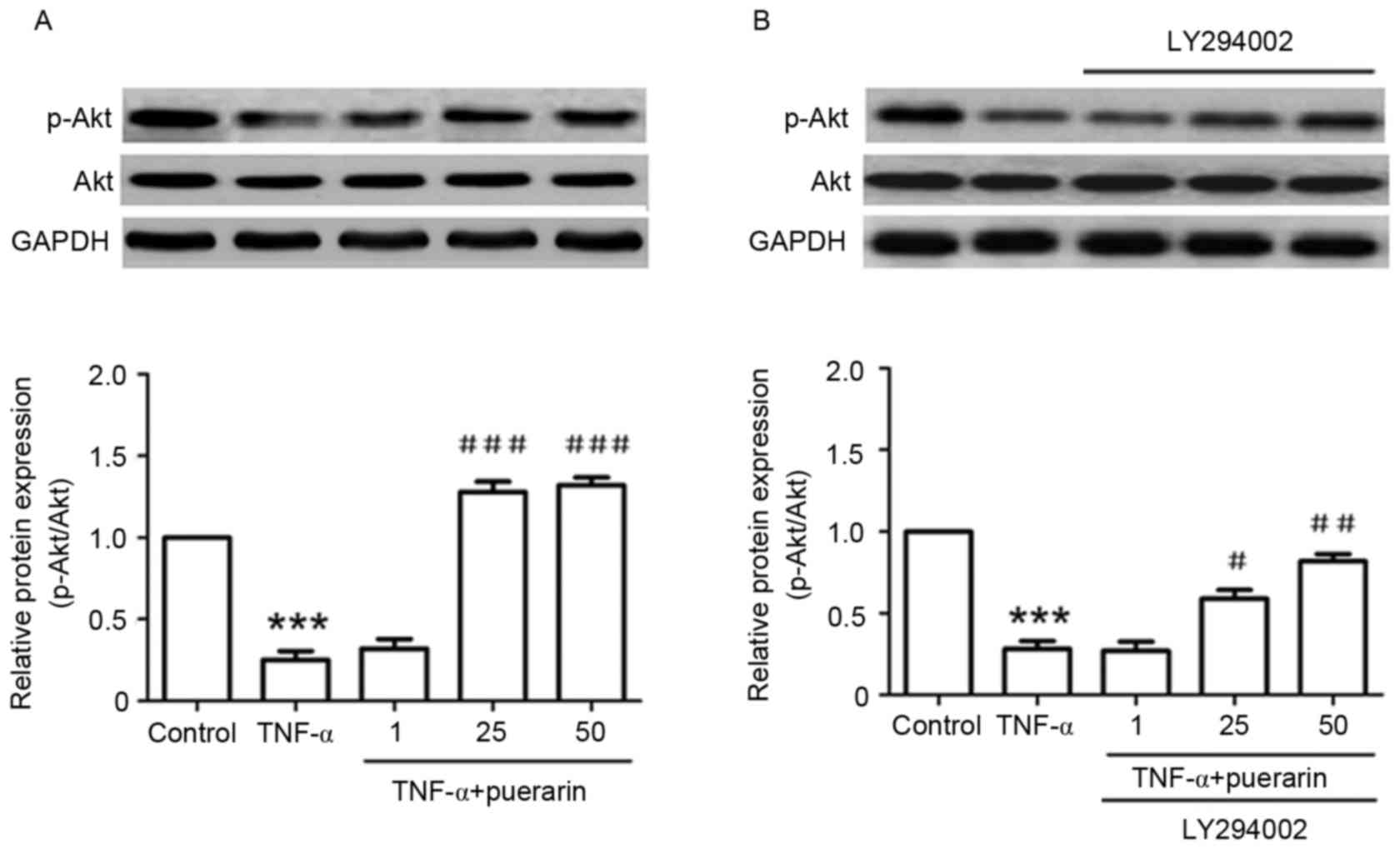
Puerarin inhibits TNF-α-induced
decreases of p-Akt (Ser473)
In addition, the detailed mechanism underlying the
suppressive effect of puerarin was investigated. TNF-α
significantly suppressed the phosphorylation of Akt (Ser473)
compared with that in the control group (P<0.001). Conversely,
pre-treatment with puerarin prior to exposure to TNF-α
significantly promoted the activation of Akt by its phosphorylation
at Ser473 (P<0.05; Fig. 5A). Of
note, the PI3K inhibitor LY294002, applied to the cells prior to
puerarin treatment, obviously reversed the p-Akt activation
achieved by puerarin treatment (P<0.05), suggesting that
puerarin prevented TNF-α-induced apoptosis in PC12 cells via
activation of the PI3K/Akt signaling pathway (Fig. 5B).
