Introduction
Lung cancer is prevalent worldwide and is the
leading cause of cancer-related mortality (1). Non-small cell lung cancer (NSCLC)
accounts for >80% of all lung cancer cases (1–3).
Furthermore, the prognosis of NSCLC is poor, with a 5-year survival
rate of <15% (4). Meanwhile,
>10% of patients with NSCLC are associated with epidermal growth
factor receptor (EGFR) mutations (5).
Gemcitabine (GEMZ) is the first-line therapy against
NSCLC that has been used for the past 10 years. Previous studies
have demonstrated that it functions against NSCLC as a single agent
(6,7). Furthermore, studies have focused on
investigating the potential effect of agent combination with GEMZ
to enhance the anticancer efficacy in NSCLC patients (8,9).
However, cellular drug resistance is a major issue that often
limits the efficacy of GEMZ chemotherapy (10). Therefore, an increased understanding
of target therapy has revealed numerous potential therapeutic
strategies, including combined GEMZ with carboplatin against NSCLC
(11), combined GEMZ with abraxane
against NSCLC (12) and combined
piceatannol with GEMZ against NSCLC (10).
Trichosanthin (TCS) is a traditional Chinese
medicine that is isolated from the root tuber of Trichosanthes
kirilowii Maxim (13). It was
reported that TCS has various pharmacological and physiological
effects, including an anti-human influenza virus enzyme, inhibition
of protein synthesis, neurotoxicity and anti-tumor activity
(14–19). Additional studies have demonstrated
that TCS may regulate numerous signaling pathways, such as
mitogen-activated protein kinase family proteins, including p38,
extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinases, proapoptotic protein B-cell lymphoma 2; and
inflammation-related factors, including nuclear factor-κB (NF-κB),
inhibitor of NF-κB, and cyclooxygenase-2 (20–23).
Furthermore, TCS also possesses antitumor activity against the
NSCLC A549 cell line (24).
The purpose of the present study was to investigate
whether TCS could increase the antitumor effect and decrease drug
resistance of GEMZ in NSCLC. A549 human NSCLC cells were used as a
model and treated with a combination of GEMZ and TCS to assess cell
viability and apoptosis, and to understand the underlying molecular
mechanism.
Materials and methods
Reagents
TCS and GEMZ were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany) with a purity of >98%. Both
drugs were dissolved in dimethylsulfoxide (DMSO) and diluted by
Dulbecco's modified Eagles medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and the final concentration of
DMSO was kept <0.05% in the cell culture in order to have no
detectable effects on cell growth and viability. MTT was purchased
from Sigma-Aldrich (Merck KGaA). Insulin-like growth factor I
(IGF1) was purchased from R&D Systems, Inc. (Minneapolis, MN,
USA). All chemical reagents in the present study were of analytical
reagent grade.
Cells and culture
A549 cells were purchased from American Type Culture
Collection (Manassas, VA, USA). The cells were cultured in DMEM
supplemented with 10% fetal bovine serum, penicillin
(105 U/l) and streptomycin (100 mg/l) (Hyclone, Logan,
UT, USA) in a humidified atmosphere of 37°C containing 5%
CO2.
MTT cell viability assay
A549 cells were plated at a density of
1×104 cells/well into 96-well cell culture plates
(Corning Incorporated, Corning, NY, USA) and cultured in a
humidified atmosphere of 37°C containing 5% CO2 for 24
h. Next, the cells were treated with TCS (0–25 µM) for 24 h at
37°C, or cells were treated with different concentrations of GEMZ
(0–10 µM), with or without 20 µM TCS for 24 h at 37°C. In total, 24
h later, the cells were rinsed twice using ice-cold PBS and
incubated in 100 ml 0.5 mg/ml MTT solution for 3 h at room
temperature. The crystal was dissolved in 150 µl DMSO and the
optical density (A490 nm) was measured using a microplate reader.
Finally, the cell inhibitory rate was calculated using the
following formula: Inhibitory rate (%) =
(A490DMSO-A490sample)/(A490DMSO
-A490blank) ×100.
Observation of morphological
changes
The cells were cultured and treated with GEMZ (0–10
µM) with or without TCS (20 µM) for 48 h at 37°C, and the cellular
morphology was observed using a phase contrast microscope (Olympus
America, Inc., Center Valley, MA, USA) at magnification, ×400.
Apoptosis measured by flow
cytometry
Cell apoptosis was performed using an Annexin
V/propidium iodide (PI) staining assay (Sigma-Aldrich; Merck KGaA).
The cells were treated with 20 µM TCS, 2.23 µM GEMZ or a
combination of both in 6-well plates for 48 h at room temperature.
The cells were then lysed using cell lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) and centrifuged at 12,000 × g
for 10 min at 4°C. Then the cells were washed with PBS three times.
Subsequently, the cells were fixed in 70% ethanol overnight at room
temperature and stained with a mixture of PI containing 20 µg/ml
RNase (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. Finally, the
cells were analyzed by flow cytometry (BD Biosciences, Franklin
Lakes, NJ, USA). Data were analyzed using FlowJo software version
10 (FlowJo LLC, Ashland, OR, USA).
