Introduction
Treating large bone defects remains a major
challenge in clinical practice. Although many osteoconductive and
osteoinductive filler materials have been used for bone defects
(1,2), annually, >800,000 patients worldwide
cannot receive this treatment but instead receive autologous bone
grafts to treat their bone defects (3). The autologous bone grafting technique
has been viewed as the ‘gold standard’ for its homogeneous bony
tissue and efficacy; however, the method has limitations, including
morbidity, limited resources and its association with
complications, such as bleeding and wound problems (4,5). On the
basis of the data from the National Hospital Discharge Survey, the
use of bone grafts decreased in the United States between 1992 and
2007, with a shift in preference from autogenous bone grafts to
substitute bone grafts (6).
Previous studies have identified the structure of
the nanofibers induced the proliferation of the mesenchymal stem
cells and osteoblasts (7,8). The structure and biological functions
of poly-L-lactic acid (PLLA) electrospun nanofibers are similar to
the natural extracellular matrix (ECM) of bone tissue and are
superior to other biomaterials, due to their beneficial
biocompatibility properties (9,10).
However, as the pores generated by electrospinning are within the
range of the fiber diameters, the pores are not large enough for
the cellular migration and tissue infiltration (11). Furthermore, the surface of PLLA is
hydrophobic and lacks bioactive signals for cell recognition, which
increases the difficulty for integrin receptors to identify binding
sites (12,13). Hence, further studies are required to
overcome the disadvantages.
Approved by the Food and Drug Administration,
low-intensity pulsed ultrasound (LIPUS) is considered as a
non-invasive and feasible modality for the treatment of the delayed
union and the nonunion of bone (14). A previous study has demonstrated that
ultrasound exposure increased the porosity and permeability of the
solid-state fabricated PLA foams (15). The findings are in agreement with
results obtained from previous results from Guo et al
(16). Moreover, other investigators
have concluded that the bioeffect of LIPUS exposure was promoted
via the integrin/FAK/MAPK pathway (17). Cell-matrix adhesions are
predominantly mediated by the members of the integrin family
(18). A previous study revealed
that the conformation of proteins, which are composed of
cell-matrix adhesions, changed and the cryptic-binding sites were
exposed while mechanical force acted on the cell-matrix adhesions
(19). Therefore, it seems
reasonable that mechanical stresses, such as LIPUS acting on the
cell-matrix adhesions, may expose the cryptic site.
The potential synergistic effect of LIPUS and PLLA
electrospun nanofibers remains unclear. The present study
investigated the hypothesis that the critical size of cortical bone
defect filled with the PLLA electrospun nanofibrous membrane would
acquire bone union with the aim of LIPUS treatment.
Materials and methods
Fabrication of PLLA electrospun
nanofibers
The PLLA nanofibrous membrane was successfully
produced by the polymer solution methodology as previously
described (20,21). The polymer solution was fabricated by
dissolving PLLA (Thermo Fisher Scientific, Ltd., Waltham, MA, USA)
in chloroform/ethanol (3:1, v/v). Then polymer was subsequently
placed into a 5 ml syringe connecting to a 50 cm Teflon tube (Bo
Jie Co., Ltd, Huatan, Taiwan). The electrospinning solution was
