Introduction
Surgery on the spine and spinal cord carries a
significant risk of injury and postoperative neurological
dysfunction. The prevention of paraplegia, a serious complication,
resulting from spine and spinal cord surgery has been well
documented (1,2). Spinal cord ischemic injury is believed
to be the primary reason for neurological dysfunction following
spine and spinal cord surgery (3).
The role of intraoperative electrophysiological monitoring of the
spinal cord functional pathways is to detect spinal cord injury at
an early and reversible stage, allowing for the application of
protective strategies (4).
Evoked potentials (EPs) are used widely in
intraoperative monitoring for the purpose of preventing spinal cord
dysfunction (5). Somatosensory EPs
(SEPs) were the first to be applied intraoperatively to prevent
spinal cord injury (6). However,
SEPs reflect only the functional integrity of the spinal sensory
pathway, and there have been reports of postoperative paralysis
despite the appearance of intact SEPs (7). In addition, SEP recording requires a
signal averaging process, which results in a time delay while the
neurophysiological physician communicates with the surgeon
(8). This lack of real-time feedback
prior to intervention may lead to an irreversible spinal cord
injury (9). Many of these problems
were solved following the adoption of transcranial electrical
motor-evoked potentials (TceMEP) intraoperative monitoring. Due to
its real-time feedback and proven clinical correlation, there have
been many reports of TceMEP leading to protective actions prior to
irreversible injury; however, there are still many questions about
the use of TceMEP (10).
The present study investigated the relationship
between changes in TceMEP amplitude 5 min after spinal cord injury
and the severity of the impact on spinal cord function following
different degrees of permanent spinal cord ischemic injury in a
rabbit animal model. Comparisons were made between TceMEP amplitude
changes and pathological changes in neurons of the spinal cord. The
aim was to determine a reliable predictor of neurologic deficits at
an early and reversible stage of different degrees of permanent
spinal cord ischemia. In the present experiments, myogenic
motor-EPs (MEPs) were generated by TceMEP to help predict the early
onset of neurological deficits when intervention is still possible
during surgery.
Materials and methods
Perioperative management
The present study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Committee on the Ethics of Animal
Experiments of the Union Hospital of Fujian Medical University
(Fuzhou, China; permit no. 12-5923).
All animals received humane care. The handling of
laboratory animals and their use conformed to the Guidelines for
Animal Experiments at Fujian Medical University, Medical Laboratory
Animal Management Regulations and other relevant laws and
regulations. All experimental animals were fed under the same
conditions at the institute, where they had access to food and
water ad libitum, and were housed individually in metal
cages in a 12-h light/dark cycle at a regulated temperature of
25–26°C and relative humidity of 50–65%.
The housing facility maintained national standards,
in compliance with the Laboratory Animal-Requirements of
Environment and Housing Facilities (GB 14925-2001) (11). If the rabbits appeared to be in
extremely poor or moribund condition following surgery, euthanasia
was considered and was conducted by intravenous sodium
pentobarbital (100 mg/kg; China Langchem, Inc., Shanghai,
China).
The management of the laboratory animals conformed
to the Laboratory Animal Regulations of the National Science and
Technology Commission (12). The
animals were monitored according to the experimental design as
follows: TceMEP was recorded prior to and within 5 min of ligation;
and during the experiment, TceMEP was monitored every 2 sec.
The rabbits in the groups with low Tarlov scores
(13) were maintained on the
premises with sufficient water and food under standard animal house
conditions; they could feed themselves with the help of an animal
administrator. The paralyzed lower limbs were moved with the help
of an animal administrator every 2 h. No incontinence was found in
the rabbits in the groups with low Tarlov scores, and all excrement
was cleaned in a timely fashion. Those animals with low Tarlov
scores of the lower limbs retained some ability of self-care, such
as the cleaning of their fur.
All surgery was performed under sodium pentobarbital
anesthesia, and all efforts were made to minimize suffering. There
was no obvious pain or distress during the experiments, as well as
no weight loss, no poor body conditions and no changes in skin or
fur conditions. The rabbits were fed under standard animal housing
conditions with free access to water and food. Experimental
procedures and animal welfare were implemented strictly in
accordance with the Use of Laboratory Animals (National Research
Council of USA, 1996) and the guide for the care and the related
ethical criteria of the Department of Neurosurgery, Fujian Medical
University Union Hospital (Fuzhou, China). All measures were made
to reduce the number of animals used and to minimize the animals'
suffering.
Study design and management
A total of 24 New Zealand white rabbits (14 males
and 10 females; weight, 3.0–3.5 kg; age, 11±1.2 months), were
randomly selected for this experiment. Rabbits were purchased from
the Center for Animal Experiments of Hubei Province (Wuhan, China).
A sham group (group A) containing 6 rabbits was used to exclude the
effects of anesthesia and surgery on EPs and to determine the
optimal stimulation intensity. The remaining 18 rabbits in the
experimental groups were used to generate models of different
levels of permanent spinal cord ischemic injury. The rabbits
underwent different levels of serial lumbar artery ligation in a
cranio-caudal direction between the renal artery and the aortic
bifurcation. In the experimental groups, different levels of lumbar
artery ligation were performed at three, four and five levels
(groups B, C and D, respectively; n=6 in each group). An
observation period of 5 min was used to detect whether spinal cord
ischemia was reflected in TceMEP waveform changes. The sham
operation group did not undergo ligation. In the sham operation
group, stimulation of different intensities was used to induce
TceMEP and to determine the most appropriate stimulation intensity.
EPs were recorded before and after the surgery and every 30 min for
3 h in the sham group.
Anesthesia management
Intravenous access was established in the marginal
ear vein, and anesthesia was infused at a dose of 1 ml/kg 3%
pentobarbital sodium and was maintained at 1/3-1/2 of the initial
dose according to the response of the animals during the
experiment. The rabbits were intubated and connected to a
respirator to control their breathing during the experiment;
nitrous oxide and oxygen at a 2:1 ratio were inhaled. Another
intravenous line of lactated Ringer's solution (Henan Huali
Pharmaceutical Co., Ltd., Pingdingshan, China) was infused
according to the amount of bleeding. During the experiment, body
temperature was monitored continuously with a rectal thermometer
and was maintained between 38–39°C with an electric blanket.
Surgical technique
Rabbits were placed in the supine position after
sterile surgical preparations. Additional local anesthesia,
containing 0.5% lidocaine hydrochloride (1 ml; Henan Huali
Pharmaceutical Co., Ltd.), was applied to the abdominal wall. A
midline abdominal incision was made, and the bowels were removed by
turning them to the left and by covering them with wet and heated
sterile gauze to reduce fluid and heat loss. After the
retroperitoneum was opened and probed, the abdominal aorta and the
five lumbar arteries between the renal artery and the aortic
bifurcation were exposed. The superior and inferior mesenteric
arteries were untouched during the surgery.