Western blot analysis
A549 cells were treated with 2.23 µM GEMZ combined
with or without 20 µM TCS for 48 h at 37°C, or cells were
pre-treated with 0.5 µM BMS-754807 for 24 h, then treated with GEMZ
and TCS for 48 h at 37°C. Next, the adherent and floating cells
were collected and centrifuged at 1,000 × g for 10 min at room
temperature and lysed with radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with phenylmethylsulfonyl fluoride (1 mM) for 30 min
at 4°C. Secondly, the suspension was centrifuged at 12,000 × g for
10 min, and the supernatant was collected. The protein
concentration was detected using a Bio-Rad protein assay reagent
(cat no. 500-0001; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Equal amounts (30 µg) of total protein were separated by 12%
SDS-PAGE and transferred onto a Millipore Immobilon®-P
Transfer Membrane (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 3% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) for 30 min at room temperature. The membranes were
incubated with primary antibodies against phosphorylated
(p)-phosphoinositide 3-kinase (PI3K) p85 (#4428), p-AKT (#4060),
p-ERK (#4370), ERK (#4695) and GAPDH (#3683) for 2 h at room
temperature. The primary antibodies were obtained from Cell
Signaling Technology, Inc., and used at 1:1,000 dilution. Then, the
membranes were incubated with HRP-linked secondary antibody (#7075;
1:1,000 dilution, Cell Signaling Technology, Inc.) for 1 h at room
temperature. The expression levels of target proteins were
visualized by electrochemiluminescence (Thermo Fisher Scientific,
Inc.). The densitometry of proteins was analyzed with Quantity One
version 4.6.2 (Bio-Rad Laboratories, Inc.).
Statistical analysis
To determine statistical significance between
groups, all results and data were detected in at least three
separate experiments. All data were analyzed using SPSS (version
13.0; SPSS, Inc., Chicago, IL, USA). Student's t-test was used to
compare two groups and one way analysis of variance and Dunnett's
post-hoc test was used to analyze multiple group comparisons.
P<0.05 was considered to indicate a statistically significant
difference. All data were expressed as the mean ± standard
deviation.
Results
TCS enhances the cytotoxicity of
GEMZ
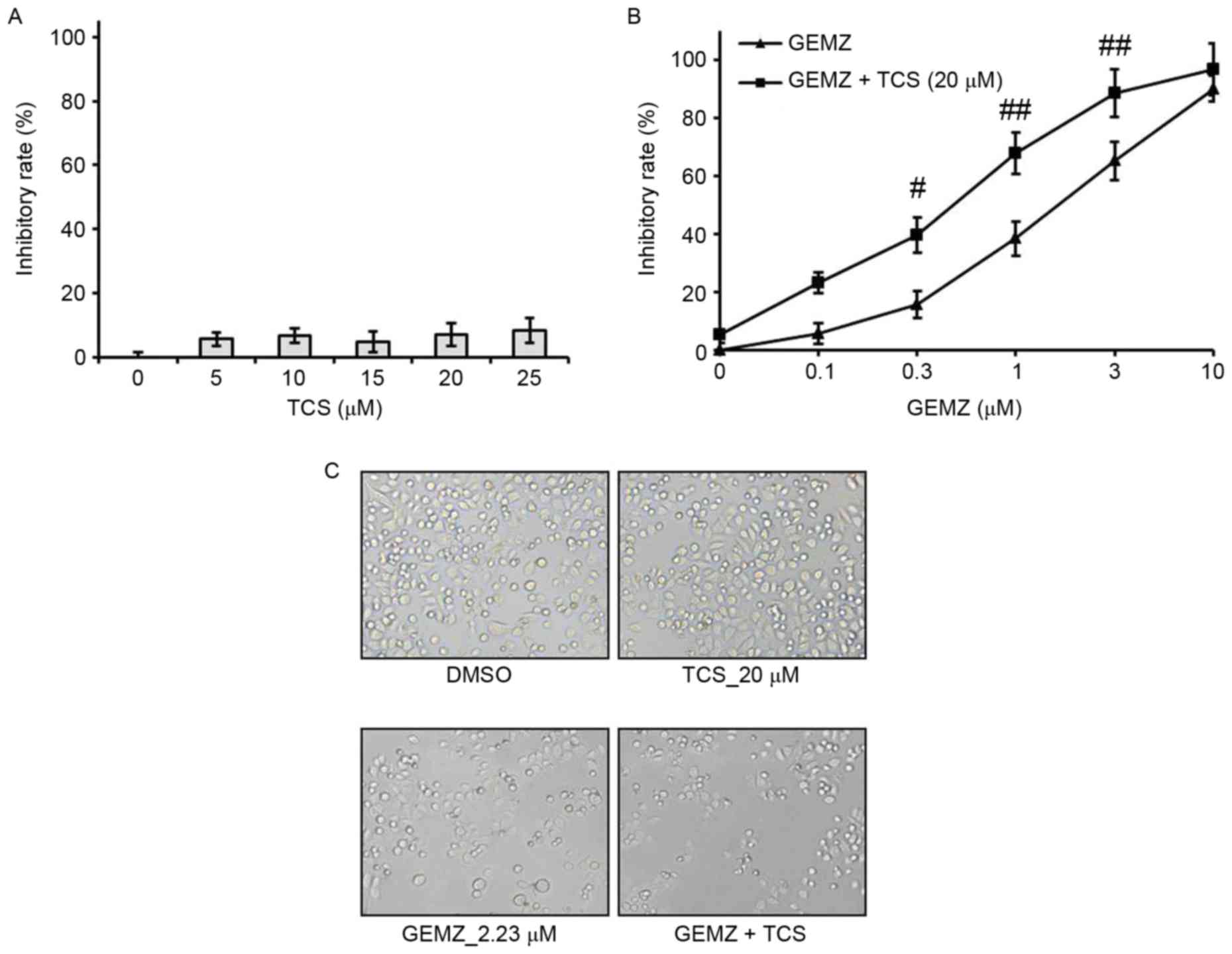
A549 cells were treated with different
concentrations of TCS ranging between 0 and 25 µM. The results of
the MTT assay demonstrated that TCS had no significant cell growth
inhibitory effect on A549 cells (Fig.