delivered with a flow rate of 1.0 ml/h via a syringe pump. The
thickness of the PLLA was 2.0 mm. All electrospun nanofibrous
membranes were stored in the desiccators prior to use.
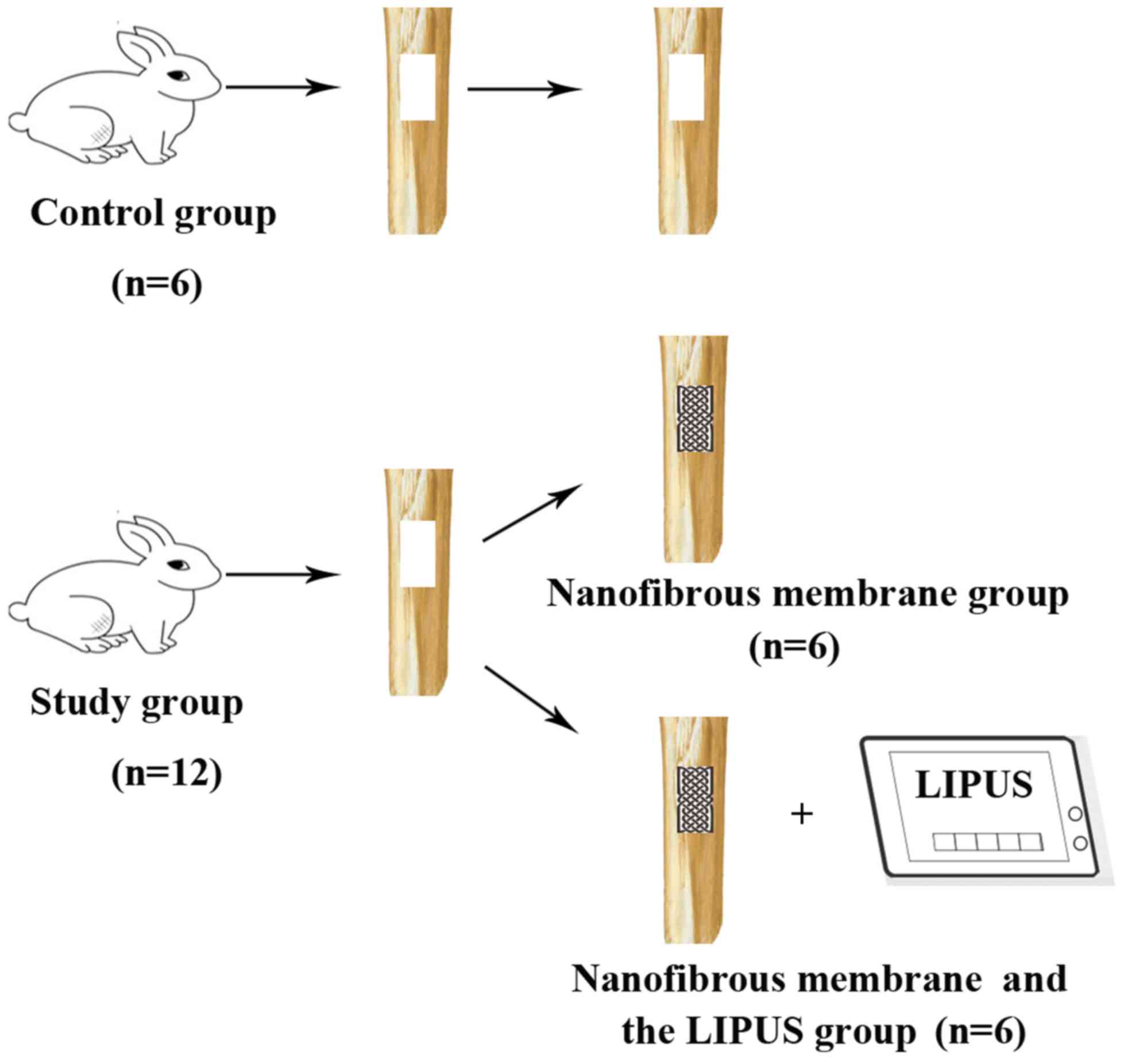
Animal experiment
The present study was approved by the Zhejiang
Institutional Animal Care and Use Committee (Hangzhou, China).
International laws and regulations for medical research with
experimental animals were followed. A total of 18 adult male New
Zealand White rabbits aged between 15 and 18 weeks and weighing
2.19±0.17 kg, were randomly divided into two groups: The control
group (n=6) and the study group (n=12). Rabbits were housed at
21–25°C, 60% humidity with a 12 h light/dark cycle. Rabbits had
full access to dry food and water in individual cages without
activity restriction. All rabbits were anesthetized by an
intravenous injection with chloral hydrate (280 mg/kg; Tokyo
Chemical Industry Co., Ltd., Tokyo, Japan) prior to surgery. The
surgical procedures are described in detail in a previous study
(21) and are briefly reported here.
While the shaft of the tibia was exposed, a segmental defect (15-mm
long and 5-mm wide) was created in the anteriolateral cortex of the
tibia with an oscillating saw. This procedure was performed
repeatedly in the bilateral tibias of each rabbit. After the bone
defect was successfully administered in the anteromedial cortex of
the bilateral proximal tibia, rabbits were treated differently
according to the study protocol. Bone defects in rabbits were not
induced in the control group. The nanofibrous membrane was cut into
15×5 mm2 membrane specimens, which were used to fill the
bone defects in the study group. All of the membrane specimens were
press-fitted into the bone defect. The left tibias of the study
group were treated with nanofibrous membranes, whereas the right
tibias of rabbits were treated with nanofibrous membranes and
LIPUS. The ultrasonic generator (Nexus; Hexin Biomedical Devices,
Hangzhou, China) was employed with one ultrasonic transducer at a
pulse frequency of 1.5 MHz, output intensity of 200
mW/cm2 and a 50% duty cycle. Ultrasound gel was placed
on the surface of the transducer and the surgical site when the
LIPUS was applied. A total of 3 rabbits from the control group and
the 6 from the study group were sacrificed at 3 and 6 weeks,
respectively, for evaluation (Fig.
1).
Radiographic assessment
Rabbits were sacrificed via overdose of 200 mg/kg
pentobarbital sodium administered intravenously via the marginal
ear vein (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) at
either 3 or 6 weeks. Tibiae from rabbits were harvested, fixed in
10% neutral formalin for 1 week at 4°C and subsequently
radiographed in the anteroposterior planes using X-ray apparatus
(Kodak, Rochester, NY, USA).
Histological evaluation
Specimens were decalcified in 10% EDTA in 0.01 M
phosphate buffer and dehydrated with an ascending series of ethanol
solutions for one week at 4°C. Following decalcification and
dehydration, the samples were embedded in paraffin. The area of
interest was defined at 10-mm proximally and distally to the center
of the defect. Along the central portion of the defect in the
anteromedial cortex, tibiae were cut horizontally on a microtome.
Sections (8-µm thick) were stained with hematoxylin and eosin and
examined under a light microscope (Olympus Corp., Tokyo, Japan).
Histological analyses were performed at a primary magnification of
×40 and a second magnification of ×200 or ×400.
