Introduction
Osteosarcoma (OS) a primary sarcoma of the bones
that primarily affects children and adolescents accounting for ~5%
of pediatric tumors (1). OS affects
the distal long bones, the femur and tibia (1) and is generally characterized by its
local invasion of bone and soft tissues, loss of the affected
extremity's functions and distant metastasis (2). Multimodal conventional therapies
including radiation, surgical resection and chemotherapy are
employed to treat OS (3,4). Despite the combination therapy
approach, limited improvement has been observed in the 5-year
survival rate (65%) of patients with OS (5). Furthermore, these therapeutic
approaches often lead to severe side effects such as
cardiotoxicity, hearing loss and nephrotoxicity, and may also cause
drug resistance and increase the risk of local relapse (6). Thus, improved targeted approaches are
required, with no or less side effects.
The Janus kinase 2 (JAK2)/signal transducer and
activator of transcription 3 (STAT3) signalling pathway serves a
critical role in cell survival and division. Activated JAK2/STAT3
signalling cascade influences the expression of numerous proteins
that are associated with various physiological functions, including
cell cycle regulation and apoptosis. The JAK/STAT3 pathway
regulates the expression level of anti-apoptotic proteins [B-cell
lymphoma-extra large (Bcl-xL) and myeloid leukemia cell
differentiation protein (Mcl-1)] (7), cell cycle regulatory proteins (cyclin
D1, p21, and p27) (8) and
mitochondrial apoptosis pathway related proteins [Bcl-2 associated
X (Bax), cytochrome c and caspase-3] (9). Furthermore, STAT3 activation promotes
angiogenesis by inducing vascular endothelial growth factor (VEGF)
(10), and also stimulates invasion
and metastasis by increasing the expression of matrix
metalloproteinases (MMPs) (11). The
pathway is considered as a major molecular target of interest in a
number of cancer types, including melanoma (12), renal carcinoma (13) and breast cancer (14).
The mitogen-activated protein kinases (MAPKs)
signalling cascades are a large family of serine/threonine kinases
that control and regulate various physiological process, including
cell survival and apoptosis, and are also involved in
tumorigenesis. The functions of MAPK signalling in cancer
development are complex, as they regulate wide range of cellular
responses (15). Activated MAPK
pathway stimulates cell growth or induces apoptosis based on the
stimuli (16,17). Numerous studies have reported that
the c-Jun N-terminal kinases (JNK), p38 and extracellular
signal-regulated kinases (ERK1/2) cascades exert a vital role in
regulating cytotoxic drug induced apoptosis in OS (18,19).
Thus, targeting the pathway may have clinical value in the
treatment of OS.
Accumulating research data indicate that
plant-derived compounds are much more effective in inhibiting
cancer cell proliferation and inducing apoptosis (20). Phytochemicals are reported to elicit
antitumor effects by inducing cellular defense system, antioxidant
enzymes system and also inhibition of anti-cell growth signalling
and anti-inflammatory pathways culminating in apoptosis and/or cell
cycle arrest (21–24).
Cucurbitacins were initially identified in the
Cucurbitaceae plant family, which includes cucumber and are also
isolated from various plant families (25). Owing to their effective
pharmacological properties, plants rich in cucurbitacins have been
widely used in traditional Chinese medicine for their analgesic,
anti-inflammatory, antimicrobial, antipyretic, antitumor activities
and hepatoprotective effects (25–28).
Researchers have reported that cucurbitacin I may inhibit cancer
cell growth by disrupting the JAK/STAT3 signaling pathway in both
in vitro and in vivo tumor models (29,30).
Studies have reported that cucurbitacin B inhibits the growth of
various human cancer cell lines and tumor xenografts including
breast, prostate, lung, uterine cervix, liver, skin and brain
cancer (18,31,32).
Considering the biological effects cucurbitacin B, the present
study aimed investigate the effects of cucurbitacin B on human OS
cells and assess whether it modulates the JAK/STAT3 and MAPK
signalling pathways.
Materials and methods
Reagents and chemicals
Cucurbitacin B, glutamine, RPMI 1640 medium, fetal
calf serum (FCS), penicillin, streptomycin and 0.25% trypsin were
purchased from Sigma Aldrich; Merck KGaA (Darmstadt, Germany).
Antibodies against ERK1/2 (cat. no. 9102), phospho-ERK1/2 (cat. no.
9101), p38 (rabbit mAb, cat. no. 8690), phospho-p38 (rabbit mAb,
cat. no. 4511), JNK (cat. no. 9252), phospho-JNK (mouse mAb, cat.
no. 9255), MMP-2 (rabbit mAb, cat. no. 87809), MMP-9 (cat. no. 852)
(all from Cell Signaling Technology, Danvers, MA, USA), Bcl-xL
(cat. no. sc-8392), Bcl-2-associated death promoter (Bad) (cat. no.
sc-8044), Bax (cat. no. sc-4239), Bcl-2 (cat. no. sc-509), VEGF
(cat. no. sc-4571), caspase-9 (cat. no. sc-81663), caspase-8 (cat.
no. sc-81656), caspase-3 (cat. no. sc-176260), p-JAK2 (cat. no.
sc-34479), JAK2 (cat. no. sc-34479), p-STAT3 (cat. no. sc-56747)
and STAT3 (cat. no. sc-482) (all from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) were used for expression analysis. All other
reagents used in the study were purchased from Sigma Aldrich; Merck
KGaA, unless otherwise stated.
Cell lines and culture
Human osteosarcoma cell line, U-2 OS was obtained
from the Cell Bank of Shanghai Institute of Biochemistry and Cell
Biology (Chinese Academy of Sciences, Shanghai, China). The cells
were cultured and maintained in RPMI-1640 medium supplemented with
10% FCS, 2 mM glutamine, streptomycin (100 µg/ml) and penicillin
(100 U/ml) at 37°C in a humidified atmosphere with 5%
CO2.
Measurement of cell viability
OS cells were seeded into 96-well plates
(5×105 cells/well) and incubated at 37°C for 24 h. On
reaching 70% confluence, the cells were treated with different
concentrations of cucurbitacin B (20, 40, 80 and 100 µM) for 24 h.
Cells were then incubated at 37°C with MTT for 4 h. The formazan
crystals formed were dissolved with dimethyl sulfoxide and the
absorbance was measured at 570 nm using a Thermo Multiskan Spectrum
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Analysis of apoptosis by Annexin V
assay
OS cells were incubated at 37°C with various
concentrations of cucurbitacin B (20, 40, 80 and 100 µM) for 24 h.
Following incubation, cells were treated with 0.25% trypsin, washed
twice with ice-cold PBS and harvested for detection of apoptosis
using an Annexin V-FITC apoptosis detection kit (BD Biosciences,
Franklin Lakes, NJ, USA), according to the manufacturer's
instructions. The cells were washed in PBS at 1×106
cells/ml and suspended at 1×105/100 µl binding buffer
(BD Biosciences) containing FITC-conjugated anti-Annexin V antibody
(provided in the kit) and PI, and analyzed for fluorescence using a
flow cytometer (FACSCalibur; BD Biosciences).
Determination of morphological changes
by Hoechst staining
Morphological changes of the U-2 OS cells following
treatment with cucurbitacin B was determined by Hoechst staining.
Briefly, the OS cells were seeded at a density of 5×104
cells per well in a 6-well plate and cultured at 37°C for 12 h and
treated with cucurbitacin B (20, 40, 80 and 100 µM) for 48 h. The
cells were then fixed with 4% paraformaldehyde in PBS for 10 min,
washed with PBS and stained with Hoechst 33258 (5 mg/l) for 15 min
at room temperature in the dark. The cells were observed under a a
fluorescence microscope (Nikon Eclipse TiS coupled with
NIS-Elements imaging software; Nikon Corporation, Tokyo, Japan).
The cells undergoing apoptosis were characterized by condensed or
fragmented nuclei.
Wound healing assay
The influence of cucurbitacin B on the cell
migration of U-2 OS cells was assessed. In brief, 5×105
cells were maintained in 10 cm Petri plates and incubated for 24–72
h until they reached complete confluency and were wounded using a
200 µl pipette tip. All cells in the plates were treated with final
concentrations of cucurbitacin B (25, 50 and 100 µM; 3 plates for
each concentration) and then were incubated at 37°C in fresh
culture medium for 24 h. The migration of the cells in the wounded
area was measured as previously described (33). Cell migration was determined as a
percentage of the cell-free area compared with the area of the
initial wounded area. The fields were photographed using an
inverted microscope (Nikon Eclipse TS2; Nikon Corporation).
