Introduction
Glioblastoma is the most widespread and malignant
brain tumor, and is often irremediable. This tumor has high
mortality rate due to low response to the currently available
treatments, resulting in short survival time that may be <1 year
between diagnosis and mortality (1).
A number of therapies are available for the treatment of
glioblastoma, including surgery, chemotherapy, photodynamic
therapy, gene therapy, treatment with traditional Chinese medicine,
radiotherapy, heat therapy and immune therapy (2–4).
However, these therapies often have insufficient efficacy in curing
glioblastoma due to its unusual appearance, powerful invasion and
resistance towards chemotherapy. Furthermore, the administration of
temozolomide, an alkylating compound, for cancer treatment in
patients is limited (met with resistance) due to increased activity
of the DNA repair enzyme O6-alkylguanine-DNA
alkyltransferase enzyme (5).
Metastatic cancer cell growth and the high resistance of cells to
conventional treatments generate difficulties in complete
eradication of the disease. Therefore, novel therapies are
essential to defeat these limitations of conventional treatments.
Furthermore, the clarification of mechanisms underlying the
occurrence and development of gliomas is also essential, since it
can contribute towards novel successful treatments.
In the field of cancer research, the evolution of
food-derived chemopreventive compounds has been receiving
increasing attention. For instance, the use of phytochemicals is an
encouraging and contemporary approach for the prevention of cancer
and its treatment (6). In addition,
traditional herbal therapy may be considered as a favorable
substitute for modern medicine (6).
Previous studies have been conducted to examine the anti-invasive
and antitumor properties of different herbal compounds (7). Researchers identified reduced
occurrence of side effects for these herbal components when
compared to chemotherapeutic agents. Prior to initiation of
expensive clinical trials, in vitro evaluation and other
studies can be conducted to produce easy, quick and satisfactory
data (8).
Plant-based compounds have served important roles in
regulating the human health and improving the quality of life for
several years (9,10). α-Tomatine, isolated from
Solanum plants such as Solanum lycopersicon L., is a
natural steroidal glycoalkaloid found in the leaves, stems and
roots of tomatoes. α-Tomatine consists of six-ring steroidal
aglycone tomatidine by which two glucose units, xylose and
galactose moieties of a tetrasaccharide unit, are linked to the
3-hydroxyl group of aglycone. Different types of tomatine, such as
β-tomatine, γ-tomatine and δ-tomatine, are generated by the partial
hydrolysis of α-tomatine that leads to the loss of different sugar
units (11). α-Tomatine provides
protection against pathogenic bacteria, fungi and viruses in plants
(12). On evaluating the mechanistic
effect of α-tomatine, it has been proven that tomatine alters
essential signaling pathways involved in the maintenance of cell
proliferation, migration and differentiation (13,14).
Inhibition in the growth of human liver cancer HepG2 and human
colon cancer HT29 cell lines was observed following in vitro
α-tomatine treatment, and the results were found to be more
effective compared with those of standard anticancer drugs, such as
camptothecin and doxorubicin (15,16). In
addition, the cytotoxicity of α-tomatine towards various other
human cancer cells has been investigated, including lung cancer
(NCI-H460 and A549) (16,17), prostate cancer (PC3) (18), leukemia (MOLT-4) (19), breast cancer (MCF-7) (20) and EL4 mouse lymphoma cell lines
(21). Furthermore,
caspase-independent cell death and apoptosis have been identified
in leukemia cancer cell lines and PC3 cell lines, respectively,
upon treatment with α-tomatine; inactivation of the FAK/PI3K/AKT
and ERK signaling pathways with reduced binding potential of
nuclear factor-κB (NF-κB) occurred upon inhibiting the migration
and invasion of cancer cells (20).
Based on the aforementioned facts, the present study
aimed to investigate the molecular mechanisms involved in the
chemotherapeutic influence of α-tomatine in two human A172 and
U-118 MG glioblastoma cell lines through the induction of cell
death. The current experiments strongly suggested that several
mechanisms are involved in the induction of apoptosis in A172 and
U-118 MG cell lines following α-tomatine treatment.
