Introduction
Sepsis is a life-threatening clinical disease
induced by infection, and is characterized by systemic inflammation
(1). With the development of sepsis,
various organs, including the lungs, liver and kidney, may become
damaged. This may finally develop into multiple organ dysfunction
syndrome (2). According to the
statistics of US Centers for Disease Control, there are 750,000
serious infection and subsequent disease cases in the United States
each year (3). In China, the
morbidity and mortality is consistent with those reported from
abroad (4). Although great attention
has been paid to basic research and clinical studies of sepsis in
China and abroad, its incidence and mortality have remained
high.
Several lipids are involved in sepsis and organ
injury. Sphingosine-1-phosphate is a sphingolipid that has been
demonstrated to significantly decrease inflammation in a murine
model of acute lung injury induced by lipopolysaccharide (LPS)
(5). The upstream pathways involved
in sphingolipid synthesis include sphingomyelin (SM) (6). The biosynthesis of SM requires a series
of enzymes, and sphingomyelin synthase (SMS) is the last critical
enzyme. This enzyme has two isoforms (SMS1 and SMS2); SMS1 is found
on the Golgi apparatus, and SMS2 exists in Golgi apparatus and
plasma membranes (7). Research has
indicated that SM participated in organ injury in sepsis (8–10). For
example, studies by Hu et al (8) and Gowda et al (9) demonstrated that the expression and
activity of SMS2 were enhanced in the lungs of mice during acute
lung injury. Furthermore, SMS2-knockout mice demonstrated lower
sensitivity to LPS, and attenuated nuclear factor (NF)-κB
activation and lung injury by suppressing mitogen-activated protein
kinase-c-Jun N-terminal kinase activation, compared to the
wild-type mice (9). However, when
D609, an inhibitor of SMS, inhibited the SMS activity or small
interfering RNA knocked down the expression of SMS2, these
treatments attenuated LPS-induced pulmonary artery endothelial cell
(HPAEC) injury (10).
Cholesterol is another lipid involved in sepsis. In
early 1993, a study by Memon et al (11) indicated that when C57BL/6J mice were
injected with LPS, after 16 h, the serum cholesterol levels were
significantly increased by ~41%. Additionally, clinical cases have
also demonstrated that cholesterol is involved in sepsis. For
example, In one study, patients with sepsis with acute bacterial
infection were enrolled and divided into two groups; one group had
been treated with statins [a type of drug that inhibits the key
enzyme activity of hydroxy-3-methylglutaryl-coenzyme A reductase
(HMGCR) required for cholesterol biosynthesis] prior to their
admission, and the other group had not been treated. Severe sepsis
developed in 19% of patients in the non-statin group and in only
2.4% of the statin-treated group (12,13).
Additionally, prior exposure to statins may have a protective
effect on the development of sepsis and decrease mortality in
critically ill surgical patients (14). It is evident that cholesterol is
involved in sepsis and may be a pro-inflammatory molecule in its
development (15,16).
The liver is an important immune and metabolic organ
that is closely linked to several major biological functions,
including synthesis of glycogen, proteins and lipids, inflammatory
response, detoxification and blood clotting (17). Liver dysfunction has been known to
occur frequently in the process of sepsis (18). Although research has indicated that
SM and cholesterol are involved in sepsis, the effects of SM and
cholesterol on liver dysfunction remain to be elucidated. To
clarify the metabolism of SM and cholesterol in the liver during
sepsis in the present study, BALB/c mice were treated with LPS (to
induce sepsis), LPS + pyrrolidine dithiocarbamate (PDTC) or PBS.
PDTC inhibits the activation of NF-κB specifically by suppressing
the release of the inhibitory subunit IκB combining with NF-κB
(19,20). SM and cholesterol content, SMS
activity and related protein levels were measured.
Materials and methods
Animal model of sepsis
A total of 18 male BALB/c mice, weighing 26±3 g (5–6
weeks old), were obtained from the Experimental Animal Center of
Nanchang University (Nanchang, China). All mice were kept under a
12-h light/dark cycle with free access to standard fodder and
water. The temperature and humidity were 21±1°C and 65%,
respectively. Sepsis was induced in the mice as previously
described (8). Briefly, the mice
were divided into the following three groups (n=6/group): Control,
LPS and L + P (LPS + PDTC). The L + P group were intraperitoneally
injected with 30 mg/kg PDTC (Beyotime Institute of Biotechnology,
Haimen, China) diluted in 50 µl PBS. The control and LPS groups
were intraperitoneally injected with the same PBS volume (50 µl).
After 1 h, the LPS and L + P groups were intraperitoneally injected
with 10 mg/kg LPS (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
dissolved in 50 µl PBS. The mice in the control group only received
the intraperitoneal injection of 50 µl PBS.
Murine survival was monitored every hour for 24 h.
Subsequently, mice were euthanized by cervical dislocation and the
serum and liver were collected for analysis. To identify the
successful establishment of the sepsis model and liver dysfunction,
plasma levels of interleukin (IL)-1β (E-EL-M0037c) and tumor
necrosis factor (TNF)-α (E-EL-M0049c) (both from Elabscience
Biotechnology Co., Ltd., Wuhan, China) were analyzed by ELISA using
commercial kits, and the levels of alanine transaminase (ALT,
C009-2) and aspartate transaminase (AST, C010-2) (both from Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) were detected
using commercial kits.
