Introduction
Cryptococcus neoformans encephalitis is a
type of fungal infection in the central nervous system caused by
C. neoformans (1).
Approximately 6–10% of AIDS patients in the USA and up to 30% in
Africa are infected with C. neoformans, and 90% develop
meningoencephalitis (1). When AIDS
patients with cryptococcal meningoencephalitis were treated with
amphotericin B or fluconazole, the meningoencephalitis resolved in
one-third, active meningoencephalitis persisted in one-third and
approximately one-third died (2) In
populations with normal immunological function, the infection rate
of Cryptococcus is ~1 in 100,000 (3), and the incidence of life-threatening
cryptococcal infections among patients with AIDS has been estimated
at 6 to 10% in the United States, western Europe, and Australia,
and 15 to 30% in sub-Saharan Africa (3). However the prevalence of this disease
in healthy people is increasing year on year (4). Furthermore, outbreaks of cryptococcosis
in which >1 patient acquires the infection at the same time and
place, presumably from exposure to a common source of the inoculum,
do not occur (5). During initial
therapy, 10–25% of these patients die, and 30–60% succumb within 12
months (3).
AmB has been successfully used to treat every type
of cryptococcosis, from pneumonia to meningitis; however, the only
comparative trials have been in cases of meningitis (3). Because of its toxicity, AmB therapy has
been reduced in dose and duration by successfully using it in
combination with flucytosine (6).
Formulations of AMB and lipids have been developed to aid its
delivery, reduce toxicity, and enable higher doses to be tolerated
(1). In an attempt to optimize
therapy for cryptococcal meningitis, AMB has also been used locally
at the site of infection (7). As AMB
penetrates the central nervous system (CNS) poorly,
intraventricular AMB has been used successfully in severe cases of
cryptococcal meningitis under the premise that local administration
will increase its concentration within the CNS (8). However, this approach commonly leads to
CNS complications and tolerance (3).
The patient with C. neoformans in the current
case study had poor tolerance for intravenous administration of
liposomal AmB, and symptoms were not relieved following 5 months
treatment with 150 mg/day liposomal AmB. In order to rapidly
control the intracranial infection, continuous intrathecal
administration of liposomal AmB was conducted over 1 month.
Case report
The present report was approved by the Ethics
Committee of Ethical Research for Zhujiang Hospital of Southern
Medical University (Guangzhou, China). The patient provided consent
for the case and related images to be used in this report.
A 28-year-old male patient, was admitted to the
Department of Neurology, Zhujiang Hospital of Southern Medical
University on 10 May 2014, having experienced a headache and fever
for >20 days. From 16 April, the patient had begun experiencing
headaches without obvious inducement, mainly presenting with
paroxysmal aggravated headache. The patient had no symptoms of
dizziness, nausea, vomiting, unsteady walking, limbs lacking in
strength, numbness of limbs, convulsion of limbs, unconsciousness,
hearing loss, blurred vision or double vision. Following 3 days of
headache, the patient developed a low-grade fever, with a maximum
temperature of 37.5°C. The patient sought treatment at a local
hospital, where the disease was regarded as an upper respiratory
infection. However, the patient did not get better after receiving
treatment suitable for this diagnosis (dextromethorphan
hydrobromide tablets; 30 mg twice daily; Guangzhou Baiyun Shan
Guanghua Pharmaceutical Co., Ltd., Guangzhou, China; and Xiaochaihu
granules, Guangzhou Baiyun Shan Guanghua Pharmaceutical Co., Ltd.;
10 g twice daily) and the patient's condition continued to decline.
The patient attended PANYU Central Hospital (Guangzhou, China) in
May 2014 for treatment. A routine blood examination indicated that
the white blood cell was 13×109 cells/l (reference value
4–10×109 cells/l). The electroencephalogram presented as
slightly abnormal, with decreased alpha wave and increased theta
wave activity. Symptoms did not improve following treatment with
piperacillin (4 g intravenously twice daily) during admission, and
the patient experienced two occurrences of foaming at the mouth,
upturning binoculus and convulsion of limbs on 5 and 6 May 2014.
