Introduction
Acute kidney injury (AKI) is a particularly common
complication in cancer patients and associated with increased
mortality (1). It may occur as a
direct or indirect consequence of the cancer itself, its treatment
or associated complications (2,3). Among
the heterogeneous etiology, nephrotoxic AKI by chemotherapeutic
drugs is one of the most frequently encountered in malignancies
(4), whereas in rare cases, renal
infiltration in chronic myeloid leukemia (CML) has also been
reported (5). Under these
circumstances, renal hypoperfusion further jeopardizes the patients
to the development of AKI or more likely exacerbates it to full
acute renal failure (ARF) (3). On
the other hand, patients with AKI are predisposed to fluid
overload, which may also increase mortality (6). Maintenance of an appropriate fluid
balance is hence considered vital to the management of AKI in
cancer patients, in which AKI usually emerges in the context of
multiple organ dysfunction (7).
More recently, it has been shown that the presence
of an underlying cancer alone should no longer deny the critically
ill patients the chance of receiving continuous renal replacement
therapy (CRRT) or other advanced life-support measures (8,9).
Continuous venovenous hemodiafiltration (CVVHDF) with its dialysis
component is thought to be the best option to treat ARF in cancer
patients (10). CRRT, however, is
not a panacea without concern. For instance, one obvious potential
well-recognized hazard is overly aggressive fluid removal evoking
hypotension, renal hypoperfusion, prerenal azotemia, and de
novo renal injury or even ARF (11). An inaccurate volume assessment, if
left unattended, may thus lead to the inappropriate implementation
of therapy and the clinical consequence may be costly. Therefore,
careful assessment of the fluid status prior to the initiation of
and during CRRT is required. In this regard, impedance cardiography
(ICG) is a subtle and non-invasive method of evaluating hemodynamic
parameters (12) and ICG-guided
CVVHDF was of bona fide value in a previous study by our
group on the management of CML-associated ARF (5).
With increasingly complex cancer treatment
protocols, novel antineoplastic drugs and a growing emphasis on AKI
in cancer patients, onconephrology has evolved into a new field of
nephrology (13). It is an
intersection between nephrology and oncology, in which
nephrologists are required to be familiar with the epidemiology,
etiology and treatment of AKI in cancer patients. As such, this
concept of onconephrology was put into practice and the present
study reported on recent clinical experience in this novel
sub-specialty field of nephrology. The study also focused on three
rare cases of biopsy-confirmed AKI in patients with various types
of malignancy.
Materials and methods
Subjects
The clinical records of 117 cancer patients with AKI
that were either admitted or referred to the onconephrology unit
between February 2014 and December 2016 at Hebei General Hospital,
a tertiary medical center in Shijiazhuang (China), were reviewed.
They were found to have irresectable tumors and 93 (79.5%) received
chemotherapy. The onset of AKI was defined by the Kidney Disease:
Improving Global Outcomes criteria (14). At the first presentation, laboratory
tests were performed according to standard procedures, followed by
electrocardiogram, echocardiography, renal ultrasound and other
auxiliary examinations when deemed necessary. Patients with overt
congestive heart failure were excluded. The estimated glomerular
filtration rate (eGFR) was deduced from the modified version of the
Modification of Diet in Renal Disease (MDRD) equation for Chinese
individuals: eGFR=175 × Serum creatinine−1.234 ×
age−0.197 (×0.79 for females). A total of 120
age-matched ‘healthy’ controls were recruited later during annual
health examinations. The term ‘healthy’ was introduced for the
descriptive purpose against the existence of cancer. They went
through an extensive work-up, including hematological screening
tests, urinalysis, chest X-ray and the abovementioned examinations.
These subjects were non-diabetic without edema, free of cardiac and
renal diseases, devoid of health problems requiring immediate
medical attention and received voluntary referral ICG testing.
Other selection criteria were similar to those in a previous study
by our group (15). Data from these
healthy controls were used for establishing the baseline impedance
as well as the magnitude and duration of the change in pertinent
parameters. The study protocol was approved by the institutional
review board and written informed consent was obtained from all
participants or their authorized kin.
Etiology of AKI
The cause of AKI was estimated collectively by three
senior nephrologists. Depending upon the patient's condition and
consent, ultrasound-guided percutaneous renal biopsy was performed
under local anesthesia. The specimen was subjected to light,
fluorescent and electron microscopy examinations. Immunofixation
electrophoresis was used when multiple myeloma was suspected.
During the fixation phase, antiserum was inoculated with the serum
samples and the presence of monoclonal protein could be detected in
the form of precipitant (16).
CRRT and ICG
In addition to the symptom-oriented treatments, 79
patients accepted CRRT. It was performed with an Aquarius machine
(Aquarius; Edwards Lifesciences, Maurepas, France) using
polysulfone hemofilters (AV 600; Fresenius, Oberursel, Germany)
through a provisional venous catheter. Substitution solutions were
delivered pre-filter at a rate of 35 ml/kg/h. The ICG (BioZ,
CardioDynamics, CA, USA) was performed as previously described
(5,12) during CRRT (initiation, for 5 h
thereafter and cessation) and in the healthy controls. The
ultrafiltration was performed in a linear decreasing manner
(17) and adjusted according to the
ICG data, particularly the thoracic fluid content (TFC). To
evaluate the utility of ICG in hemodynamic monitoring during the
CRRT, the healthy controls and cancer patients were then stratified
according to two ICG parameters, the stroke index (SI) and TFC.