Materials and methods
Materials
α-Tomatine was purchased from Extrasynthese (Genay,
France) and its purity was confirmed using high-performance liquid
chromatography. Wright staining kit (cat. no. WS16), Fura-2 (cat.
no. 47989) and trypan blue (cat. no. T6146) were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Apop Tag assay kit
(cat. no. S7100) was obtained from EMD Millipore (Billerica, MA,
USA). Anti-calpain IgG antibody (cat. no. 2556; 1:1,000), all the
utilized primary antibodies, as well as horseradish peroxidase
conjugated goat anti-mouse (cat. no. 7076; 1:2,000) and anti-rabbit
IgG (cat. no. 58802; 1:1,000) secondary antibodies, were acquired
from Cell Signaling Technology, Inc. (Denver, MA, USA).
Cell culture and treatment
A172 and U-118 MG human glioblastoma cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA). These cell lines were grown in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS), penicillin (1%) and streptomycin
(100 µg/ml) in an incubator with a humidified atmosphere of 95% air
and 5% CO2 at 37°C. The medium was replaced every 24 h.
In all the experiments, cells were collected using trypsinization
after cells were allowed to grow up to passage number 9. Prior to
drug addition, cells were starved for 1 day in the same growth
medium, followed by preservation using low serum state during
addition of α-tomatine. The 100 mM α-tomatine stock solution was
prepared using dimethyl sulfoxide (DMSO) and aliquots were diluted
serially using growth medium to obtain the desired concentrations
for the treatment of cell lines. In order to determine the suitable
α-tomatine dose to induce apoptosis, cell viability was measured by
conducting dose-dependent studies (0–50 µM). It was demonstrated
that 25 and 50 µM α-tomatine were the appropriate doses for
apoptosis induction, thus these were used for subsequent
experiments. The control group was treated with only DMSO. The
morphological and biochemical changes in cells were also analyzed
to predict the apoptosis and the mechanistic pathway.
Trypan blue assay for cell viability
determination
The cell viability was measured using a trypan blue
assay upon treatment with α-tomatine, as previously described
(22). The cell viability was
determined according to the principle that live cells do not absorb
Tryphan blue due to membrane integrity, whereas cells with damaged
cell membranes absorb the dye and are counted as dead cells. Viable
cell counts are presented in terms of the % of the total number of
cells.
Wright staining
Wright staining was performed to analyze the changes
in morphological characteristics of cells in order to predict the
apoptosis. Briefly, cells in the control and α-tomatine groups were
washed using phosphate-buffered saline (PBS) solution and then
deposited onto slides using a Hettich centrifuge and Cytobuckets
(10 min, 150 × g, room temperature; Hettich Lab Technology,
Tuttlingen, Germany). Prior to monitoring the morphological changes
using Wright staining, cells were fixed in ethanol (95% v/v)
(23). Subsequently, various
morphological characteristics of the cells were examined, including
the chromatin condensation, reduction in cell volume and presence
of membrane-bound apoptotic bodies. The percentage of apoptotic
cells was measured from three independent experiments.
Peroxidase in situ apoptosis
assay
Apoptotic DNA fragmentation upon α-tomatine
treatment was evaluated using an ApopTag Peroxidase In Situ
Apoptosis Detection kit. In brief, cells were treated with
α-tomatine as mentioned earlier, washed with PBS, centrifuged
(1,000 × g for 3 min at room temperature) and then deposited onto
microscopic slides. Cells were fixed in ethanol (95% v/v) and then
allowed to dry overnight. A protein digesting enzyme, Proteinase K
(cat. no. 21627; EMD Millipore) was used to pretreat the cells for
10 min and the washed using distilled water for ~3 min. This was
followed by quenching of the cells using hydrogen peroxide (2% v/v)
for 5 min, followed by washing twice in PBS. Next, pre-equilibrated
cells were treated with TdT-enzyme and incubated for 1 h at 37°C,
followed by addition of stop buffer in the slide, agitation for 10
sec and incubation at room temperature for a further 10 min. After
the reaction was stopped, the slides were washed with PBS twice and
horseradish peroxidase-conjugated anti-digoxigenin antibody was
added and incubated for 30 min. Slides were removed and then washed
with PBS, followed by treatment with 3,3′-diaminobenzidine at room
temperature for 5 min and finally washing with water
(ddH2O) twice. Methyl green (0.5% v/v) was used to
counterstain the slides, which were then washed by water and
n-butanol for 10 min. Cells were dehydrated by xylene and fixed in
glass coverslips. The percentage of positive apoptotic cells was
then determined by counting the brown cells under a microscope
(23,24).