Following administration with tranquilizing drugs including
diazepam (Harbin Aurora Optoelectronic Technology Co., Ltd.,
Harbin, China; 10 mg intramuscular injection, twice daily) and
phenobarbital sodium (Guangdong Bangmin Pharmaceutical Co., Ltd.,
Jianmen, China; 100 mg intramuscular injection, twice daily), the
epilepsy torsion symptoms were alleviated; however, the headache
continuously intensified. The patient also experienced low-grade
fever and gradual clouding of consciousness. The patient attended
an appointment on 10 May 2014 at the Neurology Department of
Zhujiang Hospital of Southern Medical University and was admitted
with ‘intracranial infection’. Lumbar puncture was administered to
the patient and the tested pressure of cerebrospinal fluid was 350
mmH2O. The cerebrospinal fluid tested positive for the
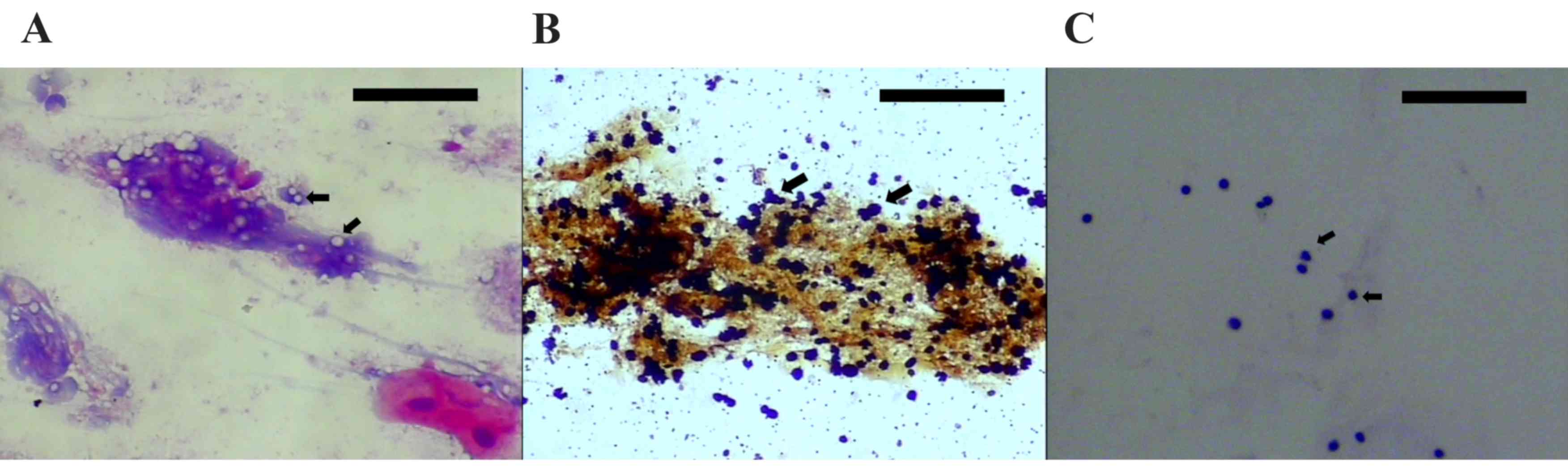
antigen of Cryptococcus (Fig.
1). C. neoformans was observed with ink staining.
The patient was treated with liposomal AmB, with an
initial dose of 0.1 mg/kg increasing up to 2.5 mg/kg daily by
intravenous injection and combined use of oral flucytosine tablets
(China Pharmaceutical University Pharmaceutical Co., Ltd., Nanjing,
China; 1.5 g/day) and fluconazole (0.4 g/day; Pfizer, Inc., New
York, NY, USA) through intravenous drip. Dehydration was also
induced to reduce intracranial pressure. Through these treatments,
the patient's temperature returned to normal, and headache did not
get worse, but the patient still had blurred vision. C.
neoformans was still observed following repeated lumbar
puncture, but when the dosage of liposomal AmB reached 150 mg/day,
the patient experienced considerable discomfort, including
xanthochromia, pruritus, poor appetite and drowsiness, thus the
dosage could not be increased.
