Introduction
Cervical cancer is one of most common cancers in
females and the second most common cause of cancer-related
mortality in women in China; its mortality varies among provinces,
with the highest mortality rates ranging from 7.28 to 11.88/10,000
females in poorly developed areas (1). The poor prognosis of cervical cancer
(including renal failure and metastasis) is associated with its
highly invasive and diffusely metastatic characteristics (2,3). Human
papillomavirus (HPV) has been recognized to cause cervical
carcinogenesis (1). The mechanism by
which HPV induces tumor formation is considered to involve the
binding of the viral oncoproteins HPV E6 and E7 to the tumor
suppressors p53 and retinoblastoma protein, respectively (4).
Cervical cancer stem cells (CCSCs) are thought to be
the ‘seed cells’ in cancer metastasis and recurrence (5–9). A
number of studies have shown that CCSCs and core stem cell
transcription factors (TFs) such as forkhead box D3 (5), sex-determining region Y-box 9 protein
(Sox9) (6), Sox2 (7,8), Nanog
(9) and octamer-binding protein 4
(Oct4) (8) are highly expressed in
cervical cancer. These TFs are recognized as stem cell markers
because they maintain the pluripotency of stem cells (10,11).
Recently, Tyagi et al (12)
reported that HPV E6 is overexpressed in CCSCs, indicating that it
plays a role in maintaining the pluripotency of these cells.
The human wings apart-like (hWAPL) gene is a
homologous sequence of the Drosophila WAPL gene, and is
closely associated with cervical carcinogenesis (13). HPV E6 and E7 are able to induce high
levels of hWAPL expression, which plays a key role during the
development of cervical cancer (14). Therefore, in the present study, the
expression of hWAPL in CCSCs was evaluated, and its effects on
invasion and colony formation in this cell population were
investigated.
Materials and methods
Culture of tumorspheres derived from
CCSCs
The study protocol was approved by the Medical
Ethics Committees of Xi'an Jiaotong University (no. H34-32-1;
Xi'an, China). All experiments were conducted in accordance with
the Declaration of Helsinki. The method of culturing tumorspheres
derived from CCSCs was conducted as previously reported (15). Briefly, 17 samples of cervical cancer
tissue (stage IB, n=11; stage IC, n=3; stage IIa, n=3; patient age,
43–65 years) were obtained by resection. These samples were
positive for HPV E6 expression, as determined by western blot
analysis. To prepare the cervical tumorspheres (CTs), tumor tissues
were washed immediately with PBS and digested overnight in
Dulbecco's modified Eagle's medium (DMEM)/F12; v/v, 1:1; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
0.5 mg/ml collagenase IV (Gibco; Thermo Fisher Scientific, Inc.).
The digested tissues were then cultured in stem cell medium
[DMEM/F12 with 10 ng/ml basic fibroblast growth factor (bFGF), 10
U/ml leukemia inhibitory factor, 1×105 U/l penicillin
and 100 mg/l streptomycin; all from Merck KGaA, Darmstadt, Germany]
at 37°C in a humidified atmosphere containing 5% CO2.
Clones of >50 cells were recognized as tumorspheres, and were
dissociated every 7–10 days by incubation in a non-enzymatic cell
dissociation solution (Sigma-Aldrich; Merck KGaA) for 2 min at
37°C, and passaged at a density of 1×103 cells/100-mm
plate. CTs were completely differentiated by 8 days after switching
to stem cell medium without bFGF.
Transduction with adenoviral
vectors
All adenoviral vectors (Ad5 serotype, E1/E3
deficiency double DNA; constructed by Beijing Nuosai Genome
Research Center Co., Ltd., Beijing, China) used had comparable
titers of 108-109 transducing U/ml.
Suspensions of the vectors were stored at −80°C until use. The
primers used for hWAPL overexpression are shown in Table I. Suspensions were briefly
centrifuged (500 × g, room temperature) and kept on ice immediately
prior to use. For transduction, 2×104 dissociated
tumorspheres were transduced 1 day after the initial seeding of
cells with a multiplicity of infection of 25. Cells were incubated
in stem cell medium containing adenoviral particles and 4 µg/ml
Polybrene (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 18
h at 37°C in a humidified atmosphere containing 5% CO2.
Adenoviral particles were removed, and the medium was replaced with
fresh stem cell medium.
 | Table I.Primers for overexpression of
hWAPL. |
Table I.
Primers for overexpression of
hWAPL.
| Gene | Primer | Sequence |
|---|
| hWAPL | Sense |
5′-TTAAGCTTTGAAACTGGTGTCAAAATGACATCCAGATT-3′ |
|
| Antisense |
5′-TTGAATTCAAGCAATGTTCCAAATATTCAATCACTCTAGAG-3′ |
| GAPDH | Sense |
5′-AAGGCTGAGAATGGGAAAC-3′ |
|
| Antisense |
5′-TTCAGGGACTTGTCATACTTC-3′ |
Colony formation assay
A colony formation assay was performed as previously
described (15). Briefly, single
cervical carcinoma cells or dissociated tumorspheres were cultured
in DMEM/F-12 medium supplemented with 10% fetal calf serum (FCS;
Thermo Fisher Scientific, Inc.), 2 mmol/l glutamine (Invitrogen;
Thermo Fisher Scientific, Inc.), 1×105 U/l penicillin
and 100 mg/l streptomycin. Cells were cultured at clonal densities
of 100–300/cm2 on 2% gelatin (Sigma-Aldrich; Merck
KGaA)-coated tissue culture dishes (BD Biosciences, San Jose, CA,
USA) at 37°C in 5% CO2 in air. Clones were monitored
every day, and the medium was changed every 2–3 days. After 28 days
of culture, plates were fixed in 10% formaldehyde/PBS for 10 min
and stained with Harris hematoxylin. Clones (>50 cells) were
counted for ≥3 plates per sample and averaged. The efficiency of
colony formation was determined as follows: Efficiency (%) = number
of colonies/number of cells seeded × 100.