Monitoring technique for TceMEPs
Rabbits were placed in a prone position, and the
skull was placed in a stereotaxic instrument (Ruanlong Technology
Development Co., Ltd., Shanghai, China). The scalp was treated with
1% lidocaine, and a 3-cm-long incision was cut in the scalp,
exposing the skull. The sagittal and coronal sutures of the
calvarium were exposed after the periosteum was removed. The
stimulating electrodes (Axon Systems, Inc., Hauppage, NY, USA) were
fixed on the skull, with the cathode placed in the C4 position and
the anode placed in the C2 position, according to the International
10–20 system (13). The stimulating
electrodes were connected to an EpochXP-2000 electrical stimulator
(Axon Systems, Inc.). Silver acupuncture needles were used as
recording electrodes and placed subcutaneously into the
gastrocnemius muscle of the hind leg. The stimulation parameters
were as follows: A stimulation train (three pulses, 120–130 V,
100-msec duration and a 2-msec interstimulus interval) was used to
elicit TceMEPs. TceMEPs were also recorded from an upper extremity
as a control, and the recording parameters were as follows: Time
base, 100 msec; bandpass filter of 30–3000 Hz; and amplified 5,000
times. TceMEPs were recorded prior to and within 5 min following
ligation, and the baseline value was determined just prior to the
initiation of lumbar artery ligation. The TceMEP amplitude was
defined as the voltage range from the most positive to the most
negative component. After the baseline values of the TceMEPs were
recorded, prepared lumbar arteries were ligated (14) in a cranio-caudal direction.
Evaluation of neurological
outcome
The hind limb motor function of all rabbits was
assessed after recovery from anesthesia and 2 days after ligation,
and the Tarlov score was assessed as follows (15): A score of 1 for spastic paraplegia,
cannot move; a score of 2 for paraparesis, slight movements; a
score of 3 for paraparesis, powerful movements in hind limbs but
not able to stand; a score of 4 for able to stand but unable to
walk; and a score of 5 for full recovery, normal walking function.
Neurological examination was carried out at the same time by two
investigators who were blinded to the groupings and who
independently assessed the animals' neurological functions.
Evaluation of pathological
outcome
All rabbits were sacrificed with deep intravenous
sodium pentobarbital anesthesia (100 mg/kg) 2 days after surgery.
The spinal cord between L2 and L4 was removed and soaked in 10%
paraformaldehyde/0.1 mol/l phosphate-buffered saline solution at
4°C for 48 h. The spinal cord was sectioned and embedded in
paraffin. The experimental slices (5-µm thick) were stained with
hematoxylin-eosin and examined by light microscopy for
histopathological observation. The neuropathologists were blinded
to the experimental groups and observed the destruction of spinal
cord motor neurons of the anterior horn. The light microscopy
findings were graded on a scale of +3 to 0, which corresponded to
no change, mild, moderate and severe changes, respectively
(16).
Statistical analysis
Data were expressed as the mean ± standard
deviation, and TceMEP data were expressed as the median and 10–90th
percentiles. Statistical analysis of neurological scores was
performed using an unpaired t-test. Multi-group variables were
compared using one-way analysis of variance; if this indicated
significance, a post hoc test was used to determine which results
were significantly different from each other. The relationship
between TceMEP and the neurological score was also analyzed using
Pearson correlation analysis. Analyses were performed with SPSS v.
17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Model success rate
A total of 24 New Zealand white rabbits were used in
the present study. During the surgery, 2 rabbits were excluded due
to intraoperative hemorrhage or variation. The other rabbits were
free to eat after recovering from anesthesia. The success rate of
this model was 92.31%.
Control group
In the control group, stimulation with different
intensities was used to induce TceMEPs to determine the most
appropriate stimulation intensity. When the stimulation intensity
was >120 V, there was no change in the amplitude or latency. The
most appropriate stimulation intensity was determined to be 130 V.
The EP recordings before and after surgery, and at different time
points after the rabbits were anesthetized are demonstrated in
Tables I and II.
 | Table I.The amplitude and latency of TceMEP
at different times after anesthesia (mean ± standard
deviation). |
Table I.
The amplitude and latency of TceMEP
at different times after anesthesia (mean ± standard
deviation).
|
| TceMEP
parameter |
|---|
|
|
|
|---|
| Time after
anesthesia, min | Latency,
mseca | Amplitude,
µVb |
|---|
| 30 | 13.03±1.12 |
5312.67±1801.85 |
| 60 | 13.12±1.15 |
5185.33±1691.75 |
| 90 | 13.00±1.15 |
5202.67±1680.04 |
| 120 | 13.02±1.10 |
5180.00±1687.67 |
| 150 | 12.98±1.09 |
5125.00±1701.80 |
| 180 | 12.97±1.12 |
5086.17±1490.85 |
 | Table II.The amplitude and latency of TceMEP
at different times before and after surgery. |
Table II.
The amplitude and latency of TceMEP
at different times before and after surgery.