Following administration of this treatment for 5
months, C. neoformans could still be detected in the
cerebrospinal fluid. Therefore, it was decided that continuous
intrathecal administration of liposomal AmB should be conducted. On
13 November 2014, a Medtronic lumbar puncture external drainage
suite (product model 27303; Medtronic, Minneapolis, MN, USA) was
used. The space between 3/4 lumbar vertebrae was selected, 20 cm of
the head end of the drainage tube was inserted; then 30–50 ml
cerebrospinal fluid was slowly discharged prior to each
administration of the drug. For 1 month, liposomal AmB was
administered for 20 h each day; an additional 4 h was required to
slowly discharge cerebrospinal fluid and change the dressing. The
dosage of liposomal AmB was increased by 0.5 mg each day. When the
dosage reached 2 mg/day, the patient started to exhibit damage to
the oculomotor nerve on both sides and damage to the facial nerve
on the left side. The dosage was subsequently decreased to 1
mg/day, after which the cranial nerve damage gradually recovered,
headache was reduced fully and temperature remained normal. The
patient received a total of 28 mg intrathecal liposomal Amb over
the course of the month.
Following the removal of the drainage tube on the
lumbar cistern, six consecutive ink stains of cerebrospinal fluid
tested negative for Cryptococcus. As indicated in Fig. 1, in the reexamination on 23 November
2014, a relatively large number of lymphocytes were observed in the
hematoxylin and eosin stain of cerebrospinal fluid, and no C.
neoformans could be detected (observed using light microscopy).
The patient was discharged on 6 February 2015. Follow-up visits
were conducted for 6 months and no reappearance of C.
neoformans was observed.
As presented in Table
I, the patient's cerebrospinal fluid was tested 30 times during
the hospitalization period. The cerebrospinal fluid in the
20th-24th examinations were samples kept from the drainage tube on
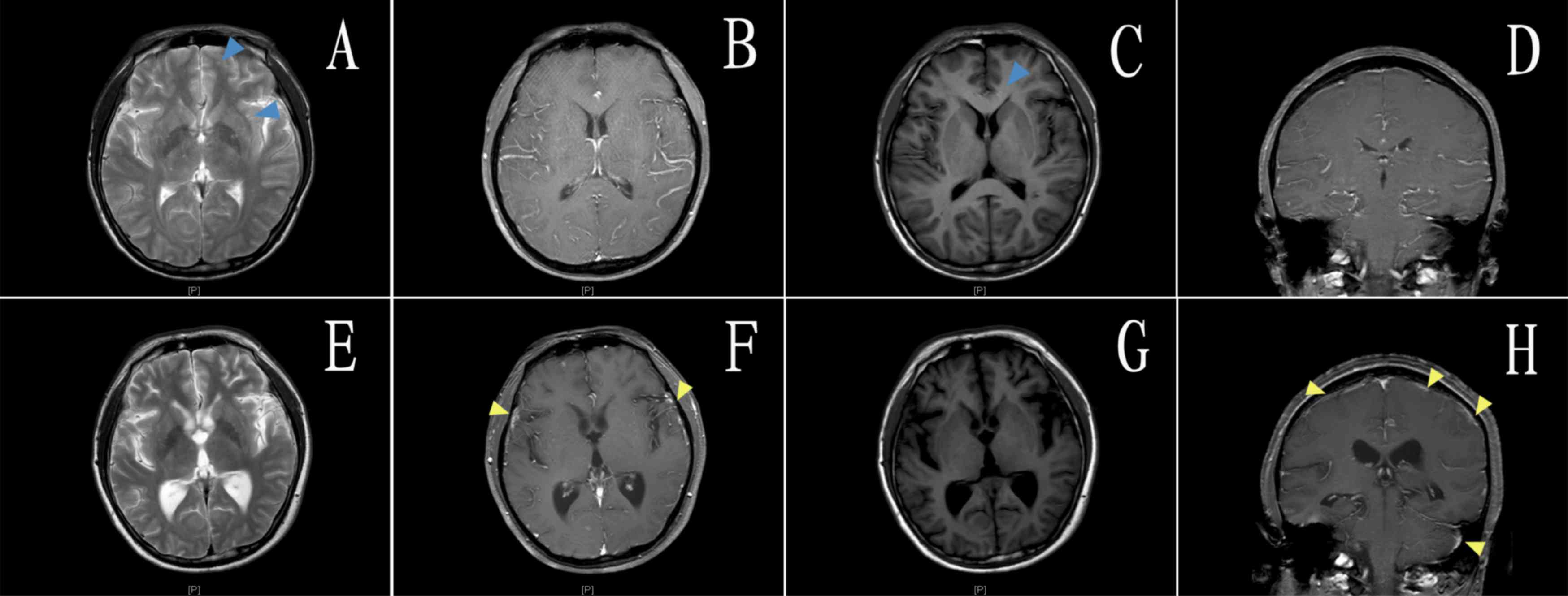
the lumbar cistern. Magnetic resonance imaging scan and enhanced
images of the patient's brain on day 3 after admission (in May
2014) and the day before leaving hospital (in February 2015) are
presented in Fig. 2.