1A). However, the MTT assay indicated that GEMZ suppressed A549
cell growth in a concentration-dependent manner (Fig. 1B). Additionally, the half maximal
inhibitory concentration for 48 h GEMZ treatment was 2.23 µM. Since
treatment with TCS alone had no toxicity, a higher dose of 20 µM
was selected as a combination strategy. The results indicated that
the inhibitory effect on cell viability of different concentrations
of GEMZ was significantly enhanced when cells were treated with 0.3
(P<0.05), 1 (P<0.01) or 3 µM (P<0.01) GEMZ combined with
20 µM TCS compared with the use of GEMZ alone (Fig. 1B). The results of the changes in cell
morphology were consistent with the cell viability assay (Fig. 1C). These data indicated that TCS
enhanced the cytotoxicity of GEMZ on NSCLC A549 cells. Since the
combination of 20 µM TCS with 2.23 µM GEMZ had a marked cell growth
inhibition effect with limited cell toxicity, it was used as a
standard combination strategy in the following studies.
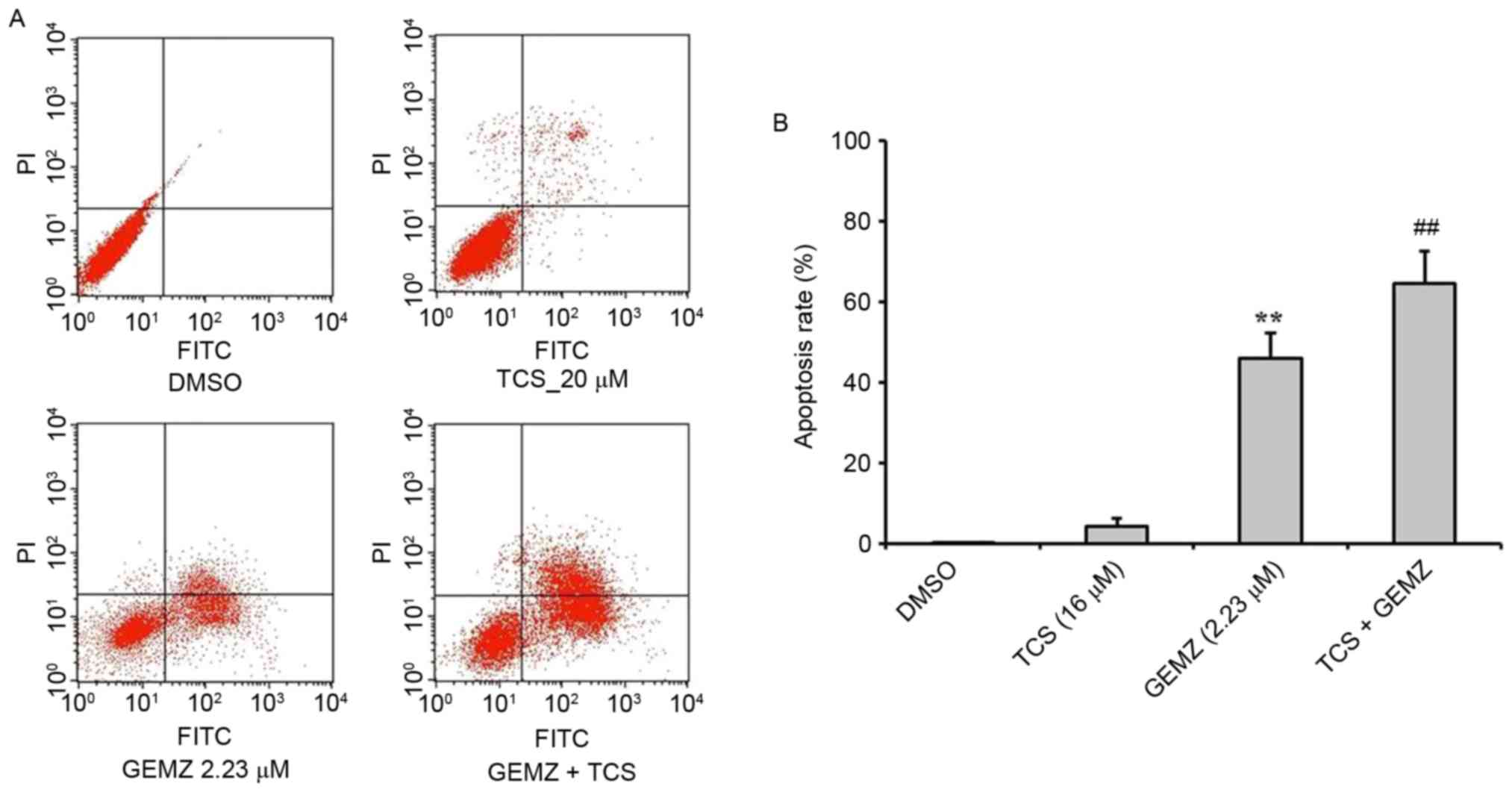
TCS increases GEMZ-induced apoptosis
in A549 cells
To evaluate the function of TCS combined with GEMZ
in A549 cells, an annexin/PI staining flow cytometry assay was
performed to detect the mechanism of cell death in A549 cells