|
| TceMEP
parameter |
|---|
|
|
|
|---|
| Time | Latency,
mseca | Amplitude,
µVb |
|---|
| Preoperative | 13.02±1.10 |
5300.00±1816.84 |
| 30 min after
surgery | 13.03±1.07 |
5213.67±1679.67 |
| 1 day after
surgery | 13.03±1.11 |
5013.17±1587.49 |
Experimental groups
Reproducible TceMEPs were found in every rabbit
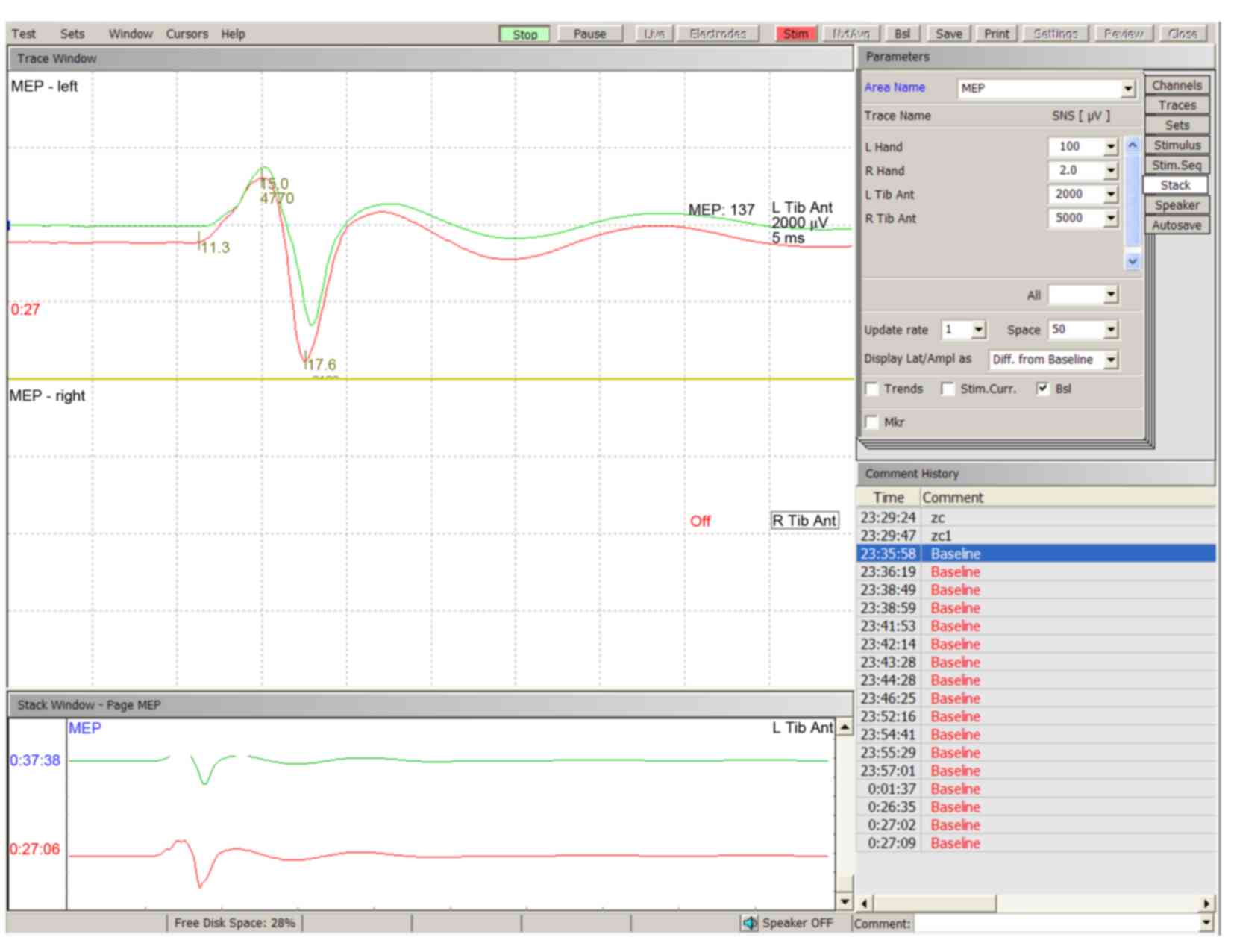
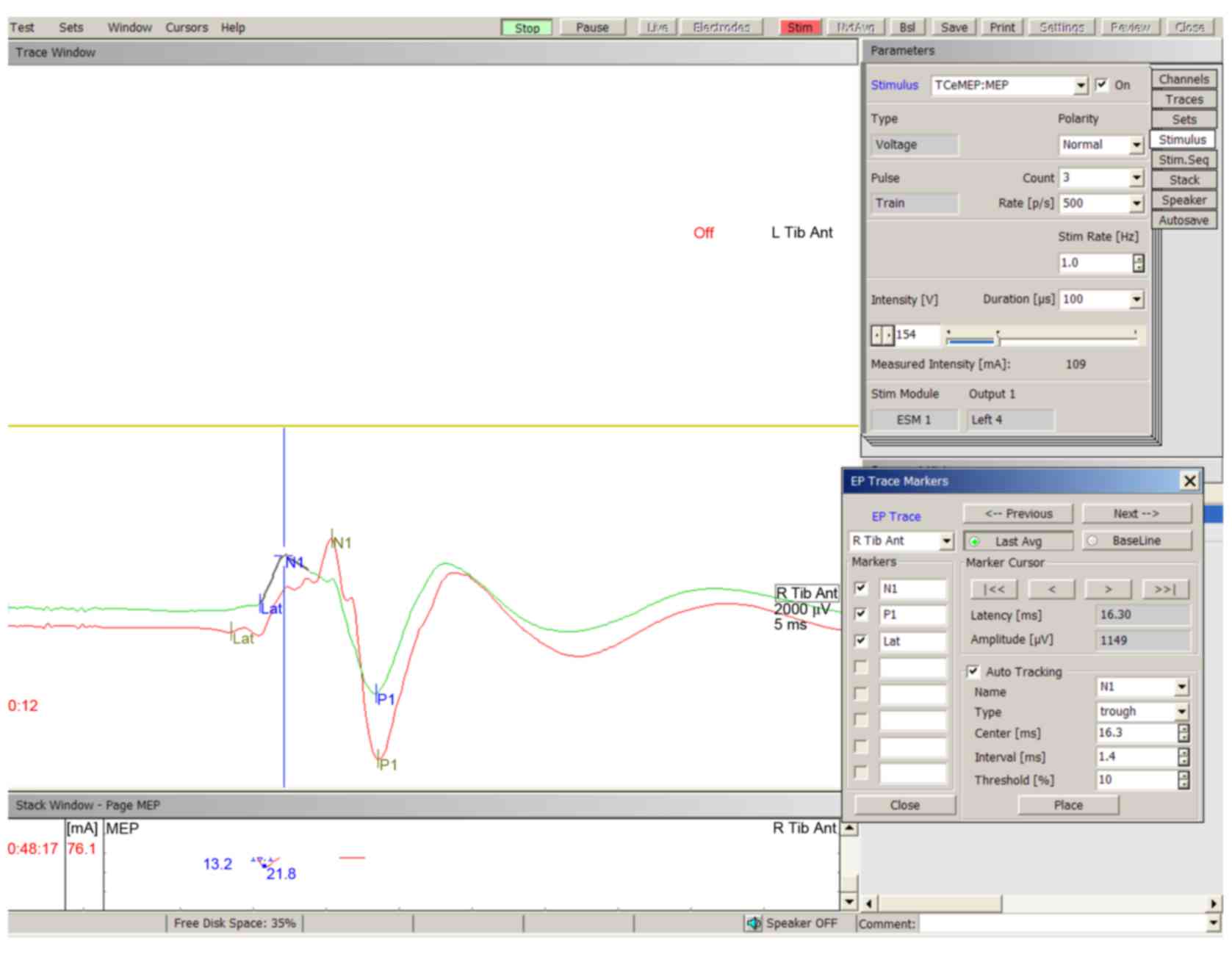
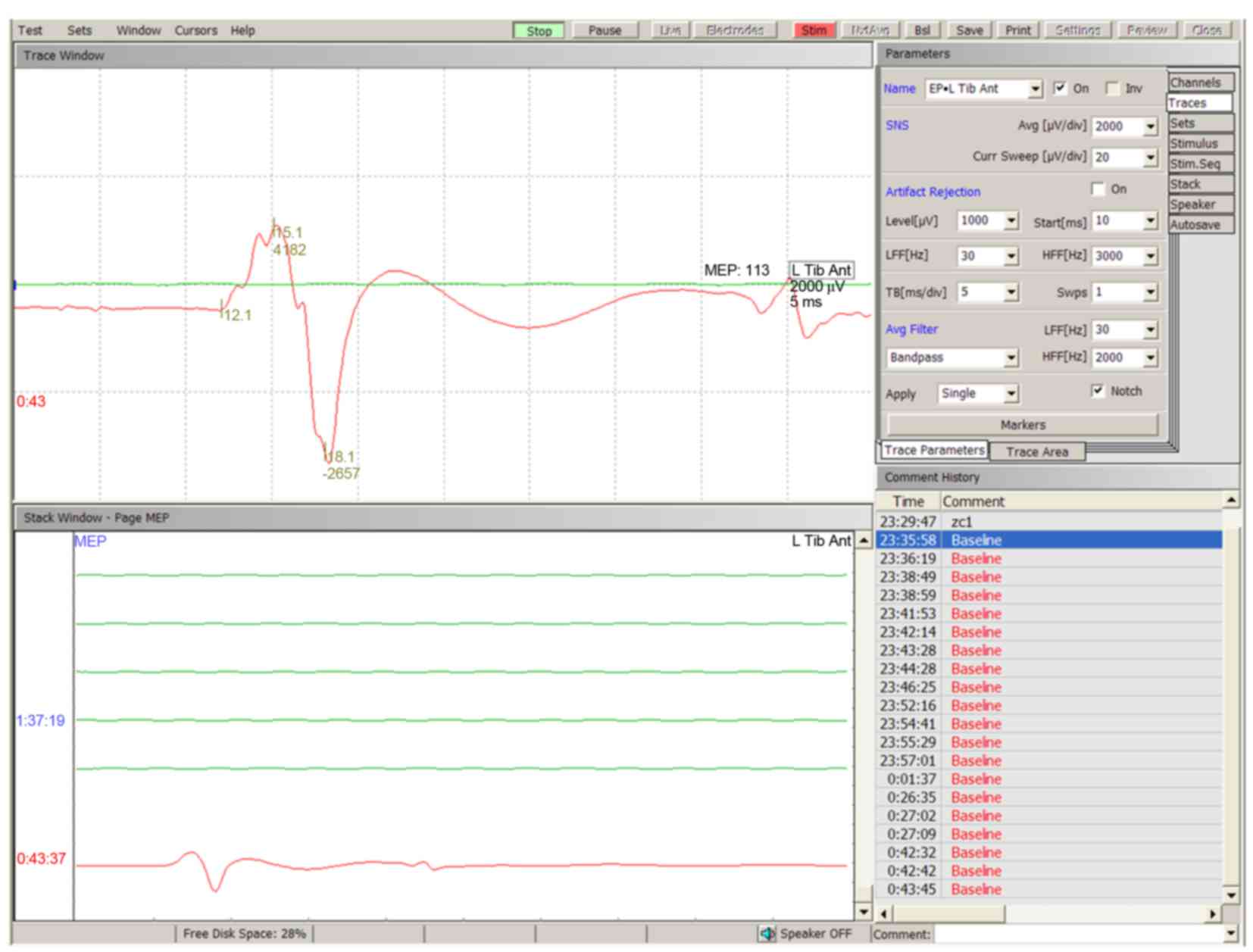
tested. Typical TceMEP wave deflections were described as N1 and P1
(Fig. 1). The baseline amplitude of
TceMEP was 5518.00 µV (3502.60-7903.60 µV, 10–90th percentile) in
all animals. A decrease in the amplitude of TceMEP was observed
following different levels of lumbar artery ligation. In the 3-, 4-
and 5-level spinal cord ischemia (SCI) groups, the amplitude
decrease was observed within 0.52±0.08, 0.58±0.15 and 0.50±0.12
min, respectively. It took 3.33±0.38, 3.18±0.41 and 2.96±0.46 min,
respectively, for the amplitude to stabilize in these groups.
With increasing levels of vascular ligation, the
amplitude gradually decreased and eventually disappeared after the
ligation of all five lumbar arteries. Once the TceMEP amplitudes
stabilized within 5 min of ligation, the ratios of the amplitudes
after the lumbar arteries were ligated compared to the baseline
amplitude in groups A, B, C and D were 95.69±5.33, 63.16±5.37,
25.55±8.44 and 0.00±0.00%, respectively (Figs. 2–4).
Functional evaluation
The animals in the sham group demonstrated no
neurological deficits after recovering from anesthesia or 2 days
after the procedure (5.0±0.0 and 5.0±0.0, respectively). In groups
B, C and D, the neurological scores were 3.80±0.45, 2.00±0.63 and
0.00±0.00, respectively, after recovering from anesthesia, and
5.0±0.0, 2.67±0.52 and 0.40±0.55, respectively, 2 days after
ligation (data not shown). Rabbits in the sham group demonstrated
significantly improved recovery compared with the other SCI groups
(P<0.05) after the animals recovered from anesthesia. There was
no significant difference between the sham group and group B 2 days
after ligation, whereas there was a significant difference between
the sham group and groups C and D (P<0.05).