Fura-2 assay
Alterations in intracellular calcium
([Ca2+]i) in human glioblastoma cell lines
(A172 and U-118 MG) were determined by Fura-2 assay, according to
the reported literature (23,24). In
brief, cells at a density of 2×104 cells/ml were grown
in phenol-red medium for 72 h, removed from the culture medium and
then centrifuged to obtain pellets for ~5 min at 2,000 rpm. Next,
cells were washed twice using PBS buffer and then returned into the
suspension, incubated for 2 h and washed twice in Locke's buffer
(containing 9 g sodium chloride, 0.4 g potassium chloride, 0.1 g
calclium chloride, 0.3 g sodium bicarbonate and 1 g glucose) which
was prepared as previously described (25). Cell density was measured with a
hemocytometer and then the cells were disseminated in FBS (10%)
containing Locke's buffer. Fura-2 stock solution was prepared by
dissolving the appropriate amount of Fura-2 in DMSO and then
diluting with FBS (10%) containing Locke's buffer to obtain a final
concentration of 2 µM, followed by incubation with cells for 30 min
at 37°C. Cells were subsequently diluted to 1×105
cells/ml using Locke's buffer solution (Ca2+ free), and
the [Ca2+]i was determined using a previously
described spectrofluorometric method (26). The fluorescence ratio was measured at
wavelengths of 340 and 380 nm using FluoroMax (Horiba Scientific,
Kyoto, Japan). Next, 200 µl digitonin (cat. no. D141;
Sigma-Aldrich; Merck KGaA) (250 µM) and EDTA (500 mM) were used to
calculate the maximal and minimal ratios of [Ca2+]
(Rmax and Rmin), respectively. The cell
specific dissociation constants for the two cell lines were
determined to be 0.378 and 0.477 µM, respectively, by following the
manufacturer's instructions provided in the calcium calibration
buffer kit (cat. no. C3008MP; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Western blotting
The adherent and suspended cells from each treatment
group were collected and centrifuged at 100 × g for 10 min at room
temperature to obtain cell pellets. The cell pellets were washed
twice with PBS and resuspended in homogenized buffer solution
containing Tris-HCl (50 mM, pH 7.4), EGTA (1 mM), sucrose (325 nM)
and phenylmethylsulfonyl fluoride (0.1 mM) for homogenization for
~30 sec at 4°C. Following the homogenization process, the protein
concentration in the samples was conducted using a Bradford Protein
reagent (Sigma-Aldrich) and measuring spectrophotometrically the
absorption at a wavelength of 595 nm (NanoVue spectrophotometer; GE
Healthcare Life Sciences, Chicago, IL, USA).
Protein samples (~50 µg) were then treated with 2X
loading buffer composed of equal amount of Tris-HCl (120 mM, pH
6.8), SDS (6%), glycerol (20%), 1,4-dithio-DL-threitol (200 mM),
β-mercaptoethanol (10 mM) and bromophenol blue (0.01%), and boiled
for 10 min. Gradient gels (4–20%) were used to load the samples for
electrophoresis at 200 V for 30 min using an electrophoretic system
(GE Healthcare Life Sciences). The α-spectrin bands were detected
using electrophoresis gel (5%) at 100 V for 2 h. Subsequently, the
gels were electroblotted onto nylon membranes with an
electroblotting apparatus (Bio-Rad Laboratories, Inc., Hercules, CA
USA). A blocking buffer solution with Tris-HCl (10 mM, pH 7.4),
non-fat powdered milk (5%) and NaCl (120 mM) was used to block the
membranes and then washed with a washing buffer composed of
Tris-HCl (10 mM, pH 7.4), NaCl (120 mM) and Tween 20 (0.1%).
Primary antibodies were then properly diluted, and added to the
blots for ~1 h. Primary antibodies against the following proteins
were used: Bcl-2 (cat. no. 2872; 1:1,000), cytochrome c (cat. no.
4272; 1:1,000), β-actin (cat. no. 4967; 1:1,000), calpain (cat. no.