This generated a plot of four quadrants with the respective cut-off
point for the SI and TFC as 35 ml/m2 and 35
kOhm−1.
Statistical analysis
Statistical analyses were performed with the SPSS
package (version 19.0; IBM Corp., Armonk, NY, USA). Values are
expressed as the mean ± standard deviation. Significantly skewed
data were log-transformed prior to the analysis. Analysis of
variance with Bonferroni's post-hoc test, Student's t-test and the
χ2 test were performed to assess differences between
groups for continuous and categorical variables, respectively.
Correlations were examined using Pearson's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Epidemiology of AKI in cancer
patients
The clinical profiles of the participants were
listed in Table I. Comparing with
the healthy controls, the cancer patients were in worse condition
with a lower body mass index, hypotension, overt anemia, elevated
serum creatinine (Scr), decreased eGFR, hypoalbuminemia and
hypolipidemia. However, the Scr and eGFR were 106.1±37.8 µmol/l and
76.4±22.1 ml/min/1.73 m2, respectively, in the cancer
patients prior to the onset of AKI. On average, the increase of Scr
was 1.68±0.38-fold within 10.2±5.7 days. Sites of primary
malignancy are presented in Table
II with the digestive, respiratory and hematological systems as
the top three sites. As illustrated in Table III, the causes of AKI were diverse
and the leading ones were nephrotoxic agents, hypotension (grouped
with extracellular dehydration) and obstructive nephropathy.
 | Table I.Clinical profiles between the healthy
controls and cancer patients with acute kidney injury. |
Table I.
Clinical profiles between the healthy
controls and cancer patients with acute kidney injury.
| Parameter | Healthy controls | Cancer patients |
|---|
| Subjects | 120 | 117 |
| Age (years) | 60.6±11.9 | 61.7±10.5 |
| Gender
(male/female) | 80/40 | 76/41 |
| Body mass index
(kg/m2) | 22.7±3.2 | 18.9±2.7a |
| Systolic blood
pressure (mmHg) | 132.7±13.3 |
112.1±20.8a |
| Diastolic blood
pressure (mmHg) | 79.0±12.5 |
63.1±15.2a |
| White blood cell
count (×109/l) | 7.3±2.1 | 8.5±3.7 |
| Neutrophils (%) | 69.7±10.6 | 76.7±23.1 |
| Hemoglobin
concentration (g/l) | 136.7±16.2 |
89.3±14.5a |
| Glutamic pyruvic
transaminase (IU)b | 22.6±10.0 | 20.1±14.0 |
| Serum creatinine
(µmol/l)b | 79.8±22.3 |
179.3±38.4a |
| eGFR (ml/min/1.73
m2) | 109.3±10.5 |
35.0±21.2a |
| Serum potassium
(mmol/l) | 4.1±1.2 | 5.0±0.9 |
| Fasting plasma
glucose (mg/dl)b | 95.9±15.0 | 87.2±16.4 |
| Albumin (g/l) | 45.3±4.5 |
25.9±5.3a |
| Total cholesterol
(mmol/l) | 6.74±1.43 |
3.49±1.00a |
| Triglycerides
(mmol/l)b | 2.78±1.20 |
1.37±0.81a |
| Fibrinogen
(g/l)b | 3.39±0.78 | 4.21±0.98 |
 | Table II.Types of primary malignancy within
the cohort. |
Table II.
Types of primary malignancy within
the cohort.
| Site/type of
malignancy | n (%) |
|---|
| Digestive
system | 51 (43.5) |
| Respiratory
system | 29 (24.8) |
| Hematology | 15 (12.9) |
| Gynaecology | 10 (8.5) |
| Urology | 9 (7.7) |
| Miscellaneous | 3 (2.6) |
| Total | 117 (100) |
 | Table III.Causes of acute kidney injury in the
cancer patients. |
Table III.
Causes of acute kidney injury in the
cancer patients.
| Cause | n (%) |
|---|
| Prerenal |
|
|
Capillary leak syndrome
(interleukin-2) | 1 (0.86) |
| Drugs
(ACEI, NSAIDs) | 6 (5.13) |
|
Extracellular dehydration
(diarrhea and vomiting) | 10 (8.55) |
|
Hepatorenal syndrome | 1 (0.86) |
|
Hypotension | 21 (17.95) |
|
Sepsis | 6 (5.13) |
| Renal |
|
|
Hypercalcemia | 2 (1.71) |
|
Intravascular hemolysis | 1 (0.86) |
| Myeloma
kidney | 5 (4.27) |
|
Nephrotoxic agents | 45 (38.46) |
|
Thrombotic
microangiopathy | 2 (1.71) |
| Tumor
infiltration | 3 (2.56) |
| Tumor
lysis syndrome | 3 (2.56) |
| Postrenal |
|
|
Ureteral or bladder outlet
occlusion | 11 (9.4) |
| Total | 117 (100) |
Hemodynamic features prior to
CRRT
Compared with the healthy controls (Table IV), the pre-CRRT data of the
patients presented a significantly higher heart rate, system
vascular resistance index (SVRI), TFC and systolic time ratio
(STR). Furthermore, markedly lower blood pressures, cardiac index
(CI), SI and velocity index (VI) were recorded. The markedly
decreased SI, VI and prolonged STR may indicate compromised cardiac
function of myocardial origin.
 | Table IV.Hemodynamic parameters by impedance
cardiography in healthy controls and cancer patients. |
Table IV.
