Introduction
Ovarian cancer is one of the most lethal
gynecological malignancies with a high case-to-mortality ratio
(1). In 2014, the American Cancer
Society reported 21,550 cases of epithelial ovarian carcinoma and
14,600 disease-related mortalities, indicating that 69% percent of
all patients with ovarian carcinoma succumbed to their disease. The
high mortality rate of this cancer is largely due to the fact that
many patients present with advanced or metastatic disease (2). In addition, conventional treatment
strategies such as chemotherapy and radiation are unlikely to
reduce the metastatic frequency in ovarian cancer (3).
Nuclear factor (NF)-κB is a major transcription
factor that is essential for the development of inflammation and
cancer (4). It is reportedly
involved in many physiological functions of cells, including
apoptosis, proliferation, invasion and angiogenesis (5). Under normal conditions, NF-κB is mainly
located in the cytoplasm with a heterotrimer complex comprising
p50, p65 and IκBα. When activated by stimulators and stress, IκBα
is rapidly phosphorylated, ubiquitinated and degraded by the
proteasome. NF-κB then translocates to the nucleus followed by p65
phosphorylation and binding to specific response elements in the
DNA, which subsequently activates target gene expression (5).
NF-κB controls the expression of an array of genes
involved in multiple features of cancer, including proliferation,
survival angiogenesis and invasion. In invasion and metastasis,
NF-κB regulates the gene transcription of proteolytic enzymes,
cytokines and signaling molecules associated with
epithelial-mesenchymal transition (EMT) (6). For example, matrix metalloproteinases
(MMPs), such as MMP-2 and −9, have been shown to be typical NF-κB
target proteins that are responsible for extracellular matrix
breakdown and cell invasion (7). In
addition, NF-κB serves as a key regulator for EMT-associated
proteins such as Snail and Slug (8–10).
Traditional herbal medicines have demonstrated great
potential in cancer treatment. Several natural materials used in
Chinese medicines, including green tea polyphenols, curcumin and
triptolide, have been intensively studied and exhibit antitumor
efficacy in preclinical models of various cancer types (11). Some leads are currently in early
stage clinical trials for a variety of cancers in combination with
other interventions. For example, curcumin shows considerable
prevention effects when used before chemotherapy in Phase I trials
in colon cancer patients (12). One
reason for the extensive application of traditional medicine in
modern cancer therapy is that, in comparison with chemotherapy,
natural agents are less toxic to normal tissues and, therefore,
patients maintain tolerance to them (13). Notably, many natural compounds have
been found to effectively suppress NF-κB signaling, providing an
experimentally validated molecular explanation for their functional
outcomes in tumor cells (14).
Celastrol is a natural triterpene derived from the
Chinese plant Thunder God Vine (Tripterygium wilfordii). It
is a pleiotropic compound showing antitumor, anti-inflammatory,
antihypertensive and antidiabetic activities (15). With regard to cancer treatment,
celastrol has been shown to exert considerable cell-killing effects
and to shrink xenografted tumors in tissue and animal models of
various malignancies including cancers of the prostate (16–18),
breast (19), liver (20,21) and
lung (22,23). However, the intervention potential of
celastrol on the growth, survival and metastatic features of
ovarian cancer cells remains largely unknown.
In the current study, the functional role of
celastrol in ovarian cancer cell migration and invasion, two key
steps in the metastatic cascade, was evaluated. Furthermore, the
possible molecular mechanism underlying the effects of celastrol on
the cells was investigated.
Materials and methods
Cell lines
OVCAR-3 and SKOV-3 cells were purchased from ATCC
(Manassas, VA, USA). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine
serum (FBS; Hyclone; GE Healthcare Life Sciences), 100 U/ml
penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), at 37°C in a humidified 5%
CO2 incubator. All cells were passaged using 0.05%
trypsin/0.02% EDTA (Gibco; Thermo Fisher Scientific, Inc.).
Reagents
Celastrol was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). The powder was reconstituted in DMSO to
generate a stock concentration of 20 mM and was stored at −20°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and MG132 were also purchased from Sigma-Aldrich (Merck KGaA).
Cells were treated with varied concentrations of celastrol (0.125,
0.25, 0.5, 1 and 2 µM) depending on the experiments. To detect
NF-κB activity, cells were pre-treated with MG132 (0.5 µM) for 1 h
and then treated with celastrol for 24 h. Matrigel was purchased
from BD Biosciences (San Diego, CA, USA). Primary antibodies for
western blotting were as follows: Antibodies against p-IκBα (Ser
32) (sc-7977), IκBα (sc-203), p65 (sc-109), poly ADP ribose
polymerase (PARP) (sc-1562), MMP-2 (sc-53630), MMP-7 (sc-8832) and
MMP-9 (sc-21733) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Anti-actin antibody (A5441) was obtained from
Sigma-Aldrich (Merck KGaA).
Cell proliferation assay
Cell growth was measured by MTT assay (24). Briefly, OVCAR-3 and SKOV-3 cells
(5×103 cells/well) were cultured with serially diluted
celastrol (0.125, 0.25, 0.5, 1 and 2 µM) in 96-well plates. Control
cells were incubated with DMSO in fresh medium. Following
incubation for 96 h, the medium was removed and the cells were
incubated with 20 µl MTT solution (5 mg/ml) at 37°C for 4 h. The
MTT was then removed, and 100 µl DMSO was added to each well. After
15 min, the absorbance of each well was measured with a microplate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA) at a wavelength
of 570 nm. The viable cell number was proportional to the
absorbance. All assays were performed in triplicate.
Wound healing assay
Cell motility was determined using a wound-healing
scratch assay. Briefly, OVCAR-3 cells were seeded in a 6-well plate
(5×104 cells/well) and grown until confluency. The
confluent monolayer was scratched using a sterilized 1-ml pipette
tip and incubated with celastrol (0.125, 0.25 and 0.5 µM) in fresh
growth medium for 24 h. The movement of cells to the denuded area
was photographed.