Bone score
Bone scoring was applied to evaluate the degree of
nascent bone formation during histological evaluation. The
semiquantitative score from 0 (no mineralized bone) to 4 (complete
bridging of the defect with mineralized bone) was used in the
present study, as described previously (22). The other score values were defined as
follows: 1, few and isolated centers of ossification; 2, increased
ossification with discontinuous novel bone formation; and 3,
notable but incomplete bridging of the defect (22). Scoring was evaluated by at least two
investigators. The score was added to give a cumulative sum to
estimate the overall bone formation in the defect at a given time.
Bone scores were compared among all groups by at least two persons
with no knowledge of the groups being graded.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 software package (GraphPad Software, Inc., La Jolla, CA,
USA). The bone formation ratio is expressed as the mean ± standard
deviation and the normality of the distribution was tested. For
multiple comparisons among groups, differences were analyzed by
one-way analysis of variance followed by Tukey's post-hoc test.
P<0.01 was considered to indicate a statistically significant
difference.
Results
Excluded results
One animal in the control group was sacrificed due
to a postoperative hematoma and secondary infection. All other
animals recovered from the bilateral surgery and completed the
study without any complications.
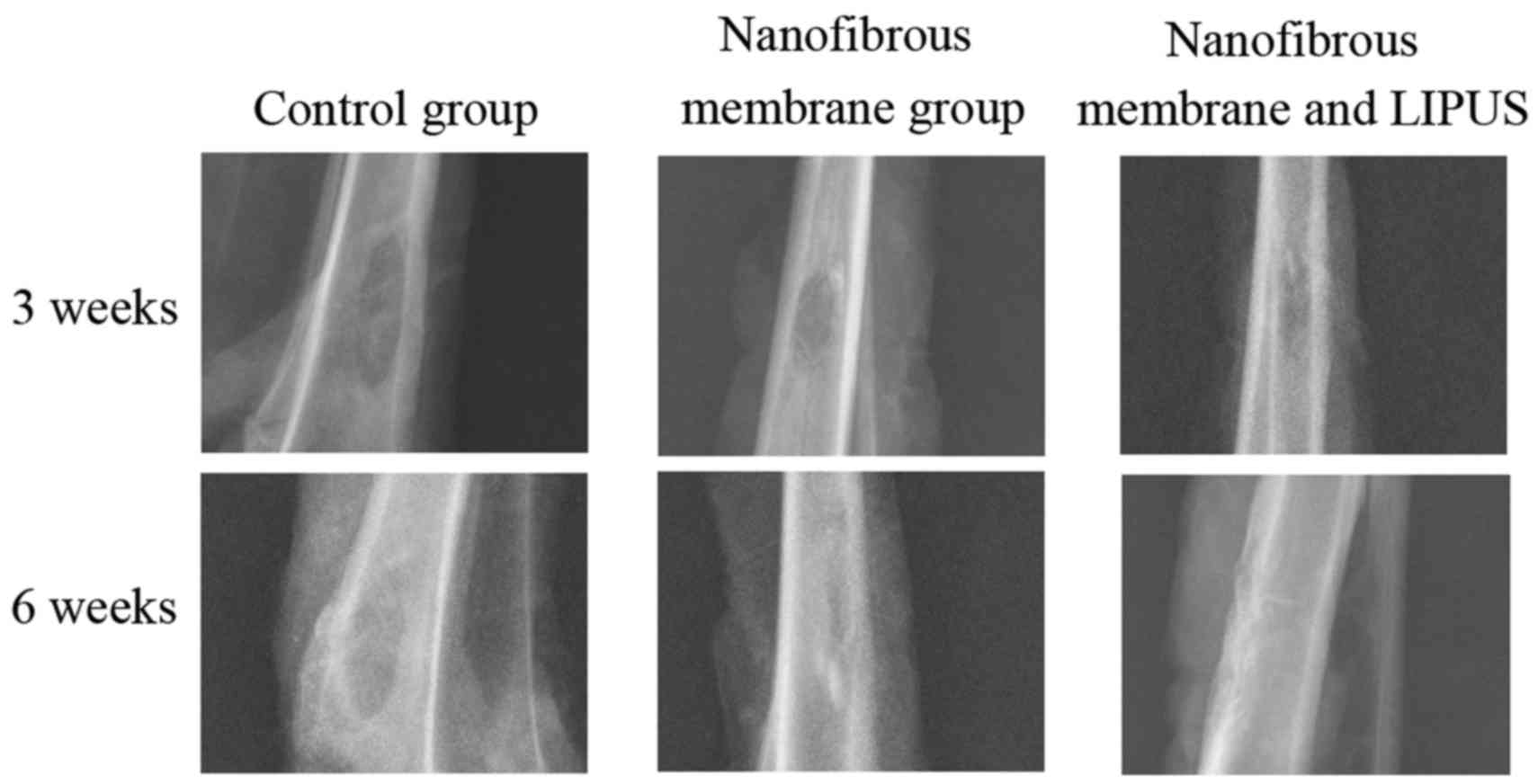
X-ray evaluation
No signs of gross adverse reactions or infection
according to the macroscopic examination of the proximal tibia at
harvest were observed. The representative conventional X-ray
photographs of the tibia cortex are shown in Fig. 2. All of the control group and the
study groups showed that bone healing did not occur completely 3
weeks after surgery. Small areas of mineralization scattered at the
bone defects margin were detectable in the nanofibrous membrane
group and nanofibrous membrane plus LIPUS group. Conversely, the
bone defects filled with nanofibrous membranes and treated with
LIPUS demonstrated increased bone formation compared with the
nanofibrous membrane group. However, in the control group, the
defect sites indicated no bone ingrowth. A total of 6 weeks after
surgery, novel bone formation in the central part of the defect
regions was observed only in the nanofibrous membrane plus LIPUS
group. The bone defect was not fully healed in the nanofibrous
membrane group and the defect was filled with radio-opaque tissue,
with the exception of the central region. The control group showed
no areas of mineralization.