The present study obtained ethical approval from the
Committee on Animal Experimentation of Nanchang University
(Nanchang, China), and the procedures complied with the NIH Guide
for the Care and Use of Laboratory Animals (21).
Cholesterol and SM measurement
The livers of the mice were homogenized with PBS and
then centrifuged at 9,659 × g at 4°C for 10 min. The hepatic
supernatant was collected and used to determine the protein
concentration using a bicinchoninic acid (BCA) assay (CW0014S;
Century Biotechnology Co., Ltd., Beijing, China). An equal volume
mixture of chloroform/methanol (2:1, vol/vol) was added to the
supernatant to extract the total lipids. The mixture was
centrifuged at 4,293 × g, at 4°C for 10 min. The supernatant was
collected and then dried by nitrogen gas. The cholesterol content
was calculated using a cholesterol assay kit (E1015; Applygen
Technologies, Inc., Beijing, China), and the SM content was
measured as previously described (22).
SMS activity assay
SMS activity of mouse liver was analyzed as
previously described (23). Briefly,
livers were homogenized in a buffer containing 50 mM Tris-HCl, 1 mM
EDTA, 5% sucrose and protease inhibitors. The homogenate was
centrifuged at 9,659 × g at 4°C for 10 min, and the supernatant was
used to analyze SMS activity. The reaction system contained 50 mM
Tris-HCl (pH 7.4), 25 mM KCl, C6-NBD-ceramide (0.1 mg/ml;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
phosphatidylcholine (0.01 mg/ml). The mixture was incubated at 37°C
for 2 h. Subsequently, lipids were extracted in chloroform:
Methanol (2:1, vol/vol), dried under nitrogen gas, and separated
using thin layer chromatography. The plate was scanned with an
autoradiography system (ChemiScope 6000 Pro; CLINX, Shanghai,
China), and the intensity of each band was measured using Image-Pro
Plus version 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Western blot analysis
Proteins from liver tissues of mice were extracted
using radioimmunoprecipitation buffer (CW2333S; Century
Biotechnology Co., Ltd.), and the protein concentration was
measured using a BCA assay. Equal amounts of clear lysates (~50 µg
protein) were separated by SDS-PAGE (10%) and then transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Equal transfer was validated by staining with Ponceau red.
The membranes were blocked with 10% skimmed milk in Tris-buffered
saline (TBS) at room temperature for 1 h and then incubated with
primary antibodies in TBS containing 0.05% Tween-20, 2% bovine
serum albumin (A8010; Solarbio Bioscience & Technology Co.,
Ltd., Beijing, China) and 0.05% sodium azide overnight at 4°C. The
following antibodies were used at the indicated dilutions: SMS1 at
1:800 (A521; ABclonal Biotech Co., Ltd., Wuhan, China), SMS2 at
1:1,000 (AP9801b; Abgent Biotech Co., Ltd., Suzhou, China),
apolipoprotein A1 (Apo A1; 14427-1-AP; Proteintech Group, Wuhan,
China) at 1:500, ABCA1 (ATP binding cassette subfamily A member 1)
at 1:300 (PB0490; Boster Biological Technology, Ltd., Wuhan,
China), scavenger receptor class B member 1 (SR-B1) at 1:1,000
(21277-1-AP; Proteintech Group), HMGCR at 1:1,000 (A1633; ABclonal,
Biotech Co., Ltd.) and β-actin at 1:10,000 (60008-1-Ig; Proteintech
Group). Secondary horseradish peroxidase-coupled antibodies [mouse
(SA00001-1) and rabbit (SA00001-2) (both from Proteintech Group)
were used at 1:10,000 in 10% skimmed milk in TBS containing 0.05%
Tween-20 (8,23). Signals were revealed using an
enhanced chemiluminescence reagent (CW0049M; Century Biotechnology
Co., Ltd.) and an autoradiography system (ChemiScope 6000 Pro).