Implantation of tumorsphere-derived
cells into nude mice
Following the dissociation of tumorspheres from 17
cervical cancer patients in a non-enzymatic cell-dissociation
solution, cells were washed in serum-free Hank's balanced salt
solution. Cells were then suspended in a 1:1 (v/v) mixture of
serum-free DMEM/F12, and 1×105 cells were injected
subcutaneously into the right and left sides of the mid-abdominal
area of nude mice using a 23-G needle. Animals were subjected to
dissection and analysis 28 days after implantation, and tumor
growth was assessed by measurement of the tumor volume (V) using
the formula: V = 1/2 × (L × W2), where L is the length
of the tumor and W is the width.
Western blotting
To extract the total protein, 1×104 cells
were lysed in lysis buffer [1.0 M Tris-HCl (pH 6.8) 1.0 ml, 10% SDS
6.0 ml, β-mercaptoethanol 0.2 ml and ddH2O 2.8 ml] on
ice for 10 min. The lysate was then subjected to centrifugation at
10,000 × g at 4°C for 10 min. Following protein denaturation at
100°C for 10 min, the protein level was normalized by measuring the
absorbance at 280 nm. Then, 80 µg protein samples were analyzed by
12.5% SDS-PAGE and gels were transferred onto an Immobilon-P
transfer membrane (polyvinylidene difluoride; EMD Millipore,
Billerica, MA, USA). The membrane was blocked with 5% non-fat dried
milk in TBST (10 mM Tris-HCl, 150 mM NaCl and 0.1% Tween-20) for 1
h at room temperature, and incubated overnight at 4°C with anti-HPV
E6, Oct4 and hWAPL antibodies (1:100 dilution in PBS, cat. nos.
sc-460, sc-101534 and sc-365189, respectively) or GAPDH (1:500
dilution, cat. no. sc-293335) (both from Santa Cruz Biotechnology,
Inc.). Following incubation with horseradish peroxidase
(HRP)-labeled rabbit anti-mouse secondary antibodies (1:2,000
dilution, cat. no. sc-358919; Santa Cruz Biotechnology, Inc.),
membranes were developed using a SuperSignal® West Pico
Trial kit (Pierce; Thermo Fisher Scientific, Inc.). Protein
quantitation was conducted from the optical density using software
for Bio-Rad's Molecular Imager® systems (Image
Lab™ 2.0 and Molecular Imager® Gel Doc™ XR
System; Bio-Rad Laboratories, Inc., Hercules, CA, USA). All
experiments were repeated three times.
Immunohistochemical analysis
Cervical cancer samples were fixed in
phosphate-buffered 10% formalin (pH 7.2), embedded in paraffin and
cut into 4-µm sections. The sections were dewaxed in xylene,
dehydrated in alcohol, and then incubated in 0.01 M sodium citrate
buffer (pH 6.0) for antigen retrieval. Sections were incubated with
3% H2O2 for 30 min to block endogenous
peroxidase activity, and with 10% milk at 37°C for 15 min to block
non-specific binding of antibodies. Sections were then incubated
with hWAPL, Oct4 or HPV E6 antibodies (1:100 dilution in PBS, cat.
nos. sc-365189, sc-101534 and sc-460, respectively; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. This was followed
by incubation with biotinylated secondary antibody (1:2,000, cat.
no. sc-358919; Santa Cruz Biotechnology, Inc.) at room temperature
for 1 h and visualization using 3,3′-diaminobenzidine under a light
microscope.
Injection of transactivating
transcriptional factor (TAT)-mediated si-hWAPL or si-HPV E6 into
tumorspheres or exograft tumors in nude mice
Synthesis of TAT was performed as described in a
previous study (16). In brief, 50
nM siRNAs (Ambion; Thermo Fisher Scientific, Inc.) were dissolved
in RNase-free ddH2O and then mixed with 10 µM TAT at a
TAT:siRNA molar ratio of 20:1 and incubated for 30 min at 37°C. The
siRNA sequences are shown in Table
II. For in vivo use, treatment of the tumors was
initiated at 2 weeks after tumorsphere implantation, as previously
described (17). In brief,
dissociated cells from tumorspheres were injected into the left and
right sides of the mouse (as described above), and palpable tumors
were formed 2 weeks later. At this time point, the tumor site on
one side of the mouse was injected with TAT-mediated si-hWAPL (100
µM TAT/siRNA) and that on the other side was injected with control
[scrambled (Scr) siRNA]. The tumor size was measured each week.
 | Table II.siRNA sequences. |
Table II.
siRNA sequences.