Hemodynamic parameters by impedance
cardiography in healthy controls and cancer patients.
| Parameter | Healthy
controls | Pre-CRRT | 5 h of CRRT | Post-CRRT | Normal range |
|---|
| n | 80 | 79 | 79 | 79 | – |
| HR (beats/min) | 76.1±15.3 |
89.2±13.4a | 85.8±11.5 | 83.1±10.1 | 58–86 |
| SBP (mmHg) | 136.0±21.8 |
110.6±23.1a | 114.6±18.1 |
123.6±17.7b | 100–140 |
| DBP (mmHg) | 77.6±12.2 |
61.5±17.3a | 64.3±12.5 |
73.4±10.5b | 60–90 |
| CI
(l/min/m2) | 3.02±0.57 |
2.30±0.61a | 2.58±0.46 |
2.87±0.55b | 2.5–4.2 |
| SI
(ml/m2) | 47.16±8.93 |
25.1±7.95a |
30.0±7.2b |
36.1±7.50b | 35–65 |
| SVRI
(dyne.sec/cm5/m2) | 1,573.5±332.3 |
2,934.8±908.8a |
2,252.5±733.0b |
1,901.3±401.6b | 1,337–2,483 |
| TFC (/kΩ) | 35.30±7.71 |
53.5±11.9a | 49.5±19.8 |
41.7±11.5b | 30.0–50.0 |
| VI (/1,000
sec) | 47.62±12.76 |
28.7±5.3a | 30.2±10.1 |
35.8±7.8b | 33–65 |
| STR | 0.33±0.08 |
0.49±0.11a | 0.45±0.13 | 0.38±0.17 | 0.30–0.50 |
Evaluation of CRRT
CRRT was performed with individualized
ultrafiltration prescription in the light of the ICG data. Changes
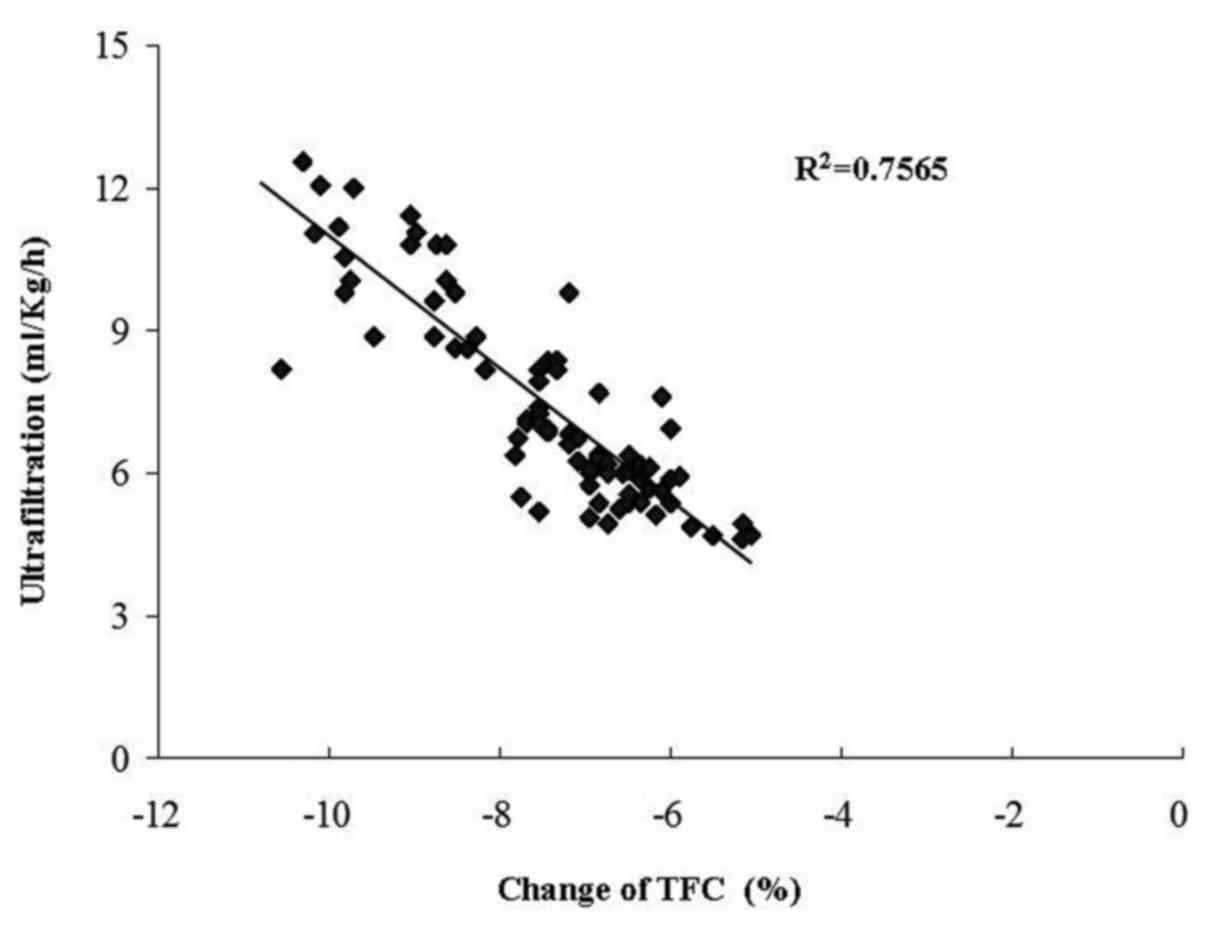
in the TFC were inversely correlated with the ultrafiltration rate
(r=0.87, P<0.01; Fig. 1). In