Statistical analysis
Data were presented as the mean ± standard
deviation. All statistical analysis was conducted using SPSS
version 17.0 software (SPSS, Inc., Chicago, IL, USA). Statistical
analysis was performed using one-way analysis of variance and
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of an animal model of
sepsis
In the process of sepsis, the levels of plasma
inflammatory factors may be increased. To identify whether the
construction of an animal model of sepsis was successful, the
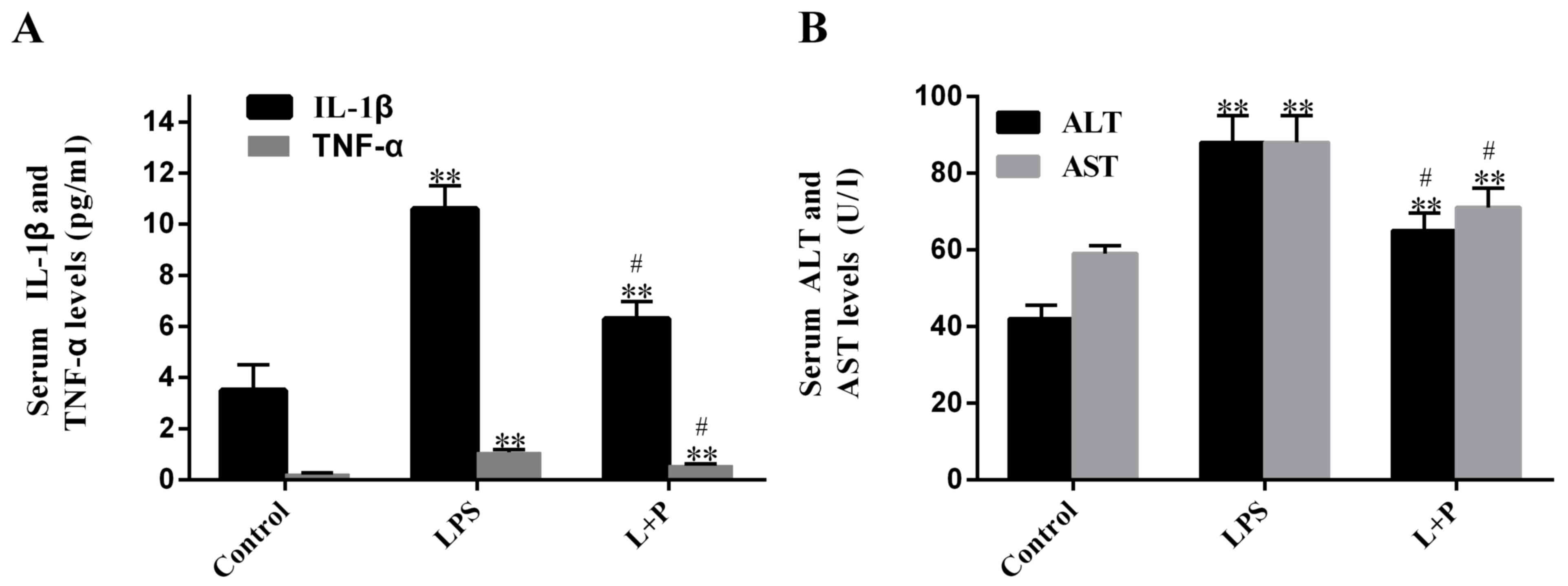
present study measured plasma levels of IL-1β and TNF-α. Results
demonstrated that LPS significantly increased (P<0.001; n=6)
plasma levels of IL-1β and TNF-α after 24 h, by 2.03- and
3.69-fold, respectively, compared to the levels in the control
groups (PBS-treated; Fig. 1A). When
the mice were injected with 30 mg/kg PDTC prior to treatment with
LPS (L + P group), plasma levels of IL-1β and TNF-α were increased
compared to the levels in the control groups (Fig. 1A; P<0.001; n=6); however, the
increase folds were significantly reduced (P<0.05; n=6; 0.80 and
1.46 folds, respectively) compared with the increases induced by
LPS alone. These results demonstrated that the LPS treatment was
successful at inducing sepsis after 24 h; however, PDTC was able to
attenuate this process.
Furthermore, to detect whether sepsis causes damage
to the liver, the AST and ALT levels were measured. Results
revealed that the plasma ALT and AST levels of the LPS-treated
group significantly rose by ~109.59 and 48.19% (P<0.001; n=6),
respectively, compared with the levels in the control group.
However, the levels in the L + P group increased by 54.18 and
21.04%, respectively, compared with the levels in the control
group, and these levels were significantly decreased compared with
the levels in the LPS group (Fig.
1B; P<0.05; n=6).
Content of cholesterol and SM in the
livers of mice
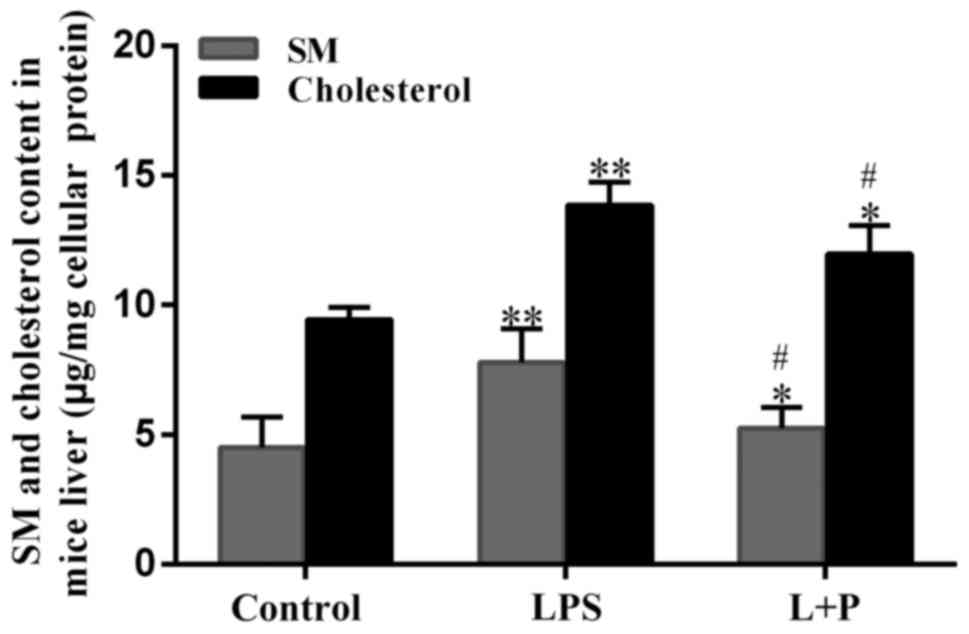
SM and cholesterol are involved in the process of
sepsis; therefore, the livers from the mice were homogenized with
PBS to isolate the total lipids and the content of SM and
cholesterol was measured. As demonstrated in Fig. 2, the SM and cholesterol content
significantly increased by 73.18 and 46.74%, respectively, in the
LPS treatment group compared with those in the control group
(P<0.001; n=6). However, the L + P treatment significantly
reduced (P<0.05; n=6) these levels compared with the LPS group;
although the levels were still significantly greater than those in
the control group (P<0.05; n=6). These results suggested that
the sepsis altered hepatic SM and cholesterol content, and that
these peptides may be involved in sepsis-associated liver
dysfunction.