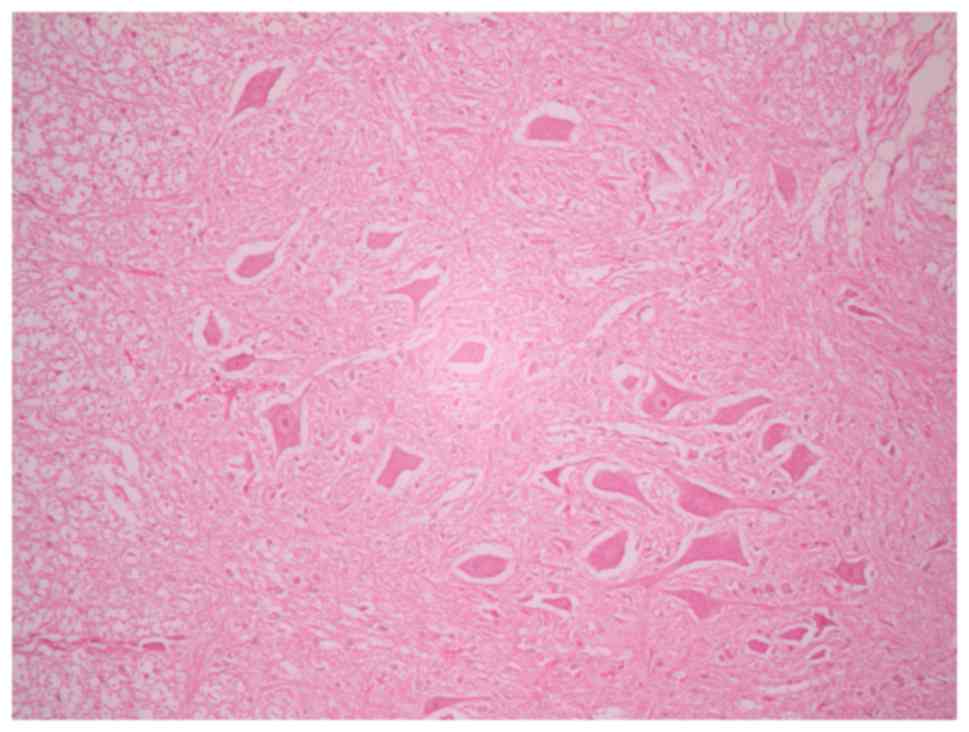
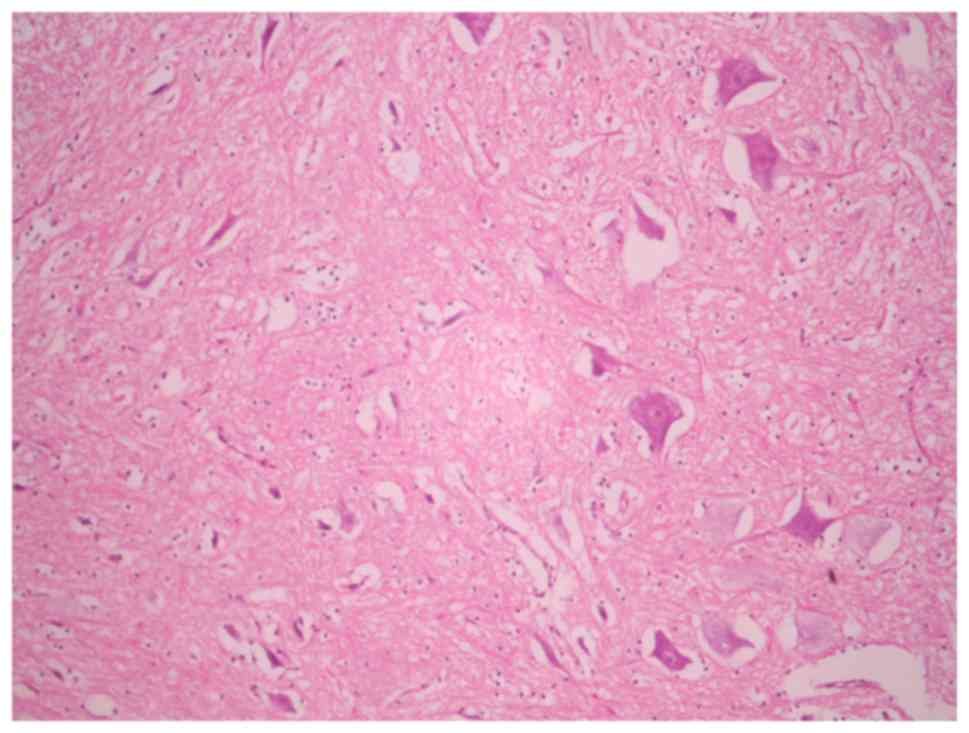
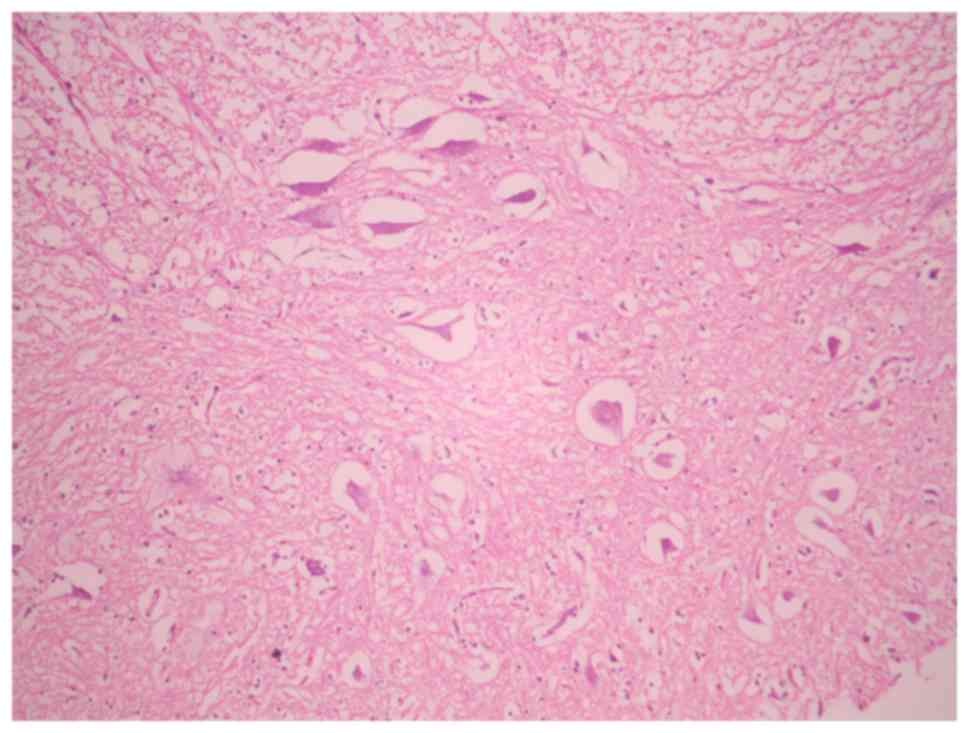
Histological assessment
In the sham-operated groups, the spinal cord was
normal with many intact motor neurons in the anterior spinal horn
(Fig. 5). Ischemic damage was
observed almost exclusively in the anterior horn spinal cord gray
matter in the 3-, 4- and 5-level ligation groups (Figs. 6 and 7). This area contained neuronal necrosis
with a typical loss of cytoplasmic structures and eosinophilic
cytoplasm, and neuronal apoptotic features, such as apoptotic
bodies, chromatin condensation, shrinkage and nuclear
fragmentation. The extent of ischemic damage was grossly
proportional to the number of ligated lumbar arteries. The
pathological score in groups A, B, C and D were 3.00±0.00,
2.40±0.55, 1.33±0.52 and 0.20±0.45, respectively (data not shown).