Discussion
It is well-known that TNF-α has an important role in
the neuroinflammatory response linked with several
neurodegenerative diseases, including Parkinson's disease,
Alzheimer's disease and multiple sclerosis, which are associated
with neuronal apoptosis (14). The
present study suggested that puerarin prevented TNF-α-induced
apoptosis in PC12 cells through anti-apoptotic mechanisms,
including activation of the PI3K/Akt signaling pathway, suggesting
its promising prospect as a neuroprotective drug candidate.
Puerarin, the major bioactive constituent of
Radix puerariae, is widely used in China for the treatment
of cardiovascular diseases and diabetes (15). The neuroprotective potential of
puerarin has been comprehensively evaluated in cell cultures and
rodent models of several neurodegenerative diseases (16). A previous study showed that puerarin
suppressed 6-hydroxydopamine-induced nitric oxide (NO) production
and neurotoxicity in PC12 cells and primary rat midbrain neurons,
which was attributed to the activation of arginase-2 in the
L-arginine-NO pathway (17). In
addition, puerarin significantly prevented
1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity
in PC12 cells through inhibition of the c-Jun N-terminal kinase
(JNK) signaling pathway (18).
Furthermore, puerarin exerted a protective effect against
epilepsy-induced brain injury through antioxidant and
anti-apoptotic mechanisms (19). In
line with these findings, puerarin also prevented amyloid β-induced
neurotoxicity in PC12 cells. In the present study, various
concentrations of puerarin (1–100 µM) showed no cytotoxicity
(Fig. 1A), while TNF-α
(3×105 U/l) obviously inhibited the proliferation of
PC12 cells (Fig. 1B). Of note,
puerarin (25 and 50 µM) significantly attenuated TNF-α-induced
apoptosis of PC12 cells. To the best of our knowledge, the present
study was the first to report that puerarin reduces TNF-α-induced
neurotoxicity in PC12 cells.
Apoptosis is a programmed cell death and is induced
via well-characterized intrinsic and extrinsic pathways. The
intrinsic pathway is initiated in response to stress within the
cell, such as DNA damage and endoplasmic reticulum stress. The
extrinsic pathway is initiated through activation of pro-apoptotic
receptors on the cell surface, which are activated by pro-apoptotic
ligands (20). Accumulating evidence
has indicated that puerarin exerts its neuroprotective effect by
inhibition of neuronal apoptosis. In PC12 cells treated with
amyloid β, suppression of apoptosis by puerarin was shown to
involve modulation of the levels of pro- and anti-apoptotic
proteins, including Bax, phosphorylated Bcl-2-associated death
promoter and Bcl-2 (21). Puerarin
significantly prevented MPP+-induced cytostatic
activities, caspase-3 activation and DNA fragmentation in PC12
cells via suppressing the activation of caspase-9 and release of
cytochrome c (22).
Consistent with these previous findings, puerarin inhibited
TNF-α-induced increases in the Bax/Bcl-2 ratio and caspase-3
activation in the present study. The suppressive effect of puerarin
was further demonstrated by the decreased enzymatic activity of
caspase-3, which acts as an essential executor and biomarker of
apoptosis in mammalian cells. In addition, the involvement of
enzymatic activity of caspase-9 suggested that TNF-α-induced
neuronal apoptosis and its inhibition by puerarin proceed via the
mitochondrial apoptotic pathway.