Transwell assays
For the migration assay, SKOV-3 cells
(5×103/insert) in DMEM with 10% FBS (Hyclone; GE
Healthcare Life Sciences) were seeded into a commercial Transwell
insert (membrane pore size, 8 µm) and incubated with celastrol
(0.125, 0.25 and 0.5 µM). After 24 h, cells that had migrated to
the bottom of the filter were stained with crystal violet and
counted under a light microscope. For the invasion assay, OVCAR-3
cells were tested using a Transwell insert pre-loaded with Matrigel
(BD Biosciences). Inserts were incubated with serum-free DMEM at
37°C for 2 h to allow rehydration of Matrigel. Cells suspended in
serum-free DMEM were then loaded onto the top chamber
(1×104/insert), and celastrol was added to both upper
and lower chambers with equal concentrations (0.125, 0.25 and 0.5
µM). Complete DMEM with 10% FBS was used in the lower chamber as a
chemo-attractant. After 24 h of incubation, the Matrigel was
removed and the inserts were stained. Invaded cells on the
underside of the filter were counted.
Western blotting
The protocol for western blotting was as previously
reported (25) with minor
modifications. Following treatment with celastrol, OVCAR-3 cells
were harvested and lysed in radioimmunoprecipitation assay lysis
buffer [50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% sodium dodecyl
sulfate (SDS), 1% NP-40, 0.25% sodium deoxycholate and 1 mM EDTA
with protease inhibitor cocktail, 1 mM NaF and 1 mM
Na3VO4] at 4°C for 20 min. Whole cell lysates
were subjected to protein quantification by Bradford assay. Equal
amounts (10 µg/lane) of lysates were resolved by SDS-polyacrylamide
gel electrophoresis (SDS-PAGE; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and then electrotransferred to a nitrocellulose
membrane (Bio-Rad Laboratories, Inc.). The membrane was probed with
primary antibodies against p-IκBα (Ser 32) (1:200), IκBα (1:200),
p65 (1:200), PARP (1:200), MMP-2 (1:100), MMP-7 (1:100), MMP-9
(1:100) or Actin (1:5,000) at 4°C overnight, then incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies
(anti-mouse IgG1-HRP, ab193651; anti-rabbit IgG1-HRP, ab191866;
Abcam, Shanghai, China) with 1:5,000 dilution at room temperature
for 1 h, and detected with an enhanced chemiluminescence substrate
(Amersham; GE Healthcare Life Sciences).
Cytosol-nuclei fractionation
To detect the subcellular distribution of NF-κB
proteins, cytoplasmic and nuclear compartments of the cells were
prepared as described previously (26). Briefly, following treatment with
celastrol, OVCAR-3 cells (3×106) were suspended in
hypotonic lysis buffer (10 mM HEPES, 5 mM KCl, 1 mM
MgCl2, 1 mM phenylmethylsulfonyl fluoride, 1 mM
dithiothreitol and protease inhibitors) and incubated at 4°C for 15
min. Nonidet P-40 (10%) was then added to a final concentration of
0.5%. Immediately after NP-40 addition, samples were vortexed and
centrifuged at 13,000 × g for 1 min, and supernatant (cytosolic
extract) was collected. For the precipitation, Laemmli lysis buffer
(Santa Cruz Biotechnology, Inc.) was used to reconstitute the
pellet followed by ultrasonic homogenization on ice to generate the
nuclear extract. Subcellular proteins were quantified by Bradford
assay and employed for western blot analysis.
Gelatin zymography
OVCAR-3 cells were treated with celastrol (0.25 or
0.5 µM) for 24 h. Supernatants were collected and loaded on a 10%
SDS-PAGE gel with gelatin (1 mg/ml). Following electrophoresis, the
gels were washed for 15 min in 2.5% Triton X-100 and then incubated
overnight in the same buffer at room temperature. After washing
with deionized water, the gels were incubated for an additional 24
h at 37°C in a calcium-zinc renaturation buffer (1610765; Bio-Rad
Laboratories, Inc.). The gel was stained with Coomassie blue R-250.
Clear bands appearing at the expected locations for matrix
metalloprotease (MMP)-9 and MMP-2 on the basis of molecular weight
markers were visualized using a transilluminating densitometer. The
number of pixels per band was used to determine the enzyme activity
in each group. The zymography experiment was repeated three times
with independent samples.
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). Inter-group analyses were performed using analysis
of variance with Prism 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Anti-proliferative effect of celastrol
on ovarian cancer cells
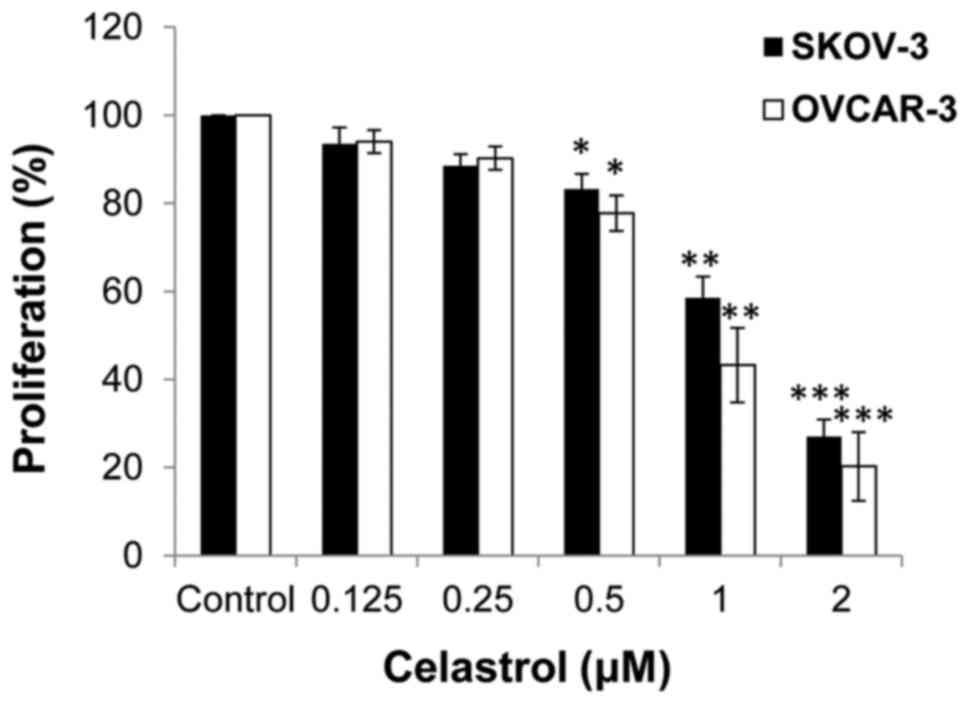
The impact of celastrol on cell proliferation was
investigated. SKOV3 and OVCAR-3 ovarian cancer cells were treated
with serial concentrations of celastrol and cell proliferation was
tested by MTT assay. Celastrol markedly inhibited cell
proliferation when concentrations ≥1 µM were used in the two cell
lines, while at <1 µM, the drug yielded only modest effects
(Fig. 1). In order to exclude the
antiproliferative effect of celastrol from its effects on cell
migration and invasion, drug concentrations <1 µM were used for
subsequent experiments.