2556; 1:1,000), caspase-12 (cat. no. 2202; 1:1,000), caspase-9
(cat. no. 9502; 1:1,000), caspase-3 (cat. no. 9662; 1:1,000),
apoptosis-inducing factor (AIF) (cat. no. 4642, 1:1,000),
Smac/Diablo (cat. no. 2954; 1:1,000), BIRC-2 (cat. no. 4952;
1:1,000), BIRC-3 (cat. no. 3130; 1:1,000), IkBa (cat. no. 9242;
1:1,000), NFkB (cat. no. 4717; 1:1,000) were purchased from Cell
Signaling Technology, Inc. Primary antibody against inhibitor of
caspase-activated DNase (ICAD) (cat. no. ab108521; 1:10,000) was
purchased from Abcam (Cambridge, MA, USA). Primary antibodies
against 145 kDa Spectrin Breakdown Products (cat. no.
CSB-EQ028022HU; 1:100) and 120 kDa Spectrin Breakdown Products
(cat. no. CSB-EQ028055HU; 1:100) were purchased from Cusabio
(Hubei, China). The blots were washed thrice with washing buffer
and then secondary antibodies were added for 1 h at a dilution of
1:2,000. Finally, the blots were then visualized with an ECL
chemiluminescence system (EMD Millipore) and the optical density of
bands was measured using a densitometric technique.
Statistical analysis
The acquired experimental data following α-tomatine
treatment of A172 and U-118 MG cells were analyzed using SPSS (ver
21) software (SPSS, Inc., Chicago, IL, USA). Results are presented
as the mean ± standard error of at least three independent
experiments (n≥3). The statistical differences between each group
were analyzed using one-way analysis of variance followed by
Tukey's honest significant difference post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell viability and apoptosis
measurements
Two different concentrations of α-tomatine were used
to determine whether it reduces cell viability and induces
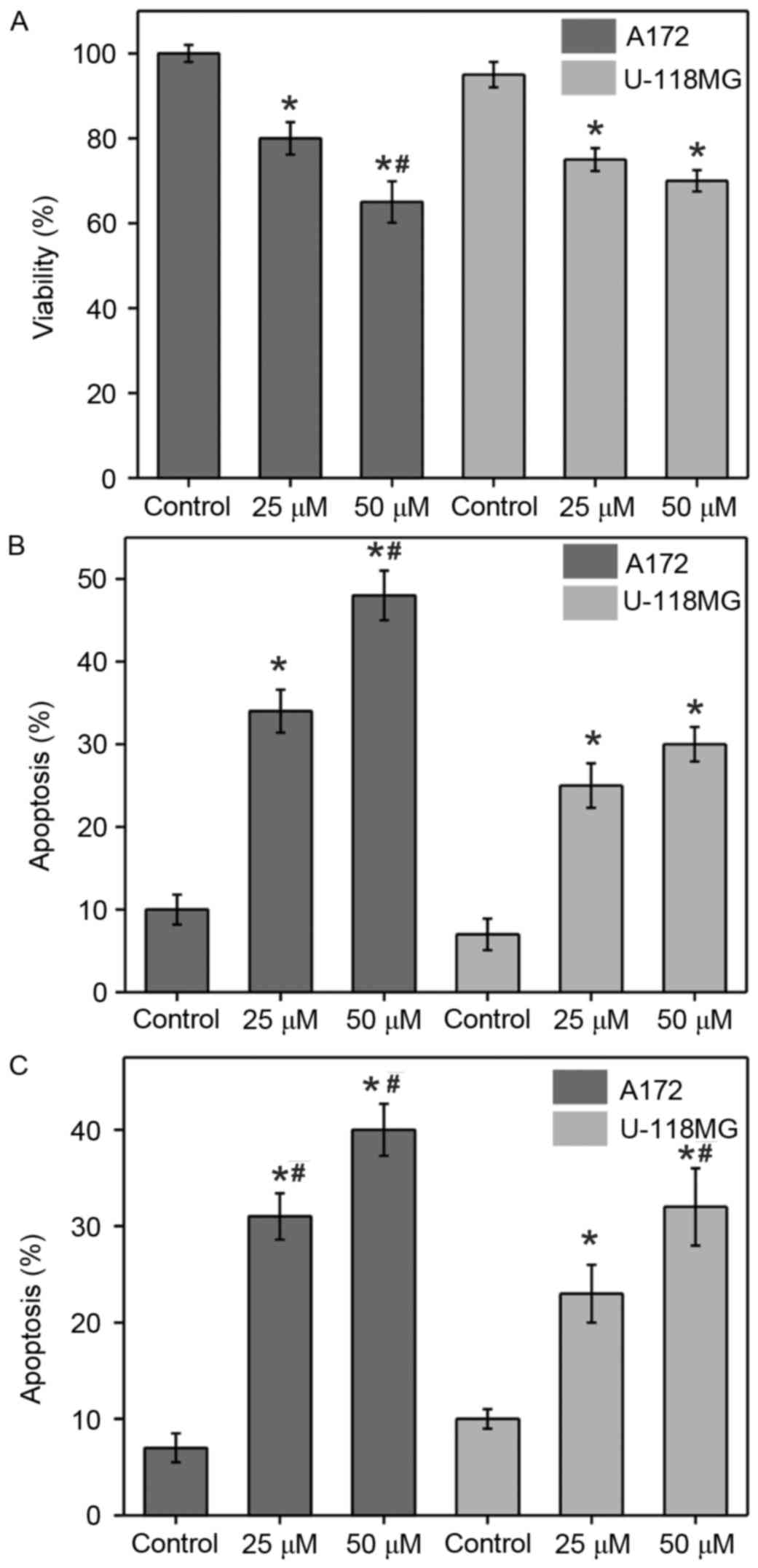
apoptosis in glioblastoma A172 and U-118 MG cell lines (Fig. 1). Cells were treated with different