patients with increased TFC, fluid was removed accordingly, while
the other parameters were concurrently monitored. SI was
significantly increased and SVRI decreased at 5 h after the
initiation of CRRT. At the end of this procedure, the patients
presented with major improvements, including increased systolic and
diastolic blood pressure, CI, SI and VI, as well as reduced TFC and
SVRI. Similarly, the central venous pressure was significantly
decreased from 17.0±2.9 to 11.0±4.1 mmH2O. The efficacy
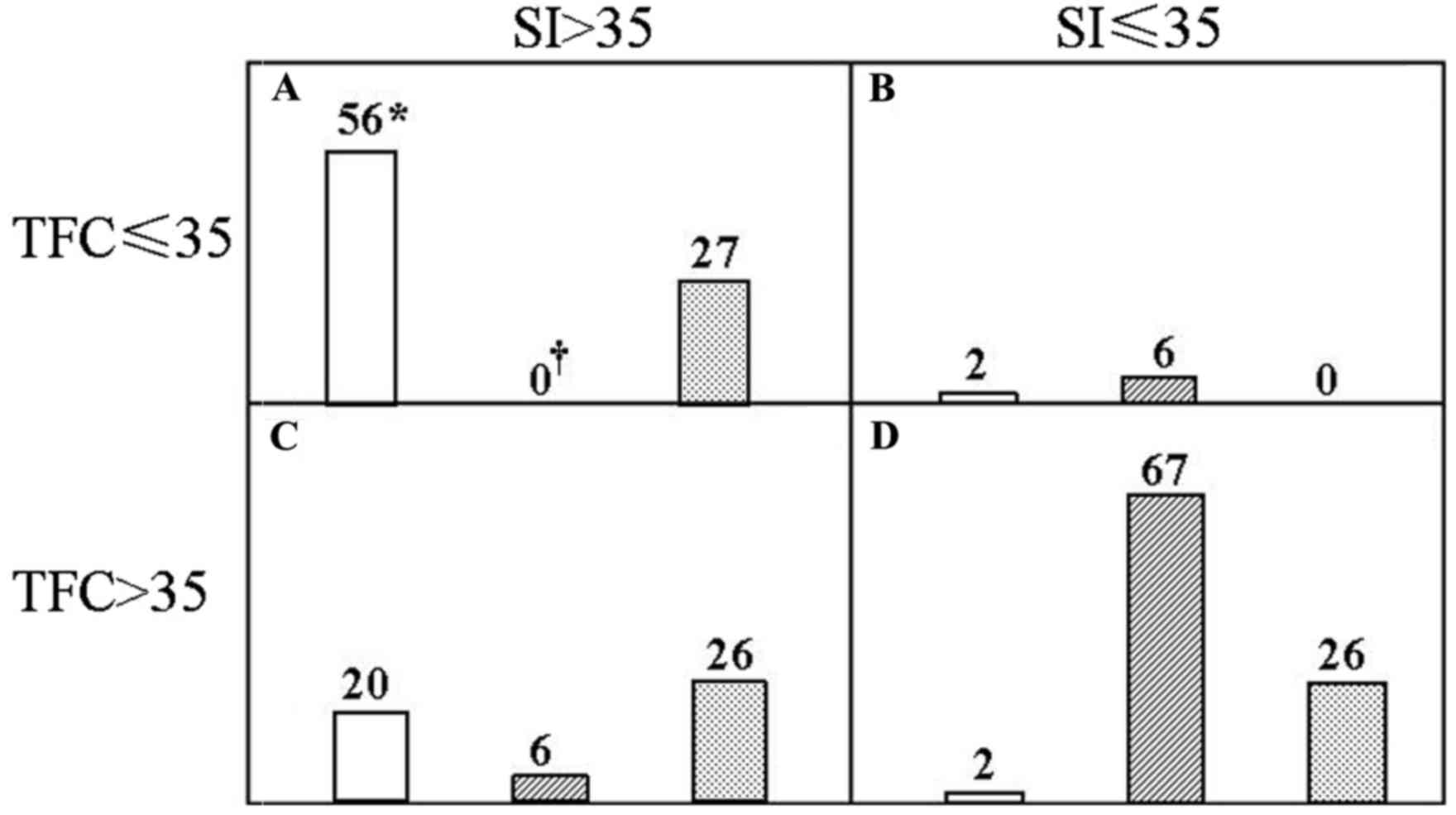
of the CRRT was then validated in Fig.
2. An SI>35 ml/m2 and TFC≤35 kOhm−1
was denoted as a hemodynamically stable group (Fig. 2A) and vice versa for the
hemodynamically unstable one (Fig.
2D). The remaining two groups were deemed to have an
intermediate risk (Fig. 2B and C).
In total, 70.0% of the healthy controls, 0% of the cancer patients
prior to CRRT and 34.2% of them after the CRRT were in the stable
group, whereas the corresponding rates were 2.5, 84.8 and 32.9% in
the unstable group, respectively. Of note, the differences between
any two groups were significant among the control, pre-and
post-CRRT values.
