Introduction
Foot-and-mouth disease (FMD) is an acute, febrile
and contagious disease caused by the FMD virus (FMDV) (1). FMDV infection primarily occurs through
the binding of FMDV to receptors on the host cell surface (2). Following cell penetration and
uncoating, FMDV undergoes replication, transcription, translation
and genome packaging (3). Receptor
binding is mediated by an arginine-glycine-aspartate (RGD) sequence
in the FMDV major capsid protein 1 (VP1), which acts as the cognate
ligand for cell surface receptors (4). Adsorption to host cells involves
specific interactions between viral proteins, including VP1, and
cell surface membrane receptors, which is a prerequisite for viral
infection of host cells (5).
Previous results have confirmed the presence of two types of
cellular receptors for FMDV: Integrins and heparin sulfate
(6,7). In particular, integrins have key roles
in mediating viral infection of host cells.
Integrins are protein heterodimers composed of α and
β subunits, and occur in at least 20 distinct types. Infection of
host cells by FMDV is principally mediated by integrin
heterodimers. Currently, four types of receptors are considered to
mediate FMDV infection, namely αvβ1, αvβ3, αvβ6 and αvβ8, with αvβ6
having a key role in the infection process (8). It has been demonstrated that the major
adsorption receptor for FMDV is cell surface-expressed αvβ6
integrin (9). Loss of the β6 subunit
has an inhibitory effect on viral infection, as the conserved motif
region within the cytoplasmic domain of integrin β6 has an
important role in the infection process.
In the present study, based on the gene sequence of
the porcine integrin receptor β6 subunit, small interfering RNA
molecules (siRNAs) were designed to target the cytoplasmic domain
of the integrin β6 subunit, and corresponding expression plasmids
were constructed. The inhibitory function of different siRNAs and
their effects on viral replication were evaluated in porcine
embryonic fibroblasts (PEFs). Ultimately, PEFs were obtained in
which viral replication was inhibited, thus providing a basis for
therapeutic targeting of the receptor-virus interactions in
FMD.
Materials and methods
Materials
The E. coli DH5α strain, PEF cells, green
fluorescent protein (GFP)-expressing RNA interference (RNAi)
expression vectors pGenesil-1 and pXL-eGFP-Neo and the pGsi-Z4
recombinant expression plasmid were generated in our laboratory as
previously described (10). The FMDV
virulent strain OS-22 was provided by Ningbo Tecon Biotechnology
Co., Ltd. (Ningbo, China). G418 (250 µg/ml) antibiotic, reverse
transcriptase PrimeScript Reverse Transcriptase kit, restriction
endonucleases and a fluorescence quantitative polymerase chain
reaction (qPCR) reagent kit were purchased from Takara
Biotechnology Co., Ltd., (Dalian, China). An X-tremeGENE HP DNA
Transfection Reagent kit was purchased from Roche Diagnostics GmbH
(Mannheim, Germany). TRIzol reagent was purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA. USA).
Cell isolation and culture
The porcine embryonic fibroblast (PEF) cells were
proved by Dr Shiwei Ma (College of Animal Science and Technology,
SHihezi University, Xinjiang, China) (11). Cells were cultivated for 24 h in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) in at
atmosphere containing 5% CO2 at 39°C.
Construction of interference
plasmids
Gene sequences of the porcine integrin β6 subunit
were retrieved from the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/). Six pairs of
positive short hairpin RNAs shRNAs and one pair of negative
(control) shRNAs were designed to target different regions of the
cytoplasmic domain of the β6 subunit gene; these primer pairs were
termed pGsi-Z1, pGsi-Z2, pGsi-Z3, pGsi-Z4, pGsi-Z5, pGsi-Z6 and
pGsi-Z7 (negative control). DNA sequences corresponding to the
hairpin structure of the shRNA were synthesized (Table I) by Beijing Liuhe Genomics
Technology Co., Ltd. (Beijing, China).
 | Table I.shRNA sequences. |
Table I.
shRNA sequences.