Gel zymography
Gel zymography was performed to evaluate the
activities of MMP-2 and MMP-9. Sample preparation was done as
previously described by Mizoguchi et al (34). Samples were run on SDS-PAGE (10%)
gels that contained 0.1% gelatin under non-reducing conditions.
Triton X-100 (2.5%) was used to wash the gels to remove SDS, then
the gels were washed further in incubation buffer (50 mM Tris HCl,
0.15 M NaCl, 10 mM CaCl2) (25±2°C) for 30 min and
incubated (24 h, 37°C). Following incubation, the gels were stained
with Coomassie Brilliant Blue (1% Coomassie Brilliant Blue G-250,
30% methanol and 10% acetic acid) for 3 h and then were destained
(7% acetic acid, 40% methanol) until clear bands representing
gelatinolysis were seen against dark background. By using an Atto
Densitograph Software Library Lane Analyser (Atto Instruments,
Inc., Rockville, MD, USA) the total activity was determined.
Western blot analysis
Following treatment with cucurbitacin B (25, 50 and
100 µM), U-2 OS cells were washed twice with ice-cold PBS and
centrifuged at 10,000 × g for 5 min at 4°C. The cells were treated
in ice-cold hypotonic lysis buffer (10 mM HEPES pH 7.9, 1.5 mM
MgCl2, 0.2 mM KCl, 0.5 mM dithiothreitol, 0.2 mM
phenylmethyl-sulphonylfluoride), vortexed for 2 min, centrifuged at
12,000 × g for 10 min at 4°C and the supernatants were collected
and assayed for protein content using the Bradford method (35). Equal amounts of protein samples (60
µg) were subjected to electrophoresis in 12% SDS-PAGE and the bands
were then transferred to nitrocellulose membranes (GE Healthcare
Lifesciences, Chalfont, UK). The membranes were washed and blocked
using blocking buffer (5% non-fat dry milk/0.1% Tween-20 in TBS)
for 1 h at room temperature. They were then incubated overnight at
4°C with respective primary antibodies followed by incubation at
room temperature for 40 min with horseradish
peroxidase-conjugated-secondary antibody (mouse mAb; Cell Signaling
Technology, cat. no. 7076; dilution 1:1,000). The immunoreactive
bands were detected by enhanced chemiluminescence procedure (GE
Healthcare Lifesciences). Protein expression levels were normalized
with the expression levels of β-actin using anti-β-actin antibody
(AC-15) from Santa Cruz Biotechnology, Inc. (cat. no. sc-69879;
dilution 1:1,000). The positive signals from the immunoblots (n=3)
were quantified using Image Lab Software (v5.1) (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data are presented as mean ± standard deviation,
from three or six individual experiments. The values were analyzed
by one-way analysis of variance followed by Duncan's Multiple Range
Test. All statistical analyses were performed using the SPSS
software (version 22.0; IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
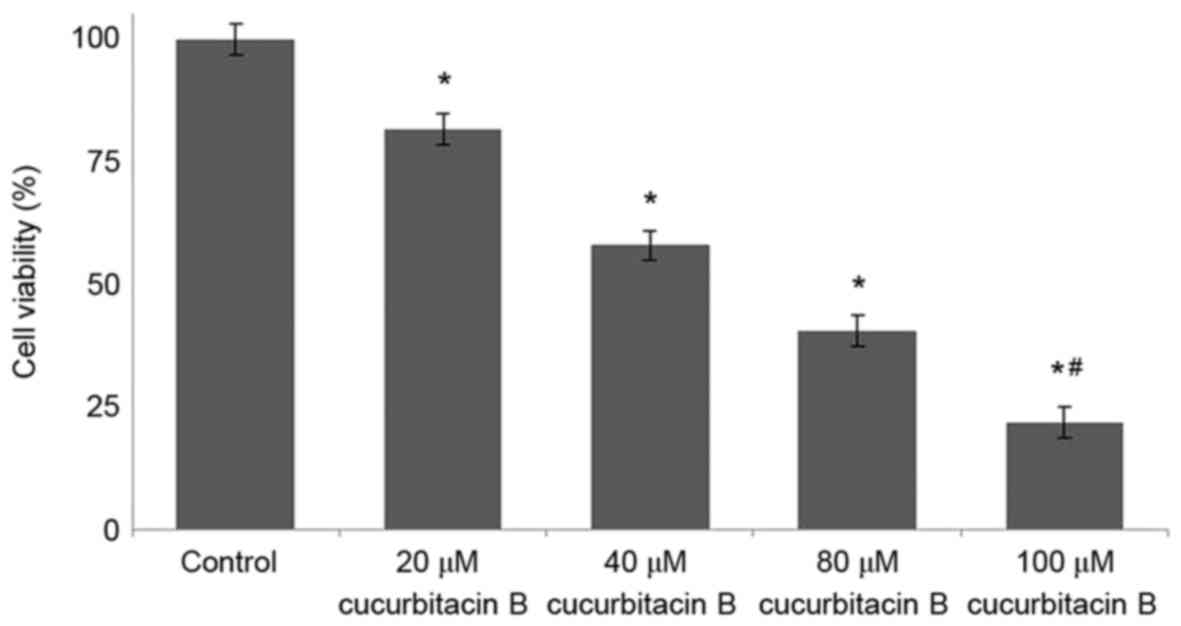
Cucurbitacin B inhibits U-2 OS cell
proliferation
The viability of osteosarcoma cells following
treatment with cucurbitacin B was determined by MTT assay, and the
findings are presented in Fig. 1.
Osteosarcoma cells treated with different concentrations of
cucurbitacin B (20, 40, 80 and 100 µM) exhibited reduced cell
proliferation in a concentration-dependent manner. The viability
percentage of U-2 OS cells treated with cucurbitacin B at 20 µM was
83%, whereas following the 100 µM dose, the cell viability
significantly decreased to 21% (P<0.05; Fig. 1).
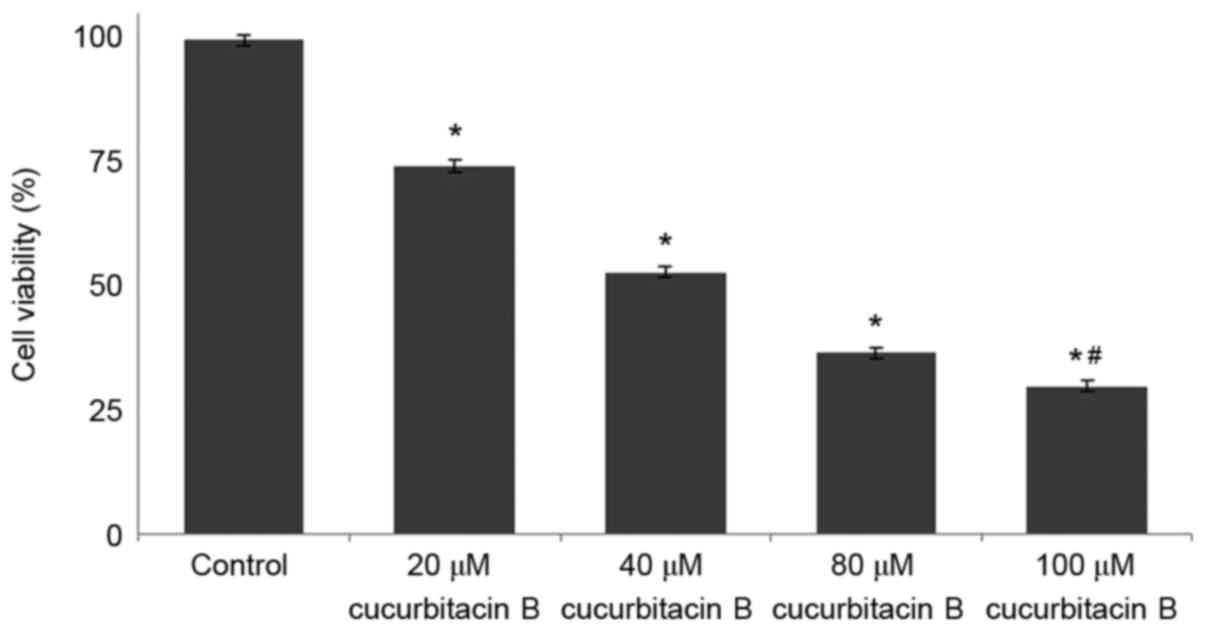
Influence of cucurbitacin B on cell
viability counts and morphological changes in U-2 OS cells
The results of MTT assay revealed that cucurbitacin
B may effectively reduce the viability of U-2 OS cells. The present
study further analyzed whether cucurbitacin B reduced viability by
inducing apoptosis. Annexin V/PI staining assay was performed to
determine apoptotic cell counts. Exposure to cucurbitacin B was
identified to significantly increase the apoptotic cell counts
(P<0.05; Fig. 2), in a
dose-dependent manner with 100 µM concentration exhibiting maximum
effects.