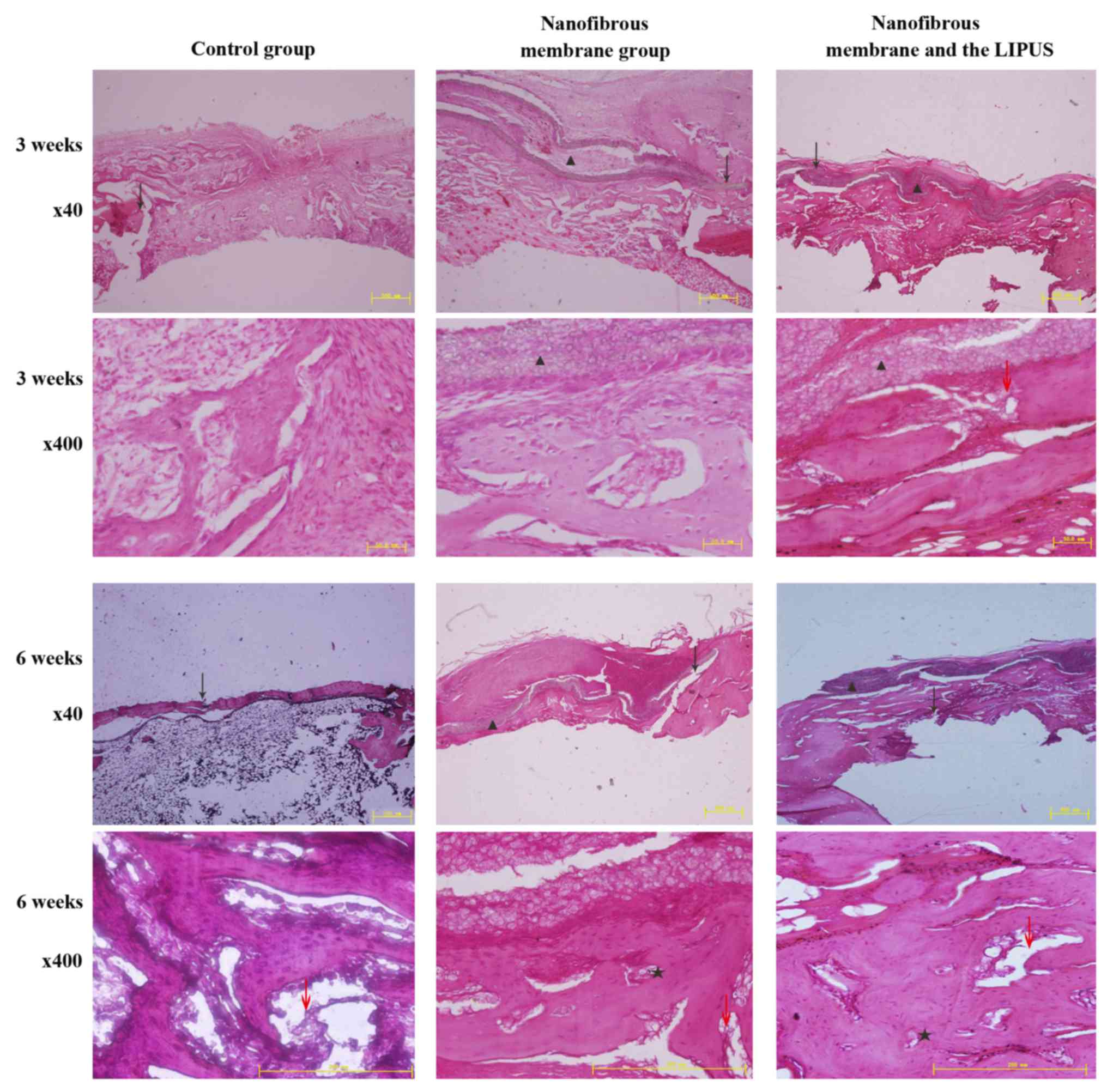
Histological evaluation
Microscopically, the residues of the nanofibrous
membranes were visible at 3 and 6 weeks; this is consistent with
previous findings (21). The results
indicated that LIPUS did not alter the resorption rate of
nanofibrous membranes according to the sections. The aligned
fibers, which covered the bone defect site, guided the newly formed
bone from the edge of the gap to the other edge. In addition, the
amount of newly formed bone and connective tissue in the study
group was different from the control group. Increased volumes of
newly formed cortical bones were indicated in the nanofibrous
membrane group and nanofibrous membrane plus LIPUS group (Fig. 3).