| shRNA name | shRNA sequence |
|---|
| pGsi-Z1 |
5′-GATCCAAATTTGAAGCAGAACGGTCATTCAAGAGATGACCGTTCTGCTTCAAATTTTTTTTTACGCGTA-3′ |
| pGsi-Z2 |
5′-GATCCAAGCAGAACGGTCAAAGGCCATTCAAGAGATGGCCTTTGACCGTTCTGCTTTTTTTTACGCGTA-3′ |
| pGsi-Z3 |
5′-GATCCAACGGTCAAAGGCCAAGTGGCTTCAAGAGAGCCACTTGGCCTTTGACCGTTTTTTTTACGCGTA-3′ |
| pGsi-Z4 |
5′-GATCCAAAGGCCAAGTGGCAAACGGGTTCAAGAGACCCGTTTGCCACTTGGCCTTTTTTTTTACGCGTA-3′ |
| pGsi-Z5 |
5′-GATCCAAGTGGCAAACGGGAACCAATTTCAAGAGAATTGGTTCCCGTTTGCCACTTTTTTTTACGCGTA-3′ |
| pGsi-Z6 |
5′-GATCCAACCAATCCACTGTACAGAGGTTCAAGAGACCTCTGTACAGTGGATTGGTTTTTTTTACGCGTA-3′ |
| pGsi-Z7 |
5′-GATCCAGTCCTGTACAGAGCGACTCTTTCAAGAGAAGAGTCGCTCTGTACAGGACTTTTTTTACGCGTA-3′ |
Single-stranded shRNA fragments were diluted to 100
µmol with distilled water. Pairs of single-stranded shRNA that
targeted the same DNA sequence were then mixed together (10 µl
each) and the mixture was incubated at 95°C for 3 sec, 72°C for 2
min, 37°C for 2 min and 25°C for 2 min for annealing. The resulting
dsRNA sequence was cloned into a pGenesil-1 vector and transformed
into DH5α competent cells for the isolation of recombinant
plasmids. Positive plasmids were identified by double-enzyme
digestion using the BamHI, HindIII, and EcoRI enzymes. Plasmids
with the correct digestion patterns were sent to Beijing Liuhe
Genomics Technology Co. Ltd. for sequencing and identification.
Cell transfection and observation of
recombinant plasmids by fluorescence microscopy
Prior to transfection, PEF cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 20% FBS in a 5% CO2 incubator
at 39°C. When cell confluence reached 80–90%, recombinant
interference plasmids (PXL-U6-Z4) were transfected into PEF cells
using the X-tremeGENE HP DNA Transfection Reagent kit, according to
the manufacturer's instructions. Cell morphology and the expression
of recombinant plasmids containing the GFP gene were observed under
a fluorescence microscope every 12 h starting 24 h after
transfection. The transfection efficiency of recombinant
interference plasmids was also evaluated using flow cytometry as
previously described (12).
Analysis of recombinant plasmid
interference
Gene sequences of the porcine integrin receptor β6
subunit and the internal control, β-actin, were retrieved from
GenBank. Primers for reverse transcription-quantitative polymerase
chain reaction PCR (RT-qPCR) were designed using Primer Premier 5.0
primer design software (PREMIER Biosoft, Palo Alto, CA, USA;
Table II) and synthesized by
Beijing Liuhe Genomics Technology Co., Ltd.
 | Table II.Primer design for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer design for reverse
transcription-quantitative polymerase chain reaction.
| Primer name | No. of amplified
bases | Primer DNA
sequences |
|---|
| Murine β-actin | 98 | F,
5′-GCTGTCCCTGTATGCCTCTG-3′ |
|
|
| R,
5′-GGAGAGCATAGCCCTCGTAG-3′ |
| Murine β6 | 186 | F,
5′-GTGTCACTGGCGATCCTG-3′ |
|
|
| R,
5′-GTGCTTGTAGGTCACGTTC-3′ |
| Porcine β-actin | 196 | F,
5′-CATTGTCATGGACTCTGGGGA-3′ |
|
|
| R,
5′-CTTCTCCTTGATGTCCCGCA-3′ |
| Porcine β6 | 180 | F,
5′GGGGTTTCACTGGCTATTCT-3′ |
|
|
| R,
5′-GGTTACATTTTTAAAGGCGC-3′ |
Total cellular RNA was extracted from PEF cells
transfected with RNAi vectors using a total RNA extraction kit
(Tiangen Biotech Co., Ltd., Beijing, China). First-strand cDNA was
synthesized from 2 µg of total RNA using a reverse transcription
kit (Tiangen Biotech Co., Ltd.) according to the manufacturer's
protocol. β6 expression was detected by RT-qPCR, with β-actin as an
internal control. The primer sequences for β6 are listed in
Table II. PCR cycling conditions
were as follows: 94°C for 5 min; followed by 40 cycles of 94°C for
30 sec, 56°C for 30 sec, 72°C for 30 sec and 72°C for 10 min. The
results were calculated and analyzed using the 2-ΔΔCq method. Three
replicates were performed (13).
Cell construction with an integrated
U6-Z4 gene
The recombinant plasmid constructed in the present
study, pGsi-Z4, was verified by double-enzyme digestion with EcoRI
and BglII. The digested fragment included the U6 promoter and Z4
RNAi fragment sequence; this fragment was termed U6-Z4. The U6-Z4
gene and PXL-EGFP-NEO vector were ligated and transformed into E.
coli DH5α competent cells for plasmid isolation. Plasmids were
digested with MluI (Takara Biotechnology Co., Ltd.) and recombinant
plasmids were identified by running digestion products on an 1%
agarose gel. DNA ladder 10,000 was used (Tiangen Biotech Co.,
Ltd.). The stored bacterial solutions were sent to Beijing Liuhe
Genomics Technology Co. Ltd., for subsequent sequencing and
identification.
Positive recombinant plasmid clones expressing U6-Z4
were subjected to single-enzyme digestion using ApaLI. DNA
fragments were obtained using an agarose gel DNA Recovery kit
(CWBIO, Beijing, China). DNA fragments (pXL-U6-Z4 plasmid) were
transfected into PEF cells using the X-tremeGENE HP DNA
Transfection Reagent kit, according to the manufacturer's protocol.