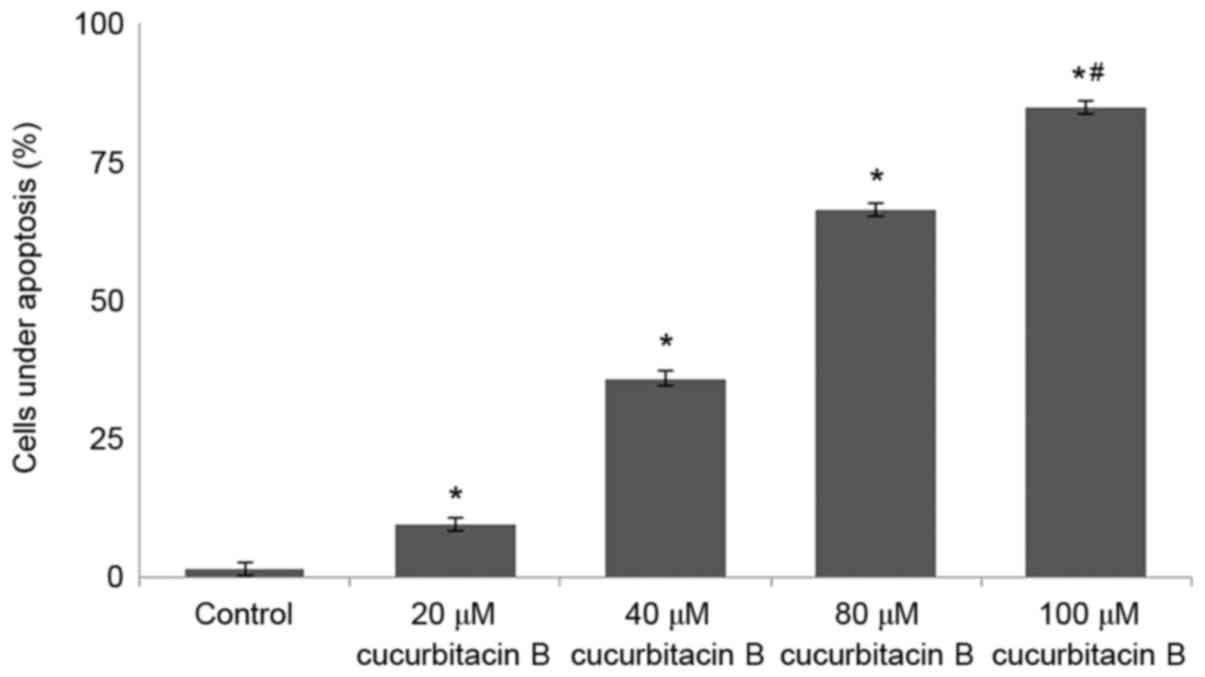
Furthermore, Hoechst 33258 staining was performed to
assess morphological changes in the U-2 OS cells following
cucurbitacin B treatment. Following treatment for 48 h, the
chromatin of U-2 OS cells was condensed and appeared to be brighter
and deeper. Notably, treatment with cucurbitacin at higher
concentrations of 80 and 100 µM demonstrated deeper staining of
nuclear chromatin than the lower doses (20 and 40 µM). Highly
condensed and stained chromatin indicates characteristics of
apoptotic cells, thus suggesting that cucurbitacin B effectively
induced apoptosis in U-2 OS cells. The percentage of apoptotic
cells increased significantly following cucurbitacin B treatment
(20, 40, 80 or 100 µM) compared with the control (P<0.05;
Fig. 3).
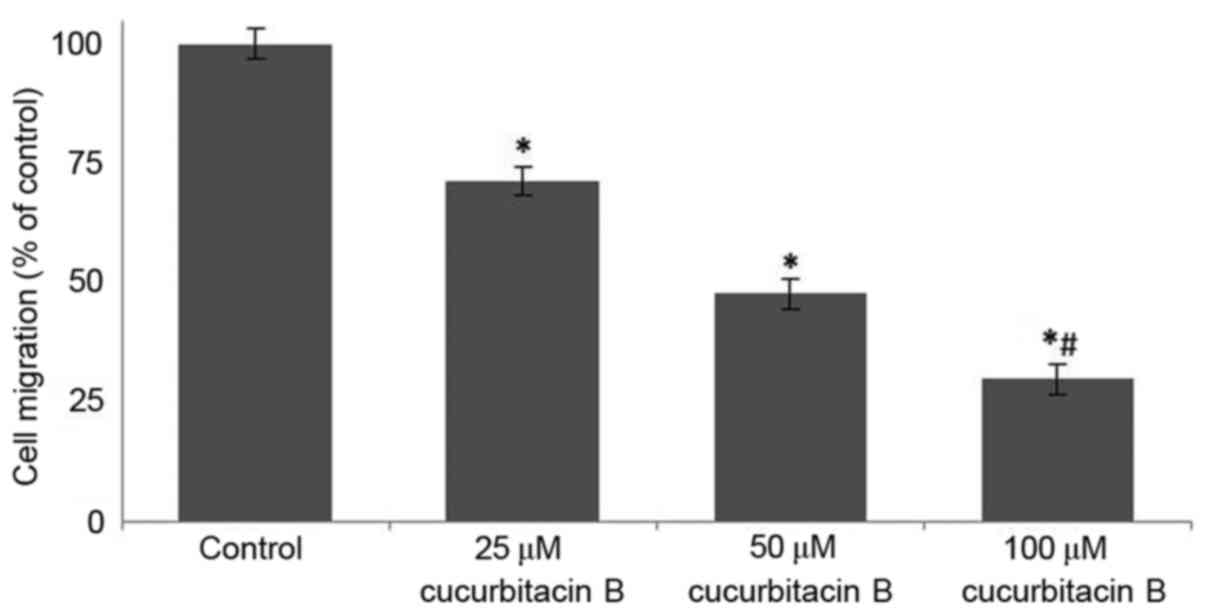
Cucurbitacin B inhibits migration of
U-2 OS cells
Following incubation with cucurbitacin B (25, 50 and
100 µM) for 24 h, the area of the wound was measured. In the cells
that were not treated with cucurbitacin B, the distance between the
scratches was significantly reduced when compared with U-2 OS cells
that were exposed to cucurbitacin, in a dose-dependent manner
(P<0.05). In the OS cells that were incubated with cucurbitacin
B the cell free area was evidently increased, the distance
increased with concentration of cucurbitacin B (Fig. 4). The growth and movement of the
cells in the cell free scratch area observed in cells that were not
treated with cucurbitacin suggests cell division and migration were
occuring, which were supressed following cucurbitacin B
treatment.
Cucurbitacin B downregulates MMPs
It has been established that MMPs principally
contribute to the invasion and metastasis of tumor cells (36–38). In
the present study, to further assess whether cucurbitacin inhibits
MMPs, the expression of MMP-2 and −9 were determined by gel
zymography and western blot analysis. The result of the current
study indicated that cucurbitacin B exposure at all assessed
concentrations caused marked inhibitions in the expressions of MMPs
(Fig. 5A). The zymography analysis
revealed significantly supressed activities of MMP-2 and −9 in a
dose-dependent manner (Fig. 5B).
Furthermore, western blot analysis revealed a markedly decreased
expression level of MMP2 and MMP9 in cucurbitacin B-treated cells.