(Fig. 2). The results revealed that
GEMZ significantly induced apoptosis in A549 cells (P<0.01 vs.
TCS treatment alone). Combined treatment of GEMZ with TCS
significantly increased the apoptosis rate of A549 compared with
GEMZ treatment alone (P<0.01). However, TCS treatment on its own
did not induce apoptosis, which was consistent with the cell
cytotoxic assay. The results revealed that TCS enhanced cell death
induced by GEMZ by inducing A549 cell apoptosis.
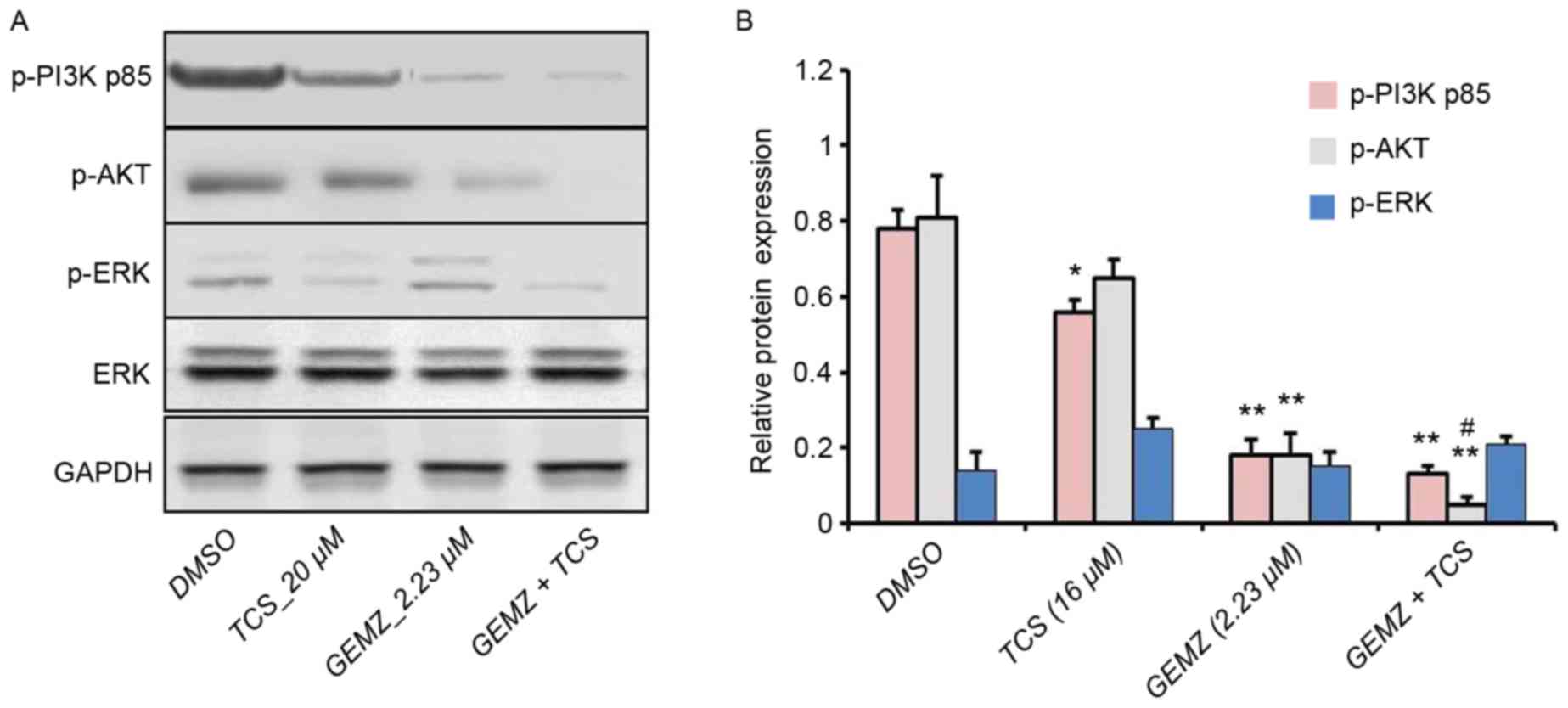
Combination treatment of GEMZ with TCS
suppresses the PI3K pathway in A549 cells
To further detect the underlying mechanism of
combination therapy of TCS and GEMZ, western blot analysis was
performed. The PI3K/AKT pathway is a classical signaling pathway
involved in cell death, tumor progression, survival, metabolism,
metastasis and drug resistance (25). Western blot analysis revealed that
the expression level of p-AKT was significantly lower in cells
treated with combination treatment of TCS and GEMZ when compared
with those treated with GEMZ alone (P<0.05; Fig. 3). However, the levels of p-P3K p85
and p-ERK were not significantly altered. Therefore, the results
indicated that PI3K signaling may be involved in TCS-enhanced
GEMZ-induced cell death and apoptosis.