 | Table I.Cerebrospinal fluid pressure and
results of laboratory tests. |
Table I.
Cerebrospinal fluid pressure and
results of laboratory tests.
| Time | Date
(dd/mm/yyyy) | Cerebrospinal fluid
pressure (mm H2O) | White blood cell
count (106 cells/lM/l) | Chloride ion
(mmol/l) | Glucose (mmol/l) | Lactic acid
(mmol/l) | Total cerebrospinal
fluid protein (mg/l) |
|---|
| 1 | 10/05/2014 | 350 | 50 | 118.2 | 2.5 | 2.5 | 459 |
| 2 | 27/05/2014 | 300 | 50 | 117.7 | 2.0 | 3.3 | 666 |
| 3 | 09/06/2014 | 400 | 50 | 115.7 | 1.4 | 3.6 | 926 |
| 4 | 20/06/2014 | 300 | 70 | 114.4 | 1.6 | 3.6 | 914 |
| 5 | 03/07/2014 | 320 | 110 | 118.6 | 3.4 | 3.1 | 541 |
| 6 | 22/07/2014 | 350 | 25 | 121.6 | 3.8 | 2.5 | 562 |
| 7 | 30/07/2014 | 350 | 20 | 117.7 | 3.9 | 2.2 | 425 |
| 8 | 07/08/2014 | 350 | 20 | 116.2 | 2.6 | 1.5 | 398 |
| 9 | 16/08/2014 | 330 | 4 | 118.5 | 3.8 | 1.5 | 465 |
| 10 | 22/08/2014 | 250 | 9 | 122 | 3.9 | 2.3 | 513 |
| 11 | 29/08/2014 | 300 | 0 | 124.2 | 3.8 | 2.1 | 519 |
| 12 | 04/09/2014 | 320 | 12 | 120.5 | 3.8 | 2.2 | 558 |
| 13 | 04/09/2014 | 320 | 2 | 121 | 3.4 | 2.7 | 633 |
| 14 | 18/09/2014 | 320 | 1 | 122.9 | 3.6 | 2.5 | 565 |
| 15 | 25/09/2014 | 320 | 3 | 124.7 | 3.4 | 2.8 | 673 |
| 16 | 08/10/2014 | 350 | 30 | 123.1 | 3.7 | 2.9 | 475 |
| 17 | 15/10/2014 | 260 | 10 | 123.6 | 3.8 | 2.3 | 522 |
| 18 | 23/10/2014 | 350 | 0 | 121.7 | 3.6 | 2.0 | 572 |
| 19 | 11/11/2014 | 350 | 40 | 124.2 | 3.0 | 2.7 | 1252 |
| 20 | 19/11/2014 | – | 50 | 111.7 | 5.6 | 7.4 | 382 |
| 21 | 21/11/2014 | – | 20 | 130.8 | 4.8 | 4.1 | 209 |
| 22 | 23/11/2014 | – | 50 | 126.3 | 4.1 | 3.4 | 754 |
| 23 | 10/12/2014 | – | 30 | 120.1 | 3.0 | 2.6 | 293 |
| 24 | 08/12/2014 | – | 0 | 122.2 | 2.6 | 2.5 | 422 |
| 25 | 18/12/2014 | 320 | 30 | 122.6 | 2.7 | 2.7 | 534 |
| 26 | 24/12/2014 | 300 | 20 | 124.3 | 2.7 | 3.0 | 516 |
| 27 | 30/12/2014 | 220 | 10 | 122.8 | 2.8 | 3.2 | 463 |
| 28 | 06/01/2015 | 230 | 20 | 125.0 | 2.5 | 3.2 | 388 |
| 29 | 13/01/2015 | 215 | 30 | 122.5 | 2.9 | 2.6 | 421 |
| 30 | 19/01/2015 | 230 | 10 | 120.9 | 3.3 | 1.6 | 378 |
Cerebrospinal fluid pressure was 325.79±34.85 mm
H2O, white blood cell count was
26.63±29.16×106 cells/l and total cerebrospinal fluid
protein was 612.53±209.74 mg/l. After the continuous intrathecal
administration of liposomal amphotericin B, the cerebrospinal fluid
pressure was 252.50±45.36 mm H2O, the white blood cell
count was 20±8.94×106 cells/l and total cerebrospinal
fluid protein was 450±65.49 mg/l. These results suggest that
administration of amphotericin B liposomes caused cerebrospinal
fluid levels and increased intracranial hypertension to gradually
return to normal, and the white blood cell levels and the total
cerebrospinal fluid protein were decreased.
Discussion
The Clinical Practice Guidelines for Cryptococcosis
(4) (hereinafter referred to as