Therapy-induced thrombotic
microangiopathy (TMA)
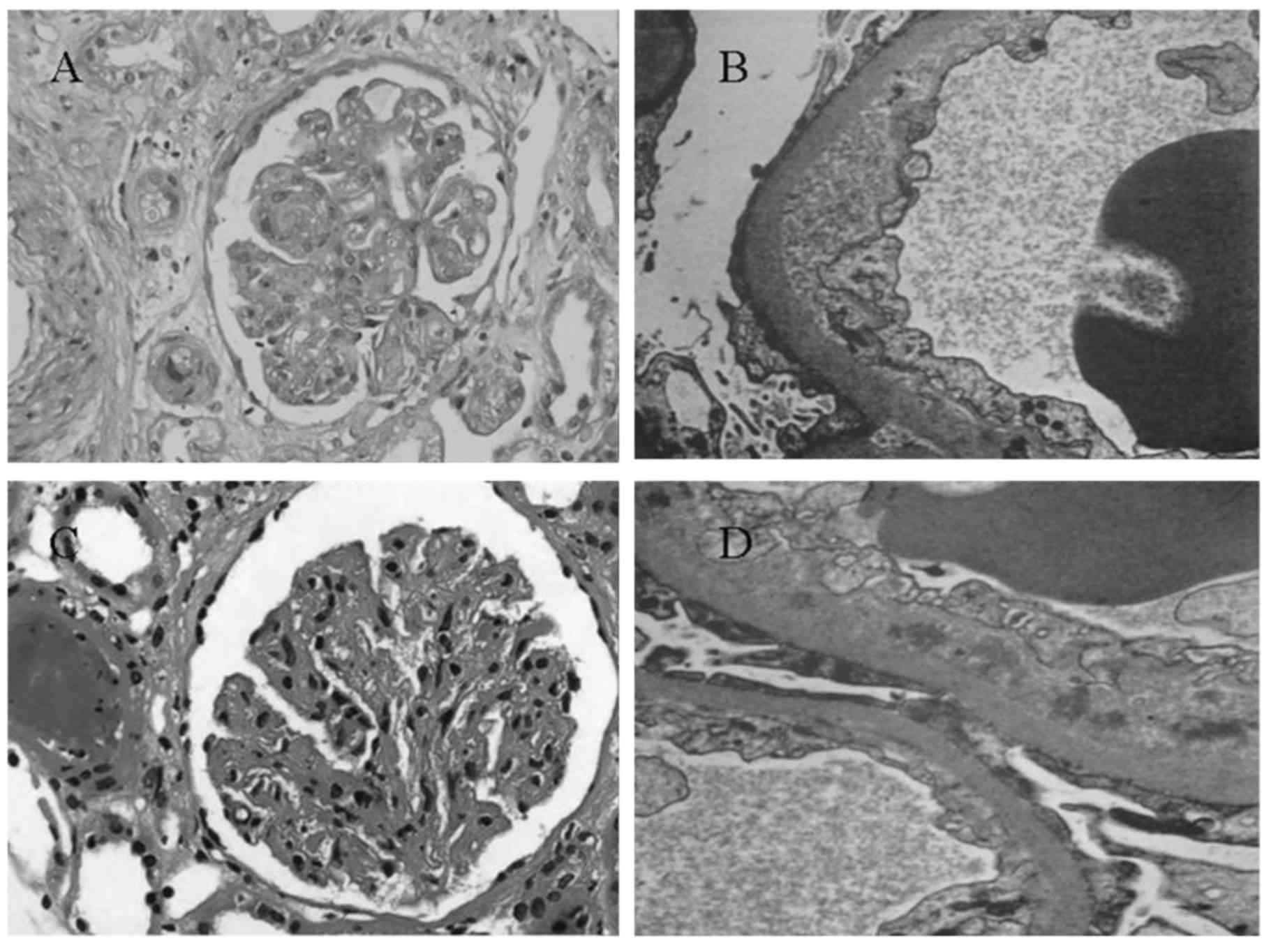
TMA occurred in a patient with chronic myeloid
leukemia at the end of 1 year of interfon-α therapy (Fig. 3A and B) and in a patient with gastric
mesothelioma at the end of 6 months of sunitinib malate treatment
(Fig. 3C and D). In each of the two
cases, diffuse and severe endothelial damage with double contours
and occlusion of capillary lumen was observed. Nodular expansion of
mesangium was also seen (Fig. 3A and
C). Each of the two patients was negative on immunofluorescence
analysis (data not shown) and electron microscopy of the glomerular
capillary wall revealed expansion of the subendothelial space by
electron-lucent debris (Fig. 3B and
D).