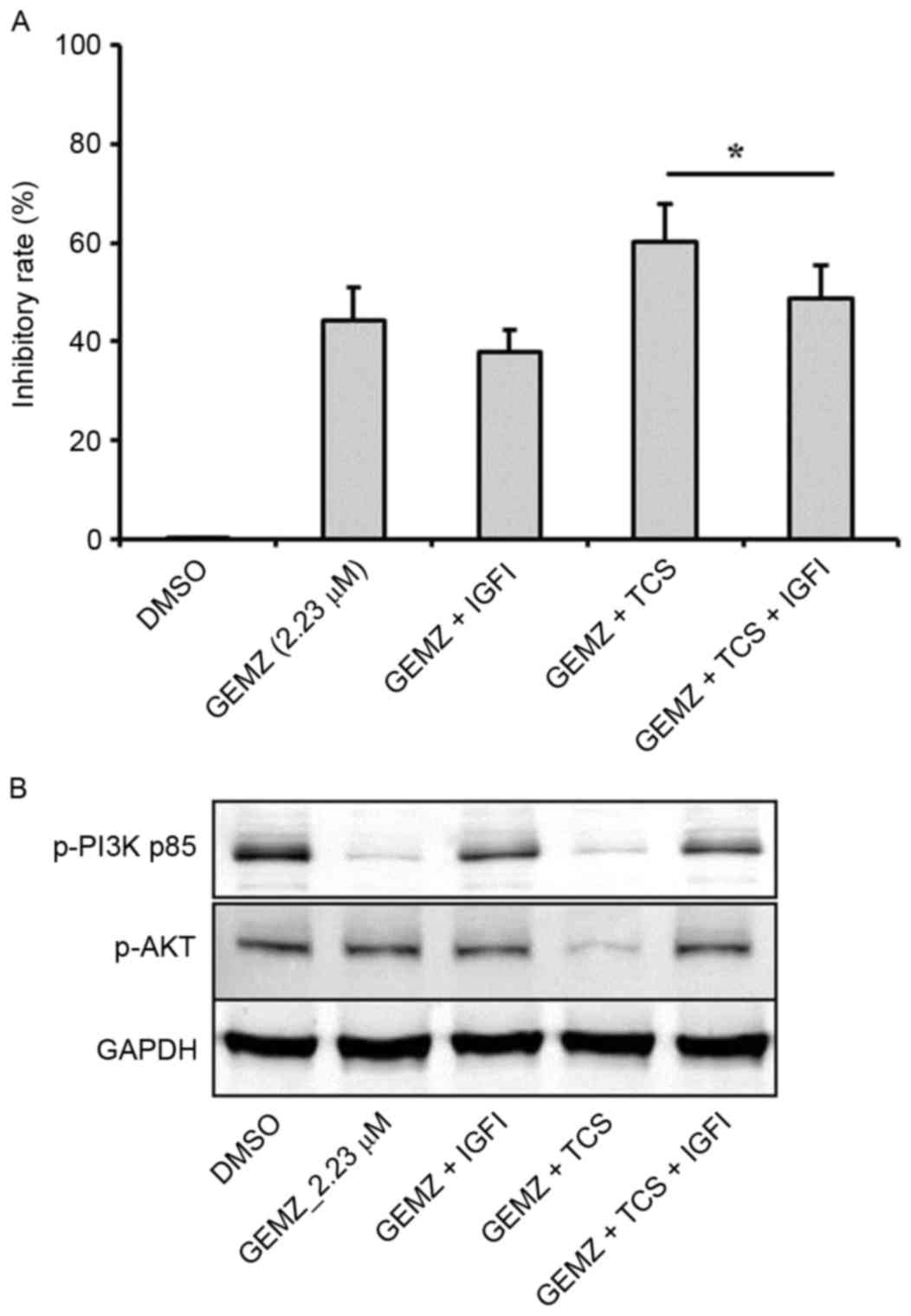
TCS combined with GEMZ suppresses the
PI3K pathway
Insulin is able to stimulate the activation of PI3K
signaling, which is involved in tumor cell survival, cell growth
and proliferation (25–28). In order to validate that IGF pathway
was involved in the effects of TCS and GEMZ combination treatment
in A549 cells, IGF1 (5 ng/ml) was used. The cell viability assay
revealed that following pretreatment with IGF1, the inhibitory rate
decreased compared with combination therapy of TCS and GEMZ
(P<0.05; Fig. 4A). Furthermore,
the expression levels of p-PI3K p85 and p-AKT were increased upon
addition of IGF1 compared with combination therapy of TCS and GEMZ
(Fig. 4B). The results indicated
that in A549 cells, the PI3K/AKT pathway was suppressed when
combination treated with TCS and GEMZ, and it was hypothesized that
TCS may enhance GEMZ exerting its pharmacological effects by
regulating the level of insulin.