| siRNA | Primer | Sequence (5′-3′) | Product (bp) |
|---|
| Si-hWAPL 1 | Sense |
ACAGUUUUUAUCACUUUGGAU | – |
|
| Antisense |
CCAAAGUGAUAAAAACUGUGA |
|
|
| Antisense |
CCAGAUUUGGGAAAACAUACA |
|
| Si-HPV E6 1 | Sense |
GCAACAGUUACUGCGACGUUU | – |
|
| Antisense |
ACGUCUCGCAGUAACUGUUGCUU |
|
|
| Antisense |
AGCUGGGUUUCUCUACGUGUU |
|
| Si-scrambled | Sense |
GACCUGUUAAUGACGGCACUU | – |
|
| Antisense |
GUGCCGUCAUUAACAGGUCUU |
|
| GAPDH | Sense |
AAGGCTGAGAATGGGAAAC | 254 |
|
| Antisense |
TTCAGGGACTTGTCATACTTC |
|
Cell invasion assay
Cell invasion was evaluated using 24-well
Transwell® culture chambers, as previously described
(17). Cells dissociated from
tumorspheres were seeded at a density of 5×104 cells per
well and cultured in stem cell medium (as mentioned above)
containing 2% fetal bovine serum (cat. no. 10082139; Thermo Fisher
Scientific, Inc.) for 24 h at 37°C in a humidified atmosphere
containing 5% CO2. Cells in the lower compartment were
then fixed in methanol and stained with 5% crystal violet for 10
min at room temperature. The cells were counted under a light
microscope. Three fields per sample were examined.
Statistical analysis
Data are shown as the mean ± standard error of the
mean. Comparisons of the groups were performed using analysis of
variance, Fisher's exact test or two-tailed Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of hWAPL and core stem cell
TFs in undifferentiated CCSCs
All cervical cancer tissue specimens from the
patients tested positive for HPV E6 expression, and the majority
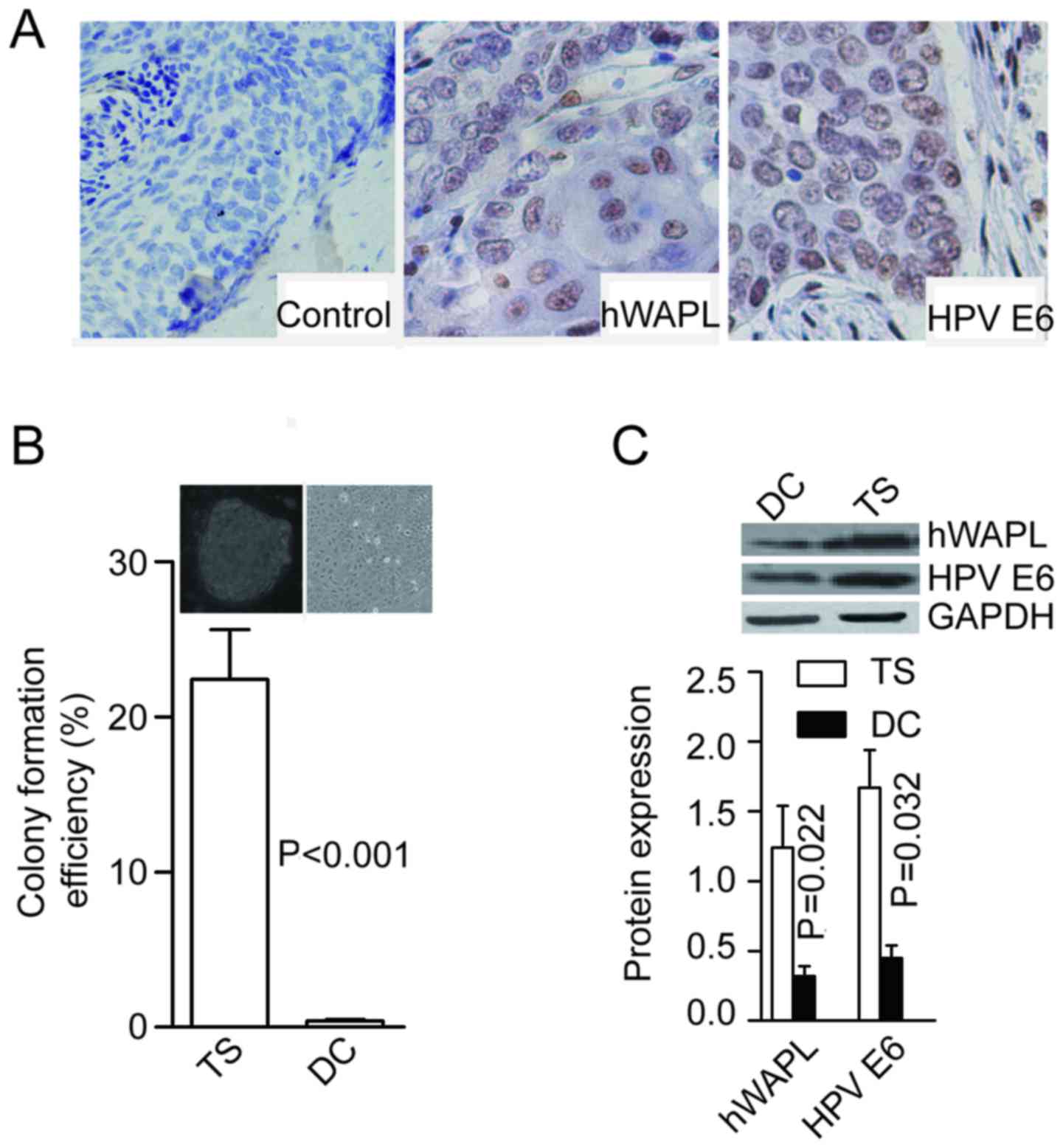
(15/17) were immunopositive for hWAPL (Fig. 1A). Following enzymatic dissociation
and culture in stem cell medium, a few colonies (tumorspheres)
formed in all of the tissues tested (17/17) after 2 weeks. Colonies
were formed at the rate of 21.53±2.64% for cells dissociated from
tumorspheres, and only 0.41±0.07% for cells dissociated from
differentiated tumorspheres (P<0.001; Fig. 1B). In addition, protein levels of
hWAPL (P=0.022) and HPV E6 (P=0.032) were significantly higher in
tumorspheres than in differentiated cells (Fig. 1C).