Cells transfected with linearized pXl without a U6-Z4 sequence were
used as a control group. Cells were cultured in DMEM supplemented
with 10% FBS in an incubator containing 5% CO2 at 39°C. When cell
confluence reached 60–70%, cell medium was replenished with G418
selection culture medium with DMEM supplemented with 10% FBS (250
µg/ml). After ~15 days of selection, antibiotic selection was
terminated and surviving (G418-resistant PEF) cells were cultured,
passaged and amplified.
Validation of integration plasmid
interference effects
The level of integrin receptor β6 subunit gene
expression in PEF cells transfected with pXL-U6-Z4 plasmid was
assessed using RT-qPCR. Titers of the OS-22 virulent FMDV strain
(Ningbo Tecon Biotechnology Co., Ltd.) were determined, and the 50%
tissue culture infective dose (TCID50) of the virus stock was
calculated using the Reed-Muench formula (14). Based on calculations, PEF cells were
infected with FMDV at 48 h after transfection with the interference
plasmid. PEF cells were monitored for cytopathic effects (CPEs) at
12, 18, 24 and 36 h after virus inoculation, and virus supernatants
were collected. Differences in the levels of FMDV replication in
the supernatants of integrated plasmid transfection and control
groups (including normal PEF cells and PEF cells with FMDV
infection without pXL-U6-Z4 plasmid) were preliminarily determined.
CPEs within cells were observed using an inverted microscope
TH4-200 (Olympus Corporation, Tokyo, Japan) and measured using a
semi-quantitative method (20% of cells exhibiting CPEs, +; 40% CPE,
++; 60% CPE, +++; 80% CPE, ++++; and 100% CPE, +++++). Virus
supernatants were collected at different time points (12, 18, 24
and 36 h) following the viral challenge, and differences in the
levels of viral replication between the integrated plasmid
transfection and control groups following FMDV infection were
determined using RT-PCR with three replicates. Total RNA was
extracted using a total RNA extraction kit (Tiangen Biotech Co.,
Ltd.). First-strand cDNA was synthesized from 2 µg of total RNA
using a reverse transcription kit (Tiangen Biotech Co., Ltd.)
according to the manufacturer's protocol. Expression of FMDV VP1
gene was detected by RT-PCR with GAPDH as an internal control. The
RT-PCR final reaction volume was 20 µl comprised of ddH2O (7.2 µl),
SYBR Fluorescent dye (10.0 µl), cDNA Template (2.0 µl), upstream
primer (0.4 µl), and downstream primer (0.4 µl). The primers used
were as follows: FMDV VP1, forward 5′GAAGATCTCCCAGTGGAAAGACGCG-3′
and reverse 5′CGGAATTCTTGGAAAAAAGCTACAGATCACC-3′; GAPDH, forward,
5′GTCACCAGGGCTGCTTT-3′ and reverse 5′TGTGCCGTTGAACTTGC-3′. Primers
were designed using Primer Premier 5.0 software. PCR cycling was as
follows: 95°C for 5 sec followed by 40 cycles of 95°C for 15 sec,
52°C for 30 sec, 72°C for 20 sec and 72°C for 10 min. The results
were calculated and analyzed using the comparative 2-ΔΔCq method
(13).
Statistical analysis
Data were analyzed with one-way analysis of variance
using SPSS Statistics version 17.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
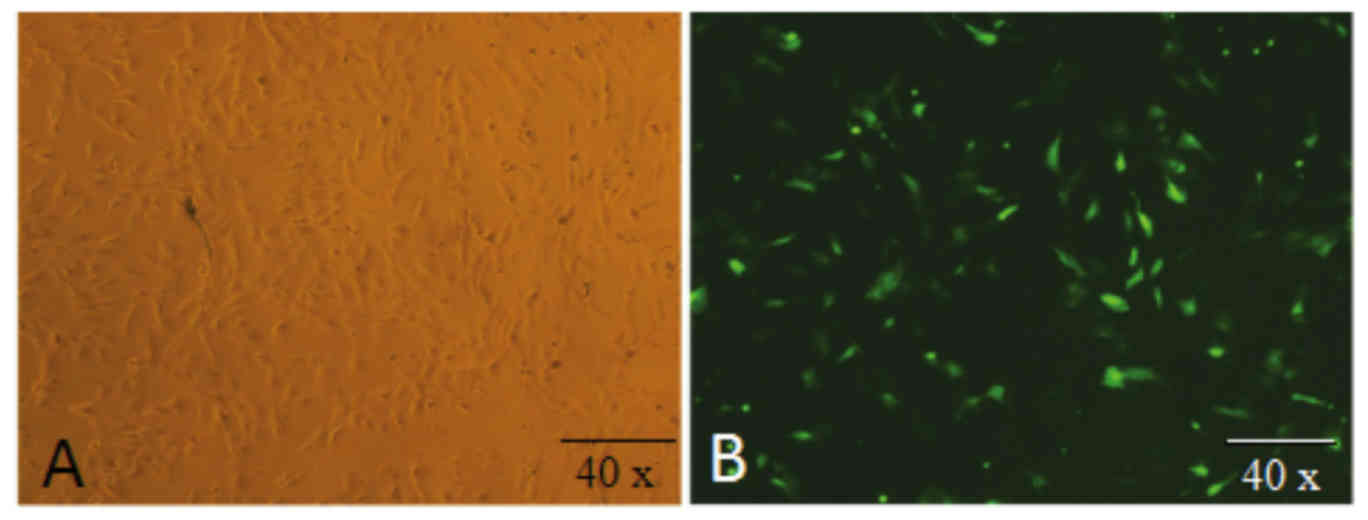
Transfection of PEF cells with
recombinant interference plasmids
PEF cells were isolated using an enzyme digestion
and tissue block culture method (Fig.