The formation of novel bone adjacent to the defect
margin was observed in all groups except for the control group at 3
weeks. The bone defect site of the control group was filled with
connective tissue. Novel bone formation was consistently identified
where the nanofibrous membranes were in direct contact with the
defect margin. Combined with nanofibrous membranes, LIPUS treatment
increased the amount of newly formed bone when compared with
nanofibrous membrane alone. An obvious increase in nascent bone
formation was observed with ultrasound treatment (Fig. 3). Based on these findings, defects
treated with nanofibrous membranes plus LIPUS demonstrated thicker
bone-like tissue.
At 6 weeks, in the nanofibrous membrane plus LIPUS
group, abundant mature lamellar bone was observed running through
the margins of the bone defect via the nanofibrous membrane.
Nascent bone formation was observed primarily in proximity with the
nanofibrous membranes. Compared with the nanofibrous membrane
group, the bone formation of the nanofibrous membrane plus LIPUS
group was thicker and more mature in the center of the defect site.
However, the spatial and temporal pattern of novel bone formation
indicated no difference between the nanofibrous membrane plus LIPUS
group and the nanofibrous membrane alone. Newly-formed capillaries
and osteoblasts were also observed in the study group, which are
responsible for the formation of bone. Newly formed bone, which
appeared to be spongy, and connective tissue were scattered in the
defect sites of the control group. Limited mature lamellar bone was
visible in the control group (Fig.
3).
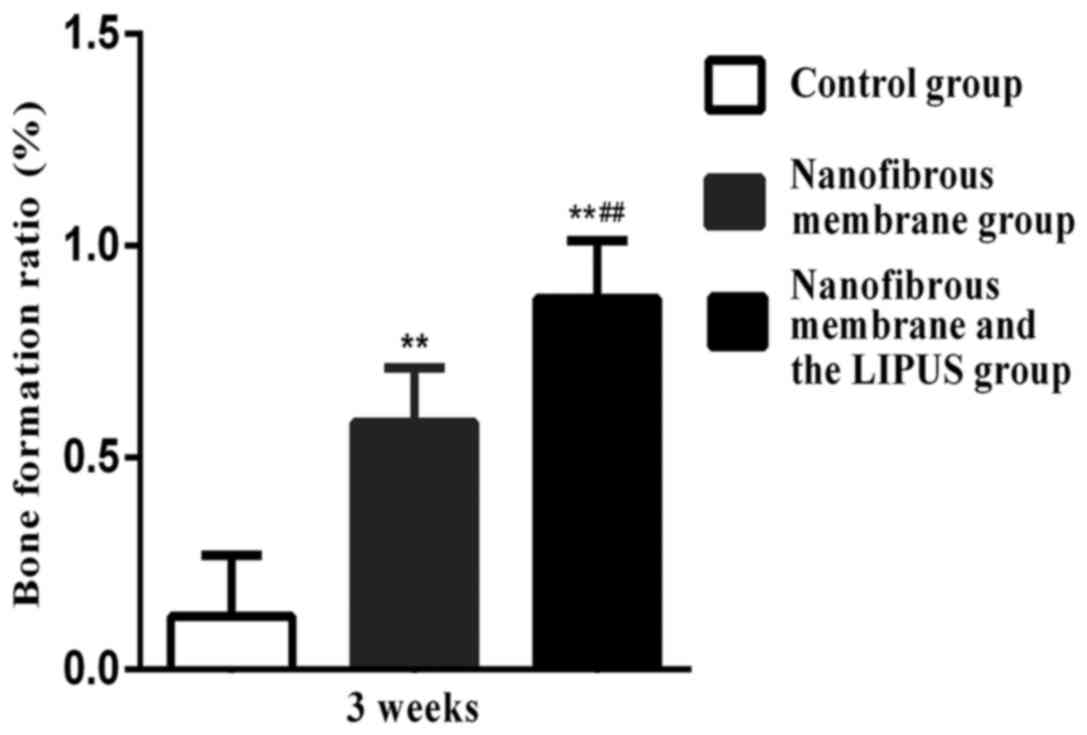
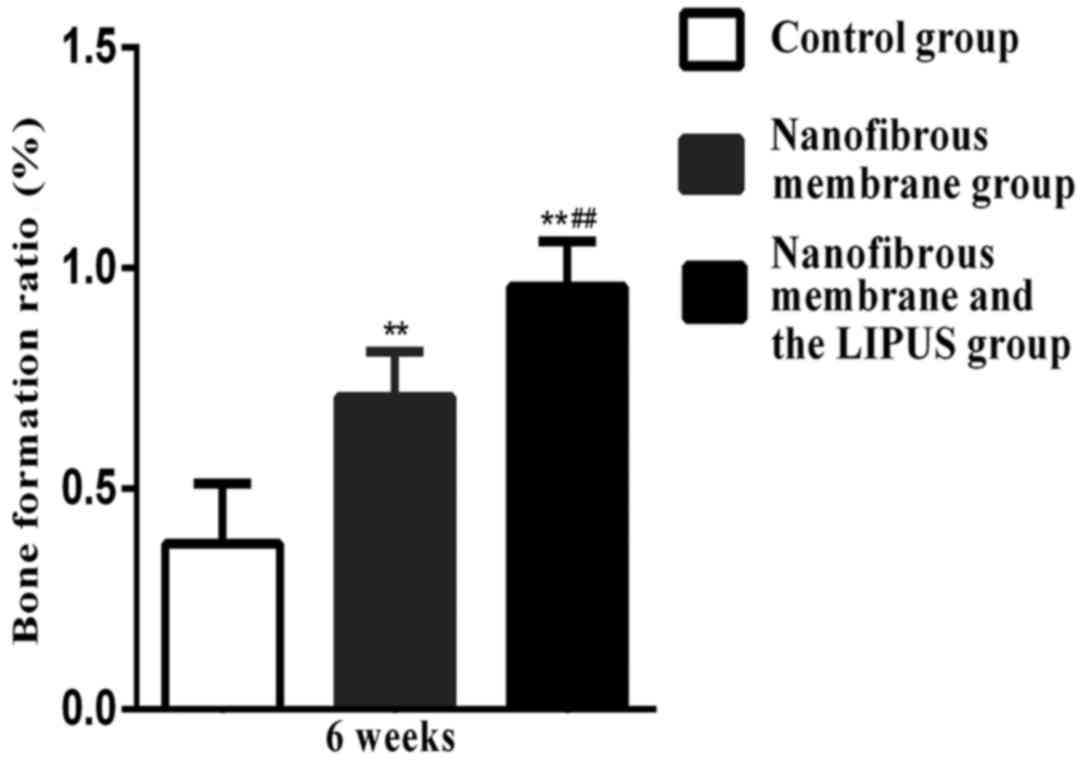
Bone score
Significant differences in bone scores were
determined between the nanofibrous membrane plus LIPUS group and
the nanofibrous membrane group at 3 and 6 weeks, respectively
(P<0.01; Figs. 4 and 5, respectively). The nanofibrous membrane
plus LIPUS group exhibited a significantly greater bone score when
compared with the nanofibrous membrane group (P<0.01; Figs. 4 and 5).
Discussion
The healing of large bone defects remains a clinical
challenge. The majority of studies have focused on the biological,
material or mechanical factors that are applied for the treatment
of bone defects. As it is difficult to create enough macropores for
cellular migration using the electrospun PLLA nanofibrous membrane
and the surface of the PLLA lacks bioactive signals for cell
recognition, we believe the LIPUS may be utilized to overcome these
disadvantages.
The present study evaluated the efficacy of LIPUS