There were significant differences in pathological score between
the sham operation group (group A) and groups C and D (P<0.05),
with group A demonstrating improved pathological scores compared
with groups C and D; however, there was no significant difference
between the sham operation group (group A) and group B
(P>0.05).
The relationship between TceMPE
amplitude changes within 5 min following different levels of
permanent SCI and motor function
The correlation between the changes in TceMEP
amplitude and motor function after recovery from anesthesia was
significant (P<0.001), and the Pearson correlation coefficient
was 0.980. The relationship between the TceMEP amplitude changes
and motor function 2 days after lumbar artery ligation was also
significant (P<0.001), and the Pearson correlation coefficient
was 0.923.
The relationship between changes in
TceMEP amplitude and the pathological score
The correlation between changes in TceMEP amplitude
and the pathological score was significant (P<0.001), and the
Pearson correlation coefficient was 0.945.
Discussion
The spinal cord has a particularly complex blood
supply network on the surface that includes three main arteries: A
single anterior spinal artery and paired posterior arteries. These
arteries are joined by a mesh-like pial plexus surrounding the
spinal cord. The arterial blood supply of the spine and spinal cord
is segmentally provided by lumbar and intercostal arteries
branching from the aorta. These rich anastomotic vascular supply
network channels offer alternative pathways for supplying the
spinal cord with blood and may preserve spinal cord circulation
following the ligation of segmental arteries at certain levels
(17). The present study
demonstrated that, in rabbits, the ligation of lumbar arteries at
1–2 levels does not lead to spinal cord dysfunction, which reveals
the presence of a dense and complex collateral arterial network
feeding the spinal cord.
Rabbits were used in the present experiment because
of their unique lumbar arterial blood supply to the spinal cord
from the infrarenal aorta (18). The
lumbar artery of rabbits demonstrated fewer variations and is more
convenient for ligation, making it particularly suitable as an
animal model of spinal cord ischemia, and this model is important
for the study of the dynamics of blood supply to the spinal cord
and the reaction of the spinal cord to different levels of
permanent spinal cord ischemia (19). Furthermore, this model is useful in
refining the design and verifying the safety of intraoperative
protective measures in protecting the function of the spinal
cord.
With advances in microsurgical and visualization
techniques, complicated surgeries on the spine and spinal cord may
be performed more easily (20). In
spite of these advances, techniques to monitor the functional
integrity of the nerve pathways are lacking. This problem has led
to many neurosurgeons trying to identify novel neurophysiological
monitoring techniques to protect neurological function and decrease
the incidence of surgical complications. Cortical (C)SEPs and MEPs
have been widely used (21,22). SEPs reflect the ascending sensory
pathway of the posterior columns, which are supplied by the paired
posterior arteries (23). SEP
monitoring has been reported to be a useful and non-invasive method
for protecting spinal cord function and detecting spinal cord
ischemic injury (24). SEPs have
been used frequently because they may be recorded intraoperatively
under general anesthesia (25).
However, SEPs primarily examine the sensory pathway of the dorsal
column, and the specificity and sensitivity of SEPs for monitoring
the motor neuron pathways has been questioned (26,27).
Furthermore, SEP monitoring cannot be conducted in real-time
because each signal is so small that they must be averaged to
create an SEP wave (28).
TceMEPs are suitable for the intraoperative
monitoring of the spinal cord motor pathway (29,30).
TceMEPs reflect the transmission of the spinal cord motor pathway,
which is supplied by the anterior spinal artery. Research has
indicated that changes in TceMEPs are able to predict motor
function (31). TceMEPs are
considered a direct reflection of the function of descending motor
tracts (32). Recently, research has
recommended a unified evaluation of spinal cord function using
CSEPs and TceMEPs during surgery (5). The present study examined the value of
TceMEP monitoring intraoperatively during spine and spinal cord
surgery. A decrease in the amplitude of TceMEP was observed after
different levels of lumbar arterial ligation; the 3-, 4- and
5-level SCI groups took 0.52±0.08, 0.58±0.15 and 0.50±0.12 min,
respectively, to reflect this decrease in amplitude. Therefore, it
was concluded that TceMEP is sensitive to spinal cord ischemic
damage.
A study by Zivin and DeGirolami (33) has reported classic and traditional
animal models for use in evaluating the value of
neuroelectrophysiological intraoperative monitoring techniques.
There are many serious complications of congestive and ischemic
injury in these spinal cord ischemic injury animal models. Ischemia
in muscle and peripheral nerves, occurring as a result of clamping
of the abdominal aorta, interferes with the interpretation of
myogenic TceMEP amplitude and SEP recordings. Some researchers have
excluded the effects of muscle and peripheral nerve ischemia in
lower extremities as a cause of changes in myogenic TceMEP
amplitude by ligating the right femoral artery; in these
experiments, myogenic TceMEP amplitudes were preserved for 30 min
(15). However, it remains unclear
whether there are any effects that appear after 30 min. The present
experiment utilized a novel technique of producing permanent spinal
cord ischemic injury in rabbits by ligating the lumbar arteries at
different levels to evaluate the value of neuroelectrophysiological
intraoperative monitoring. The present study did not identify any
congestive or ischemic complications that affected the
interpretation of myogenic TceMEP amplitude and SEP recordings,
particularly in muscle and peripheral nerve ischemia of the lower
limbs. As the blood flow to the muscle and peripheral nerves of the
lower extremities was unchanged, we believe that this rabbit animal
model is ideal for evaluating changes in neurophysiology resulting
from different levels of permanent spinal cord ischemic injury.
It is generally believed that 5 min of spinal cord
ischemic injury will not result in irreversible damage (34). Therefore, the aim of the present
study was to better understand the relationship between changes in
MEP at an early and reversible stage of ischemia and the motor
function after different levels of permanent spinal cord ischemic
injury. The relationship between changes in MEP at an early
reversible stage and the pathological damage of anterior horn motor
neurons was also investigated. Previous studies have demonstrated
that MEPs are highly sensitive to SCI when recorded from muscles in
the lower extremities (35,36). A study by de Haan et al
(15) demonstrated that myogenic
responses disappeared within 2 min after the initiation of arterial
occlusion in rabbits. A study by Reuter et al (37) observed that the peripheral nerve
responses disappeared within 1 min after the onset of spinal cord
ischemia. A study by Kakinohana et al (38) demonstrated that TceMEPs are lost
almost instantaneously after the cross-clamping of the thoracic
aorta. During the present study, it was observed that the amplitude
of MEP began to decrease within 1 min following the ligation of
different levels of lumbar arteries and began to stabilize within 5
min. It was concluded that TceMEP may detect spinal cord ischemia
during spine and spinal cord surgery at an early and reversible
stage, which would allow surgeons to modify surgical techniques and
apply protective measures. A study by Nemoto (39) demonstrated that dogs showed no sign
of spinal cord damage when the MEP amplitude was retained at a
minimum of 50% of baseline, and this conclusion is supported by the
findings of the present study. When the lumbar arteries are ligated
at three continuous levels, motor function was fully restored after
2 days if the amplitude remained at or above 50% of baseline.