Celastrol inhibits ovarian cancer cell
migration
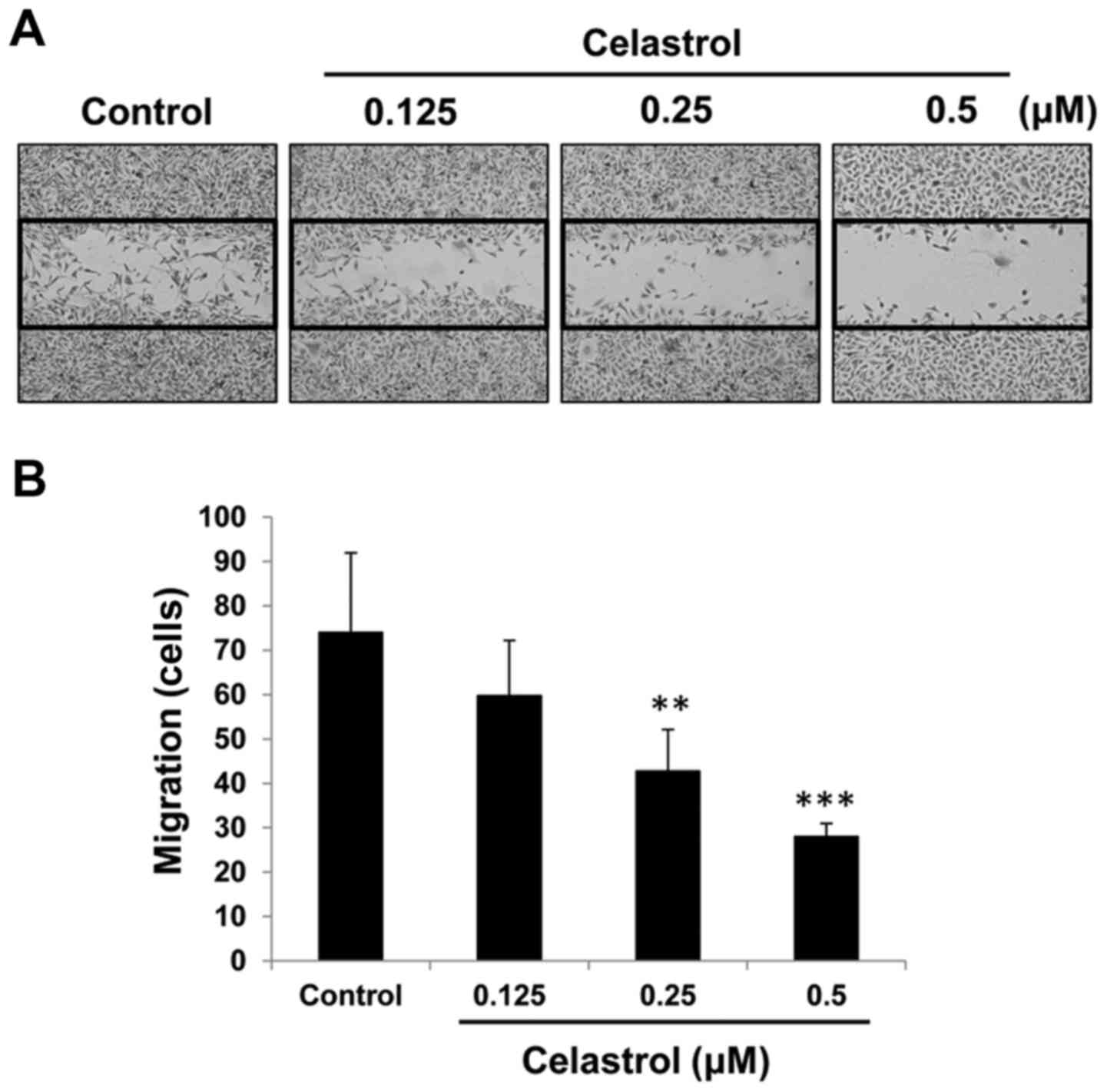
The effect of celastrol on cell movement was
evaluated by wound-healing scratch assay and Transwell assays. When
used at subtoxic concentrations (0.125–0.5 µM), celastrol clearly
decreased the number of OVCAR-3 cells that moved into the denuded
area (Fig. 2A). Consistent with
this, in the Transwell migration assay, the number of SKOV-3 cells
that passed through the Transwell membrane was significantly
reduced by celastrol in a concentration-dependent manner (Fig. 2B).