1A). PEF cells were transfected with recombinant interference
plasmids expressing a green fluorescent protein marker, and
expression of green fluorescent protein was used to identify
successful transfectants (Fig.
1B).
Selection of recombinant interference
plasmids
Recombinant interference plasmids were digested with
enzymes and subjected to agarose gel electrophoresis. It was
observed that a 320 bp band obtained from BamHI and EcoRI digestion
included the U6 promoter, while a 390 bp band obtained from HindIII
and EcoRI digestion contained the U6 promoter and shRNA identified
as previously described (15)
(Fig. 2). Recombinant plasmids
containing the U6 promoter and shRNA were selected as positive
clones. Positive plasmids identified by double-enzyme digestion
were also sent to the Beijing Genomics Institute for sequencing.
The sequencing results were consistent with the experimental
findings.
 | Figure 2.Identification of RNAi recombinant
plasmids using enzyme digestion. Lanes 1, 4, 7, 10 and 13 show the
full RNAi recombinant plasmids (controls), lanes 2, 5, 8, 11 and 14
show the U6 promoter digestion product (~320 bp) obtained from
BamHI and EcoRI digestion and lanes 3, 6, 9, 12 and 15 show the the
U6 promoter and short hairpin RNA (~390 bp) digestion products
obtained from a HindIII and EcoRI digestion. Lane M indicates the
Marker 5,000 DNA ladder. RNAi, RNA interference. |
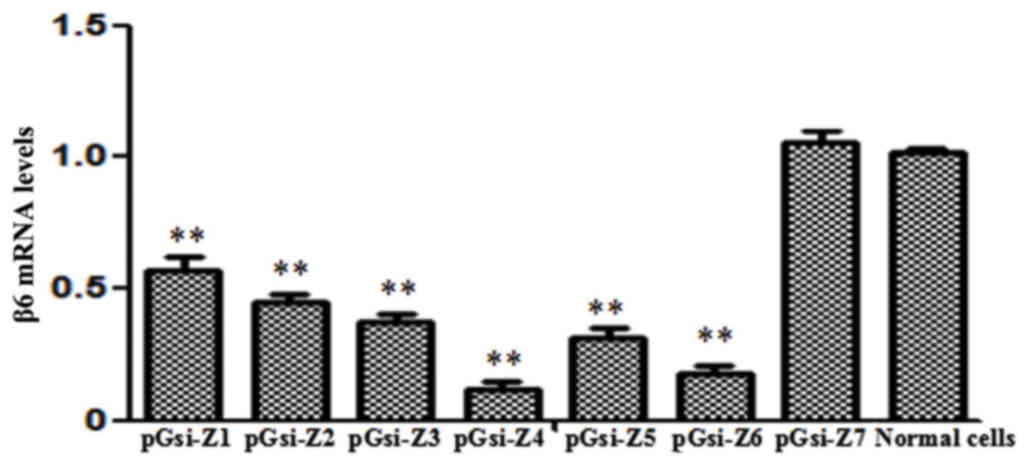
Fluorescence RT-qPCR detection of the
FMDV integrin receptor β6 subunit gene
In PEF cells, the pGsi-Z4 recombinant plasmid
exhibited marked inhibitory effects on the expression of the β6
subunit gene, whereby inhibition efficiency reached 91.7%
(P<0.01 vs. control). The interference effects of the other
recombinant plasmids were not significant (Fig. 3).