Knockdown of hWAPL induces tumorsphere
differentiation and HPV E6 downregulation
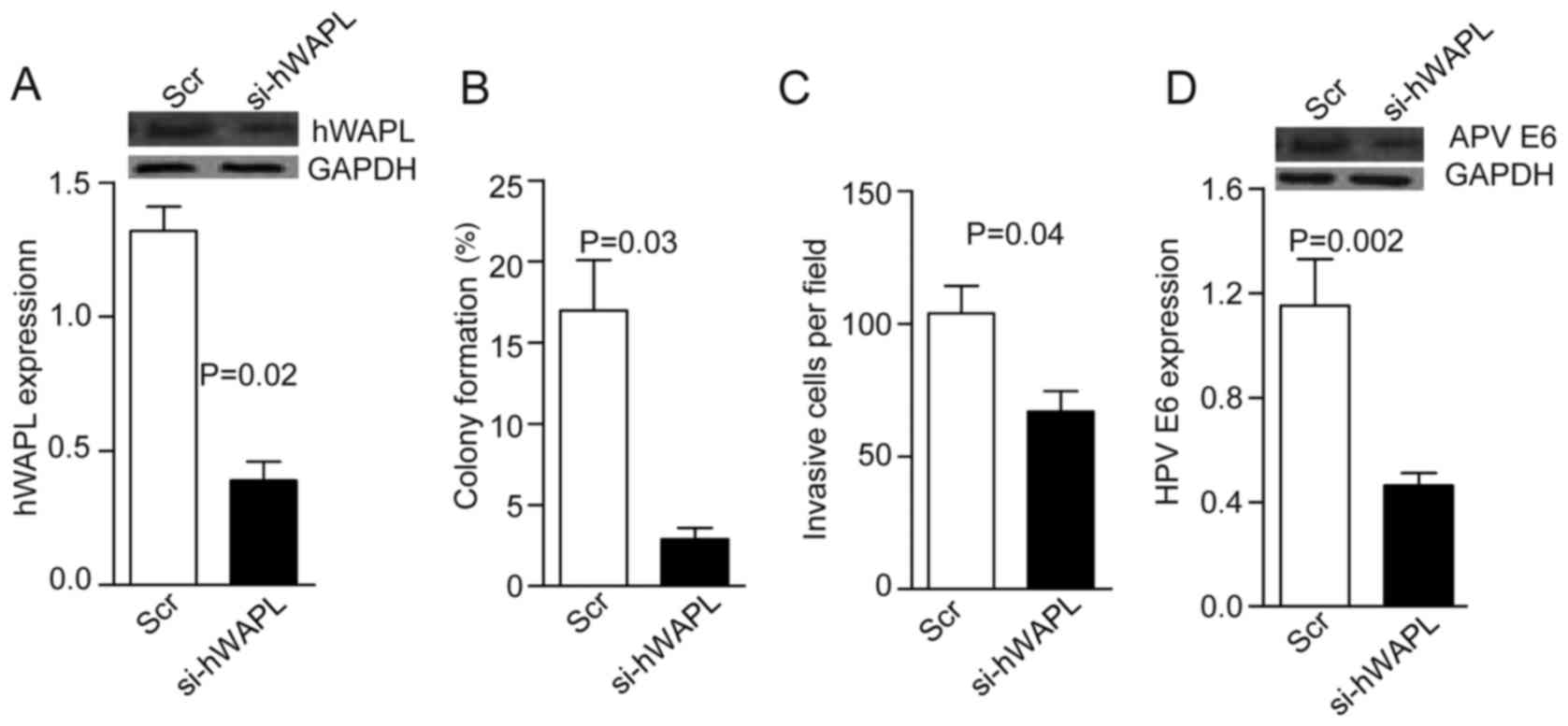
The role of hWAPL in cervical cancer was
investigated. TAT-mediated si-hWAPL was co-cultured with
tumorspheres for 30 min. After 24 h, western blot analyses showed
that hWAPL levels decreased by ~3-fold in the si-hWAPL group
compared with the controls (P=0.02; Fig.
2A). Notably, in addition to decreased tumorsphere formation
(P=0.03; Fig. 2B) and invasion
(P=0.04; Fig. 2C), HPV E6 expression
also decreased following the knockdown of hWAPL (P=0.002; Fig. 2D).