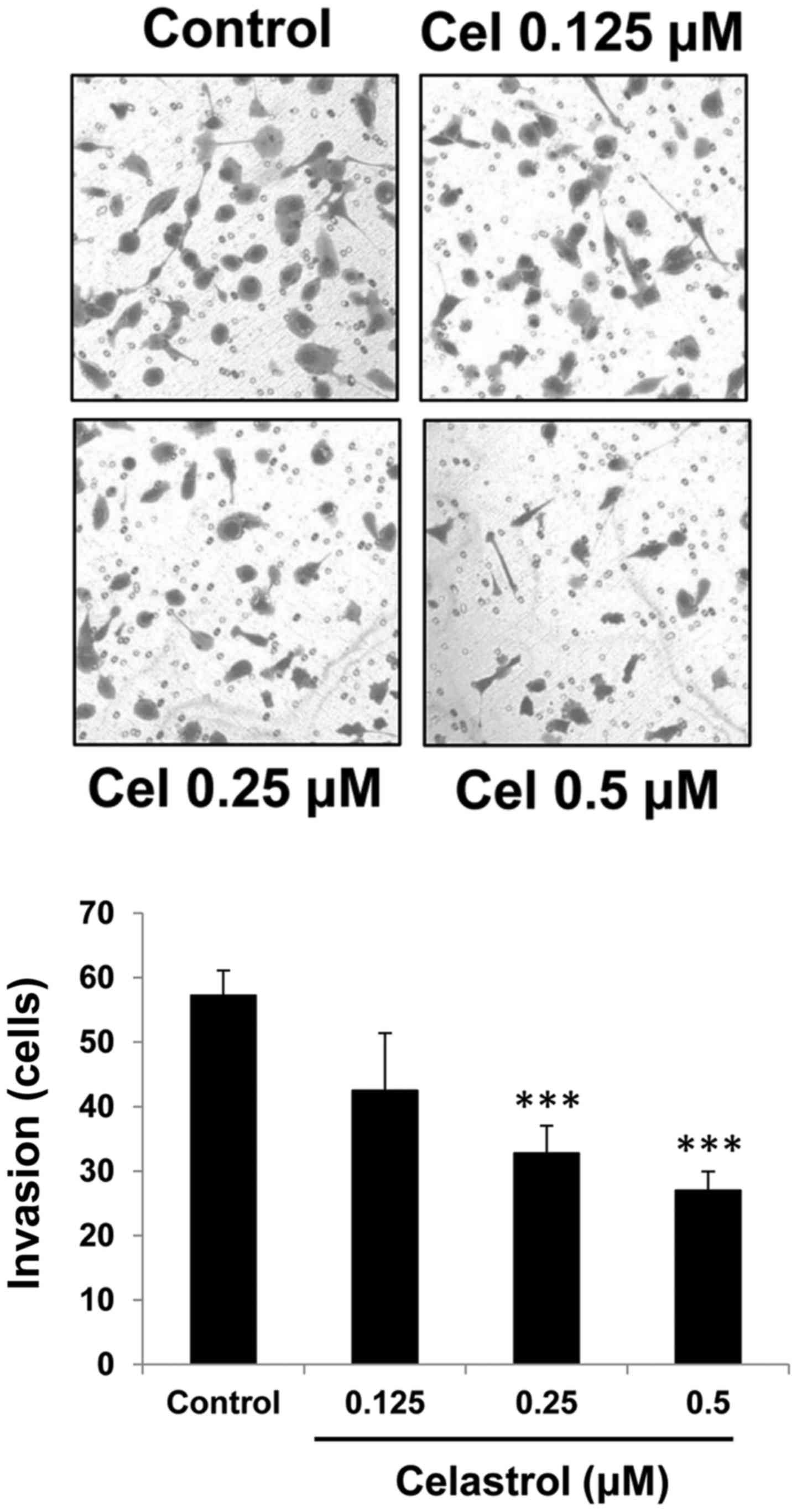
Celastrol suppresses ovarian cancer
cell invasion
The effect of celastrol on the invasive capacity of
OVCAR-3 cells was investigated. In a Matrigel-coated Transwell
assay, celastrol at concentrations of 0.25 and 0.5 µM significantly
inhibited cell invasion (Fig. 3).
The inhibition rates were 39.6±5.2 and 51.7±3.5% for 0.25 and 0.5
µM celastrol, respectively.
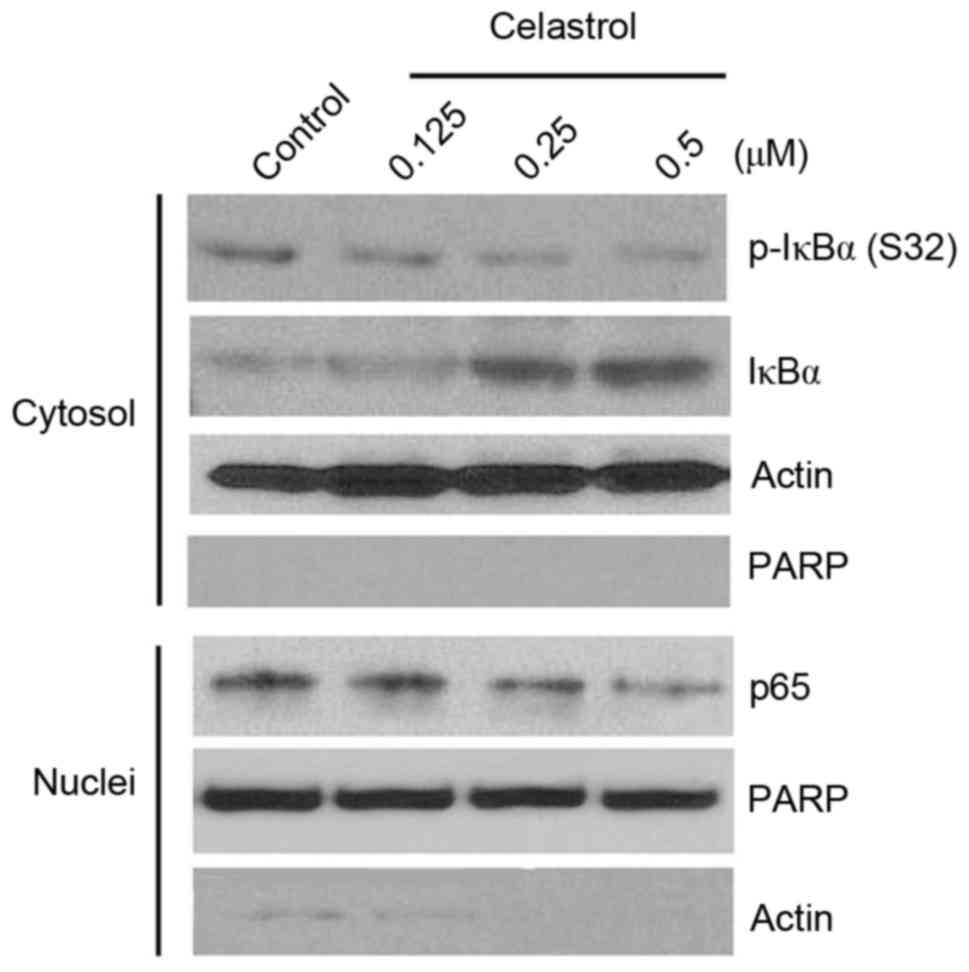
Celastrol strongly blocks the NF-κB
pathway
To elucidate the molecular mechanisms by which
celastrol affects ovarian cancer cells, proteins in the NF-κB
pathway were analyzed, since this pathway is indicated to be one of
the predominant mechanisms underlying the antitumor efficacy of
celastrol (27). As shown in
Fig. 4, celastrol clearly inhibited
p-IκBα (S32) and induced IκBα accumulation in the cytosol, and
consistent with this, reduced nuclear p65 recruitment in OVCAR-3
cells, suggesting blockade of the canonical NF-κB pathway. The
levels of cytosolic p65 and nuclear IκBα were undetectable, with or
without drug treatment, indicating that celastrol did not affect