However, relatively little inhibition was observed following
treatment with 25 µM cucurbitacin when compared with higher doses,
50 and 100 µM. Furthermore, marked suppression of VEGF levels was
also observed following cucurbitacin B exposure in a dose-dependent
manner (Fig. 5C). These observations
suggest that cucurbitacin B was able to markedly supress cell
migration and inhibit angiogenesis, potentially by downregulating
the expression of MMP2, MMP9 and VEGF.
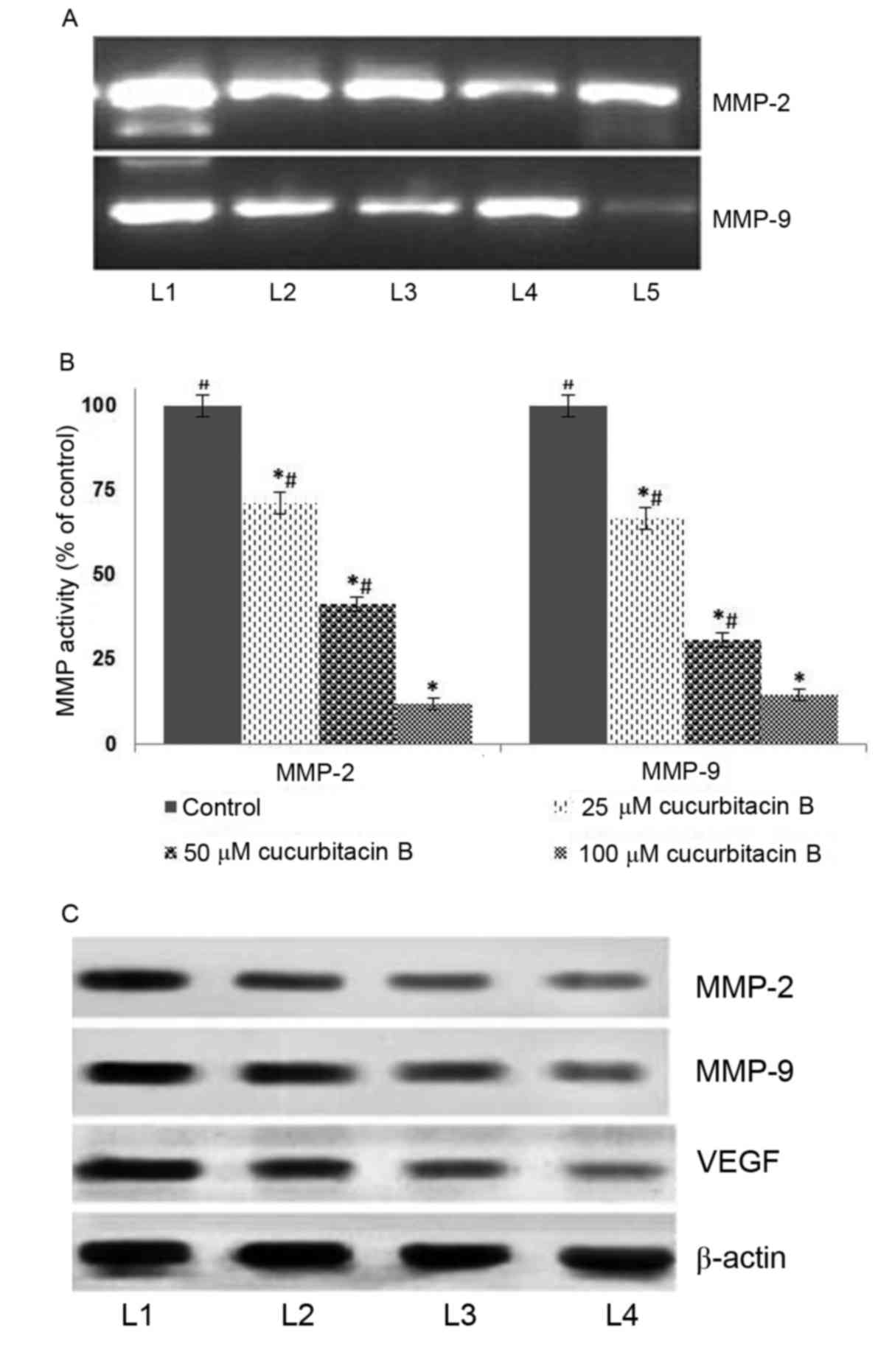 | Figure 5.Cucurbitacin B reduces activities of
MMP-2, MMP-9 and VEGF in U-2 OS cells. This was determined by (A)
gelatin zymography following exposure to cucurbitacin B, indicating
marked downregulation in the expressions of MMP-2 and −9, (B)
quantified results from the gelatin zymography indicating a
significant downregulation of MMP activity. (C) Western blot
analysis indicates a marked reduction in the expression of MMP-2,
MMP-9 and VEGF. *P<0.05 vs. control; #P<0.05 vs.
25 µM. Data are presented as mean ± standard deviation, n=3. MMP,
matrix metalloproteinases; VEGF, vascular endothelial growth
factor; OS, osteosarcoma; L1, control; L2, 20 µM cucurbitacin B;
L3, 40 µM cucurbitacin B; L4, 80 µM cucurbitacin B; L5, 100 µM
cucurbitacin B. |
Influence of cucurbitacin B on the
expressions of apoptotic pathway proteins
Apoptosis is a coordinated network of genes that
lead to cell death and is the target pathway in many anticancer
therapies. Anticancer drugs are known to induce apoptosis by
targeting cells that harbour genetic damage or that divide
inappropriately (39). The present
study assessed the influence of cucurbitacin B on the expression of
apoptotic pathway proteins in U-2 OS cells to investigate the
molecular events associated with cucurbitacin-induced cell death. A
significant increase in the expression of caspase-3, −8 and −9 in
the U-2 OS cells exposed to cucurbitacin B was observed following
western blot analysis (P<0.05; Fig.
6). The activation of caspase-9 and −8 have previously been
documented, suggesting the involvement of intrinsic and extrinsic
apoptotic pathways that subsequently lead to the activation of
caspase-3 (40). Therefore, in the
present study the enhanced expression of caspases by cucurbitacin B
indicates an increased level of apoptosis in U-2 OS cells.
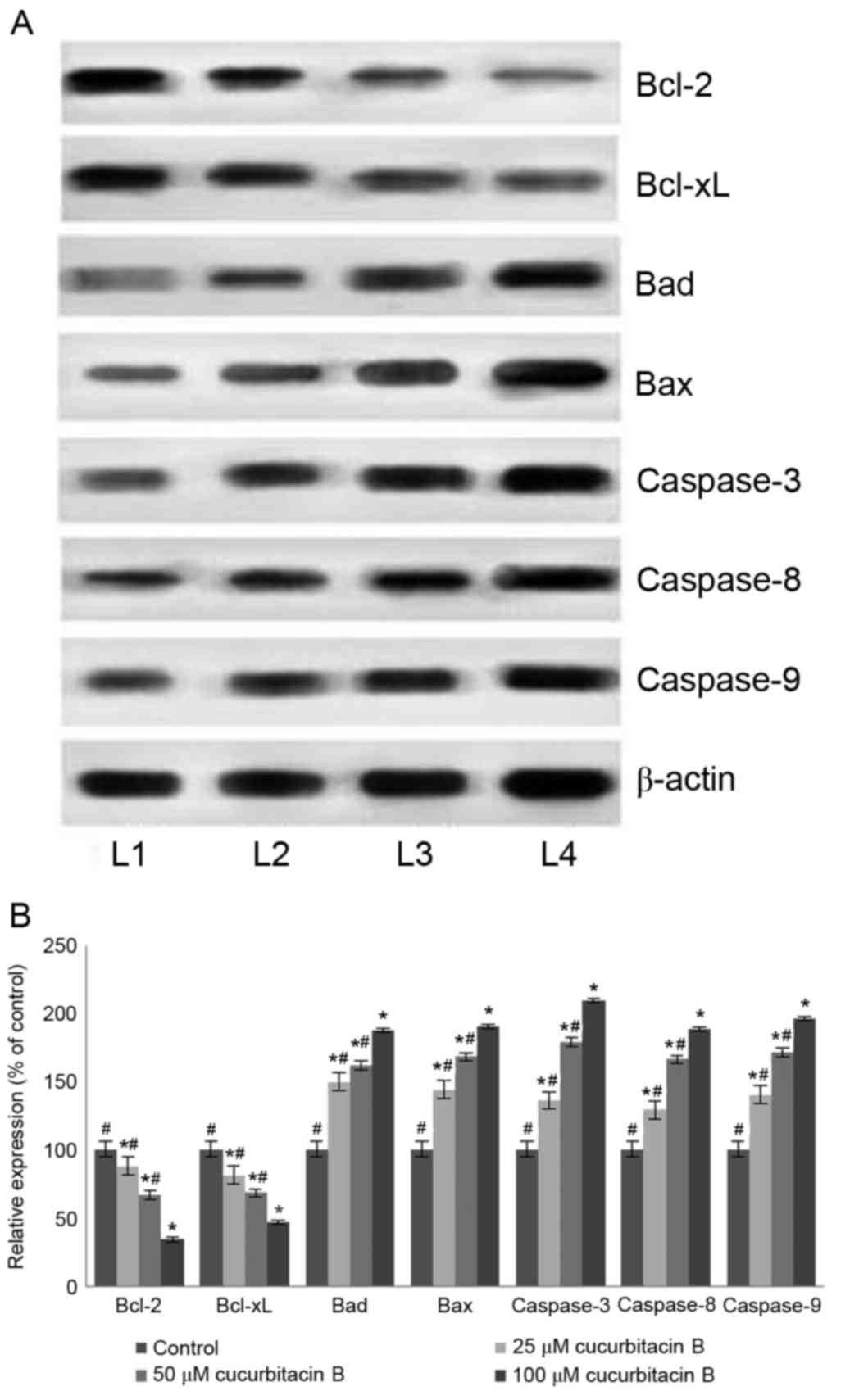 | Figure 6.Cucurbitacin B modulates the
expression of apoptosis pathway proteins. Cucurbitacin B caused
effective upregulation of caspase-3, −8 and −9 in addition to an
increase in the expression of Bax and Bad, while suppressing the
expression of Bcl-2 and Bcl-xL, demonstrated in (A) western blot
analysis and (B) quantification of the results. *P<0.05 vs.