combined with the cell-impermeable PLLA nanofibrous membrane on the
healing of large cortical bone defects. The present findings
suggested that LIPUS was able to increase cell ingrowth and expose
the cryptic site when acting on cell-matrix adhesions. Furthermore,
the present study demonstrated that LIPUS improved the efficacy of
PLLA. A predominant challenge of the widespread use of electrospun
nanofibers has been their innate hydrophobic nature and the
difficulty in creating enough macropores for cellular migration
(11,13). The pores generated by the
electrospinning are approximately the same order of the fiber sizes
(11,23). A previous study hypothesized that the
surrounding fibers were dynamically moved by cells entering the
pores (24). Few studies have
explored this hypothesis and further investigation is required.
When implanted material is placed in the bone
defect, cells from the surrounding tissues interact with the
membrane material in a process, which is mediated by the protein
that is adsorbed to the surface (25). Mesenchymal stem cells are able to
detect and migrate along the surface of the membrane. In the
present study, thicker and more mature bone formed in the bone
defect site adjacent to the membrane in the nanofibrous membrane
plus LIPUS group. Detection of mesenchymal stem cells infiltrating
the membrane was limited; however, the aligned fibers guided the
newly formed bone from the edge of the gap to the center of the
defect. Previous investigation has demonstrated that aligned
nanofibers increased cell migration along the direction of fiber
orientation, but did not influence osteogenic differentiation
(26). Therefore, this suggests that
the efficacy of bone formation in the present study may be
attributed to LIPUS rather than the PLLA nanofibrous membrane.
Although the morphology and cell viability were not
measured by scanning electron microscopy, the findings of the
present study suggested that LIPUS may increase cell ingrowth into
porous membranes. Previous results have indicated that LIPUS
treatment accelerated bone ingrowth into the porous tricalcium
phosphate bioceramic scaffold (27)
and increased cell ingrowth into the pores of the three-dimensional
silicon carbide scaffold (28).
These findings suggest that the increased bone formation observed
in the PLLA nanofibrous membrane plus LIPUS group in the present
study may be attributed to the capacity for LIPUS to increase cell
ingrowth into porous nanofibrous membranes. Further investigations
and scanning electron microscopy should be performed to clarify the
cell ingrowth depths inside the nanofibrous membrane.