However, this function was not restored if the amplitude decreased
below 50%.
During resection of intramedullary, subdural and
epidural tumors, MEP monitoring has become a true surgical
technology (40). Recording and
interpretation of MEPs is straightforward and fast. During tumor
separation procedures and during tumor resections, spinal cord and
nerve roots may be damaged by vascular compromise, compression,
traction or electric coagulation hemostasis (40). For detection of such potentially
reversible damage, the function of the motor pathways must be
assessed continuously with MEP monitoring. The correct prediction
of the clinical motor function at a given time during surgery is
possible with a very high certainty (41). Loss of muscle MEPs reflects a pattern
of MEP change, indicating a reversible injury to the essential
motor pathways. Using this information, protective measures may be
adopted before irreversible neurological damage is caused, which
will allow the surgeon to confidently proceed with a tumor
resection. The sensitivity of muscle MEPs for postoperative motor
deficits is nearly 100%, with a specificity of ~90% (41).
In conclusion, TceMEPs are an ideal spinal cord
monitoring method for use during spine and spinal cord surgery, and
TceMEP is sensitive to spinal cord ischemic damage. Following
different levels of permanent spinal cord ischemic injury, the
amplitude changes of TceMEP that occur within 5 min are positively
correlated with motor function and pathological damage. The
detection of acute spinal cord ischemia by TceMEP monitoring occurs
without a time delay, which would allow protective measures to be
used in a timely fashion, which may prevent irreversible spinal
cord injury.
Acknowledgements
The present study was supported by funds from the
Health Department of Shandong Province of China (grant nos.
2011QW008 and 2015WS0375).
References
|
1
|
Legatt AD, Fried SJ, Amaral TD, Sarwahi V
and Moguilevitch M: Loss of lower limb motor evoked potentials and
spinal cord injury during the initial exposure in scoliosis
surgery. J Clin Neurophysiol. 31:e1–e5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basoglu H, Kurtoglu T, Cetin NK, Bilgin MD
and Kiylioglu N: Assessment of in vivo spinal cord conduction
velocity in rats in an experimental model of ischemic spinal cord
injury. J Spinal Cord. 51:616–622. 2013. View Article : Google Scholar
|
|
3
|
Mesquita RC, D'Souza A, Bilfinger TV,
Galler RM, Emanuel A, Schenkel SS, Yodh AG and Floyd TF: Optical
monitoring and detection of spinal cord ischemia. PLoS One.
8:e833702013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taskiran E, Brandmeier S, Ozek E, Sari R,
Bolukbasi F and Elmaci I: Multimodal intraoperative
neurophysiologic monitoring in the spinal cord surgery. Turk
Neurosurg. 27:436–440. 2017.PubMed/NCBI
|
|
5
|
Jahangiri FR, Sheryar M and Al Okaili R:
Neurophysiological monitoring of the spinal sensory and motor
pathways during embolization of spinal arteriovenous
malformations-propofol: A safe alternative. Neurodiagn J.
54:125–137. 2014.PubMed/NCBI
|
|
6
|
Ando M, Tamaki T, Yoshida M, Kawakami M,
Kubota S, Nakagawa Y, Iwasaki H, Tsutsui S and Yamada H:
Intraoperative spinal cord monitoring using combined motor and
sensory evoked potentials recorded from the spinal cord during
surgery for intramedullary spinal cord tumor. Clin Neurol
Neurosurg. 133:18–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lesser RP, Raudzens P, Lüders H, Nuwer MR,
Goldie WD, Morris HH III, Dinner DS, Klem G, Hahn JF, Shetter AG,
et al: Postoperative neurological deficits may occur despite
unchanged intraoperative somatosensory evoked potentials. Ann
Neurol. 19:22–25. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang SH, Park YG, Kim DH and Yoon SY:
Monitoring of motor and somatosensory evoked potentials during
spine surgery: Intraoperative changes and postoperative outcomes.
Ann Rehabil Med. 40:470–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pillai JB, Pellet Y, Panagopoulos G, Sadek
MA, Abjigitova D, Weiss D and Plestis KA: Somatosensory-evoked
potential-guided intercostal artery reimplantation in
thoracoabdominal aortic aneurysm surgery. Innovations (Phila).
8:302–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Min HK, Sung K, Yang JH, Kim WS, Jun TG,
Lee YT, Park PW and Park BJ: Can intraoperative motor-evoked
potentials predict all the spinal cord ischemia during moderate
hypothermic beating heart descending thoracic or thoraco-abdominal
aortic surgery? J Card Surg. 25:542–547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tu XK, Zhang HB, Shi SS, Liang RS, Wang
CH, Chen CM and Yang WZ: 5-LOX inhibitor zileuton reduces
inflammatory reaction and ischemic brain damage through the
activation of PI3K/Akt signaling pathway. Neurochem Res.
41:2779–2787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi SS, Yang WZ, Chen Y, Chen JP and Tu
XK: Propofol reduces inflammatory reaction and ischemic brain
damage in cerebral ischemia in rats. Neurochem Res. 39:793–799.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gürer B, Kertmen H, Kasim E, Yilmaz ER,
Kanat BH, Sargon MF, Arikok AT, Ergüder BI and Sekerci Z:
Neuroprotective effects of testosterone on ischemia/reperfusion
injury of the rabbit spinal cord. Injury. 46:240–248. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
LeMaire SA, Ochoa LN, Conklin LD, Widman
RA, Clubb FJ Jr, Undar A, Schmittling ZC, Wang XL, Fraser CD Jr and
Coselli JS: Transcutaneous near-infrared spectroscopy for detection
of regional spinal ischemia during intercostal artery ligation:
Preliminary experimental results. J Thorac Cardiovasc Surg.
132:1150–1155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Haan P, Kalkman CJ, Ubags LH, Jacobs MJ
and Drummond JC: A comparison of the sensitivity of epidural and
myogenic transcranial motor-evoked responses in the detection of
acute spinal cord ischemia in the rabbit. Anesth Analg.