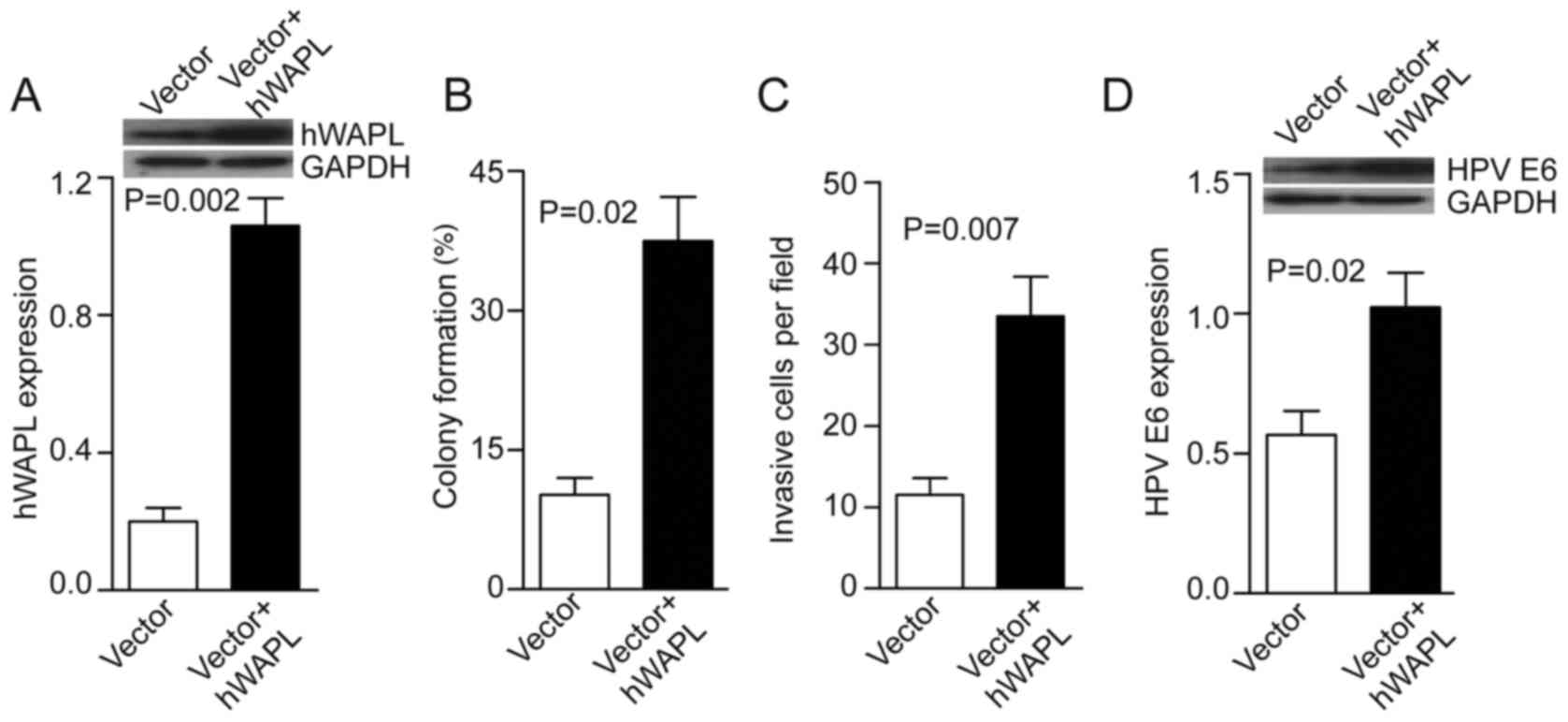
Overexpression of hWAPL promotes
tumorsphere tumorigenicity by increasing HPV E6 expression
Next, the tumorspheres were transfected with either
Ad-hWAPL (Ad-hWAPL-GFP) vector for overexpression of hWAPL, or its
control (Ad-scr-GFP). In the Ad-hWAPL transfected tumorspheres,
hWAPL expression levels were increased 4-fold compared with those
in the control (P=0.002; Fig. 3A),
colony formation (P=0.02; Fig. 3B)
and cell invasion (P=0.007; Fig. 3C)
were also increased. HPV E6 expression also increased following
hWAPL overexpression (P=0.023; Fig.
3D).
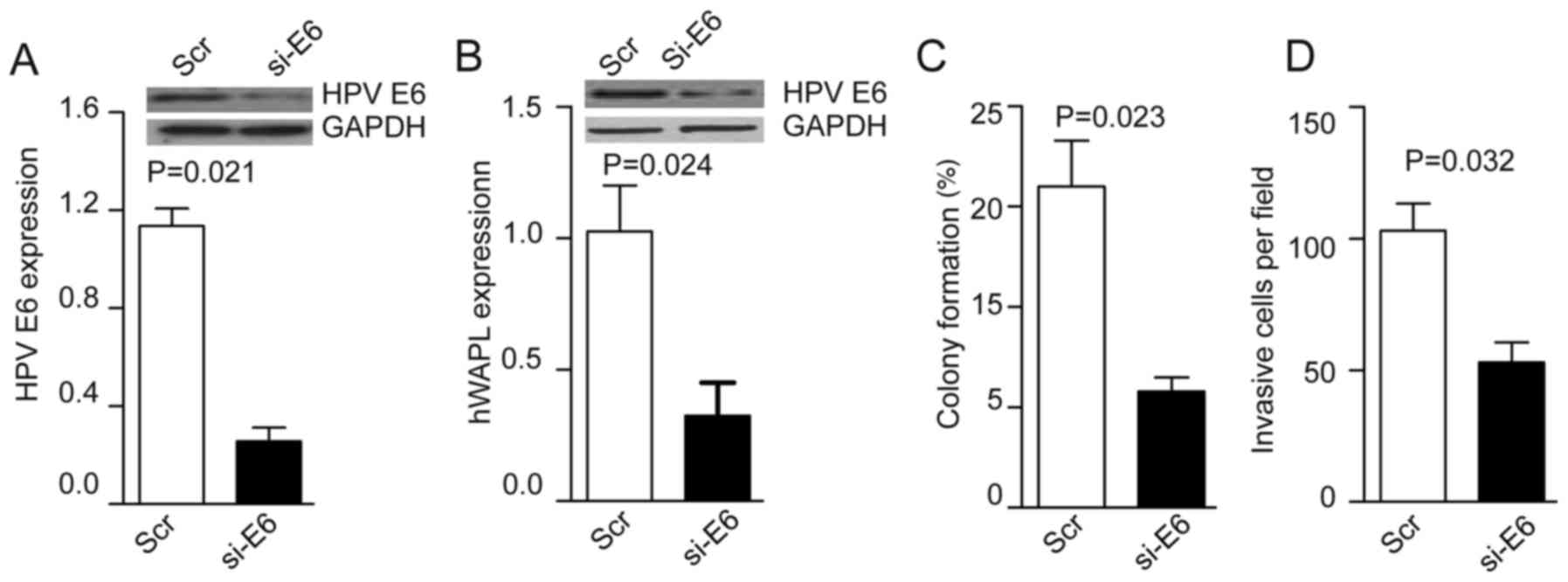
Knockdown of HPV E6 inhibits the
invasion and colony formation of CTs
Following the coculture of TAT-mediated si-HPV E6
with tumorspheres for 30 min, the levels of HPV E6 were decreased
~4-fold in the si-HPV E6 group compared with the control group
(P=0.021; Fig. 4A). HPV E6 knockdown
inhibited hWAPL expression (P=0.024; Fig. 4B), and also reduced colony formation
(P=0.023; Fig. 4C) and cell invasion
(P=0.032; Fig. 4D) compared with
that of control CTs.