‘Guidelines’), which were updated by the Infectious Disease Society
of America in 2010, suggest using AmB deoxycholic acid (AmBd;
0.7–1.0 mg/kg/day, via intravenous drip) and flucytosine (100
mg/kg/day, orally) for inductive treatment for at least 4 weeks,
followed by treatment with fluconazole (400 mg/day) for 8 weeks for
patients with normal immunological functions. If patients cannot
tolerate AmBd, treatment with liposomal AmB (3–4 mg/kg/day,
intravenous drip) or AmB lipid complex (5 mg/kg/day, intravenous
drip) should be conducted (4). In
the current case study, the patient could not tolerate an expected
dosage of liposomal AmB, and the dose administered did not reach
the target dose of 3–4 mg/kg/day. This suggests that liposomal AmB
did not reached the minimal inhibitory concentration over the
5-month treatment period. Although three types of drug were
administered and the clinical symptoms of the patient stabilized,
Cryptococcus was still present and the disease had a
protracted course.
Damage to the cranial nerve occurred when the
intrathecal dosage of liposomal AmB was increased to 2 mg/day. When
the dosage was decreased to 1 mg/day, the damage to the cranial
nerve was reduced and patient experienced an effective reduction of
symptoms. It has been proposed that during the circulation of
cerebrospinal fluid of the patient, the drug is distributed in the
cerebrospinal fluid and is absorbed into the blood along with
arachnoid granulations. In an infected state, the rate and quantity
of absorption of arachnoid granulations may have significant
individualized differences and the simple application of the drug
metabolism rule during cerebrospinal fluid circulation under
physiological status is not appropriate (9,10).
Hence, it is proposed that a safe dosage of liposomal AmB should
begin at 0.5 mg/day, increasing by 0.5 mg/day every 1–2 days, with
close monitoring of any damage to the cranial nerve and etiological
examination of cerebrospinal fluid, based on which an
individualized concentration of liposomal AmB can be
determined.
The Guidelines do not recommend intrathecal
injection of liposomal AmB, as the interval time of intrathecal
injection is too long (4). It is
also impossible to form a stable drug concentration in the
cerebrospinal fluid - this is unsafe and does not help with
controlling the infection. The current authors propose that
continuous intrathecal administration may compensate for the
weaknesses of conventional intrathecal administration in the
following ways: i) The duration and interval of administration may
be more effectively controlled; ii) extreme peaks in drug
concentration are avoided, reducing the risk of a toxic reaction of
the nervous system occurring; and iii) the stable drug
concentration can control the infection more effectively.