Although the precise molecular mechanisms by which
puerarin exerts its anti-apoptotic effects remain to be fully
clarified, the results of the present and previous studies
indicated that the PI3K/Akt signaling pathway has a pivotal role in
the process. A recent study indicated that puerarin restrained the
progression of cardiac hypertrophy and apoptosis, which was
probably mediated by the blockade of PI3K/Akt and JNK signaling
pathways (23). In diabetic mice,
puerarin exerted a protective effect on pancreatic β-cell function
and survival, which was mediated via the PI3K/Akt pathway (24). Treatment with puerarin effectively
inhibited lead-induced apoptosis in the kidney, which was
associated with its antioxidant activity and its ability to
modulate the PI3K/Akt/endothelial NO synthase signaling pathway
(25). Furthermore,
puerarin-mediated attenuation of amyloid β-induced microglial
apoptosis was dependent upon activation of the PI3K survival
pathway and phosphorylation of Akt (26). Puerarin inhibited lead
acetate-induced oxidative stress in PC12 cells, associated with
inhibiting PI3K and Akt phosphorylation, through increasing
glutathione synthesis (27). In line
with these results, the present study also showed that puerarin
inhibited TNF-induced apoptosis and decreases of Akt activation in
PC12 cells, which was associated with its neuroprotective
effect.
In conclusion, the present study showed that
puerarin prevented TNF-α-induced apoptosis of PC12 cells via the
PI3K/Akt signaling pathway, resulting in inhibition of
TNF-α-mediated caspase-3 activation and increases in the Bax/Bcl-2
ratio. These findings partly explained the mechanisms underlying
the neuroprotective effect of puerarin, suggesting its potential
application in the clinical treatment of neuroinflammation involved
in neurodegenerative diseases such as Parkinson's disease,
Alzheimer's disease and multiple sclerosis.
References
|
1
|
Carson MJ, Thrash JC and Walter B: The
cellular response in neuroinflammation: The role of leukocytes,
microglia and astrocytes in neuronal death and survival. Clin
Neurosci Res. 6:237–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu WM: Tumor necrosis factor. Cancer
Lett. 328:222–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okouchi M, Ekshyyan O, Maracine M and Aw
TY: Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal.
9:1059–1096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin TF and Grishanin RN: PC12 cells as
a model for studies of regulated secretion in neuronal and
endocrine cells. Methods Cell Biol. 71:267–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Liu J, Jin NA, Xu D, Wang J, Han Y
and Yin N: Fructus Corni extract-induced neuritogenesis in PC12
cells is associated with the suppression of stromal interaction
molecule 1 expression and inhibition of Ca2+ influx. Exp Ther Med.
9:1773–1779. 2015.PubMed/NCBI
|
|
6
|
Yin N, Hong X, Han Y, Duan Y, Zhang Y and
Chen Z: Cortex Mori Radicis Extract induces neurite outgrowth in
PC12 cells activating ERK signaling pathway via inhibiting Ca (2+)
influx. Int J Clin Exp Med. 8:5022–5032. 2015.PubMed/NCBI
|
|
7
|
Maji AK, Pandit S, Banerji P and Banerjee
D: Pueraria tuberosa A review on its phytochemical and therapeutic
potential. Nat Prod Res. 28:2111–2127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie N, Wang C, Lian Y, Wu C, Zhang H and
Zhang Q: Puerarin protects hippocampal neurons against cell death
in pilocarpine-induced seizures through antioxidantand
anti-apoptotic mechanisms. Cell Mol Neurobiol. 34:1175–1182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Xie N, Zhang H, Li Y and Wang Y:
Puerarin protects against β-amyloid-induced microglia apoptosis via
a PI3K-dependent signaling pathway. Neurochem Res. 39:2189–2196.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu LJ, Liu LQ, Bo T, Li SJ, Zhu Z, Cui RR
and Mao DA: Puerarin suppress apoptosis of human osteoblasts via
ERK signaling pathway. Int J Endocrinol. 2013:7865742013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan Y, Zong J, Zhou H, Bian ZY, Deng W,
Dai J, Gan HW, Yang Z, Li H and Tang QZ: Puerarin attenuates
pressure overload-induced cardiac hypertrophy. J Cardiol. 63:73–81.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang HY, Liu YH, Wang HQ, Xu JH and Hu
HT: Puerarin protects PC12 cells against beta-amyloid-induced cell
injury. Cell Biol Int. 32:1230–1237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q, Huang WD, Lv XY and Yang YM:
Puerarin protects differentiated PC12 cells from H2O2-induced
apoptosis through the PI3K/Akt signalling pathway. Cell Biol Int.