TAT-mediated si-hWAPL inhibits the
growth of tumors derived from CTs
Tumor cells were dissociated from tumorspheres and
injected into null mice. The resulting tumors were visible or
palpable 2 weeks after injection. Subsequently, 100 µM/mg TAT/siRNA
or its control (Scr) were injected into tumors once per week. Mice
were sacrificed at day 28 and the tumor volume was measured. Tumors
in the TAT-mediated si-hWAPL group were smaller compared with those
in the Scr group (P=0.007; Fig. 5A),
and the weak expression of hWAPL and HPV E6 was detected after
si-hWAPL treatment (Fig. 5B). The
expression of hWAPL was decreased in the si-hWAPL group compared
with that in the control group, as determined by western blotting
(P=0.021; Fig. 5C). Moreover, in the
si-hWAPL group compared with the control group, colony formation
(P=0.005; Fig. 5D) and cell invasion
(P=0.015; Fig. 5E) was
decreased.
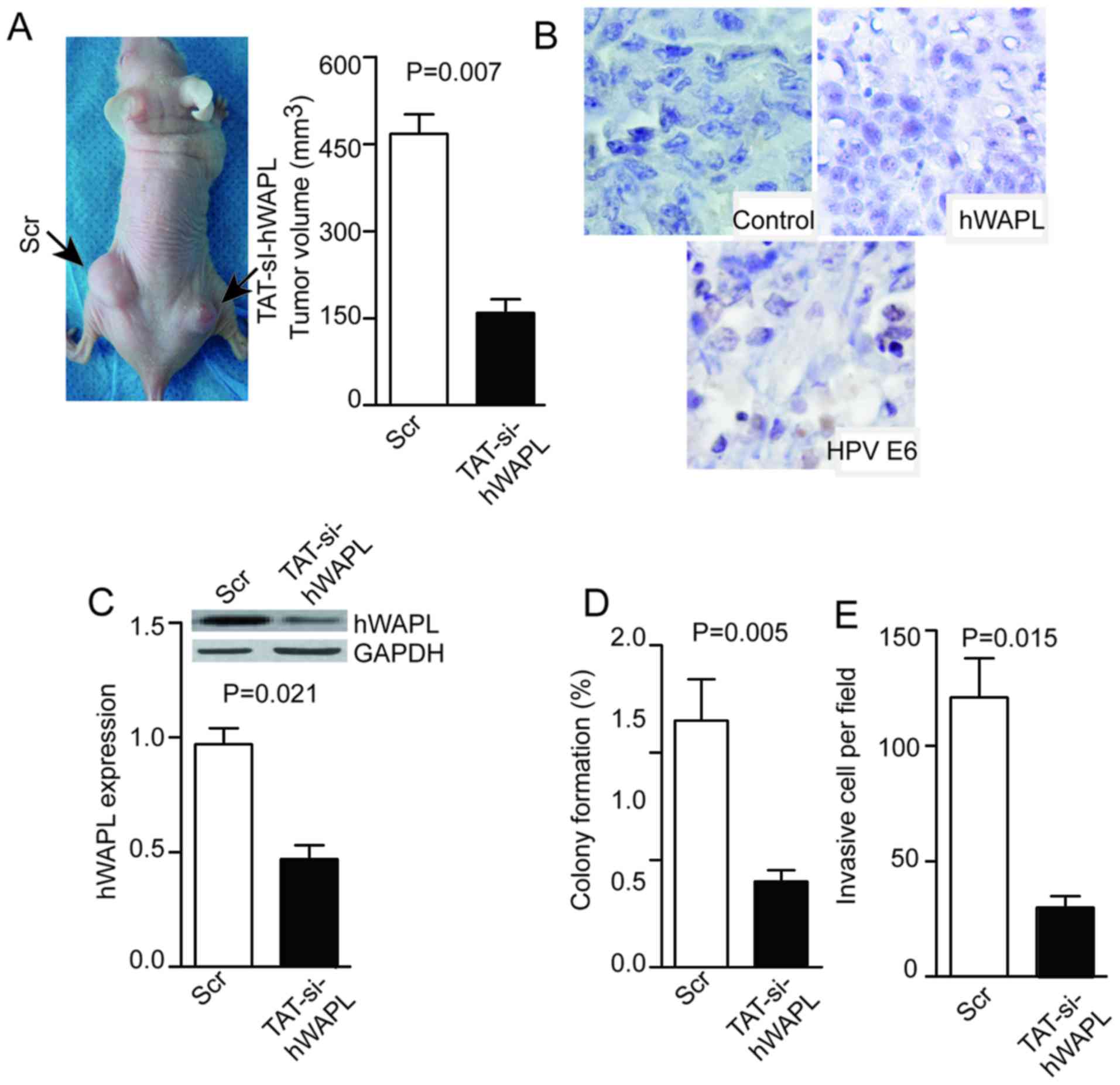 | Figure 5.Injection of TAT-si-hWAPL into
transplanted tumors inhibits tumor growth, hWAPL expression, colony
formation, and tumorsphere invasion. (A) Injection of TAT-si-hWAPL
into transplanted tumors inhibited tumor growth, (B) suppressed the
expression of hWAPL and HPV E6 as shown by immunohistochemistry
(magnification, ×400), (C) decreased hWAPL expression as detected
by western blot analysis and decreased (D) colony formation and (E)
cell invasion (n=3). hWAPL, human wings apart-like; HPV, human
papillomavirus; TAT-si-hWAPL, TAT-mediated short interfering RNA
against hWAPL; Scr, scrambled control. |
Discussion
Cervical cancer is caused by HPV infection (18). The HPV E6 oncoprotein induces
proteasome-dependent p53 degradation and inhibits expression of the
p53 tumor suppressor protein. The HPV E6 protein targets the
cellular E3 ubiquitin ligase E6AP to p53, resulting in the transfer
of ubiquitin peptides from E6AP to p53, which induces degradation
of p53 by the 26S proteasome (19).
Knockdown of HPV E6 has been shown to efficiently kill HPV-positive
cancer cells (20). In addition, it
has been reported that HPV E6 is selectively overexpressed in
CCSCs, and that the silencing of HPV E6 using siRNA abolishes CT
formation and induces tumorsphere re-differentiation (21). In the present study, it was observed
that HPV E6 was expressed in all the cervical cancer samples
tested, indicating that it plays an important role in the
maintenance of CCSC proliferation.
The WAPL gene was first identified in fruit flies
(22,23). The human WAPL (hWAPL) gene is
homologous in sequence to WAPL, is 30,793 base pairs in length, and
is located on chromosome 10q23.2. The hWAPL gene encodes an
aggregated anchored protein that disaggregates the polymerization
of chromosome arms in the early stage of mitosis (24). The hWAPL protein is highly expressed
in cervical cancer patients, and HPV E6 and E7 oncoproteins induce
hWAPL expression (25). Moreover,
HPV E6 is associated with cervical carcinogenesis, and as such, is