The course of treatment for continuous intrathecal
administration was 1 month. Cerebrospinal fluid can easily effuse
from the sinus tract during puncture, which may lead to
catheter-related infections (11,12).
However, this risk can be reduced by careful disinfection by iodine
tincture and ethyl alcohol every day. Prior to each continuous
intrathecal administration, 30–50 ml cerebrospinal fluid should be
slowly discharged to relieve the increase in cerebrospinal fluid
pressure. If any necrosis at the pipe orifice is observed, the
catheter should be removed immediately and a new catheter placed at
the space between other lumbar vertebrae. If infection symptoms
appear, broad-spectrum antibiotics with high efficiency should be
used immediately to control the infection (13).
In conclusion, conventional intravenous
administration may be suitable for most C. neoformans
encephalitis cases, although continuous intrathecal administration
is not recommended for all patients. However, if an increasing
dosage of liposomal AmB is not tolerated by the patient, continuous
intrathecal administration is an effective way to treat patients
with refractory C. neoformans encephalitis.
References
|
1
|
Furukawa K, Sasaki H, Pollard RB and
Suzuki F: Lanoconazole, a new imidazole antimycotic compound,
protects MAIDS mice against encephalitis caused by Cryptococcus
neoformans. J Antimicrob Chemother. 46:443–450. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saag MS, Powderly WG, Cloud GA, Robinson
P, Grieco MH, Sharkey PK, Thompson SE, Sugar AM, Tuazon CU, Fisher
JF, et al: Comparison of amphotericin B with fluconazole in the
treatment of acute AIDS-associated cryptococcal meningitis. N Engl
J Med. 326:83–89. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitchell TG and Perfect JR: Cryptocoecosis
in the era of AIDS-100 years after the discovery of Cryptococcus
neoformans. Clin Microbiol Rev. 8:515–548. 1995.PubMed/NCBI
|
|
4
|
Desalermos A, Kourkoumpetis TK and
Mylonakis E: Update on the epidemiology and management of
cryptococcal meningitis. Expert Opini Pharmacother. 13:783–789.
2012. View Article : Google Scholar
|
|
5
|
Levitz SM: The ecology of Cryptococcus
neoformans and the epidemiology of cryptococcosis. Rev Infect Dis.
13:1163–1169. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coker RJ, Viviani M, Gazzard BG, Du Pont
B, Pohle HD, Murphy SM, Atouguia J, Champalimaud JL and Harris JR:
Treatment of cryptococcosis with liposomal amphotericin B
(AmBisome) in 23 patients with AIDS. Aids. 7:829–835. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mwaba P, Mwansa J, Chintu C, Pobee J,
Scarborough M, Portsmouth S and Zumla A: Clinical presentation,
natural history, and cumulative death rates of 230 adults with
primary cryptococcal meningitis in Zambian AIDS patients treated
under local condition. Postgrad Med J. 77:769–773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pappas PG, Kontoyiannis DP, Perfect JR and
Chiller TM: Real-time treatment guidelines: Considerations during
the Exserohilum rostratum outbreak in the United States. Antimicrob
Agents Chemother. 57:1573–1576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greitz D and Hannerz J: A proposed model
of cerebrospinal fluid circulation: Observations with radionuclide
cisternography. AJNR Am J Neuroradiol. 17:431–438. 1996.PubMed/NCBI
|
|
10
|
Brinker T, Stopa E, Morrison J and Klinge
P: A new look at cerebrospinal fluid circulation. Fluids Barriers
CNS. 11:102014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lemaire X, Morena M, Leray-Moragués H,
Henriet-Viprey D, Chenine L, Defez-Fougeron C and Canaud B:
Analysis of risk factors for catheter-related bacteremia in 2000
permanent dual catheters for hemodialysis. Blood Purif. 28:21–28.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jean G, Charra B, Chazot C, Vanel T,
Terrat JC, Hurot JM and Laurent G: Risk factor analysis for
long-term tunneled dialysis catheter-related bacteremias. Nephron.
91:399–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weber DJ and Rutala WA: Central
line-associated bloodstream infections: Prevention and management.
Infect Dis Clin North Am. 25:77–102. 2011. View Article : Google Scholar : PubMed/NCBI
|