the translocation of these proteins. Notably, nuclear-specific
protein PARP was undetectable in the cytosolic compartment,
indicating the precision of the cytosol-nuclei fractionation.
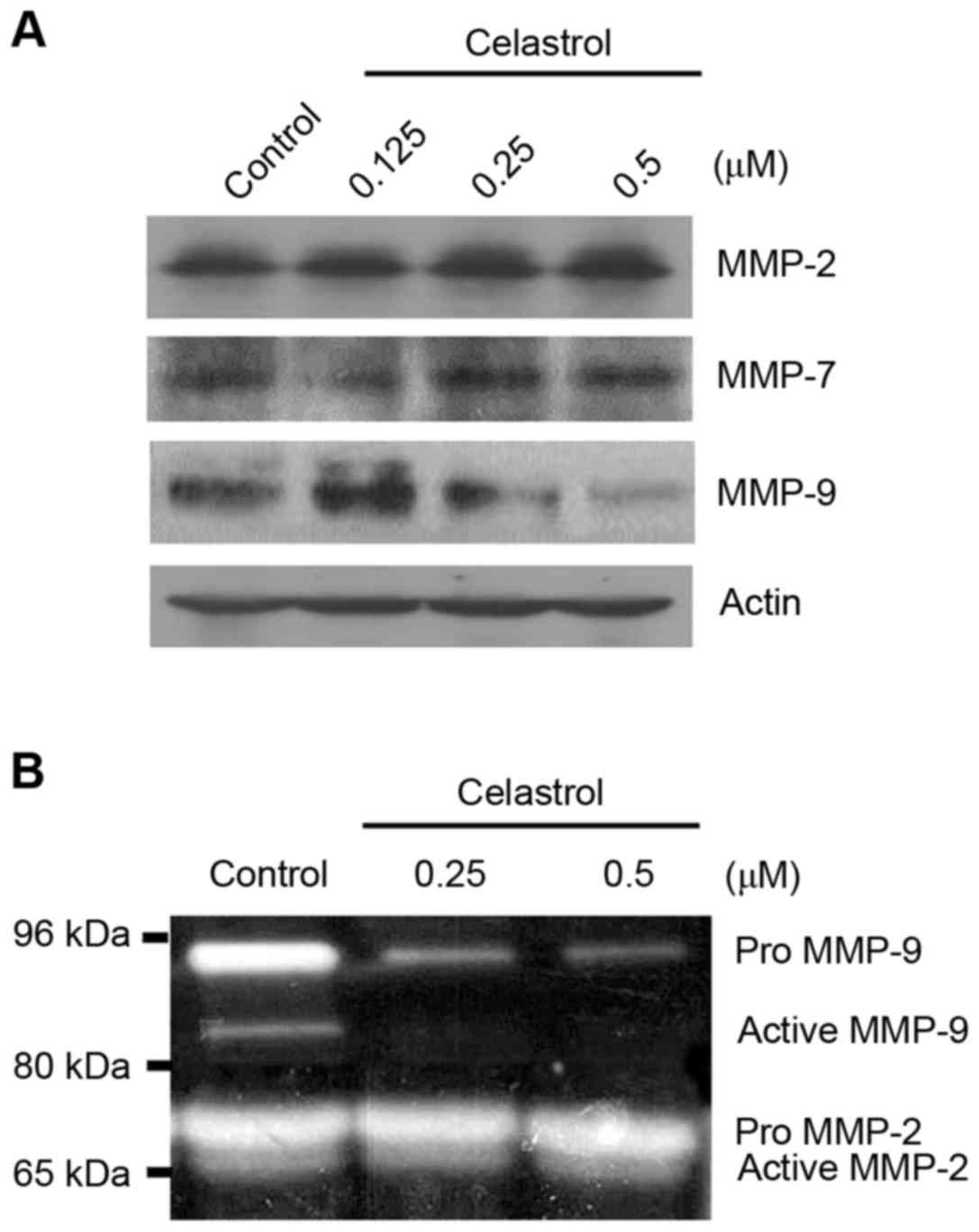
MMP-9 expression and activity are
reduced by celastrol
NF-κB controls the gene expression of many
proteolytic enzymes involved in cell invasion and metastasis, such
as urokinase-type plasminogen activator and MMP-9 (5). To test whether celastrol impacts the
expression of MMPs, three typical MMPs (MMP-2, MMP-7 and MMP-9)
were detected in OVCAR-3 cells following celastrol treatment. MMP-9
protein was clearly inhibited by celastrol, particularly at higher
concentrations (Fig. 5A). By
contrast, no inhibition of MMP-2 and MMP-7 was observed (Fig. 5A). Furthermore, in the zymography
assay, the expression of gelatin (the substrate of MMP-2 and
MMP-9), was decreased at 92 kDa (for Pro MMP-9) and 83 kDa (for
active MMP-9), but not 72 kDa (for Pro MMP-2) and 66 kDa (for
active MMP-2) by celastrol treatment, suggesting that celastrol
inhibits the proteolytic activity of MMP-9, but not that of MMP-2
(Fig. 5B).