83:1022–1027. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ehrlich M, Knolle E, Ciovica R, Böck P,
Turkof E, Grabenwöger M, Cartes-Zumelzu F, Kocher A, Pockberger H,
Fang WC, et al: Memantine for prevention of spinal cord injury in a
rabbit model. J Thorac Cardiovasc Surg. 117:285–291. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strauch JT, Lauten A, Spielvogel D, Rinke
S, Zhang N, Weisz D, Bodian CA and Griepp RB: Mild hypothermia
protects the spinal cord from ischemic injury in a chronic porcine
model. Eur J Cardiothorac Surg. 25:708–715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koçogullari CU, Becit N, Erkut B, Keleş
MS, Ceviz M, Ates A, Gündoğdu C, Kaygin MA and Koçak H: Prevention
of reperfusion injury of the spinal cord in aortic surgery: An
experimental study. Surg Today. 38:237–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazensky D, Radonak J, Danko J, Petrovova
E and Frankovicova M: Anatomical study of blood supply to the
spinal cord in the rabbit. Spinal Cord. 49:525–528. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noh JH, Cho KR, Yeon JY, Seol HJ and Shin
HJ: Microsurgical treatment and outcome of pediatric supratentorial
cerebral cavernous malformation. J Korean Neurosurg Soc.
56:237–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin DP, Bhalla T, Thung A, Rice J,
Beebe A, Samora W, Klamar J and Tobias JD: A preliminary study of
volatile agents or total intravenous anesthesia for
neurophysiological monitoring during posterior spinal fusion in
adolescents with idiopathic scoliosis. Spine (Phila Pa 1976).
39:e1318–e1324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
James WS, Rughani AI and Dumont TM: A
socioeconomic analysis of intraoperative neurophysiological
monitoring during spine surgery: National use, regional variation,
and patient outcomes. Neurosurg Focus. 37:E102014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stöckl B, Wimmer C, Innerhofer P, Kofler M
and Behensky H: Delayed anterior spinal artery syndrome following
posterior scoliosis correction. Eur Spine J. 14:906–909. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji Y, Meng B, Yuan C, Yang H and Zou J:
Monitoring somatosensory evoked potentials in spinal cord
ischemia-reperfusion injury. Neural Regen Res. 8:3087–3094.
2013.PubMed/NCBI
|
|
25
|
Mônaco BA, Benício A, Contreras IS,
Mingrone LE, Ballester G and Moreira LF: Ischemic preconditioning
and spinal cord function monitoring in the descending thoracic
aorta approach. Arq Bras Cardiol. 88:291–296. 2007.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gunnarsson T, Krassioukov AV, Sarjeant R
and Fehlings MG: Real-time continuous intraoperative
electromyographic and somatosensory evoked potential recordings in
spinal surgery: Correlation of clinical and electrophysiologic
findings in a prospective, consecutive series of 213 cases. Spine
(Phila Pa 1976). 29:677–684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiedemayer H, Sandalcioglu IE, Armbruster
W, Regel J, Schaefer H and Stolke D: False negative findings in
intraoperative SEP monitoring: Analysis of 658 consecutive
neurosurgical cases and review of published reports. J Neurol
Neurosurg Psychiatry. 75:280–286. 2004.PubMed/NCBI
|
|
28
|
Stecker MM: A review of intraoperative
monitoring for spinal surgery. Surg Neurol Int. 3 Suppl
3:S174–S187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clark AJ, Ziewacz JE, Safaee M, Lau D,
Lyon R, Chou D, Weinstein PR, Ames CP, Clark JP III and Mummaneni
PV: Intraoperative neuromonitoring with MEPs and prediction of
postoperative neurological deficits in patients undergoing surgery
for cervical and cervicothoracic myelopathy. Neurosurg Focus.
35:E72013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Szelényi A, Heukamp C, Seifert V and
Marquardt G: S100B, intraoperative neuromonitoring findings and
their relation to clinical outcome in surgically treated intradural
spinal lesions. Acta Neurochir (Wien). 156:733–739. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simó M, Szirmai I and Arányi Z: Superior
sensitivity of motor over somatosensory evoked potentials in the
diagnosis of cervical spondylotic myelopathy. Eur J Neurol.
11:621–626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kobayashi S, Matsuyama Y, Shinomiya K,
Kawabata S, Ando M, Kanchiku T, Saito T, Takahashi M, Ito Z,
Muramoto A, et al: A new alarm point of transcranial electrical
stimulation motor evoked potentials for intraoperative spinal cord
monitoring: A prospective multicenter study from the spinal cord
monitoring working group of the Japanese Society for spine surgery
and related research. J Neurosurg Spine. 20:102–107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zivin JA and DeGirolami U: Spinal cord
infarction: A highly reproducible stroke model. Stroke. 11:200–202.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Haan P, Meylaerts SA, Lips J and Jacobs
MJ: Development of spinal cord ischemia after clamping of
noncritical segmental arteries in the pig. Ann Thorac Surg.
68:1278–1284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Legatt AD: Current practice of motor
evoked potential monitoring: Results of a survey. J Clin
Neurophysiol. 19:454–460. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu LY, Callahan B, Peterss S, Dumfarth J,
Tranquilli M, Ziganshin BA and Elefteriades JA: Neuromonitoring
using motor and somatosensory evoked potentials in aortic surgery.
J Card Surg. 31:383–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reuter DG, Tacker WA Jr, Badylak SF,
Voorhees WD III and Konrad PE: Correlation of motor evoked
potential response to ischemic spinal cord damage. J Thorac
Cardiovasc Surg. 104:262–272. 1992.PubMed/NCBI
|
|
38
|
Kakinohana M, Abe M, Miyata Y, Oshiro M,
Saikawa S, Arakaki K, Kuniyoshi Y and Sugahara K: Delayed response
of transcranial myogenic motor-evoked potential monitoring to
spinal cord ischemia during repair surgery for descending thoracic
aortic aneurysm. J Anesth. 22:304–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nemoto M: Animal experiment on spinal cord
ischemia with evoked potentials by transcranial electrical
stimulation. J Juntendo Igaku. 43:586–598. 1998. View Article : Google Scholar
|
|
40
|
Kim DG, Son YR, Park YS, Hyun SJ, Kim KJ,
Jahng TA, Kim HJ and Park KS: Differences in multimodality
intraoperative neurophysiological monitoring changes between spinal
intramedullary ependymoma and hemangioblastoma. J Clin
Neurophysiol. 33:120–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kothbauer KF: Intraoperative
neurophysiologic monitoring for intramedullary spinal-cord tumor
surgery. Neurophysiolo Clin. 37:407–414. 2007. View Article : Google Scholar
|