α-tomatine concentrations (25 and 50 µM) and tryptan blue assay was
used to determine the cell viability by measuring the absorbance
under a light microscope. Treatment with α-tomatine (25 and 50 µM)
significantly reduced the cell viability in A172 cells to 80%
(P<0.05) and 65% (P<0.01), respectively, whereas the
viability in U-118 MG cells was significantly reduced to 75%
(P<0.05) and 70% (P<0.05), respectively, when compared with
the control cells (Fig. 1A).
The changes in morphological characteristics due to
α-tomatine-induced apoptosis were determined using Wright staining
(Fig. 1B). Wright stained cells
typically exhibit characteristics including reduction of cell size,
membrane blebbing or chromatin condensation as a sign of apoptotic
cell death. Treatment with α-tomatine (25 and 50 µM) in A172 cells
resulted in a significant increase in apoptosis of 34% (P<0.05)
and 47% (P<0.01), respectively, whereas an increase of 25%
(P<0.05) and 30% (P<0.05) was observed in the case of U-118
MG cells, respectively (Fig. 1B).
ApopTag assay (Fig. 1C) was further
used in order to confirm the Wright staining data. In this assay,
brown color stained cells were identified to be apoptotic when
presenting one of the morphological changes mentioned earlier. The
results revealed that very few control cells exhibited brown
staining, indicating a low number of positive ApopTag cells. By
contrast, the α-tomatine-treated groups exhibited marked staining
and thus apoptosis, which was confirmed by the ApopTag assay and
changes in the morphological characteristics. The percentage of
apoptotic cells were found to be significantly increased by 33%
(P<0.01) and 41% (P<0.01) in the A172 cell line after the
treatment with 25 and 50 µM α-tomatine, respectively, whereas
apoptosis of 24% (P<0.05) and 33% (P<0.01) was observed in
the U-118 MG cell line, respectively (Fig. 1C).