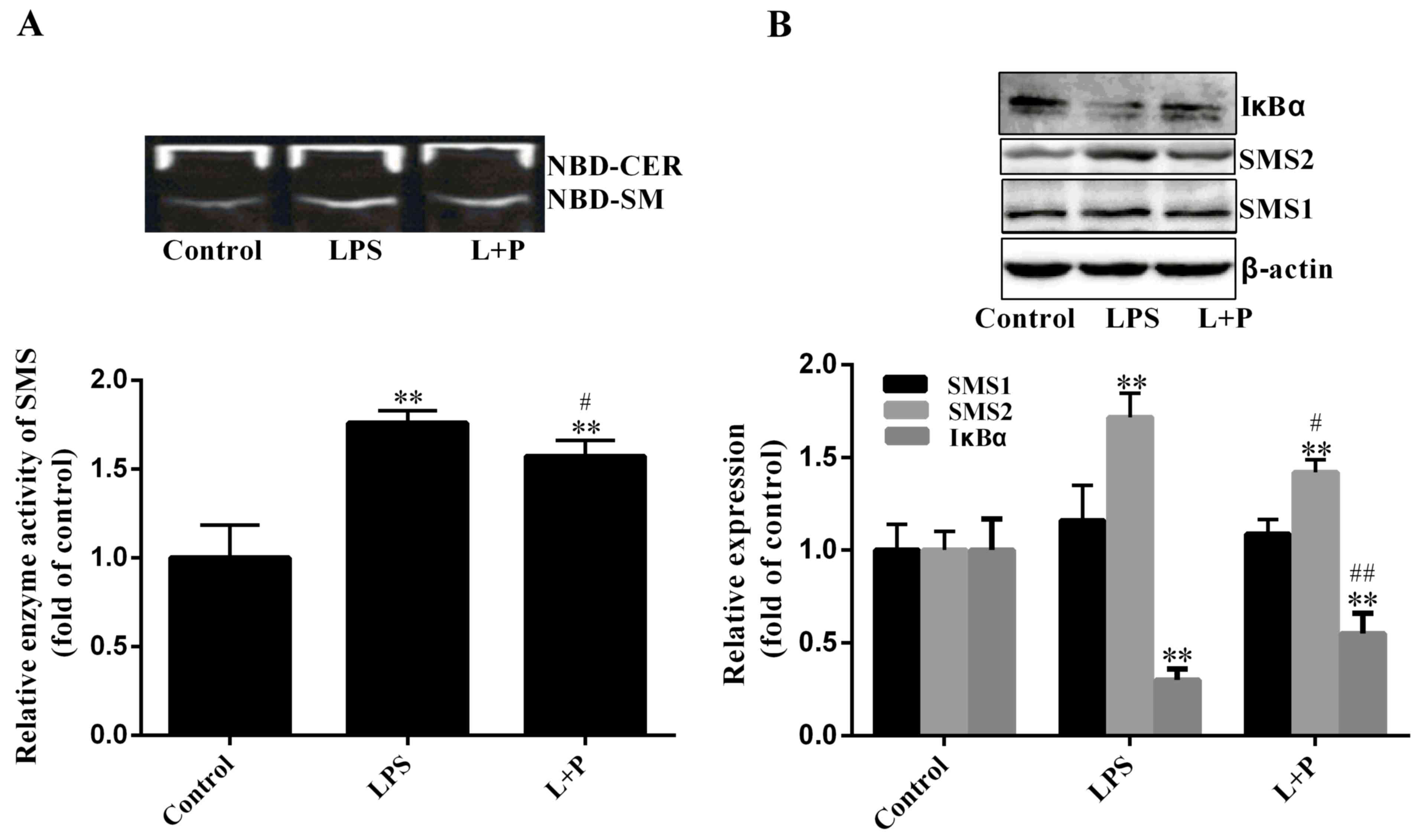
SMS activity
SMS is a key enzyme of SM biosynthesis in a series
of enzymatic reactions (7).