Cast nephropathy in immunoglobulin D
myeloma
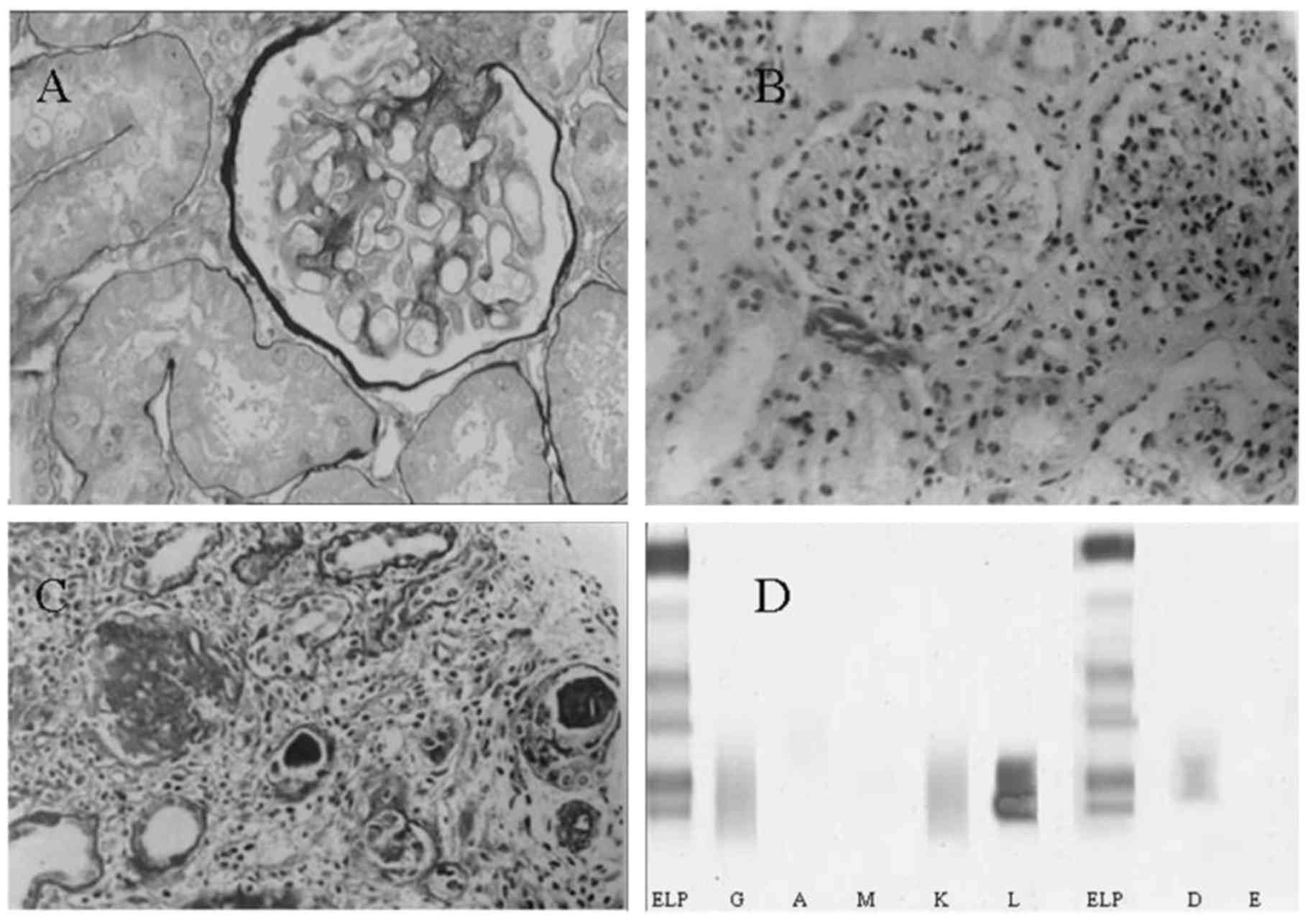
Analysis of bone marrow aspirate revealed 12.5% of
plasma cells in a patient referred to us whom we later diagnosed
with multiple myeloma. Based on the first biopsy he received
elsewhere 3 months earlier, the patient was initially considered to
have an ischemic lesion (Fig. 4A).
The second biopsy was performed at our hospital and cast
nephropathy was identified (Fig. 4B and
C). Immunofixation electrophoresis then clearly detected
immunoglobulin (Ig) D and light chain λ precipitation (Fig. 4D).
Discussion
The present study identified that cancer patients
may develop AKI when presenting with a decrease in Scr within a
short period and a deterioration of the general condition is
thought to enhance the susceptibility (4). The altered ICG parameters further
revealed that AKI was accompanied by circulatory detriments, which
improved substantially after CRRT. In addition, a change of TFC
during CRRT was significantly correlated with the ultrafiltration
rate. Furthermore, AKI was more likely in patients with a
malignancy of the digestive, respiratory and hematological system,
in general agreement with a previous study reporting that patients
with liver cancer and multiple myeloma were more susceptible to AKI
(18).
The onset of AKI in cancer patients greatly
increases the short-term risk of mortality (13). Furthermore, delayed nephrology
consultation of patients with ARF is also associated with increased
mortality (19). At the same time,
there is a paucity of information regarding the non-invasive
hemodynamic monitoring of cancer patients with renal complications,
particularly those requiring CRRT. The onconephrology unit, with
higher competence in oncology and nephrology, may be called upon to
services as recently advocated by Thajudeen and Salahudeen
(20). In this respect, the present
study may be of interest to clinicians in general and nephrologists
in particular.