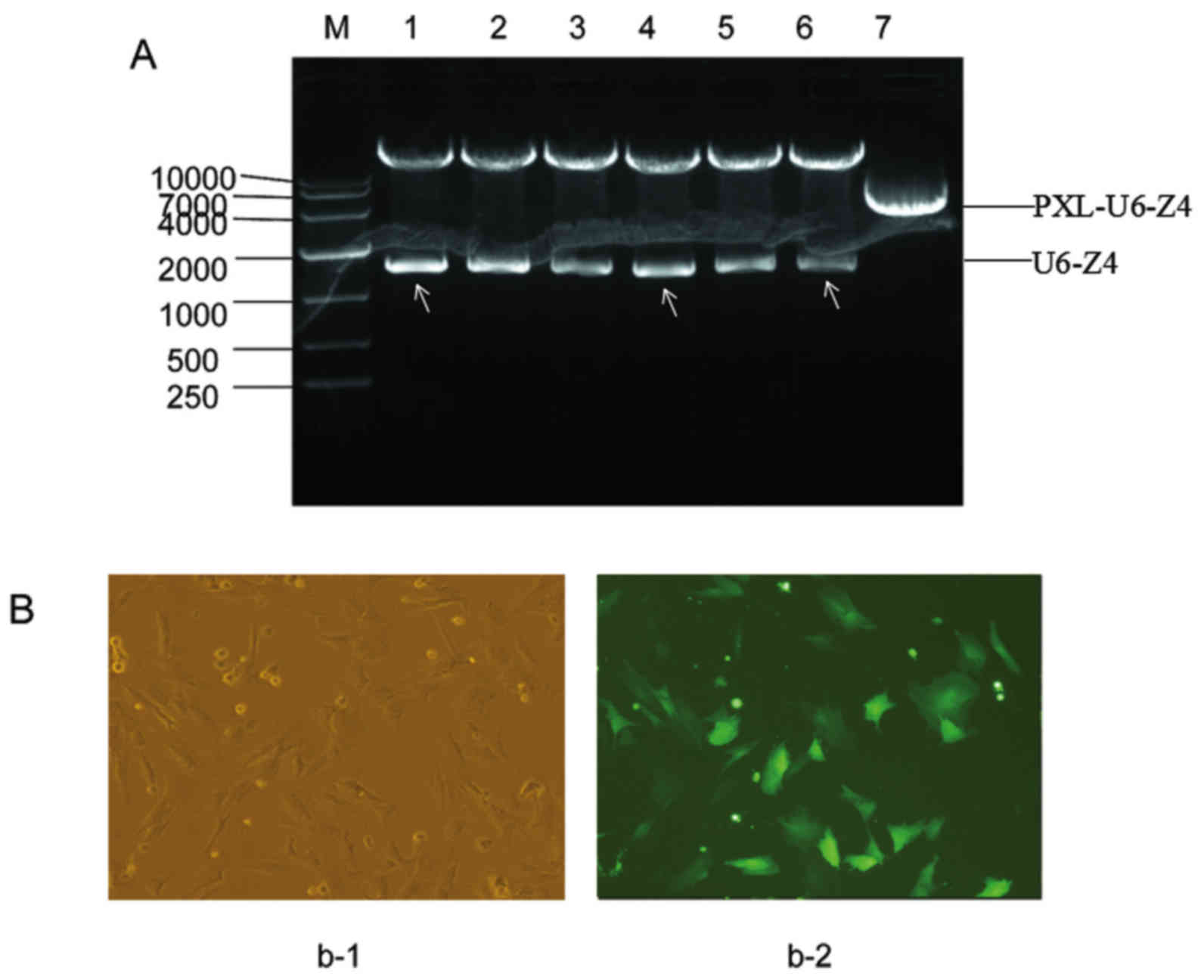
Linearizing pXL-U6-Z4 and cell
transfection
Bacterial supernatant with the U6-Z4 sequencing
results was amplified as a pXL-U6-Z4 plasmid in the E. coli DH5α
strain for plasmid isolation. PXL-U6-Z4 was linearized using ApaLI
enzyme digestion and plasmid fragments were detected by
electrophoresis. The DNA sequence that was ~1,240 bp in size and
contained the Amp resistance gene with a prokaryotic replication
initiation site was removed. A linearized pXL-U6-Z4 sequence of
~6,580 bp was recovered (Fig. 4A)
and transfected into PEF cells. Subsequent GFP expression was
observed under an inverted fluorescence microscope and transfection
efficiency was estimated to be ~90%. (Fig. 4B).
Validation of pXL-U6-Z4 interference
effects
Fluorescence qPCR indicated that the interference
effect of the integrated pXL-U6-Z4 plasmid increased after
transfection in a time-dependent manner. The plasmid exhibited
inhibitory effects (P<0.01) on the β6 subunit gene, with
inhibition rates of 56.5, 59.5 and 78.5% observed at 12, 24 and 36
h, respectively (Fig. 5).
Detection of integrated plasmid
transfection and viral challenge
After dilution of an FMDV stock, a virus challenge
assay was performed using 2.1×103 TCID50 of virus solution/well.
The following three groups were included in the assay: A virus
challenge after pXL-U6-Z4-transfection group, a virus challenge
without transfection group and a normal (untreated) cell group. At
12, 28, 24 and 36 h after the virus challenge, PEF cells were
observed for CPE. Results were determined using a semi-quantitative
method according to cell lesion conditions (Table III). Normal cells exhibited regular
long spindle morphologies, while virally challenged cells exhibited
cellular lesions, a round and/or shrunken morphology and clumped
structures (Fig. 6).
 | Table III.CPE of foot-and-mouth disease virus
infection in PEF cells. |
Table III.