control; #P<0.05 vs. 100 µM. Data are presented as
mean ± standard deviation, n=3. Bcl-2, B-cell lymphoma 2; Bcl-xL,
Bcl-2 extra large; Bad, Bcl-2-associated death promoter; Bax, Bcl-2
associated X; L1, control; L2, 25 µM cucurbitacin B; L3, 50 µM
cucurbitacin B; L4, 100 µM cucurbitacin B. |
Furthermore, the expression of Bcl-2 family members
was also assessed under the influence of cucurbitacin B. The Bcl-2
family are key proteins in the regulation of intrinsic
mitochondrial pathways of apoptosis, specifically controlling the
release of cytochrome c, which further regulates the
caspases cascade (41,42). The Bcl-2 family comprises
anti-apoptotic (Bcl-2 and Bcl-xL) and pro-apoptotic proteins [Bax,
Bcl-2 antagonist killer (Bak) and Bad] (43). Cucurbitacin B exposure resulted in a
multi-fold increase in the Bad and Bax protein expression
(P<0.05; Fig. 6B) with
significant downregulation of Bcl-2 and Bcl-xL expression
(P<0.05; Fig. 6B). The
upregulated expression of Bax and Bad indicates the activation of
the apoptotic pathway. Therefore, with enhanced levels of
pro-apoptotic proteins, cucurbitacin B aids in elevated levels of
caspases, which eventually induces apoptosis.
Cucurbitacin modulates the MAPK
signalling cascades
It has been well documented that activation of MAPK
pathways serves a critical role in inhibiting apoptosis. In the
present study, in order to elucidate the mechanisms underlying
cucurbitacin B-induced apoptosis, the expression levels of MAPK
cascade proteins, JNK, p-JNK, p38, p-p38, ERK1/2, and p-ERK1/2 were
analyzed by western blot analysis. The results demonstrated an
enhanced expression of the proteins in the U-2 OS cells that were
not exposed to cucurbitacin (Fig.
7). However, U-2 OS cells treated with cucurbitacin B exhibited
marked downregulation in the phosphorylated levels of ERK1/2, p38
and JNK. Whereas substantial decreases were observed in the
expression levels of p38 and ERK1/2, JNK and p-JNK levels were
decreased to a lesser extent. However, marked inhibitions were
observed following cucurbitacin B treatment at all the three
assessed concentrations, with 100 µM exerting maximum effects. The
results indicated that the downregulation of the MAPK signalling
pathway may have aided in induction of apoptosis of the U-2 OS
cells.
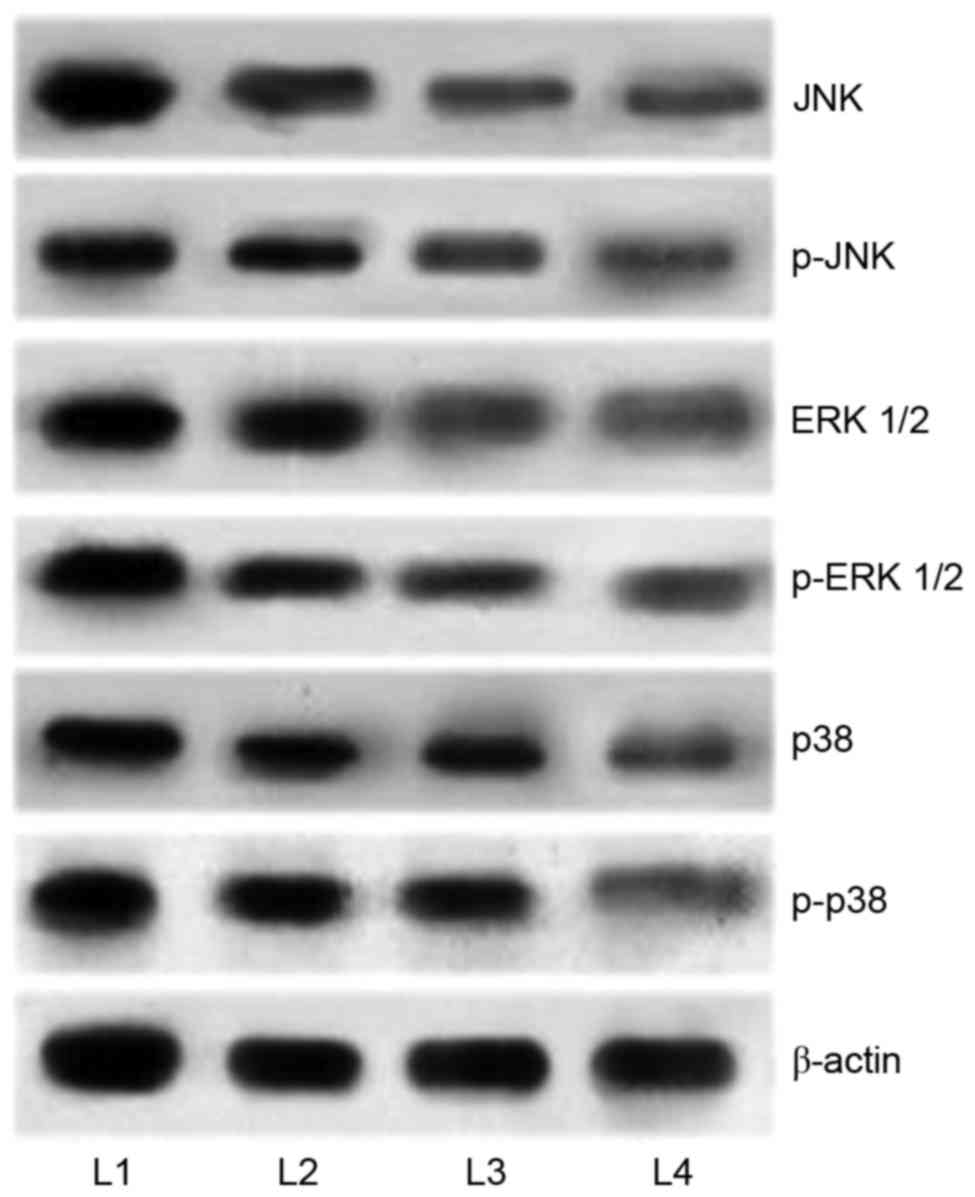 | Figure 7.Cucurbitacin B downregulates the
expression of MAPK signalling cascades. Cucurbitacin B exposure
markedly inhibited the activation of JNK, ERK1/2 and p38, thus
inhibiting the signaling pathway, demonstrated by western blot
analysis. MAPK, mitogen activated protein kinases; JNK, c-Jun
N-terminal kinases; ERK, extracellular signal-regulated kinases;
p-JNK, phosphorylated-JNK; p-ERK, phosphorylated ERK; L1, control;
L2, 25 µM cucurbitacin B; L3, 50 µM cucurbitacin B; L4, 100 µM
cucurbitacin B. |
Effects of cucurbitacin B on the
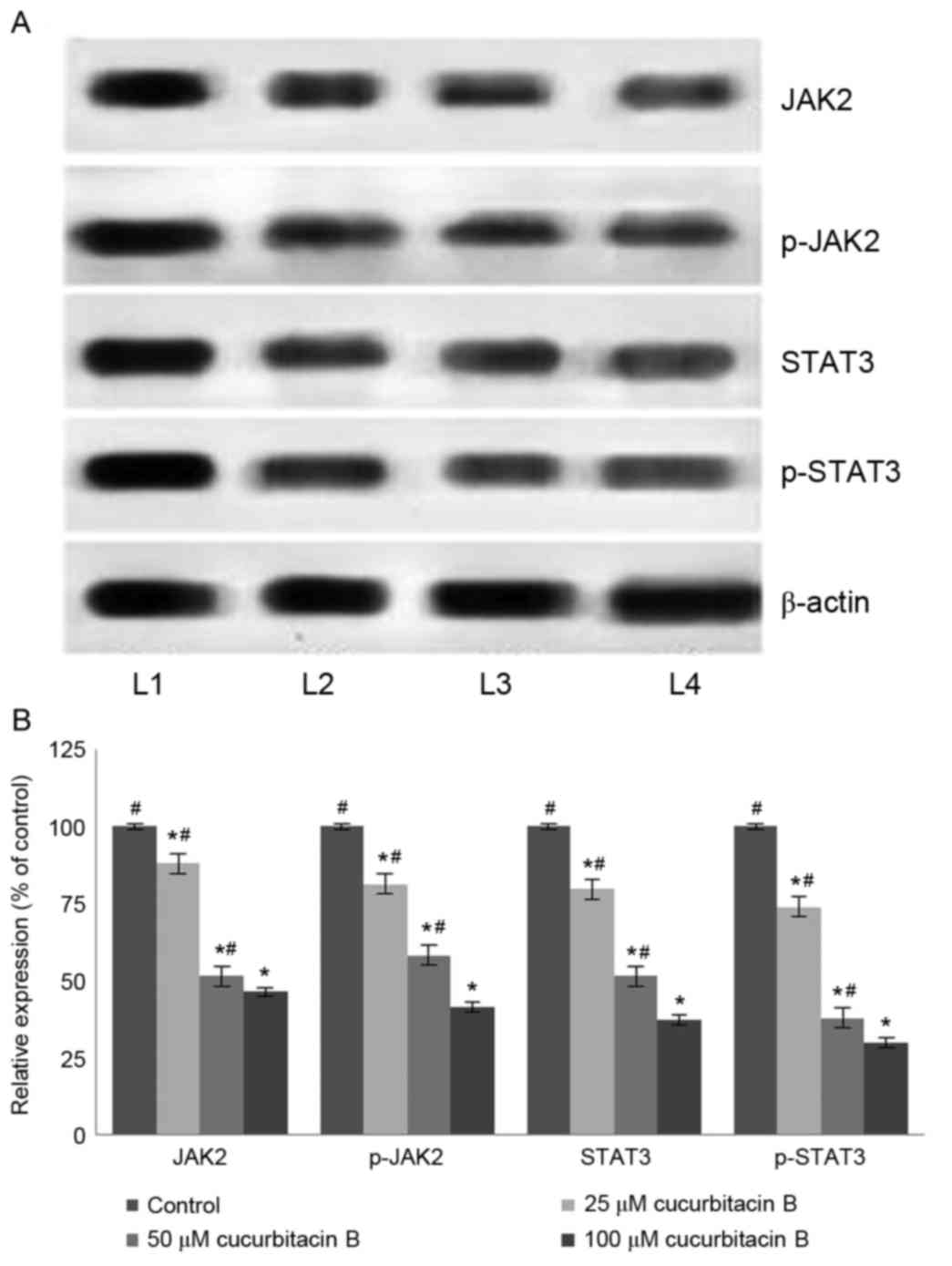
JAK2/STAT3 signalling pathway
The JAK/STAT3 pathway serves a pivotal role in the
transduction of a multitude of signals that are critically involved
in the development and homeostasis in mammals (44). STAT3 has been reported to be
constitutively activated in different types of cancer in humans
(45). It has been demonstrated that
the inhibition of STAT3 leads to apoptosis and inhibition of cancer
cell proliferation (46). In the
present study, western blot analysis was performed to assess the
expression level of phosphorylated forms of JAK2 and STAT3
following exposure to cucurbitacin B. The results of the current