Cancer patients with AKI are often hemodynamically
unstable (10). This was proved by
the different ICG data between the healthy controls and the cancer
patients. Dynamic and vigilant monitoring of blood volume is thus
conceivably imperative. The ICG could readily meet this requirement
by yielding a broad panel of parameters that reflect blood flow,
systemic vascular resistance, myocardial contractility and fluid
status. While the empirical assessment of ultrafiltration using the
dry weight is error-prone with a deviation of ~50% (21) and detection of non-specific symptoms
via small undulations of volume is far less sensitive, the good
correlation of the changes in TFC with the ultrafiltration rate
allows for ‘fine-tuning’ the latter during CRRT. Combined with the
SI, it may also be used for evaluating the outcomes of CRRT. After
CRRT, the stable, intermediate and unstable groups each accounted
for one third of the cancer patients. Their risk features indicated
that these groups may respectively require close monitoring,
further volume removal and extra CRRT sessions with possible
isotropic agents. These combined analyses were hence prognostically
more powerful and discriminating than any clinical assessment
considered alone. Careful interpretation of the data during CRRT
may allow the clinician to detect the emergence of potentially
adverse hemodynamic fluctuations, particularly during periods of
apparent clinical stability, and optimize the ultrafiltration
prescription.
A better understanding of the etiology of AKI in
cancer patients may help expedite the diagnosis and treatment. The
mechanisms of AKI in cancer are diverse in origin: Infiltration
(22) and urinary tract obstruction
(23) due to the cancer per
se; acute tumor lysis syndrome (24) and nephrotoxicity (25) caused by the treatment; sepsis
(2) and hypercalcemia (26) associated with severe complications.
With rapid advances in cancer therapy, chemotherapy-induced AKI has
drawn increasing attention (4).
Indeed, AKI may occur in up to 30% of cancer patients receiving
cisplatin (27). The diversity in
the etiology was exemplified in the cases of the present study. Of
note, chemotherapy was not only the leading cause of AKI in the
cohort of the present study, but was observed in nearly one half of
the patients who received anticancer treatment (45 out of 93). This
high prevalence may be due to the fact that a lesser amount of
patients without AKI were followed up. As modern oncology has been
rapidly turning into a multidisciplinary service, clinicians should
be familiar with these cancer-associated aspects prior to making
appropriate prophylactic and therapeutic decisions.
The present study observed three biopsy-confirmed
rare cases: TMA in a patient with CML taking interfon-α and in a
patient with gastric mesothelioma taking sunitinib malate. The
mechanisms by which interfon-α induced TMA remain elusive but are
thought to be an immune-mediated process (28). It is noteworthy that the underlying
CML may also be contributory, since chronic hepatitis C patients
receiving interfon-α seldom develop TMA. Sunitinib malate is a
novel tyrosine kinase inhibitor that simultaneously abrogates the
function of nitric oxide synthetase and vascular endothelial growth
factor (VEGF). The action of VEGF-targeted therapy may impair the
integrity of renal endothelium and lead to the resultant TMA. In
both cases, double contours and nodular expansion of mesangium may
be seen, indicating rather chronic changes. Therefore, close
monitoring of renal function should be mandatory, particularly in
patients on long-term inferton-α use or VEGF inhibition.
AKI caused by lymphomatous infiltration of the
kidneys, cast nephropathy in multiple myeloma or tumor lysis
syndrome are unique to the cancer population. IgD multiple myeloma
represents ~2% of multiple myeloma cases with an incidence of renal
involvement of 70% (29). By
contrast, these numbers in IgG multiple myeloma are 59 and 15%,
respectively. Patients are usually younger than those with IgG or
IgA myeloma and are more likely to present with nonspecific
systemic symptoms, a more rapid progressive clinical course, a
higher rate of extramedullary involvement and shorter survival time
(30,31). Unfortunately, the majority of
clinical laboratories do not include IgD in the initial workup of
monoclonal gammopathies (32). It is
prudent for clinicians to test for IgD and IgE when cast
nephropathy is suspected or serum light chain levels demonstrate a
pronounced elevation without a corresponding up-shift of monoclonal
IgG, IgA and IgM, as in the case of the present study.
In conclusion, the present study highlighted the
fact that AKI in cancer patients may have different underlying
mechanisms, manifest as a discrepant volume status and require
distinct fluid therapy. As such, the introduction of ICG may
improve the safety and efficacy of CRRT in these patients,
particularly those with a cardiac comorbidity. The establishment of
onconephrology may assuredly answer the challenges of expeditious
diagnosis and effective management of cancer patients with AKI.
Acknowledgements
The authors thank Iain Forem (Bournemouth, UK) for
English language editing. This study was supported by a grant (no.
20170315) from the Health and Family Planning Commission of Hebei
Province.
References
|
1
|
Lameier NH, Flombaum CD, Moreau D and
Ronco C: Acute renal failure in cancer patients. Ann Med. 37:13–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finkel KW and Foringer JR: Renal disease
in patients with cancer. Nat Clin Pract Nephrol. 3:669–678. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Humphreys BD, Soiffer RJ and Magee CC:
Renal failure associated with cancer and its treatment: An update.
J Am Soc Nephrol. 16:151–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perazella MA and Moeckel GW:
Nephrotoxicity from chemotherapeutic agents: Clinical
manifestations, pathobiology, and prevention/therapy. Semin
Nephrol. 30:570–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang T, Ma HC, Bai YL, Zhang JX and Xu JS:
Continuous venovenous hemodiafiltration, impedance cardiography and
critical care nephrology: A case study of chronic myeloid
leukemia-associated acute renal failure. Crit Care Shock. 14:19–23.