CPE of foot-and-mouth disease virus
infection in PEF cells.
|
| Time after
transfection, h |
|---|
|
|
|
|---|
| PEF cell group | 12 | 18 | 24 | 36 |
|---|
| Non-transfected
cells | + | ++ | ++++ | +++++ |
| Transfected
cells | + | + | +++ | + |
| Normal cells | − | − | − | − |
Detection of virus replication using
RT-qPCR
In a fluorescence RT-qPCR assay (Fig. 7), the level of FMDV VP1 antigen, as
an indicator of FMDV levels, measured in normal cells at 36 h
post-viral challenge was defined as 1. The levels of FMDV measured
in the transfection group was higher than that in the
non-transfection group at 12 after the viral challenge, and the
non-transfection group had higher levels of virus at 18 h after
viral challenge, though these differences were not significant. At
24 and 36 h post-viral challenge, the level of viral replication in
the transfection group was lower than that in the non-transfection
group (P<0.05; Fig. 7). Viral
replication was reduced by 24.2 and 12.8%, after 24 and 36 h,
respectively. Collectively, these data indicate that transfection
with pXL-U6-Z4 may have inhibited expression of the FMDV integrin
receptor, thus reducing the ability of FMDV to invade and replicate
within PEF cells. These results suggest that the pXL-U6-Z4
interference plasmid may inhibit intracellular FMDV
replication.
Discussion
FMDV expresses five antigenic sites on the surface
of its viral capsid. The main viral antigen, VP1, contains a G-H
loop on its surface with a highly conserved
arginine-glycine-aspartate (Arg-Gly-Asp, RGD) sequence, which
promotes viral infection of target cells (4,16). A
number of interactions between host cell integrins, namely αvβ1,
αvβ3, αvβ, αvβ6, αv5β8, αIIβ3b, α5β1, α1β8, and the RGD sequences
within the FMDV VP1 antigen have been proposed, though currently
only integrins αvβ1, αvβ3, αvβ6 and αvβ have been confirmed as a
receptor for FMDV (17). In
particular, the integrins αvβ and αvβ6 may have a key role in viral
infection (18).
Integrins are a family of cell surface receptor
proteins that have important roles in cell development, immune
responses, blood coagulation and inflammatory reactions (19). More than 20 combinations of integrin
αβ heterodimers are expressed on the cell-extracellular matrix
boundary. These heterodimers participate in signal transduction
between cells and also serve intermediary roles in the process of
viral invasion of cells (20). The β
subunit and a number of α subunits exhibit an α-I subunit structure
domain. This is composed of five amino acids and adopts a structure
that relies upon metal ion adsorption sites (also known as metal
ion-dependent adhesion site, MIDAS) to generate a ligand binding
site (21). A previous study of the
porcine integrin αv subunit, as a receptor for FMDV, demonstrated
that lentiviral RNAi technology inhibited target gene and
corresponding protein expression of the α subunit. RT-qPCR analysis
also indicated that this approach decreased viral replication by
>3-fold (22). Results in cattle
have also suggested that avβ6, rather than avβ3, has a major role
in determining the tropism of FMDV for the epithelia, as the
primary organ targeted by the virus (23). Indeed, αvβ6 is currently considered
to function as a primary FMDV receptor that contributes to FMDV
tropism and viral invasion (24).
Furthermore, αvβ6 exerts regulatory functions in the process of
coagulation (25). Jacobsen et
al (26) documented that
β6-knockout mice exhibit significantly delayed wound healing at the
early stage of disease when compared with wild-type diabetic mice.
In addition, expression of αvβ6 in keratinocytes promoted adhesion
and migration in cultured cells (26). Sullivan et al (27) and Miller et al (28) knocked out regions of the β6 subunit
cytoplasmic domains containing the RGD site, and observed that
modified αvβ6 was unable to mediate FMDV infection. Therefore, the
β6 subunit was the focus of the current study due to its potential
involvement in FMDV infection. In the current study, the function
of the β6 subunit was confirmed by evaluating the effect of β6
subunit knockdown on FMDV infection. It was observed that RNAi of
the β6 subunit influenced viral replication, thus indicating that
the β6 subunit may be required for viral infection.
RNAi is an effective method for inhibiting viral
replication, and previous studies have investigated the effects of
anti-viral RNAi methods on FMDV (29,30). The
initial stage of host cell infection by FMDV involves interaction
with a cognate cell-surface receptor, which subsequently enables
virus particles to enter cells in a receptor-mediated manner. Thus,
cell-surface receptors are key in determining viral host range and
tissue tropism (31). The present
study targeted the αvβ6 receptor, specifically by designing siRNA
molecules and RNAi expression plasmids that targeted the
cytoplasmic domain region of the β6 subunit. The plasmids were
introduced into PEF cells to inhibit the expression of the αvβ6
receptor, as a mediator of FMDV infection. Results of RT-qPCR
demonstrated that the pGsi-Z4 recombinant plasmid had an inhibitory
effect on the expression of the β6 subunit within PEF cells,
indicating that RNAi successfully inhibited receptor gene
expression. Measurements in PEF cells transfected with the
pXL-U6-Z4 integration plasmid indicated that an interference effect
was present; however, the interference efficiency was lower than
that of cells transfected with the initial pGsi-Z4 interference
expression plasmid. This may have been due to random integration
effects of the integration plasmid. For instance, expression of the
interference fragment or the efficiency of integration into the
plasmid target site may have been sub-optimal, thus reducing the
interference effect. Following transfection of the pXL-U6-Z4
integration into cells, the interference fragment likely integrated
into a random site in the genome. Further study is required to
identify these random plasmid integration site(s) within the
genome.