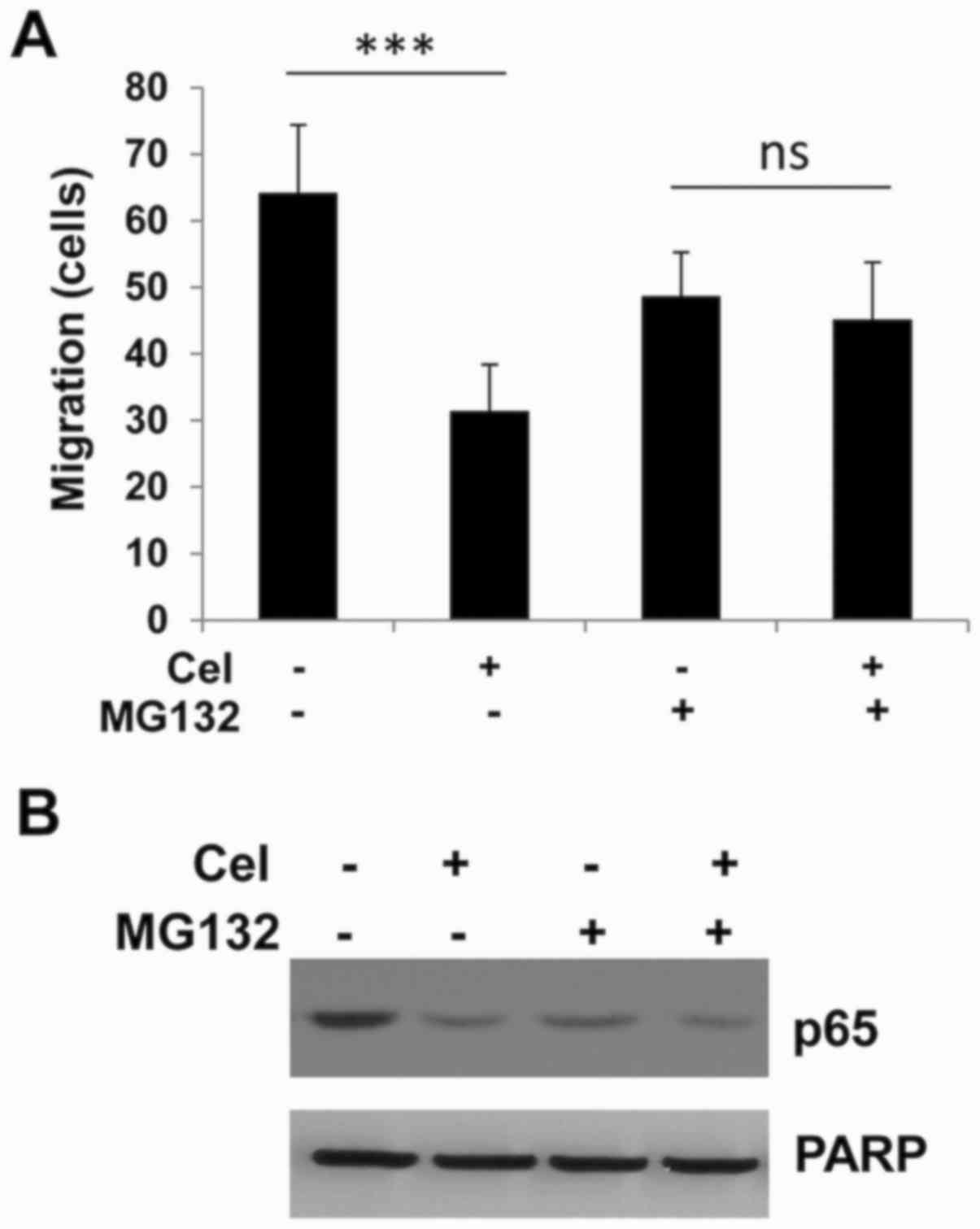
NF-κB pathway inhibitor MG132
attenuates celastrol-induced cell migration and p65 inhibition
In order to investigate whether the mechanism for
the inhibition of migration and invasion by celastrol involves
NF-κB, the proteasome inhibitor MG132 was used as an NF-κB
inhibitor; MG132 is widely used as a tool to study NF-κB signaling
and associated molecular mechanisms (28). As shown in Fig. 6A, both celastrol and MG132 inhibited
cell migration when applied alone. However, when these two agents
were used together, the celastrol-induced suppression was not
further increased by MG132; the celastrol-induced movement of tumor
cells was attenuated by MG132. Consistently, at the molecular
level, MG132 together with celastrol did not show stronger p65
suppression compared with either celastrol or MG132 alone,
suggesting that the celastrol-mediated functions proceed via the
same pathway as MG132, that is, through NF-kB pathway (Fig. 6B).