36:419–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frankola KA, Greig NH, Luo W and Tweedie
D: Targeting TNF-α to elucidate and ameliorate neuroinflammation in
neurodegenerative diseases. CNS Neurol Disord Drug Targets.
10:391–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Lam TN and Zuo Z: Radix
PuerariaeAn overview of its chemistry, pharmacology,
pharmacokinetics, and clinical use. J Clin Pharmacol. 53:787–811.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou YX, Zhang H and Peng C: Puerarin: A
review of pharmacological effects. Phytother Res. 28:961–975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao J, Cheng Y, Yang C, Lau S, Lao L,
Shuai B, Cai J and Rong J: Botanical drug puerarin attenuates
6-hydroxydopamine (6-OHDA)-induced neurotoxicity via upregulating
mitochondrial enzyme arginase-2. Mol Neurobiol. 53:2200–2201. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bo J, Ming BY, Gang LZ, Lei C and Jia AL:
Protection by puerarin against MPP+-induced neurotoxicity in PC12
cells mediated by inhibiting mitochondrial dysfunction and
caspase-3-like activation. Neurosci Res. 53:183–188. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie N, Wang C, Lian Y, Wu C, Zhang H and
Zhang Q: Puerarin protects hippocampal neurons against cell death
in pilocarpine-induced seizures through antioxidant and
anti-apoptotic mechanisms. Cell Mol Neurobiol. 34:1175–1182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lockshin RA and Zakeri Z: Programmed cell
death and apoptosis: Origins of the theory. Nat Rev Mol Cell Biol.
2:545–550. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing G, Dong M, Li X, Zou Y, Fan L, Wang
X, Cai D, Li C, Zhou L, Liu J and Niu Y: Neuroprotective effects of
puerarin against beta-amyloid-induced neurotoxicity in PC12 cells
via a PI3K-dependent signaling pathway. Brain Res Bull. 85:212–218.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, Zhou L, Zhang Y, Dong M, Li X, Liu
J and Niu Y: Implication of the c-Jun-NH2-terminal kinase pathway
in the neuroprotective effect of puerarin against
1-methyl-4-phenylpyridinium (MPP+)-induced apoptosis in PC-12
cells. Neurosci Lett. 487:88–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang YP and Jeong HG: Mechanism of
phytoestrogen puerarin-mediated cytoprotection following oxidative
injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and
HO-1. Toxicol Appl Pharmacol. 233:371–381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Shangguan Z, Liu Y, Wang J, Li X,
Yang S and Liu S: Puerarin protects pancreatic β-cell survival via
PI3K/Akt signaling pathway. J Mol Endocrinol. 53:71–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu CM, Ma JQ and Sun YZ: Puerarin
protects rat kidney from lead-induced apoptosis by modulating the
PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol. 258:330–342. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhuiyan MM Haque, Mohibbullah M, Hannan
MA, Hong YK, Han CH, Kim YK and Moon IS: The neuritogenic and
synaptogenic effects of the ethanolic extract of radix Puerariae in
cultured rat hippocampal neurons. J Ethnopharmacol. 173:172–182.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li C, Pan Z, Xu T, Zhang C, Wu Q and Niu
Y: Puerarin induces the upregulation of glutathione levels and
nuclear translocation of Nrf2 through PI3K/Akt/GSK-3β signaling
events in PC12 cells exposed to lead. Neurotoxicol Teratol. 46:1–9.
2014. View Article : Google Scholar : PubMed/NCBI
|