The findings obtained from the present study
indicated that only the nanofibrous membrane plus LIPUS group
displayed near-complete regeneration of the cortical bone 6 weeks
after surgery. Newly formed bone was visible in the center of the
bone defect site in contact with the nanofibrous membrane in the
presence or absence of LIPUS. Therefore, the nanofibrous membrane
acted as the bridge that guided the osteoblasts or the progenitors
to the bone defect site. The results suggested that the nanofibrous
membrane improved the bone formation and induced osteogenic
differentiation. The present findings was in accordance with the
findings presented by Kolambkar et al (26), who reported aligned nanofiber meshes
with increased cell migration along the direction of fiber
orientation.
Compared with the nanofibrous membrane group, the
bone formation of the nanofibrous membrane plus LIPUS group was
thicker and more mature; however, the spatial and temporal pattern
of the newly formed bone suggested that there was no difference
between these groups. It predicted that LIPUS increased bone
formation rather than accelerated the bone healing. Based on the
present findings, we believe the efficacy of LIPUS first acted on
the integrin, which indicates that this is the starting point of
mechanosensing.
Electrospun nanofibers mimic the native
extracellular tissue structures and exhibit suitable
biocompatibility (24). In spite of
this, aligned nanofibers may not influence osteogenic
differentiation or provide a limited contribution to the process.
The mechanisms involved in the limited influence of aligned
nanofibers and osteogenic differentiation remain unclear. However,
the lack of bioactive sites for cell recognition and could cause
difficulties for cells interacting with the extracellular matrix
(12,13). When cells attach to extracellular
tissue structures, the process by which cells probe the environment
and translate mechanical signal into biochemical signals is called
mechanosensing (29). During the
process, integrins, which initiate mechanosensing, are necessary
elements in the focal adhesions for the majority of mechanosensing
models (30,31). The cytoskeletal force generated by
myosin II combines with the ECM stiffness triggers the α5β1
integrin switch (31). Subsequently,
the α5β1 integrin is able to mediate downstream biochemical signals
that control cell fate and adhere to a fibronectin substrate. Once
the fibronectin interacts with integrin receptors, polymerization
of fibronectin initiates and the cryptic site for
fibronectin-fibronectin polymerization is exposed (19). The process of the conformation change
may expose cryptic sites to overcome the drawbacks of nanofibrous
membranes.
Notably, a previous study indicated that LIPUS
stimulation may activate α5β1 integrin and induce a significant
upregulation of marker proteins as the representation of the
osteoblasts (32). Furthermore, the
integrin was also highly activated under fluid flow stimulation as
well as LIPUS (33). A recent study
investigated the effect of LIPUS stimulation on mesoangioblasts and
demonstrated that LIPUS may induce a conformational change in β1
integrin to the active form (34).
In conclusion, the present study demonstrated that
the PLLA electrospun nanofibrous membranes combined with the LIPUS
improved the formation of novel bone in rabbit tibiae defects. As
the critical size of the cortical bone defect acquired
near-complete regeneration of cortical bone tissue, PLLA
electrospun nanofibrous membranes combined with the LIPUS may be a
novel potential therapy for the application of bone tissue
engineering. Moreover, further studies are required to fully
investigate the cell ingrowth depths inside the nanofibrous
membrane with scanning electron microscopy and the molecular
effects of LIPUS on integrin and fibronectin.
References
|
1
|
Bhatt RA and Rozental TD: Bone graft
substitutes. Hand Clin. 28:457–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurien T, Pearson RG and Scammell BE: Bone
graft substitutes currently available in orthopaedic practice: The
evidence for their use. Bone Joint J. 95-B:583–597. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang G, Yang H, Li M, Lu S, Chen X and Cai
X: The use of silk fibroin/hydroxyapatite composite co-cultured
with rabbit bone-marrow stromal cells in the healing of a segmental
bone defect. J Bone Joint Surg Br. 92:320–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bucholz RW: Nonallograft osteoconductive
bone graft substitutes. Clin Orthop Relat Res. 44–52. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van der Stok J, van Lieshout EM,
El-Massoudi Y, Van Kralingen GH and Patka P: Bone substitutes in
the Netherlands- a systematic literature review. Acta Biomater.
7:739–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinaci A, Neuhaus V and Ring DC: Trends in
bone graft use in the United States. Orthopedics. 37:e783–e788.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woo KM, Jun JH, Chen VJ, Seo J, Baek JH,
Ryoo HM, Kim GS, Somerman MJ and Ma PX: Nano-fibrous scaffolding
promotes osteoblast differentiation and biomineralization.