Observation of
[Ca2+]i changes upon α-tomatine
treatment
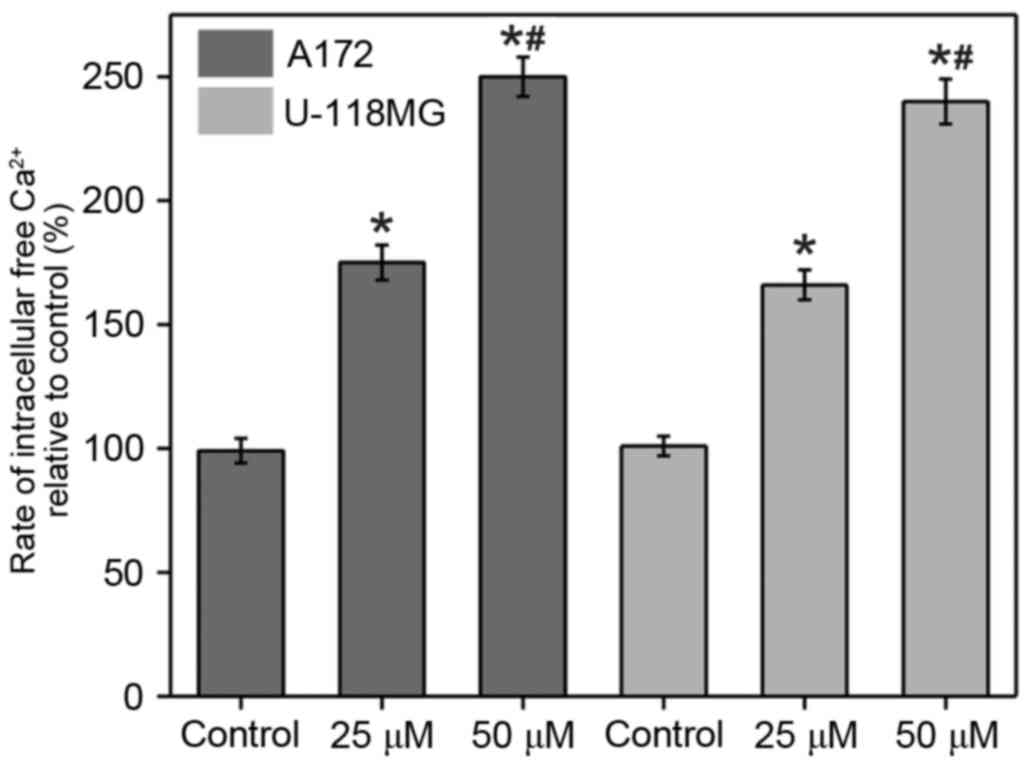
Fura-2 assay was conducted in order to monitor the
[Ca2+]i changes upon α-tomatine treatment in
A172 and U-118 MG cells (Fig. 2).
The results revealed that a marked increase in
[Ca2+]i was observed in the two cell lines
upon the addition of α-tomatine (25 and 50 µM). The percentages of
change in A172 cells were determined to be 75% (P<0.05) and 150%
(P<0.01) following treatment with 25 and 50 µM of α-tomatine,
respectively, compared with the control group. In the case of U-118
MG cells, the percentages of change were found to be 66%
(P<0.05) and 138% (P<0.01) for the treatment with 25 and 50
µM α-tomatine, respectively (Fig.
2). In the two cell lines, treatment of α-tomatine (25 and 50
µM) was observed to significantly increase the intracellular
calcium levels [Ca2+]i as compared with the
control. These [Ca2+]i enhancements are a
strong indication of apoptosis induction through endoplasmic
reticulum (ER) stress and of participation of calpain in the
degradation of cytosolic substrate.
Identification of α-tomatine-induced
apoptosis
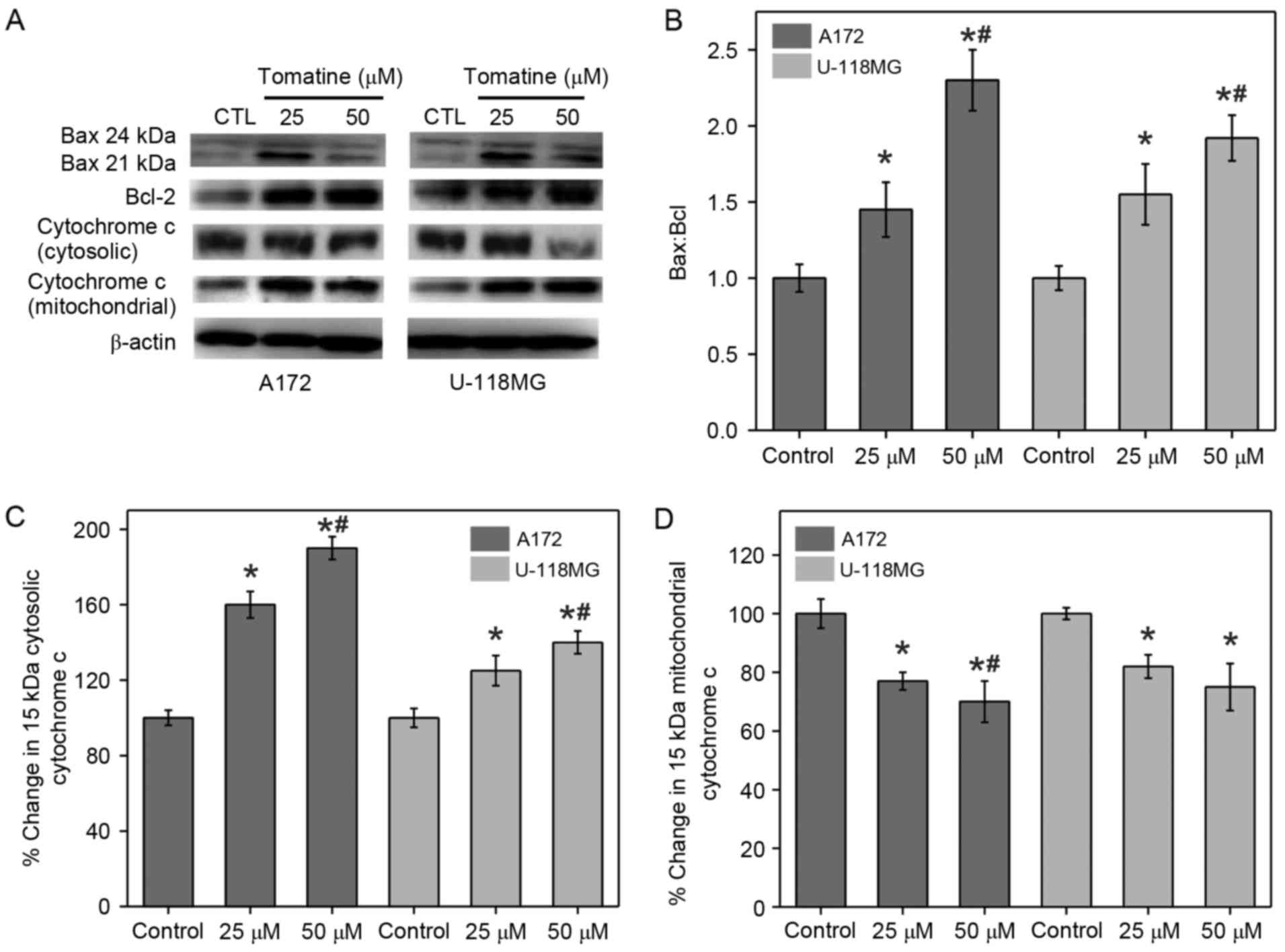
It is known that the changes in the expression
levels of Bcl-2 proteins are an indicator of cell apoptosis.
Western blotting was conducted in the present study to measure the
expression levels of Bax (pro-apoptotic) and Bcl-2
(anti-apoptotic), as well as to examine the alterations in
Bax:Bcl-2 ratio (Fig. 3A and B).
β-actin served as an internal control. It was identified that
treatment with α-tomatine resulted in an elevation in Bax
expression, whereas Bcl-2 expression was reduced. The calculated