Discussion
In recent studies, researchers have aimed to
identify the potential targets of GEMZ as well as the
identification of potential agents that may be used in combination
with GEMZ chemotherapy (10,12). It has been previously reported that
dihydroartemisinin combined with GEMZ has a synergistic interaction
in A549 cells to induce apoptosis (29), and GEMZ and sorafenib has synergistic
interaction in A549 cells to inhibit epidermal growth factor
receptor-tyrosine kinase inhibitor (EGFR-TKI)-sensitive and
EGFR-TKI-resistant NSCLC (30).
Furthermore, piceatannol enhances the antitumor efficacy of GEMZ in
A549 cells (10). In the present
study, the potential effects and underlying mechanisms of
combination therapy of GEMZ and TCS were investigated in A549
cells. It was demonstrated that in the A549 cell line, TCS enhanced
the antitumor effects of GEMZ. Additionally, the combination
treatment resulted in a significant increase in the apoptotic cell
population compared with GEMZ treatment only.
The PI3K/AKT pathway is a canonical signaling
pathway involved in proliferation, metabolism, protein synthesis
and cell survival (25). It was also
reported that the PI3K/AKT pathway was involved in cell apoptosis
in numerous cell lines (31–33), such as lung cancer cell lines
(34). In addition, a study by Mu
et al (35) reported that
GEMZ had antitumor effects in pancreatic cancer by regulating the
PI3K/AKT pathway. With this in mind, the potential role of the
PI3K/AKT pathway in mediating the enhancing effects of TCS on
GEMZ-induced cell death was investigated in the present study. The
present study revealed that TCS treatment resulted in an evident
increase of apoptosis in GEMZ-treated A549 cells, and this was
associated with significant downregulation of the PI3K/AKT pathway.
However, the expression levels of ERK were not significantly
altered. This suggests that ERK may not be involved in the cell
apoptosis of A549 cells induced by GEMZ and TCS combination
therapy. Then, the upstream factors of the PI3K/AKT pathway were
evaluated using IGF1. Insulin signaling employs the kinase-linked
cascades of PI3K/AKT and ERK (36,37).
Furthermore, IGF1 may mediate apoptosis and cell survival by
regulating the PI3K-AKT pathway (38). In the present study, following
addition of IGF1, the inhibitory rate was decreased compared with
the GEMZ + TCS group, which was consistent with the results of the
western blot analysis. Additionally, these results further
confirmed the hypothesis that TCS exerted its synergistic
pharmacological effect in GEMZ-treated NSCLC via inhibition of the
PI3K/AKT pathway.
Drug resistance is a major problem for treatment
with GEMZ. A large number of studies have indicated that PI3K/AKT
signaling is involved in chemotherapy drug sensitivity or drug
resistance (39–41). In the present study, TCS enhanced
GEMZ to suppress the expression level of PI3K and AKT, and thus it
may be hypothesized that, on one hand, TCS enhances GEMZ-induced
apoptosis via regulating the PI3K/AKT pathway; and on the other
hand, TCS combined with GEMZ could reduce drug resistance via the
PI3K/AKT pathway. However this hypothesis requires further
investigation. Various clinical research ranging between phase I
and III trails have focused on combination therapy of GEMZ with
insulin inhibitors (42,43) in order to reduce GEMZ
chemoresistance. Insulin has been demonstrated to be capable of
regulating PI3K/AKT activity (43).
This may indicate that TCS combined with GEMZ could not only induce
apoptosis via PI3K/AKT signaling, but also reduce GEMZ-resistance
in A549 cells via this signaling.
In conclusion, the present study demonstrated that
TCS was capable of significantly enhancing the cytotoxic and
apoptotic effects of GEMZ in A549 NSCLC cells via regulating the
PI3K/AKT pathway. These results may provide a potential rational
basis for a combination strategy for chemotherapy treatment of
NSCLC.