Biomaterials. 28:335–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xin X, Hussain M and Mao JJ: Continuing
differentiation of human mesenchymal stem cells and induced
chondrogenic and osteogenic lineages in electrospun PLGA nanofiber
scaffold. Biomaterials. 28:316–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong S, Sun J, Li Y, Li J, Cui W and Li B:
Electrospun nanofibrous scaffolds of poly (L-lactic acid)-dicalcium
silicate composite via ultrasonic-aging technique for bone
regeneration. Mater Sci Eng C Mater Biol Appl. 35:426–433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stevens MM and George JH: Exploring and
engineering the cell surface interface. Science. 310:1135–1138.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang JH, Castano O and Kim HW: Electrospun
materials as potential platforms for bone tissue engineering. Adv
Drug Deliv Rev. 61:1065–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schofer MD, Boudriot U, Bockelmann S, Walz
A, Wendorff JH, Greiner A, Paletta JR and Fuchs-Winkelmann S:
Effect of direct RGD incorporation in PLLA nanofibers on growth and
osteogenic differentiation of human mesenchymal stem cells. J Mater
Sci Mater Med. 20:1535–1540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Zhan J, Su Y, Wu T, Wu C,
Ramakrishna S, Mo X, Al-Deyab SS and El-Newehy M: Effects of plasma
treatment to nanofibers on initial cell adhesion and cell
morphology. Colloids Surf B Biointerfaces. 113:101–106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehta S, Long K, DeKoven M, Smith E and
Steen RG: Low-intensity pulsed ultrasound (LIPUS) can decrease the
economic burden of fracture non-union. J Med Econ. 18:542–549.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo G, Lu L, Ji H, Ma Y, Dong R, Tu J, Guo
X, Qiu Y, Wu J and Zhang D: Low intensity pulse ultrasound
stimulate chondrocytes growth in a 3-D alginate scaffold through
improved porosity and permeability. Ultrasonics. 58:43–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo G, Ma Q, Zhao B and Zhang D:
Ultrasound-assisted permeability improvement and acoustic
characterization for solid-state fabricated PLA foams. Ultrason
Sonochem. 20:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato M, Nagata K, Kuroda S, Horiuchi S,
Nakamura T, Karima M, Inubushi T and Tanaka E: Low-intensity pulsed
ultrasound activates integrin-mediated mechanotransduction pathway
in synovial cells. Ann Biomed Eng. 42:2156–2163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kadler KE, Hill A and Canty-Laird EG:
Collagen fibrillogenesis: Fibronectin, integrins, and minor
collagens as organizers and nucleators. Curr Opin Cell Biol.
20:495–501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Ouyang H, Lim CT, Ramakrishna S
and Huang ZM: Electrospinning of gelatin fibers and gelatin/PCL
composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater.
72:156–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai YZ, Wang LL, Cai HX, Qi YY, Zou XH and
Ouyang HW: Electrospun nanofibrous matrix improves the regeneration
of dense cortical bone. J Biomed Mater Res A. 95:49–57. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarkar MR, Augat P, Shefelbine SJ,
Schorlemmer S, Huber-Lang M, Claes L, Kinzl L and Ignatius A: Bone
formation in a long bone defect model using a platelet-rich
plasma-loaded collagen scaffold. Biomaterials. 27:1817–1823. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YZ, Ouyang H, Lim CT, Ramakrishna S
and Huang ZM: Electrospinning of gelatin fibers and gelatin/PCL
composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater.
72:156–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhattarai SR, Bhattarai N, Yi HK, Hwang
PH, Cha DI and Kim HY: Novel biodegradable electrospun membrane:
Scaffold for tissue engineering. Biomaterials. 25:2595–2602. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu XD, Fan HS, Xiao YM, Li DX, Zhang HJ,
Luxbacher T and Zhang XD: Effect of surface structure on protein
adsorption to biphasic calcium-phosphate ceramics in vitro and in
vivo. Acta Biomater. 5:1311–1318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kolambkar YM, Bajin M, Wojtowicz A,
Hutmacher DW, García AJ and Guldberg RE: Nanofiber orientation and
surface functionalization modulate human mesenchymal stem cell
behavior in vitro. Tissue Eng Part A. 20:398–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hui CF, Chan CW, Yeung HY, Lee KM, Qin L,
Li G, Leung KS, Hu YY and Cheng JC: Low-intensity pulsed ultrasound
enhances posterior spinal fusion implanted with mesenchymal stem
cells-calcium phosphate composite without bone grafting. Spine
(Phila Pa 1976). 36:1010–1016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu L, Lin LJ and Qin YX: Enhancement of
cell ingrowth, proliferation, and early differentiation in a
three-dimensional silicon carbide scaffold using low-intensity
pulsed ultrasound. Tissue Eng Part A. 21:53–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schiller HB and Fässler R:
Mechanosensitivity and compositional dynamics of cell-matrix
adhesions. EMBO Rep. 14:509–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y
and Li Y: Mechanism of regulation of stem cell differentiation by
matrix stiffness. Stem Cell Res Ther. 6:1032015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Friedland JC, Lee MH and Boettiger D:
Mechanically activated integrin switch controls alpha5beta1
function. Science. 323:642–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watabe H, Furuhama T, Tani-Ishii N and
Mikuni-Takagaki Y: Mechanotransduction activates α5β1 integrin and
PI3K/Akt signaling pathways in mandibular osteoblasts. Exp Cell
Res. 317:2642–2649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vaughan TJ, Mullen CA, Verbruggen SW and
McNamara LM: Bone cell mechanosensation of fluid flow stimulation:
A fluid-structure interaction model characterising the role
integrin attachments and primary cilia. Biomech Model Mechanobiol.
14:703–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernal A, Pérez LM, De Lucas B, Martín NS,
Kadow-Romacker A, Plaza G, Raum K and Gálvez BG: Low-intensity
pulsed ultrasound improves the functional properties of cardiac
mesoangioblasts. Stem Cell Rev. 11:852–865. 2015. View Article : Google Scholar : PubMed/NCBI
|