Viral challenge experiments were performed on PEF
cells transfected with the pXL-U6-Z4 plasmid. Observations of cell
morphology identified more marked cellular lesions in the
non-transfection group than in the transfection group, indicating
that transfection with the integration plasmid may have reduced
viral replication. However, results of RT-qPCR at 12 h
post-infection demonstrated that the levels of FMDV in the
transfection group were higher than that in the non-transfection
group. As transfection potentially reduces cell growth, the
resistance of transfected cells may have been lower than that of
non-transfected cells. Therefore, a number of transfected cells may
have died earlier in the assay compared with non-transfected cells,
resulting in relatively higher levels of viral replication in
remaining transfectants. At 18 h after viral inoculation, levels of
viral replication in both groups were similar. Despite a potential
loss of damaged transfected cells, surviving transfectants retained
the ability to reduce viral replication. This was indicated by
lower levels of virus in the transfection group, relative to the
non-transfection group, at 24 h after viral inoculation. At 36 h
after viral inoculation, the level of FMDV in the transfection
group remained lower than that in the non-transfection group,.
Using the TCID50 method to inoculate cells with
FMDV, it was demonstrated that siRNA had inhibitory effects on the
replication capacity of FMDV. This validated that RNAi of FMDV
integrin receptors, including the previously studied porcine
integrin αv subunit receptor (8),
may exert effects at the cellular level.
Using RNAi technology, the present study
successfully inhibited expression of the integrin β6 sub-domain I
at the mRNA level. This potentially blocked cell invasion by FMDV
and prevented viral replication and dissemination. Viral
replication was reduced by 24.2 and 12.8%, after 24 and 36 h,
respectively. Future studies are now warranted to determine whether
RNAi of integrin α subunits, similar to RNAi of the β6 subunit, has
inhibitory effects on viral replication.
Acknowledgements
The present study was supported by the National
Science and Technology Major Projects in the Cultivation of New
Varieties of Genetically Modified Organisms (grant no.
2009ZX08005-003B).
References
|
1
|
Li X, Wang J, Liu J, Li Z, Wang Y, Xue Y,
Li X, Cao H and Zheng SJ: Engagement of soluble resistance-related
calcium binding protein (sorcin) with foot-and-mouth disease virus
(FMDV) VP1 inhibits type I interferon response in cells. Vet
Microbiol. 166:35–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johns HL: Regulation of FMDV infection by
cellular rab GTPases. University of Surrey. uk.bl.ethos.486086.
2007
|
|
3
|
Shen XY, Chang HY, G Z Cong, Liu Y-S, Wang
JH and Xie QG: Advance in foot-and-mouth disease virus infectious
cycle. Progress in Veterinary Medicine. (6): 1–5. 2005.
|
|
4
|
Sekiguchi K, Franke AJ and Baxt B:
Competition for cellular receptor sites among selected
aphthoviruses. Arch Virol. 74:53–64. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lea S, Hernández J, Blakemore W, Brocchi
E, Curry S, Domingo E, Fry E, Abu-Ghazaleh R, King A, Newman J, et
al: The structure and antigenicity of a type C foot-and-mouth
disease virus. Structure. 2:123–139. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Donnell V, Pacheco JM, Gregg D and Baxt
B: Analysis of foot-and-mouth disease virus integrin receptor
expression in tissues from naïve and infected cattle. J Comp
Pathol. 141:98–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson T, F M Ellard, Ghazaleh RA,
Brookes SM, Blakemore WE, Corteyn AH, Stuart DI, Newman JW and King
AM: Efficient infection of cells in culture by type O
foot-and-mouth disease virus requires binding to cell surface
heparan sulfate. J Virol. 70:5282–5287. 1996.PubMed/NCBI
|
|
8
|
Fox G, Parry NR, Barnett PV, McGinn B,
Rowlands DJ and Brown F: The cell attachment site on foot-and-mouth
disease virus includes the amino acid sequence RGD
(arginine-glycine-aspartic acid). Gen Virol. 70:625–637. 1989.