study indicate that cucurbitacin B treatment significantly reduced
the levels of activated JAK2 and STAT3 (P<0.05; Fig. 8). Cucurbitacin B at 100 µM
concentration caused a multi-fold decrease in the expression of
STAT3 and JAK2. Furthermore, the inhibition of STAT3 and JAK2
expression was dose-dependent. Therefore, these observations
indicate that cucurbitacin significantly inhibited the STAT3/JAK2
pathway and this may have a part in the increase rate of apoptosis
observed.
Discussion
Cucurbitacins are widely used in traditional Chinese
medicines and are the bitter principles of Cucurbitaceae (25,47).
Cucurbitacin B, which is one of the most abundant cucurbitacins
(27) exhibits anti-inflammatory
activity and is used traditionally to treat hepatitis (28,48).
Previous studies have demonstrated the antitumor activities of
cucurbitacin B in various human cancer cell lines and tumor
xenografts (49,50). However, the mechanisms underlying the
anticancer activities are yet to be elucidated. Studies are
currently focussing on the identification and development of small
molecules that inhibit major cell signalling pathways, as an
important strategy in anticancer drug research (51–53). The
present study investigated the effects of cucurbitacin B on cell
proliferation and the MAPK and JAK2/STAT3 pathways in U-2 OS
cells.
Inhibition of cell proliferation is an important
indicator of anticancer activity. Treatment with cucurbitacin B
(20–100 µM) exhibited evident growth inhibition. Furthermore,
cucurbitacin B markedly induced the apoptosis of U-2 OS, as
observed by Hoechst staining and flow cytometry analysis followed
by Annexin V/PI staining. The events underlying the induction of
apoptosis were also analysed. Apoptosis occurs via the intrinsic
mitochondrial pathway and the extrinsic membrane death receptor
pathways. Cucurbitacin B exposure caused marked activation and
enhanced expression of caspase-9, −8 and −3. These observations
suggest that cucurbitacin B induces apoptosis via the intrinsic
pathway, which is in accordance with a previous study using
chemotherapeutic drugs as gallic acid (54). Furthermore, Liu et al
(55) reported that the majority of
chemotherapeutic drugs were able to induce apoptosis through the
intrinsic mitochondrial pathway.
The mitochondria-dependent apoptotic pathway is
governed by Bcl-2-family proteins that includes pro-apoptotic
(BH3-interacting domain death agonist, Bax, and Bak) and
anti-apoptotic (Bcl-2 and Bcl-xL) proteins (56,57). Bax
promotes apoptotic factors and induces apoptosis, whereas Bcl-2
inhibits the release of pro-apoptotic proteins (58). Cucurbitacin B notably enhanced the
expression of Bad and Bax and supressed the levels of Bcl-xL and
Bcl-2 indicating activation of the intrinsic apoptotic cascade.
Cancer metastasis presents a huge challenge in
cancer therapy and thus, blocking cancer cell metastasis is an
important approach. MMPs serve crucial roles in metastasis,
angiogenesis and also cause the release of growth factors from the
extra cellular matrix (59). MMP-2
and −9 are well documented to be associated with the invasive
metastatic potential of tumor cells (60). In U-2 OS cells a markedly enhanced
expression of MMP-2 and −9 was observed. Cucurbitacin B supressed
the levels of MMP-2 and −9 at all the tested doses and inhibited
the activities of the enzymes, thus exhibiting anti-metastatic
activity. The observations of the cell migration assay also reveal
the potent effects of cucurbitacin B on the inhibition of cell
migration and therefore, metastasis.