2011.
|
|
6
|
Yerram P, Karuparthi PR and Misra M: Fluid
overload and acute kidney injury. Hemodial Int. 14:348–354. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soares M, Salluh JI, Carvalho MS, Darmon
M, Ronco JR and Spector N: Prognosis of critically ill patients
with cancer and acute renal dysfunction. J Clin Oncol.
24:4003–4010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benoit DD, Hoste EA, Depuydt PO, Offner
FC, Lameire NH, Vandewoude KH, Dhondt AW, Noens LA and Decruyenaere
JM: Outcome in critically ill medical patients treated with renal
replacement therapy for acute renal failure: Comparison between
patients with and those without haematological malignancies.
Nephrol Dial Transplant. 20:552–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darmon M, Thiery G, Ciroldi M, Porcher R,
Schlemmer B and Azoulay E: Should dialysis be offered to cancer
patients with acute kidney injury? Intensive Care Med. 33:765–772.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berghmans T, Meert AP, Markiewicz E and
Sculier JP: Continuous venovenous haemofiltration in cancer
patients with renal failure: A single-centre experience. Support
Care Cancer. 12:306–311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kazory A and Ross EA: Contemporary trends
in the pharmacological and extracorporeal management of heart
failure: A nephrologic perspective. Circulation. 117:975–983. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Packer M, Abraham WT, Mehra MR, Yancy CW,
Lawless CE, Mitchell JE, Smart FW, Bijou R, O'Connor CM, Massie BM,
et al: Utility of impedance cardiography for the identification of
short-term risk of clinical decompensation in stable patients with
chronic heart failure. J Am Coll Cardiol. 47:2245–2252. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salahudeen AK and Bonventre JV:
Onconephrology: The latest frontier in the war against kidney
disease. J Am Soc Nephrol. 24:26–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kidney Disease: Improving Global Outcomes
(KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice
guideline for acute kidney injury. Kidney Int Suppl. 2:19–36.
2012.
|
|
15
|
Wang T, Zhang Y, Ma J, Feng Z, Niu K and
Liu B: Additive effect of polymorphisms in the β2-adrenoceptor and
NADPH oxidase p22 phox genes contributes to the loss of estimated
glomerular filtration rate in Chinese. Clin Exp Pharmacol Physiol.
41:657–662. 2014.PubMed/NCBI
|
|
16
|
Dimopoulos M, Kyle R, Fermand JP, Rajkumar
SV, San Miguel J, Chanan-Khan A, Ludwig H, Joshua D, Mehta J, Gertz
M, et al: Consensus recommendations for standard investigative
workup: Report of the international myeloma workshop consensus
panel 3. Blood. 117:4701–4705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donauer J, Kölblin D, Bek M, Krause A and
Böhler J: Ultrafiltration profiling and measurement of relative
blood volume as strategies to reduce hemodialysis-related side
effects. Am J Kidney Dis. 36:115–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Christiansen CF, Johansen MB, Langeberg
WJ, Fryzek JP and Sørensen HT: Incidence of acute kidney injury in
cancer patients: A Danish population-based cohort study. Eur J
Intern Med. 22:399–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehta RL, McDonald B, Gabbai F, Pahl M,
Farkas A, Pascual MT, Zhuang S, Kaplan RM and Chertow GM:
Nephrology consultation in acute renal failure: Does timing matter?
Am J Med. 113:456–461. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thajudeen B and Salahudeen AK: Role of
tolvaptan in the management of hyponatremia in patients with lung
and other cancers: Current data and future perspectives. Cancer
Manag Res. 8:105–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jaeger JQ and Mehta RL: Assessment of dry
weight in hemodialysis: An overview. J Am Soc Nephrol. 10:392–403.
1999.PubMed/NCBI
|
|
22
|
Seo-Mayer P, Kenney B, McNamara J, Stein J
and Moeckel GW: Hematuria and decreased kidney function as initial
signs of acute B-cell lymphoblastic leukemia. Am J Kidney Dis.
56:1001–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Segal A: A case of acute kidney injury due
to complex, partial, multifocal ureteral strictures. Nat Clin Pract
Nephrol. 4:102–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perazella MA: Renal vulnerability to drug
toxicity. Clin J Am Soc Nephrol. 4:1275–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller RP, Tadagavadi RK, Ramesh G and
Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins (Basel).
2:2490–2518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Jonge MJ and Verweij J: Renal
toxicities of chemotherapy. Semin Oncol. 33:68–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Badid C, McGregor B, Faivre JM, Guerard A,
Juillard L, Fouque D and Laville M: Renal thrombotic
microangiography induced by interfon-alpha. Nephrol Dial
Transplant. 16:846–848. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kyle RA, Remstein ED, Therneau TM,
Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, Plevak MF,
Jelinek DF, Fonseca R, et al: Clinical course and prognosis of
smoldering (asymptomatic) multiple myeloma. N Engl J Med.
356:2582–2590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jancelewicz Z, Takatsuki K, Sugai S and
Pruzanski W: IgD multiple myeloma: review of 133 cases. Arch Intern
Med. 135:87–93. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimamoto Y, Anami Y and Yamaguchi M: A
new risk grouping for IgD myeloma based on analysis of 165 Japanese
patients. Eur J Haematol. 47:262–267. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sinclair D and Cranfield T: IgD myeloma: A
potential missed diagnosis. Ann Clin Biochem. 38:564–565. 2001.
View Article : Google Scholar : PubMed/NCBI
|