View Article : Google Scholar
|
|
9
|
Mason PW, Baxt B, Brown F, Harber J,
Murdin A and Wimmer E: Antibody-complexed foot-and-mouth disease
virus, but not poliovirus, can infect normally insusceptible cells
via the Fc receptor. Virology. 192:568–577. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai K J, Ma SW, Qiao J, Meng WL, Chen CF,
Huang J, Zhang ZC and Yang HB: Anti-viral activity of cells from
foot-and-mouth disease virus shRNA transgenic pig. Chinese Journal
of Preventive Veterinary Medicine. 1:5–9. 2013.(In Chinese).
|
|
11
|
Ma SW, Chen CF and Qiao J: The shRNA
screen and test of high effective inhibition from type O FMDV.
Chinese Journal of veterinary science. 113–17. (22)2012.(In
Chinese).
|
|
12
|
Zhang J, Guo S, Zhang W, Niu D and Gong J:
Large-pore mesoporous silica nanospheres as vehicles for delivering
TRAF3-shRNA plasmids to Kupffer cells. Biochem Biophys Res Commun.
469:196–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reed LJ and Muench H: A simple method of
estimating fifty per cent endpoints12. Am J Epidemiol. 27:493–497.
1938. View Article : Google Scholar
|
|
15
|
Dong W, Hu H, Feng G, Yang G and Wang Q:
Construction and identification of eukaryotic expression vector of
small interfering RNA specific for BACE. Journal of Xi‘an Jiaotong
University (Medical Sciences). 26:413–416, 423. 2005.
|
|
16
|
Pierschbacher MD and Ruoslahti E: Variants
of the cell recognition site of fibronectin that retain
attachment-promoting activity. Proc Natl Acad Sci USA. 81:pp.
5985–5988. 1984; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fox G, Parry NR, Barnett PV, McGinn B,
Rowlands DJ and Brown F: The cell attachment site on foot-and-mouth
disease virus includes the amino acid sequence RGD
(arginine-glycine-aspartic acid). J Gen Virol. 70:625–637. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whitton JL, Cornell CT and Feuer R: Host
and virus determinants of picornavirus pathogenesis and tropism.
Nat Rev Microbiol. 3:765–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barczyk M, Carracedo S and Gullberg D:
Integrins. Cell Tissue Res. 339:269–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, He X, Corbett SA, Lowry SF, Graham
AM, Fässler R and Li S: Integrins are required for the
differentiation of visceral endoderm. J Cell Sci. 122:233–242.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wegener KL, Partridge AW, Han J, Pickford
AR, Liddington RC, Ginsberg MH and Campbell ID: Structural basis of
integrin activation by talin. Cell. 128:171–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo J, Du J, Gao S, Zhang G, Sun J, Cong
G, Shao J, Lin T and Chang H: Lentviral-mediated RNAi to inhibit
target gene expression of the porcine integrin αv subunit, the FMDV
receptor, and against FMDV infection in PK-15 cells. Virol J.
8:4282011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monaghan P, Gold S, Simpson J, Zhang Z,
Weinreb PH, Violette SM, Alexandersen S and Jackson T: The alpha
(v)beta6 integrin receptor for Foot-and-mouth disease virus is
expressed constitutively on the epithelial cells targeted in
cattle. J Gen Virol. 86:2769–2780. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monaghan P, Gold S, Simpson J, Zhang Z,
Weinrab PH, Violette SM, Alexandersen S and Jackson T: The
alpha(v)beta6 integrin receptor for Foot-and-mouth disease virus is
expressed constitutively on the epithelial cells targeted in
cattle. J Gen Virol. 86:2769–2780. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jackson T, Clark S, Berryman S, Burman A,
Cambier S, Mu D, Nishimura S and King AM: Integrin alphavbeta8
functions as a receptor for foot-and-mouth disease virus: role of
the beta-chain cytodomain in integrin-mediated infection. J Virol.
78:4533–4540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jacobsen JN, Steffensen B, Häekkinen L,
Krogfelt KA and Larjava HS: Skin wound healing in diabetic ß6
integrin deficient mice. APMIS. 118:753–764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sullivan BP, Weinreb PH, Violette SM and
Luyendyk JP: The coagulation system contributes to alphaVbeta6
integrin expression and liver fibrosis induced by cholestasis. Am J
Pathol. 177:2837–2849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller LC, Blakemore W, Sheppard D,
Atakilit A, King AM and Jackson T: Role of the cytoplasmic domain
of the beta-subunit of integrin alpha(v)beta6 in infection by
foot-and-mouth disease virus. J Virol. 75:4158–4164. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kahana R, Kuznetzova L, Rogel A, Shemesh
M, Hai D, Yadin H and Stram Y: Inhibition of foot-and-mouth disease
virus replication by small interfering RNA. J Gen Virol.
85:3213–3217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohapatra JK, Sanyal A, Hemadri D, Tosh C,
Kumar RM and Bandyopadhyay SK: Evaluation of in vitro inhibitory
potential of small interfering RNAs directed against various
regions of foot-and-mouth disease virus genome. Biochem Biophys Res
Commun. 329:1133–1138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schneider-Schaulies J: Cellular receptors
for viruses: Links to tropism and pathogenesis. J Gen Virol.
81:1413–1429. 2000. View Article : Google Scholar : PubMed/NCBI
|