Discussion
Celastrol has been shown to have anti-invasive and
antimetastatic activities in preclinical models of prostate cancer
(17), breast cancer (19), colon cancer and pancreatic cancer
(29). The current study provides
new evidence for the inhibitory effect of celastrol on certain
functions of ovarian cancer cells. The in vitro data
indicate that celastrol may be a suitable candidate for preventing
tumor cell migration and invasion in ovarian cancer. Furthermore,
such functional consequences are associated with blockade of the
NF-κB/MMP-9 pathway, which is widely associated with malignancy and
aggressiveness (30,31).
Natural medicines have been studied in cancer
management for many years, usually in combination with traditional
interventions. Products from the plant Tripterygium
wilfordii, including celastrol and triptolide, have exhibited
impressive anticancer activities in a variety of cancer models, and
therefore are amongst the traditional herb medicines considered to
have the most potential in modern cancer therapy (11). For the treatment of ovarian cancer,
while very limited studies have focused on celastrol, triptolide,
which exhibits similar biological activities to celastrol, has been
demonstrated to exert efficacy in preclinical models (32). Ou et al reported that
triptolide effectively inhibits ovarian cancer cell proliferation
by blocking the HER2/PI3K/Akt/NF-κB pathway (33). In addition, triptolide has been shown
to potently inhibit the migration and invasion of ovarian cancer
cells, and such suppression is strongly associated with the reduced
transcription and translation of several MMPs (34). The results of the present study
indicate that celastrol, an analog of triptolide, impairs ovarian
cancer cell migration and invasion, and inhibits the NF-κB/MMP-9
pathway; such molecular findings are consistent with previous
studies in other cancers (7,19). Together, these findings provide
information useful for future studies on these active ingredients
from the plant Tripterygium wilfordii, in cancer
treatment.
Various molecular mechanisms have been described for
the antitumor effects of celastrol. While many signaling pathways
are highly associated with survival and apoptosis, NF-κB is the
predominant pathway that plays a pleiotropic role in controlling
multiple cell functions, including proliferation, survival, cell
death, invasion and angiogenesis (5). The NF-κB pathway serves as one of the
major mechanisms underlying the cancer-killing effects of natural
compounds.
In the current study, the results indicated that the
NF-κB pathway may be an important in the functional changes induced
by celastrol. In particular, they suggest that celastrol may
inhibit the classical NF-κB pathway by preventing IκBα degradation,
blocking p65 translocation and suppressing MMP-9 expression
downstream of NF-κB. These results are consistent with those of
previous studies (18,19,27),
although other studies have suggested different mechanisms for the
effects of celastrol on invasiveness (22,35–38). It
should be noted that alternative mechanisms to the NF-κB pathway
are also likely to contribute to the effects of celastrol on
migration and invasion in the current models. For example, Rac/Rho
GTPase is a key player involved in cell skeletal rearrangement and
cell polarity, while the Src/FAK axis is a major pathway
controlling focal adhesion, cell movement and invasion (39). Notably, celastrol is able to suppress
the constitutive phosphorylation of Src kinase at least in multiple
myeloma cells (40), suggesting that
Src may serve as another potential molecular contributor. Future
studies will focus on detailed delineation of the signaling
pathways interrupted by celastrol.
In summary, the results of the present study
illustrate the potential of celastrol for impairing tumor
invasiveness in ovarian cancer, therefore broadening the possible
applications of this natural agent. The results of these in
vitro experiments supports the use of celastrol as a potential
clinical intervention modality for preventing and delaying ovarian
cancer metastasis. Therefore, celastrol warrants further
preclinical investigation.
References
|
1
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Naora H and Montell DJ: Ovarian cancer
metastasis: Integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ,
Bast RC Jr, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et
al: Rethinking ovarian cancer II: Reducing mortality from
high-grade serous ovarian cancer. Nat Rev Cancer. 15:668–679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malaponte G, Signorelli SS, Bevelacqua V,
Polesel J, Taborelli M, Guarneri C, Fenga C, Umezawa K and Libra M:
Increased levels of NF-kB-dependent markers in cancer-associated
deep venous thrombosis. PLoS One. 10:e01324962015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu K and Bonavida B: The activated
NF-kappaB-Snail-RKIP circuitry in cancer regulates both the
metastatic cascade and resistance to apoptosis by cytotoxic drugs.
Crit Rev Immunol. 29:241–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Storci G, Sansone P, Mari S, D'Uva G,
Tavolari S, Guarnieri T, Taffurelli M, Ceccarelli C, Santini D,
Chieco P, et al: TNFalpha up-regulates SLUG via the
NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a
stem cell-like phenotype. J Cell Physiol. 225:682–691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Do SI, Kim JY, Kang SY, Lee JJ, Lee JE,
Nam SJ and Cho EY: Expression of TWIST1, Snail, Slug, and NF-κB and
methylation of the TWIST1 promoter in mammary phyllodes tumor.
Tumour Biol. 34:445–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Corson TW and Crews CM: Molecular
understanding and modern application of traditional medicines:
Triumphs and trials. Cell. 130:769–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnson JJ and Mukhtar H: Curcumin for
chemoprevention of colon cancer. Cancer Lett. 255:170–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sak K: Chemotherapy and dietary
phytochemical agents. Chemother Res Pract.
2012:2825702012.PubMed/NCBI
|
|
14
|
Li-Weber M: Targeting apoptosis pathways
in cancer by Chinese medicine. Cancer Lett. 332:304–312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong KF, Yuan Y and Luk JM: Tripterygium
wilfordii bioactive compounds as anticancer and anti-inflammatory
agents. Clin Exp Pharmacol Physiol. 39:311–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiang KC, Tsui KH, Chung LC, Yeh CN, Chen
WT, Chang PL and Juang HH: Celastrol blocks interleukin-6 gene
expression via downregulation of NF-κB in prostate carcinoma cells.