Therefore, SMS activity was measured in the present study. As
demonstrated in Fig. 3A, SMS
activity increased significantly by 75.91% (P<0.001; n=6) in the
LPS group compared to that in the control group, and PDTC
significantly reduced this increase (P<0.05; n=6). As SMS1 and
SMS2 may affect SMS activity, the expression levels of SMS1 and
SMS2 were investigated. As indicated in Fig. 3B, the expression level of SMS2 was
significantly upregulated (P<0.001; n=6) by ~71.62% in the LPS
treatment group compared with that in the control group. Although
the expression level of SMS1 had slightly increased in the LPS
group compared with the level in the control group, this difference
was not statistically significant (P>0.05; n=6). Notably, the
increase of SMS activity (75.91%) was similar to the increase of
SMS2 expression (71.62%). Therefore, the increase of SM content may
be predominantly influenced by SMS2.
Furthermore, the protein expression level of IκBα
was also measured in the present study. Results indicated that the
degradation level of IκBα was significantly increased in the
LPS-treated group (Fig. 3B;
P<0.001; n=6) compared to that in the control group; however,
PDTC significantly inhibited (P<0.001; n=6) the IκBα degradation
compared to the LPS group. These results suggested that PDTC
inhibition of NF-κB activity may reduce hepatic inflammation.
Expression of cholesterol-related
proteins
In cells, HMGCR is a key enzyme in the biosynthesis
of cholesterol, however, the transportation of cholesterol is
associated with Apo A1, ABCA1 and SR-B1 (24). Therefore, the expression levels of
these proteins in the livers of mice were analyzed. As demonstrated
in Fig. 4, HMGCR was significantly
upregulated (P<0.05; n=6) by ~37.63% in the LPS group compared
with the level in the control group. Furthermore, ABCA1 and SR-B1
expression levels were significantly upregulated (P<0.001; n=6)
in the LPS group, with an increase of ~57.82 and 111.70%,
respectively, compared to the levels in the control group. The
expression level of Apo A1 was significantly downregulated
(P<0.001; n=6) by ~43.31% in the LPS group compared with the
level in the control group. However, when PDTC was administered
prior to LPS treatment, the trend of upregulation or downregulation
was significantly reversed (P<0.05; n=6) compared with the LPS
group. These results suggested that depression of NF-κB by PDTC may
reduce the alterations induced by LPS.
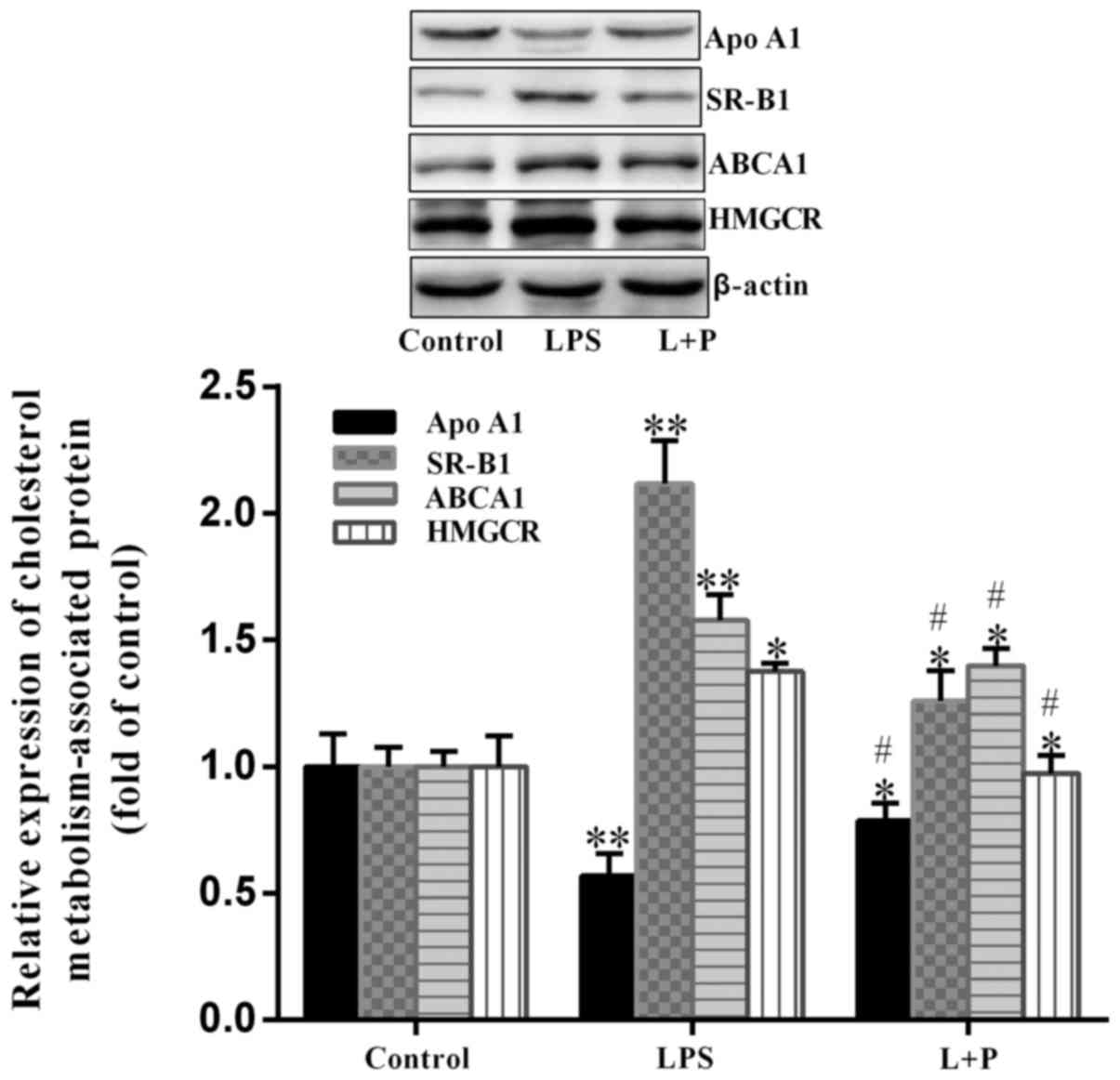 | Figure 4.Expression of HMGCR, ABCA1, SR-B1 and
Apo A1 in the livers of mice. Protein expression levels of ABCA1,
HMGCR, SR-B1 and Apo A1 were analyzed by western blot analysis.
Data are presented as the mean ± standard deviation (n=6).
*P<0.05 and **P<0.001 vs. control group;
#P<0.05 vs. LPS group. L + P, LPS + pyrrolidine
dithiocarbamate; LPS, lipopolysaccharide; HMGCR,
hydroxy-3-methylglutaryl-coenzyme A reductase; ABCA1, ATP binding
cassette subfamily A member 1; SR-B1, scavenger receptor class B
member 1; Apo A1, apolipoprotein A1. |
Discussion
LPS-induced sepsis is a complex process that may
damage many organs, particularly the lungs, liver and kidney, and
several lipids are involved in this process (1). However, in murine livers, the effect of
sepsis on regulation of SM and cholesterol metabolism remains
unknown, and it is necessary to clarify it. The results of the
present study demonstrated that LPS treatment was able to induce