The JAK/STAT3 pathway is one of the major pathways
that regulate and control various vital physiological processes.
JAK activation exerts critical roles in cell proliferation,
differentiation, migration and apoptosis (44,61).
Activated JAKs phosphorylate cellular substrates, including the
STAT family, which are vitally associated with oncogenic signalling
pathways (62). Constitutive
activation of STAT3 is typically observed in cancer cells and has
been demonstrated to serve a critical role in tumor cell growth and
survival in human solid tumors (62,63).
STAT3 also upregulates anti-apoptotic proteins as Mcl-1 and Bcl-xL
(63,64). Therefore, blocking the pathway may
aid in the inhbition of cancer cell growth and promotion of
apoptosis. Treatment with cucurbitacin B was observed to markedly
downregulate the level of phosphorylated STAT3 and JAK2 in a
dose-dependent manner. The reduced level of Bcl-2 and Bcl-xL were
also in line with STAT3 levels. Treatment with cucurbitacin B
exhibited a marked reduction in the level of phosphorylated STAT3
and its downstream targets, such as cyclin B1 and Bcl-2 in the
human laryngeal cell line Hep-2 (65). Furthermore, downregulated expression
of VEGF was observed, which may be due to potent inhibition of
STAT3. In the present study cucurbitacin B treatment downregulated
JAK2/STAT3 signalling and also significantly upregulated
mitochondrial apoptotic pathway-related proteins (Bax, Bad and
cleaved caspases).
It has also been demonstrated that members of the
MAPK family are important regulators of stress responses, in
addition to being associated with cell survival and modulating the
induction of apoptosis (66,67). Cucurbitacin B was identified to
effectively inhibit the activation of JNK, ERK1/2 and p38 in U-2 OS
cells. Studies have reported that ERK1/2, the key molecule of the
MAPK signalling pathway (68), is
associated with promotion of tumor invasion and metastasis
(69). Therefore, by inhibiting the
activation of ERK1/2, cucurbitacin B effectively contributes to the
inhibition of metastasis in line with MMP suppression.
In conclusion, cucurbitacin B significantly
downregulates MAPK signalling and JAK2/STAT3 cascades and induces
apoptosis in U-2 OS cells. The downregulation of MMPs and VEGF aid
in the inhibition of invasion, metastasis and angiogenesis.
Therefore, these findings indicate that cucurbitacin B is a
potential potent candidate for OS therapy in the future. Further
experiments under in vivo conditions will be required to
confirm the effects of cucurbitacin B. Furthermore, the mechanisms
associated with the protective effects of cucurbitacin B should be
explored in more detail.
References
|
1
|
Thayanithy V, Park C, Sarver AL, Kartha
RV, Korpela DM, Graef AJ, Steer CJ, Modiano JF and Subramanian S:
Combinatorial treatment of DNA and chromatin-modifying drugs cause
cell death in human and canine osteosarcoma cell lines. PLoS One.
7:e437202012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guijarro MV, Ghivizzani SC and Gibbs CP:
Animal models in osteosarcoma. Front Oncol. 4:1892014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multidisciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaffe N: Osteosarcoma: Review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han XR, Sun Y and Bai XZ: The anti-tumor
role and mechanism of integrated and truncated PDCD5 proteins in
osteosarcoma cells. Cell Signal. 24:1713–1721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rajendran P, Li F, Manu KA, Shanmugam MK,
Loo SY, Kumar AP and Sethi G: γ-tocotrienol is a novel inhibitor of
constitutive and inducible STAT3 signalling pathway in human
hepatocellular carcinoma: Potential role as an antiproliferative,
pro-apoptotic and chemosensitizing agent. Br J Pharmacol.
163:283–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang JS, Chuang LY, Guh JY, Huang YJ and
Hsu MS: Antioxidants attenuate high glucose-induced hypertrophic
growth in renal tubular epithelial cells. Am J Physiol Renal
Physiol. 293:F1072–F1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du W, Hong J, Wang YC, Zhang YJ, Wang P,
Su WY, Lin YW, Lu R, Zou WP, Xiong H, et al: Inhibition of
JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via
mitochondrial pathway. J Cell Mol Med. 16:1878–1888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie TX, Huang FJ, Aldape KD, Kang SH, Liu
M, Gershenwald JE, Xie K, Sawaya R and Huang S: Activation of stat3
in human melanoma promotes brain metastasis. Cancer Res.
66:3188–3196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nam S, Xie J, Perkins A, Ma Y, Yang F, Wu
J, Wang Y, Xu RZ, Huang W, Horne DA, et al: Novel synthetic
derivatives of the natural product berbamine inhibit Jak2/Stat3
signaling and induce apoptosis of human melanoma cells. Mol Oncol.
6:484–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Um HJ, Min KJ, Kim DE and Kwon TK:
Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of
human renal carcinoma Caki cells. Biochem Biophys Res Commun.
427:24–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JH, Darvin P, Lim EJ, Joung YH, Hong
DY, Park EU, Park SH, Choi SK, Moon ES, Cho BW, et al:
Hwanggeumchal sorghum induces cell cycle arrest, and suppresses
tumor growth and metastasis through Jak2/STAT pathways in breast
cancer xenografts. PLoS One. 7:e405312012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park WH: MAPK inhibitors differentially
affect gallic acid-induced human pulmonary fibroblast cell growth
inhibition. Mol Med Rep. 4:193–204. 2011.PubMed/NCBI
|
|
17
|
You BR and Park WH: The effects of
mitogen-activated protein kinase inhibitors or small interfering
RNAs on gallic acid induced HeLa cell death in relation to reactive
oxygen species and glutathione. J Agric Food Chem. 59:763–771.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YC, Chang CN, Hsu HC, Chiou SJ, Lee
LT and Hseu TH: Sennoside B inhibits PDGF receptor signaling and
cell proliferation induced by PDGF-BB in human osteosarcoma cells.
Life Sci. 84:915–922. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noh K, Kim KO, Patel NR, Staples JR,
Minematsu H, Nair K and Lee FY: Targeting inflammatory kinase as an
adjuvant treatment for osteosarcomas. J Bone Joint Surg Am.
93:723–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pezzuto JM: Plant-derived anticancer
agents. Biochem Pharmacol. 53:121–133. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmad A, Sakr WA and Rahman KM: Novel
targets for detection of cancer and their modulation by
chemopreventive natural compounds. Front Biosci (Elite Ed).
4:410–425. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hijová E, Szabadosova V, Štofilová J and
Hrčková G: Chemopreventive and metabolic effects of inulin in colon
cancer development. J Vet Sci. 14:387–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park EJ and Pezzuto JM: Antioxidant marine
products in cancer chemoprevention. Antioxid Redox Signal.
19:115–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen JC, Chiu MH, Nie RL, Cordell GA and
Qiu SX: Cucurbitacins and cucurbitane glycosides: Structures and
biological activities. Nat Prod Rep. 22:386–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geissman TA: New substances of plant
origin. Annu Rev Pharmacolog. 4:305–316. 1964. View Article : Google Scholar
|
|
27
|
Farias MR, Schenkel EP, Mayer R and Rücker
G: Cucurbitacins as constituents of Wilbrandia ebracteata. Planta
Med. 59:272–275. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peters RR, Farias MR and Ribeiro-do-Valle
RM: Anti-inflammatory and analgesic effects of cucurbitacins from
Wilbrandia ebracteata. Planta Med. 63:525–528. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blaskovich MA, Sun J, Cantor A, Turkson J,
Jove R and Sebti SM: Discovery of JSI-124 (cucurbitacin I), a
selective Janus kinase/signal transducer and activator of
transcription 3 signaling pathway inhibitor with potent antitumor
activity against human and murine cancer cells in mice. Cancer Res.