a therapeutic target for cervical cancer (26). Nevertheless, little is known about
the function of hWAPL in cervical cancer. The results of the
present study indicate the potential of hWAPL as a marker of CCSC
proliferation and suggest that hWAPL may play a role in maintaining
the proliferation potential of CCSCs. It has previously been
reported that HPV E6 induces hWAPL expression (25). In the present study, it was found
that hWAPL has a counteractive effect on HPV E6 expression,
indicating that HPV E6 and hWAPL interact in cervical
carcinogenesis.
Cell-penetrating peptides (CPPs), which are short
cationic polypeptides comprising ≤30 amino acids, have been used
for the intracellular delivery of various macromolecules (27). TAT and MPG proteins from HIV-1, as
well as penetratin and polyarginine, have been used as CPPs to
facilitate the intracellular delivery of proteins and nucleic acids
(28). TAT-CPPs enabled the safe and
effective delivery of siRNAs for the knockdown of hWAPL in CCSCs to
inhibit CCSC invasion and proliferation in the present study. In a
previous study, Zhang et al (29) constructed a peptide that was able to
deliver si-hWAPL to HeLa cervical cancer cells and successfully
reduced hWAPL expression in those cells. As an extension of that
previous study, the present study indicated that hWAPL interacts
with HPV E6 and may be a marker of CCSC proliferation. Notably, the
knockdown of hWAPL reduced cervical cancer proliferation,
metastasis and recurrence. However, the present study has certain
limitations. The experiments were only performed in mice, and
should be repeated in higher level species. In addition, immune
maintenance and whether the local injection has any effect on other
organs requires investigation in future studies.
Acknowledgements
This study was supported by the Natural Science
Foundation of Shanxi Province, China (grant no. 2013JM4012) and
Natural Science Foundation of Shiyan (grant no. 15Y38).
References
|
1
|
Li J, Kang LN and Qiao YL: Review of the
cervical cancer disease burden in mainland China. Asian Pac J
Cancer Prev. 12:1149–1153. 2011.PubMed/NCBI
|
|
2
|
Shepherd JH: Cervical cancer. Best Pract
Res Clin Obstet Gynaecol. 26:293–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi JF, Canfell K, Lew JB and Qiao YL: The
burden of cervical cancer in China: Synthesis of the evidence. Int
J Cancer. 130:641–652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yim EK and Park JS: The role of HPV E6 and
E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer
Res Treat. 37:319–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D, Mei H, Qi M, Yang D, Zhao X, Xiang
X, Pu J, Huang K, Zheng L and Tong Q: FOXD3 is a novel tumor
suppressor that affects growth, invasion, metastasis and
angiogenesis of neuroblastoma. Oncotarget. 4:2021–2044. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang HY, Lian P and Zheng PS: SOX9, a
potential tumor suppressor in cervical cancer, transactivates
p21WAF1/CIP1 and suppresses cervical tumor growth.