sepsis and liver dysfunction in mice. Following LPS treatment, SM
and cholesterol content was increased, and the protein expression
levels of HMGCR, ABCA1, SR-B1 and SMS2 were increased.
Contrastingly, the expression of Apo A1 was downregulated compared
to the control group. However, when mice were intraperitoneally
injected with PDTC, which may attenuate the inflammatory response
by inhibiting NF-κB, all of the changes induced by LPS were
reversed (19,20). These results indicated that sepsis
directly affected the SM and cholesterol content, and the
expression of these proteins in the livers of mice.
Various reports have demonstrated that SMS was
involved in the inflammatory process. Studies by Hailemariam et
al (25) and Gowda et al
(9) indicated that LPS treatment
significantly increased SMS2 enzyme activity in HPAEC cells.
Furthermore, adding the SMS inhibitor (D609) or knocking out SMS2
may attenuate inflammation in macrophages and lung injury of mice
induced by LPS (9,10). In other words, SMS2 participates in
and promotes sepsis. In the present study, it was indicated that
hepatic SMS activity increased following LPS treatment, and this
implied that the cause of the increase in SMS activity and SM
content was the overexpression of SMS2, but not of SMS1. SMS2 is
the principal enzyme that takes charge of the biosynthesis of SM in
the liver (26).
Cholesterol is a main lipid in cells that
participates in sepsis. For example, modified low-density
lipoprotein (oxLDL) treatment of macrophages increases cholesterol
content and triggers inflammation in vivo, and mice fed with
a high-fat diet also demonstrate inflammatory properties in
macrophages (27). However,
simvastatin may depress the inflammatory response by reducing
cholesterol content (27). When mice
are injected with LPS, the sepsis and liver dysfunction would be
induced in the body of the mice, and the cholesterol content would
be enhanced in the liver. The cholesterol content is tightly
associated with cholesterol efflux in the livers of mice (24). ABCA1 is a transmembrane protein that
is able to mediate cholesterol efflux; however, Apo A1 may accept
the cholesterol by interacting with ABCA1 to form the high-density
lipoprotein (HDL), with a series of changes, and finally, the HDL
may become the mature HDL (24,28).
Therefore, the overexpression of ABCA1 and Apo A1 may strengthen
the cholesterol efflux and decrease the content of cholesterol in
the liver (24). However, in the
present study, the cholesterol content and the expression of ABCA1
were increased following LPS treatment. In early 2002, a study by
Kaplan et al (29)
demonstrated the same results as the present study. They believed
that LPS may rapidly increase the expression of ABCA1 in the liver
and THP-1 cells through a liver X receptor-independent mechanism
(29). However, we suggest that the
upregulation of ABCA1 expression in liver cells may reflect a
feedback inhibition mechanism by elevating cellular cholesterol
content. Furthermore, reasonable explanations for the increase of
the cholesterol content may be as follows: i) Elevated expression
of HMGCR may increase the biosynthesis of cholesterol; ii) as an
HDL receptor on liver cell membranes, SR-B1 may take up cholesterol
into the liver (25), and the
increasing SR-B1 expression may promote cholesterol uptake and
cholesterol influx; and iii) the downregulation of Apo A1 may also
attenuate the cholesterol efflux, and accumulate the cholesterol in
the livers of mice.