References
|
1
|
Gompelmann D, Eberhardt R and Herth FJ:
Advanced malignant lung disease: What the specialist can offer.
Respiration. 82:111–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katlic MR, Facktor MA, Berry SA, McKinley
KE, Bothe A Jr and Steele GD Jr: ProvenCare lung cancer: A
multi-institutional improvement collaborative. CA Cancer J Clin.
61:382–396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malapelle U, Pisapia P, Rocco D, Smeraglio
R, di Spirito M, Bellevicine C and Troncone G: Next generation
sequencing techniques in liquid biopsy: Focus on non-small cell
lung cancer patients. Transl Lung Cancer Res. 5:505–510. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langer F, Helsberg K, Schütte WH and
Leschinger MI: Gemcitabine in the first line therapy of advanced
and metastatic non-small-cell lung carcinoma (NSCLC): Review of the
results of phase III studies. Onkologie. 28 Suppl 1:S1–S28.
2005.(In German).
|
|
7
|
Laskin JJ and Sandler AB: First-line
treatment for advanced non-small-cell lung cancer. Oncology
(Williston Park). 19:1671–1680. 2005.PubMed/NCBI
|
|
8
|
Gallelli L, Nardi M, Prantera T, Barbera
S, Raffaele M, Arminio D, Pirritano D, Colosimo M, Maselli R,
Pelaia G, et al: Retrospective analysis of adverse drug reactions
induced by gemcitabine treatment in patients with non-small cell
lung cancer. Pharmacol Res. 49:259–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang B and Cui J: Treatment of mid-late
stage NSCLC using sodium cantharidinate/vitamin B6/GP regimen in
clinic. J Cancer Res Ther. 10 Suppl 1:C79–C81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu B and Tao ZZ: Piceatannol enhances the
antitumor efficacy of gemcitabine in human A549 non-small cell lung
cancer cells. Oncol Res. 22:213–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karampeazis A, Vamvakas L, Kentepozidis N,
Polyzos A, Chandrinos V, Rigas G, Christofyllakis C, Kotsakis A,
Hatzidaki D, Pallis AG and Georgoulias V: Biweekly carboplatin plus
gemcitabine as first-line treatment of elderly patients with
advanced squamous non-small-cell lung cancer: A multicenter phase
I–II trial by the hellenic oncology research group. Clin Lung
Cancer. 17:543–549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshitomi S, Taira N, Doihara H, Mizoo T,
Nogami T, Iwamoto T, Motoki T, Shien T, Ogasawara Y, Matsuoka J, et
al: A phase 1, dose-finding and pharmacokinetic study of
gemcitabine with nab-paclitaxel in patients with metastatic breast
cancer. Cancer Chemother Pharmacol. 78:289–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sha O, Niu J, Ng TB, Cho EY, Fu X and
Jiang W: Anti-tumor action of trichosanthin, a type 1
ribosome-inactivating protein, employed in traditional Chinese
medicine: A mini review. Cancer Chemother Pharmacol. 71:1387–1393.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Au TK, Collins RA, Lam TL, Ng TB, Fong WP
and Wan DC: The plant ribosome inactivating proteins luffin and
saporin are potent inhibitors of HIV-1 integrase. FEBS Lett.
471:169–172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Byers VS, Levin AS, Malvino A, Waites L,
Robins RA and Baldwin RW: A phase II study of effect of addition of
trichosanthin to zidovudine in patients with HIV disease and
failing antiretroviral agents. AIDS Res Hum Retroviruses.
10:413–420. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sha O, Yew DT, Ng TB, Yuan L and Kwong WH:
Different in vitro toxicities of structurally similar type I
ribosome-inactivating proteins (RIPs). Toxicol In Vitro.
24:1176–1182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ng TB, Wong JH and Wang H: Recent progress
in research on ribosome inactivating proteins. Curr Protein Pept
Sci. 11:37–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Y, Sun Y, Cai Y, Sha O and Jiang W:
Trichosanthin reduces the viability of SU-DHL-2 cells via the
activation of the extrinsic and intrinsic apoptotic pathways. Mol
Med Rep. 13:403–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ouyang DY, Chan H, Wang YY, Huang H, Tam
SC and Zheng YT: An inhibitor of c-Jun N-terminal kinases
(CEP-11004) counteracts the anti-HIV-1 action of trichosanthin.
Biochem Biophys Res Commun. 339:25–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang H, Chan H, Wang YY, Ouyang DY, Zheng
YT and Tam SC: Trichosanthin suppresses the elevation of p38 MAPK
and Bcl-2 induced by HSV-1 infection in Vero cells. Life Sci.
79:1287–1292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang P, Yan H and Li JC: CREB-mediated
Bcl-2 expression in trichosanthin-induced Hela cell apoptosis.