Oncotarget. 6:20711–20722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen L, Huang X, Xie X, Su J, Yuan J and
Chen X: High expression of SOX2 and OCT4 indicates radiation
resistance and an independent negative prognosis in cervical
squamous cell carcinoma. J Histochem Cytochem. 62:499–509. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji J, Wei X and Wang Y: Embryonic stem
cell markers Sox-2 and OCT4 expression and their correlation with
WNT signal pathway in cervical squamous cell carcinoma. Int J Clin
Exp Pathol. 7:2470–2476. 2014.PubMed/NCBI
|
|
9
|
Ding Y, Yu AQ, Li CL, Fang J, Zeng Y and
Li DS: TALEN-mediated Nanog disruption results in less
invasiveness, more chemosensitivity and reversal of EMT in HeLa
cells. Oncotarget. 5:8393–8401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang ML, Chiou SH and Wu CW: Targeting
cancer stem cells: Emerging role of Nanog transcription factor.
Onco Targets Ther. 6:1207–1220. 2013.PubMed/NCBI
|
|
11
|
Pei D: Regulation of pluripotency and
reprogramming by transcription factors. J Biol Chem. 284:3365–3369.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tyagi A, Vishnoi K, Mahata S, Verma G,
Srivastava Y, Masaldan S, Roy BG, Bharti AC and Das BC: Cervical
cancer stem cells selectively overexpress HPV oncoprotein E6 that
controls stemness and self renewal through upregulation of HES1.
Clin Cancer Res. 22:4170–4184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oikawa K, Ohbayashi T, Kiyono T, Nishi H,
Isaka K, Umezawa A, Kuroda M and Mukai K: Expression of a novel
human gene, human wings apart-like (hWAPL), is associated with
cervical carcinogenesis and tumor progression. Cancer Res.
64:3545–3549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oikawa K, Akiyoshi A, Tanaka M, Takanashi
M, Nishi H, Isaka K, Kiseki H, Idei T, Tsukahara Y, Hashimura N, et
al: Expression of various types of alternatively spliced WAPL
transcripts in human cervical epithelia. Gene. 423:57–62. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Gao Q, Wang J, Zhang X, Liu K and
Duan Z: Linc-RNA-RoR acts as a ‘sponge’ against mediation of the
differentiation of endometrial cancer stem cells by microRNA-145.
Gynecol Oncol. 133:333–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JY, Choi YS, Suh JS, Kwon YM, Yang VC,
Lee SJ, Chung CP and Park YJ: Cell-penetrating
chitosan/doxorubicin/TAT conjugates for efficient cancer therapy.
Int J Cancer. 128:2470–2480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Lv L, Xiao W, Gong C, Yin J, Wang D
and Sheng H: Leptin activates STAT3 and ERK1/2 pathways and induces
endometrial cancer cell proliferation. J Huazhong Univ Sci
Technolog Med Sci. 31:365–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beaudenon S and Huibregtse JM: HPV E6,
E6AP and cervical cancer. BMC Biochem. 9 Suppl 1:S42008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Butz K, Ristriani T, Hengstermann A, Denk
C, Scheffner M and Hoppe-Seyler F: siRNA targeting of the viral E6
oncogene efficiently kills human papillomavirus-positive cancer
cells. Oncogene. 22:5938–5945. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tyagi A, Vishnoi K, Mahata S, Verma G,
Srivastava Y, Masaldan S, Roy BG, Bharti AC and Das BC: Cervical
cancer stem cells selectively overexpress HPV oncoprotein E6 that
controls stemness and self-renewal through upregulation of HES1.
Clin Cancer Res. 22:4170–4184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verni F, Gandhi R, Goldberg ML and Gatti
M: Genetic and molecular analysis of wings apart-like (wapl), a
gene controlling heterochromatin organization in Drosophila
melanogaster. Genetics. 154:1693–1710. 2000.PubMed/NCBI
|
|
23
|
Dobie KW, Kennedy CD, Velasco VM, McGrath
TL, Weko J, Patterson RW and Karpen GH: Identification of
chromosome inheritance modifiers in Drosophila melanogaster.
Genetics. 157:1623–1637. 2001.PubMed/NCBI
|
|
24
|
Kueng S, Hegemann B, Peters BH, Lipp JJ,
Schleiffer A, Mechtler K and Peters JM: Wapl controls the dynamic
association of cohesin with chromatin. Cell. 127:955–967. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuroda M, Kiyono T, Oikawa K, Yoshida K
and Mukai K: The human papillomavirus E6 and E7 inducible oncogene,
hWAPL, exhibits potential as a therapeutic target. Br J Cancer.
92:290–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Abbad S, Zhang Z, Wang S, Zhou J
and Lv H: Cell-penetrating peptides for cancer-targeting therapy
and imaging. Curr Cancer Drug Targets. 15:337–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heitz F, Morris MC and Divita G: Twenty
years of cell-penetrating peptides: From molecular mechanisms to
therapeutics. Br J Pharmacol. 157:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Munyendo WL, Lv H, Benza-Ingoula H, Baraza
LD and Zhou J: Cell penetrating peptides in the delivery of
biopharmaceuticals. Biomolecules. 2:187–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Mao Y, Zhang F, Ye C, Tong H, Su
Y and Zhu J: The inhibitory effect of a new scFv/tP protein as
siRNA delivery system to target hWAPL in cervical carcinoma. Mol
Cell Biochem. 391:77–84. 2014. View Article : Google Scholar : PubMed/NCBI
|