SM and cholesterol are main components of lipid
rafts, which are sites for numerous cellular processes, including
signaling, vesicular transporting, interaction with pathogens and
viral infection (30). In lipid
rafts, SM and cholesterol may be anchored with each other,
therefore, they may simultaneously increase in the livers of mice,
and only SM and cholesterol were detected in the liver in the
present study. For example, studies by Ding et al (31) and Yan et al (32) demonstrated that SMS overexpression
may cause SM accumulation in cells and lipid rafts and increase the
cholesterol content. Furthermore, patients with Niemann-Pick
disease cannot hydrolyze SM due to defective SMase, resulting in
the accumulation of SM and cholesterol in the liver and the nervous
system (33). Additionally, because
ABCA1 is a transmembrane protein that is located in or near the
lipid raft, some researchers have implied that the enhancement of
SM and cholesterol may also increase the ABCA1 expression in cells
(32).
In conclusion, in the livers of mice, both SM and
cholesterol content were increased during sepsis induced by LPS;
however, PDTC was able to attenuate these alterations. Further
investigation indicated that the change of SM and cholesterol were
tightly associated with the overexpression of SMS2, HMGCR, SR-B1
and ABCA1, and the downregulation of Apo A1. These results
suggested that lipid metabolism is a key factor affecting
sepsis.
Acknowledgements
The present research was supported by a grant from
the Jiangxi Provincial Department of Science and Technology (grant
no. 20142BAB205014).
References
|
1
|
Zotova NV, Chereshnev VA and Gusev EY:
Systemic inflammation: Methodological approaches to identification
of the common pathological process. PLoS One. 11:e01551382016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dombrovskiy VY, Martin AA, Sunderram J and
Paz HL: Rapid increase in hospitalization and mortality rates for
severe sepsis in the United States: A trend analysis from 1993 to
2003. Crit Care Med. 35:1244–1250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tadros T, Traber DL, Heggers JP and
Herndon DN: Effects of interleukin-1alpha administration on
intestinal ischemia and reperfusion injury, mucosal permeability,
and bacterial translocation in burn and sepsis. Ann Surg.
237:101–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen XC, Yang YF, Wang R, Gou HF and Chen
XZ: Epidemiology and microbiology of sepsis in mainland China in
the first decade of the 21st century. Int J Infect Dis. 31:9–14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng X, Hassoun PM, Sammani S, McVerry BJ,
Burne MJ, Rabb H, Pearse D, Tuder RM and Garcia JG: Protective
effects of sphingosine 1-phosphate in murine endotoxin-induced
inflammatory lung injury. Am J Respir Crit Care Med. 169:1245–1251.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bektas M, Allende ML, Lee BG, Chen W, Amar
MJ, Remaley AT, Saba JD and Proia RL: Sphingosine-1-phosphatelyase
deficiency disrupts lipid homeostasis in liver. J Biol Chem.
285:10880–10889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeang C, Ding T, Chirico WJ and Jiang XC:
Subcellular targeting domains of sphingomyelin synthase 1 and 2.
Nutr Metab (Lond). 8:892011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu S, Ding Y, Gong J and Yan N:
Sphingomyelin synthase 2 affects CD14-associated induction of NF-κB
by lipopolysaccharides in acute lung injury in mice. Mol Med Rep.
14:3301–3306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gowda S, Yeang C, Wadgaonkar S, Anjum F,
Grinkina N, Cutaia M, Jiang XC and Wadgaonkar R: Sphingomyelin
synthase 2 (SMS2) deficiency attenuates LPS-induced lung injury. Am
J Physiol Lung Cell Mol Physiol. 300:L430–L440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anjum F, Joshi K, Grinkina N, Gowda S,
Cutaia M and Wadgaonkar R: Role of sphingomyelin synthesis in
pulmonary endothelial cell cytoskeletal activation and
endotoxin-induced lunginjury. Am J Respir Cell Mol Biol. 47:94–103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Memon RA, Grunfeld C, Moser AH and
Feingold KR: Tumor necrosis factor mediates the effects of
endotoxin on cholesterol and triglyceride metabolism in mice.
Endocrinology. 132:2246–53. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozguler IM, Burma O, Uysal A and Akbulut
H: Rosuvastatin lowers systemic inflammatory response in coronary
artery bypass graft accompanied by cardiopulmonary bypass surgery:
A randomized controlled study. Clin Invest Med. 38:E154–E163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Almog Y, Shefer A, Novack V, Maimon N,
Barski L, Eizinger M, Friger M, Zeller L and Danon A: Prior statin
therapy is associated with a decreased rate of severe sepsis.