Biochem Biophys Res Commun. 363:101–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Chen F, Liu CP, Li DM, Li X, Wang C
and Li JC: Dexamethasone enhances trichosanthin-induced apoptosis
in the HepG2 hepatoma cell line. Life Sci. 86:10–16. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang D, Chen B, Zhou J, Zhou L, Li Q, Liu
F, Chou KY, Tao L and Lu LM: Low concentrations of trichosanthin
induce apoptosis and cell cycle arrest via c-Jun N-terminal protein
kinase/mitogen-activated protein kinase activation. Mol Med Rep.
11:349–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li CT, Lin CH, Kao TY, Wu MF, Yeh CS, Yeh
KT and Ko JL: The mechanisms of action of Tianhua(™) on antitumor
activity in lung cancer cells. Pharm Biol. 48:1302–1309. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhaskar PT and Hay N: The two TORCs and
Akt. Dev Cell. 12:487–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J: Is Src the key to understanding
metastasis and developing new treatments for colon cancer? Nat Clin
Pract Gastroenterol Hepatol. 5:306–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao C, Gao W and Chen T: Synergistic
induction of apoptosis in A549 cells by dihydroartemisinin and
gemcitabine. Apoptosis. 19:668–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Pan YY and Zhang Y: Synergistic
interaction between sorafenib and gemcitabine in EGFR-TKI-sensitive
and EGFR-TKI-resistant human lung cancer cell lines. Oncol Lett.
5:440–446. 2013.PubMed/NCBI
|
|
31
|
Li T: Pacilitaxel induces human
nasopharyngeal carcinoma cell line CNE2 apoptosis and growth
inhibition by suppressing PI3K/AKT/p53 signaling pathway. Lin Chung
Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 29:2147–2150. 2015.(In
Chinese). PubMed/NCBI
|
|
32
|
Lu D, Qian J, Li W, Feng Q, Pan S and
Zhang S: β-hydroxyisovaleryl-shikonin induces human cervical cancer
cell apoptosis via PI3K/AKT/mTOR signaling. Oncol Lett.
10:3434–3442. 2015.PubMed/NCBI
|
|
33
|
Zhao J, Zhang ZR, Zhao N, Ma BA and Fan
QY: VEGF silencing inhibits human osteosarcoma angiogenesis and
promotes cell apoptosis via PI3K/AKT signaling pathway. Cell
Biochem Biophys. 73:519–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin H, Qiao F, Wang Y, Xu Y and Shang Y:
Curcumin inhibits cell proliferation and induces apoptosis of human
non-small cell lung cancer cells through the upregulation of
miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol
Rep. 34:2782–2789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mu GG, Zhang LL, Li HY, Liao Y and Yu HG:
Thymoquinone pretreatment overcomes the insensitivity and
potentiates the antitumor effect of gemcitabine through abrogation
of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic
cancer. Dig Dis Sci. 60:1067–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoon SH, Ramalingam M and Kim SJ: Insulin
stimulates integrin-linked kinase in UMR-106 cells: Potential role
of heparan sulfate on syndecan-1. J Recept Signal Transduct Res.
35:613–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ha Ju H and Kim SJ: Association of insulin
receptor and syndecan-1 by insulin with activation of ERK I/II in
osteoblast-like UMR-106 cells. J Recept Signal Transduct Res.
33:37–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang QY, Wang L, Song ZY and Qu XJ:
Knockdown of type I insulin-like growth factor receptor inhibits
human colorectal cancer cell growth and downstream PI3K/Akt,
WNT/β-catenin signal pathways. Biomed Pharmacother. 73:12–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Q, Li X, Li C, Zheng Y and Peng G:
1-Deoxynojirimycin alleviates insulin resistance via activation of
insulin signaling PI3K/AKT pathway in skeletal muscle of db/db
mice. Molecules. 20:21700–21714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma Y, Zhang P, Gao Y, Fan H, Zhang M and
Wu J: Evaluation of AKT phosphorylation and PTEN loss and their
correlation with the resistance of rituximab in DLBCL. Int J Clin
Exp Pathol. 8:14875–14884. 2015.PubMed/NCBI
|
|
41
|
Deng QF, Su BO, Zhao YM, Tang L, Zhang J
and Zhou CC: Integrin β1-mediated acquired gefitinib resistance in
non-small cell lung cancer cells occurs via the phosphoinositide
3-kinase-dependent pathway. Oncol Lett. 11:535–542. 2016.PubMed/NCBI
|
|
42
|
Awasthi N, Zhang C, Ruan W, Schwarz MA and
Schwarz RE: BMS-754807, a small-molecule inhibitor of insulin-like
growth factor-1 receptor/insulin receptor, enhances gemcitabine
response in pancreatic cancer. Mol Cancer Ther. 11:2644–2653. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Okusaka T, Ikeda M, Fukutomi A, Kobayashi
Y, Shibayama K, Takubo T and Gansert J: Safety, tolerability,
pharmacokinetics and antitumor activity of ganitumab, an
investigational fully human monoclonal antibody to insulin-like
growth factor type 1 receptor, combined with gemcitabine as
first-line therapy in patients with metastatic pancreatic cancer: A
phase 1b study. Jpn J Clin Oncol. 44:442–447. 2014. View Article : Google Scholar : PubMed/NCBI
|