PLoS One. 9:e931512014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai Y, Desano J, Tang W, Meng X, Meng Y,
Burstein E, Lawrence TS and Xu L: Natural proteasome inhibitor
celastrol suppresses androgen-independent prostate cancer
progression by modulating apoptotic proteins and NF-kappaB. PLoS
One. 5:e141532010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao L, Zhou Z, Cai Y, Castro P, Dakhov O,
Shi P, Bai Y, Ji H, Shen W and Wang J: Celastrol suppresses tumor
cell growth through targeting an AR-ERG-NF-κB pathway in
TMPRSS2/ERG fusion gene expressing prostate cancer. PLoS One.
8:e583912013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim Y, Kang H, Jang SW and Ko J: Celastrol
inhibits breast cancer cell invasion via suppression of
NF-kB-mediated matrix metalloproteinase-9 expression. Cell Physiol
Biochem. 28:175–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li PP, He W, Yuan PF, Song SS, Lu JT and
Wei W: Celastrol induces mitochondria-mediated apoptosis in
hepatocellular carcinoma Bel-7402 cells. Am J Chin Med. 43:137–148.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajendran P, Li F, Shanmugam MK, Kannaiyan
R, Goh JN, Wong KF, Wang W, Khin E, Tergaonkar V, Kumar AP, et al:
Celastrol suppresses growth and induces apoptosis of human
hepatocellular carcinoma through the modulation of STAT3/JAK2
signaling cascade in vitro and in vivo. Cancer Prev Res (Phila).
5:631–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang GZ, Liu YQ, Cheng X and Zhou GB:
Celastrol induces proteasomal degradation of FANCD2 to sensitize
lung cancer cells to DNA crosslinking agents. Cancer Sci.
106:902–908. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan XX, Li N, Wu JL, Zhou YL, He JX, Liu L
and Leung EL: Celastrol induces apoptosis in gefitinib-resistant
non-small cell lung cancer cells via caspases-dependent pathways
and Hsp90 client protein degradation. Molecules. 19:3508–3522.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie
T, Zhang N and Ye ZM: Celastrol induces apoptosis and autophagy via
the ROS/JNK signaling pathway in human osteosarcoma cells: An in
vitro and in vivo study. Cell Death Dis. 6:e16042015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ling C, Wang Y, Feng YL, Zhang YN, Li J,
Hu XR, Wang LN, Zhong MF, Zhai XF, Zolotukhin I, et al: Prevalence
of neutralizing antibodies against liver-tropic adeno-associated
virus serotype vectors in 100 healthy Chinese and its potential
relation to body constitutions. J Integr Med. 13:341–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bolshakova A, Magnusson KE, Pinaev G and
Petukhova O: Functional compartmentalisation of NF-kB-associated
proteins in A431 cells. Cell Biol Int. 37:387–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ni H, Zhao W, Kong X, Li H and Ouyang J:
NF-kappa B modulation is involved in celastrol induced human
multiple myeloma cell apoptosis. PLoS One. 9:e958462014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zanotto-Filho A, Braganhol E, Battastini
AM and Moreira JC: Proteasome inhibitor MG132 induces selective
apoptosis in glioblastoma cells through inhibition of PI3K/Akt and
NFkappaB pathways, mitochondrial dysfunction and activation of
p38-JNK1/2 signaling. Invest New Drugs. 30:2252–2262. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yadav VR, Sung B, Prasad S, Kannappan R,
Cho SG, Liu M, Chaturvedi MM and Aggarwal BB: Celastrol suppresses
invasion of colon and pancreatic cancer cells through the
downregulation of expression of CXCR4 chemokine receptor. J Mol Med
(Berl). 88:1243–1253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang L, Wu J, Yang Y, Liu L, Song L, Li J
and Li M: Bmi-1 promotes the aggressiveness of glioma via
activating the NF-kappaB/MMP-9 signaling pathway. BMC Cancer.
12:4062012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin J, Shen X, Chen L, Bao LW and Zhu LM:
TMPRSS4 promotes invasiveness of human gastric cancer cells through
activation of NF-κB/MMP-9 signaling. Biomed Pharmacother. 77:30–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, Ma L and Zhou GB: The main
anticancer bullets of the Chinese medicinal herb, thunder god vine.
Molecules. 16:5283–5297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ou CC, Chen YW, Hsu SC, Sytwu HK, Loh SH,
Li JW and Liu JY: Triptolide transcriptionally represses HER2 in
ovarian cancer cells by targeting NF-κB. Evid Based Complement
Alternat Med. 2012:3502392012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao H, Yang Z, Wang X, Zhang X, Wang M,
Wang Y, Mei Q and Wang Z: Triptolide inhibits ovarian cancer cell
invasion by repression of matrix metalloproteinase 7 and 19 and
upregulation of E-cadherin. Exp Mol Med. 44:633–641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JH, Won YS, Park KH, Lee MK, Tachibana
H, Yamada K and Seo KI: Celastrol inhibits growth and induces
apoptotic cell death in melanoma cells via the activation
ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT
signaling. Apoptosis. 17:1275–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang H, Lee M and Jang SW: Celastrol
inhibits TGF-β1-induced epithelial-mesenchymal transition by
inhibiting Snail and regulating E-cadherin expression. Biochem
Biophys Res Commun. 437:550–556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chakravarthy R, Clemens MJ, Pirianov G,
Perdios N, Mudan S, Cartwright JE and Elia A: Role of the eIF4E
binding protein 4E-BP1 in regulation of the sensitivity of human
pancreatic cancer cells to TRAIL and celastrol-induced apoptosis.
Biol Cell. 105:414–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee HW, Jang KS, Choi HJ, Jo A, Cheong JH
and Chun KH: Celastrol inhibits gastric cancer growth by induction
of apoptosis and autophagy. BMB Rep. 47:697–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kannaiyan R, Hay HS, Rajendran P, Li F,
Shanmugam MK, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, et
al: Celastrol inhibits proliferation and induces chemosensitization
through down-regulation of NF-κB and STAT3 regulated gene products
in multiple myeloma cells. Br J Pharmacol. 164:1506–1521. 2011.
View Article : Google Scholar : PubMed/NCBI
|