Circulation. 110:880–885. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schurr JW, Wu W, Smith-Hannah A, Smith CJ
and Barrera R: Incidence of sepsis and mortality with prior
exposure of HMG-COA reductase inhibitors in a surgical intensive
care population. Shock. 45:10–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung S, Cuffe H, Marshall SM, McDaniel
AL, Ha JH, Kavanagh K, Hong C, Tontonoz P, Temel RE and Parks JS:
Dietary cholesterol promotes adipocyte hypertrophy and adipose
tissue inflammation in visceral, but not in subcutaneous, fat in
monkeys. Arterioscler Thromb Vasc Biol. 34:1880–1887. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis GF and Rader DJ: New insights into
the regulation of HDL metabolism and reverse cholesterol transport.
Circ Res. 96:1221–1232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo K, Ren J, Wang G, Gu G, Li G, Wu X,
Chen J, Ren H, Hong Z, Wu L, et al: Early liver dysfunction in
patients with intra-abdominal infections. Medicine (Baltimore).
94:e17822015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding R, Han J, Zhao D, Hu Z and Ma X:
Pretreatment with Rho-kinase inhibitor ameliorates lethal
endotoxemia-induced liver injury by improving mitochondrial
function. Int Immunopharmacol. 40:125–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabay S, Ozden H, Guven G, Burukoglu D,
Ustuner MC, Topal F, Gunes HV, Ustuner D and Ozbayer C: Protective
effects of the nuclear factor kappa B inhibitor pyrrolidine
dithiocarbamate on experimental testicular torsion and detorsion
injury. Korean J Physiol Pharmaco l. 18:321–326. 2014. View Article : Google Scholar
|
|
20
|
Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo
L, Serraino I, Britti D, Mazzullo G, Caputi AP and Thiemermann C:
Pyrrolidine dithiocarbamate attenuates the development of acute and
chronic inflammation. Br J Pharmacol. 135:496–510. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. National
Academies Press (US); Washington, DC: 2011
|
|
22
|
Dong J, Liu J, Lou B, Li Z, Ye X, Wu M and
Jiang XC: Adenovirus-mediated overexpression of sphingomyelin
synthase1 and 2 increases the atherogenic potential in mice. J
Lipid Res. 47:1307–1014. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu AQ, Xie Z, Chen XN, Feng J, Chen JW,
Qin FJ and Ge LY: Fas-associated factor 1 inhibits tumor growth by
suppressing Helicobacter pylori-induced activation of NF-κB
signaling in human gastric carcinoma. Oncotarget. 8:7999–8009.
2017.PubMed/NCBI
|
|
24
|
Lewis GF and Rader DJ: New insights into
the regulation of HDL metabolism and reverse cholesterol transport.
Circ Res. 96:1221–1232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hailemariam TK, Huan C, Liu J, Li Z, Roman
C, Kalbfeisch M, Bui HH, Peake DA, Kuo MS, Cao G, et al:
Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation.
Arterioscler Thromb Vasc Biol. 28:1519–1526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Zhang H, Li Z, Hailemariam TK,
Chakraborty M, Jiang K, Qiu D, Bui HH, Peake DA, Kuo MS, et al:
Sphingomyelin synthase 2 Is one of the determinants for plasma and
liver sphingomyelin levels in mice. Arterioscler Thromb Vasc Biol.
29:850–856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho PC, Chang KC, Chuang YS and Wei LN:
Cholesterol regulation of receptor interacting protein 140 via
microRNA-33 in inflammatory cytokine production. FASEB J.
25:1758–1766. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Segrest JP, Jones MK, De Loof H,
Brouillette CG, Venkatachalapathi YV and Anantharamaiah GM: The
amphipathic helix in the exchangeable apolipoproteins: A review of
secondary structure and function. J Lipid Res. 33:141–166.
1992.PubMed/NCBI
|
|
29
|
Kaplan R, Gan X, Menke JG, Wright SD and
Cai TQ: Bacterial lipopolysaccharide induces expression of ABCA1
but not ABCG1 via an LXR-independent pathway. J Lipid Res.
43:952–959. 2002.PubMed/NCBI
|
|
30
|
Simons K and Ikonen E: Functional rafts in
cell membranes. Nature. 387:569–572. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding TB, Li ZQ, Hailemariam T, Mukherjee
S, Maxfield FR, Wu MP and Jiang XC: SMS overe-xpression and
knockdown: Impact on cellular sphingomyelin and diacylglycerol
metabolism and cell apoptosis. J Lipid Res. 49:376–385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan N, Ding T, Dong J, Li Y and Wu M:
Sphingomyelin synthase overexpression increases cholesterol
accumulation and decreases cholesterol secretion in liver cells.
Lipids Health Dis. 10:462011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee CY, Lesimple A, Denis M, Vincent J,
Larsen A, Mamer O, Krimbou L, Genest J and Marcil M: Increased
sphingomyelin content impairs HDL biogenesis and maturation in
human Niemann-Pick disease type B. J Lipid Res. 47:622–632. 2006.
View Article : Google Scholar : PubMed/NCBI
|