63:1270–1279. 2003.PubMed/NCBI
|
|
30
|
Shi X, Franko B, Frantz C, Amin HM and Lai
R: JSI-124 (cucurbitacin I) inhibits Janus kinase-3/signal
transducer and activator of transcription-3 signalling,
downregulates nucleophosmin-anaplastic lymphoma kinase (ALK), and
induces apoptosis in ALK-positive anaplastic large cell lymphoma
cells. Br J Haematol. 135:26–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tannin-Spitz T, Grossman S, Dovrat S,
Gottlieb HE and Bergman M: Growth inhibitory activity of
cucurbitacin glucosides isolated from Citrullus colocynthis on
human breast cancer cells. Biochem Pharmacol. 73:56–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yin D, Wakimoto N, Xing H, Lu D, Huynh T,
Wang X, Black KL and Koeffler HP: Cucurbitacin B markedly inhibits
growth and rapidly affects the cytoskeleton in glioblastoma
multiforme. Int J Cancer. 123:1364–1375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ho YT, Yang JS, Li TC, Lin JJ, Lin JG, Lai
KC, Ma CY, Wood WG and Chung JG: Berberine suppresses in vitro
migration and invasion of human SCC-4 tongue squamous cancer cells
through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and
-9. Cancer Lett. 279:155–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mizoguchi H, Nakade J, Tachibana M, Ibi D,
Someya E, Koike H, Kamei H, Nabeshima T, Itohara S, Takuma K, et
al: Matrix metalloproteinase-9 contributes to kindled seizure
development in pentylenetetrazole-treated mice by converting
pro-BDNF to mature BDNF in the hippocampus. J Neurosci.
31:12963–12971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Güllü IH, Kurdoğlu M and Akalin I: The
relation of gelatinase (MMP-2 and -9) expression with distant site
metastasis and tumour aggressiveness in colorectal cancer. Br J
Cancer. 82:2492000.PubMed/NCBI
|
|
37
|
Kilian M, Gregor JI, Heukamp I, Hanel M,
Ahlgrimm M, Schimke I, Kristiansen G, Ommer A, Walz MK, Jacobi CA
and Wenger FA: Matrix metalloproteinase inhibitor RO 28–2653
decreases liver metastasis by reduction of MMP-2 and MMP-9
concentration in BOP-induced ductal pancreatic cancer in Syrian
Hamsters: Inhibition of matrix metalloproteinases in pancreatic
cancer. Prostaglandins Leukot Essent Fatty Acids. 75:429–434. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mizutani K, Kofuji K and Shirouzu K: The
significance of MMP-1 and MMP-2 in peritoneal disseminated
metastasis of gastric cancer. Surg Today. 30:614–621. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qazi A, Pal J, Maitah M, Fulciniti M,
Pelluru D, Nanjappa P, Lee S, Batchu RB, Prasad M, Bryant CS, et
al: Anticancer activity of a broccoli derivative, sulforaphane, in
barrett adenocarcinoma: Potential use in chemoprevention and as
adjuvant in chemotherapy. Transl Oncol. 3:389–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hartojo W, Silvers AL, Thomas DG, Seder
CW, Lin L, Rao H, Wang Z, Greenson JK, Giordano TJ, Orringer MB, et
al: Curcumin promotes apoptosis, increases chemosensitivity, and
inhibits nuclear factor kappaB in esophageal adenocarcinoma. Transl
Oncol. 3:99–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Monian P and Jiang X: Clearing the final
hurdles to mitochondrial apoptosis: Regulation post cytochrome C
release. Exp Oncol. 34:185–191. 2012.PubMed/NCBI
|
|
43
|
Soriano ME and Scorrano L: The interplay
between BCL-2 family proteins and mitochondrial morphology in the
regulation of apoptosis. Adv Exp Med Biol. 687:97–114. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kiu H and Nicholson SE: Biology and
significance of the JAK/STAT signalling pathways. Growth Factors.
30:88–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chau MN and Banerjee PP: Development of a
STAT3 reporter prostate cancer cell line for high throughput
screening of STAT3 activators and inhibitors. Biochem Biophys Res
Commun. 377:627–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yue P and Turkson J: Targeting STAT3 in
cancer: How successful are we? Expert Opin Investig Drugs.
18:45–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chambliss OL and Jone CM: Cucurbitacins:
Specific insect attractants in Cucurbitaceae. Science.
153:1392–1393. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yesilada E, Tanaka S, Sezik E and Tabata
M: Isolation of an anti-inflammatory principle from the fruit juice
of Ecballium elaterium. J Nat Prod. 51:504–508. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang M, Zhang H, Sun C, Shan X, Yang X,
Li-Ling J and Deng Y: Target constitutive activation of signal
transducer and activator of transcription 3 in human hepatocellular
carcinoma cells by cucurbitacin B. Cancer Chemother Pharmacol.
63:635–642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wakimoto N, Yin D, O'Kelly J, Haritunians
T, Karlan B, Said J, Xing H and Koeffler HP: Cucurbitacin B has a
potent antiproliferative effect on breast cancer cells in vitro and
in vivo. Cancer Sci. 99:1793–1797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Y, Li W, Chen X, Jiang H, Sun J, Chen H
and Lv S: Integrated analysis identifies interaction patterns
between small molecules and pathways. Biomed Res Int.
2014:9318252014.PubMed/NCBI
|
|
52
|
Wen D, Danquah M, Chaudhary AK and Mahato
RI: Small molecules targeting MicroRNA for cancer therapy: Promises
and obstacles. J Control Release. 219:237–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wondrak GT, Villeneuve NF, Lamore SD,
Bause AS, Jiang T and Zhang DD: The cinnamon-derived dietary factor
cinnamic aldehyde activates the Nrf2-dependent antioxidant response
in human epithelial colon cells. Molecules. 15:3338–3355. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liang CZ, Zhang X, Li H, Tao YQ, Tao LJ,
Yang ZR, Zhou XP, Shi ZL and Tao HM: Gallic acid induces the
apoptosis of human osteosarcoma cells in vitro and in vivo via the
regulation of mitogen-activated protein kinase pathways. Cancer
Biother Radiopharm. 27:701–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu Z, Li D, Zhao W, Zheng X, Wang J and
Wang E: A potent lead induces apoptosis in pancreatic cancer cells.
PLoS One. 7:e378412012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gross A, McDonnell JM and Korsmeyer SJ:
Bcl-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Reed JC: Apoptosis-regulating proteins as
targets for drug discovery. Trends Mol Med. 7:314–319. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang SH, Chien CM, Lu MC, Lin YH, Hu XW
and Lin SR: Upregulation of Bax and endonuclease G, and
down-modulation of Bcl-XL involved in cardiotoxin III-induced
apoptosis in K562 cells. Exp Mol Med. 38:435–444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Coussens LM and Werb Z: Matrix
metalloproteinases and the development of cancer. Chem Biol.
3:895–904. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang L, Shi J, Feng J, Klocker H, Lee C
and Zhang J: Type IV collagenase (matrix metalloproteinase-2 and
-9) in prostate cancer. Prostate Cancer Prostatic Dis. 7:327–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
O'Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: New surprises in the Jak/Stat pathway.
Cell. 109 Suppl:121–131. 2002. View Article : Google Scholar
|
|
62
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Epling-Burnette PK, Liu JH,
Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang
JM, Yang-Yen HF, Karras J, et al: Inhibition of STAT3 signaling
leads to apoptosis of leukemic large granular lymphocytes and
decreased Mcl-1 expression. J Clin Invest. 107:351–362. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu T, Zhang M, Zhang H, Sun C and Deng Y:
Inhibitory effects of cucurbitacin B on laryngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 265:1225–1232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim BM and Chung HW: Desferrioxamine (DFX)
induces apoptosis through the p38-caspase8-Bid-Bax pathway in PHA
stimulated human lymphocytes. Toxicol Appl Pharmacol. 228:24–31.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mendes O, Kim HT, Lungu G and Stoica G:
MMP2 role in breast cancer brain metastasis development and its
regulation by TIMP2 and ERK1/2. Clin Exp Metastasis. 24:341–351.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Peng L, Xing X, Li W, Qu L, Meng L, Lian
S, Jiang B, Wu J and Shou C: PRL-3 promotes the motility, invasion,
and metastasis of LoVo colon cancer cells through PRL-3-integrin
beta1-ERK1/2 and-MMP2 signaling. Mol Cancer. 8:1102009. View Article : Google Scholar : PubMed/NCBI
|