ratio of Bax:Bcl-2 was significantly increased in A172 and U-118 MG
cells following α-tomatine treatment of 25 (P<0.05) and 50 µM
(P<0.01), respectively (Fig. 3B).
These alterations in the ratio of Bax:Bcl-2 upon α-tomatine
treatment indicated that the apoptosis induction was mediated
through the mitochondrial pathway.
Release of mitochondrial cytochrome
c
Apoptosis is initiated by various apoptotic stimuli,
which result in the release of cytochrome c from mitochondria. This
results in caspase activation through biochemical reactions,
leading to cell death (20). Hence,
the protein fractions of the cytosolic and mitochondrial parts in
all treatment groups were separated to determine the levels of
cytochrome c using western blot analysis (Fig. 3A). The results demonstrated that
α-tomatine treatment in A172 and U-118 MG cell lines significantly
upregulated the cytochrome c protein level in the cytosolic
fraction with a consequent reduction in the corresponding
mitochondrial portion (Fig. 3C and
D). The cytosolic cytochrome c level was increased by
60% (P<0.05) and 90% (P<0.01) in A172 cells and by 25%
(P<0.05) and 40% (P<0.01) in U-118 MG cells following 25 and
50 µM α-tomatine treatment, respectively (Fig. 3C). In the case of mitochondrial
cytochrome c levels, 25 and 50 µM α-tomatine treatment
caused a significant 22% (P<0.05) and 32% (P<0.01) decrease
in A172 cells, respectively, whereas the decrease in U-118 MG cells
was observed to be 19% (P<0.05) and 24% (P<0.05),
respectively (Fig. 3D). These
results revealed that α-tomatine treatment caused cytochrome
c release from the mitochondria into the cytosol.
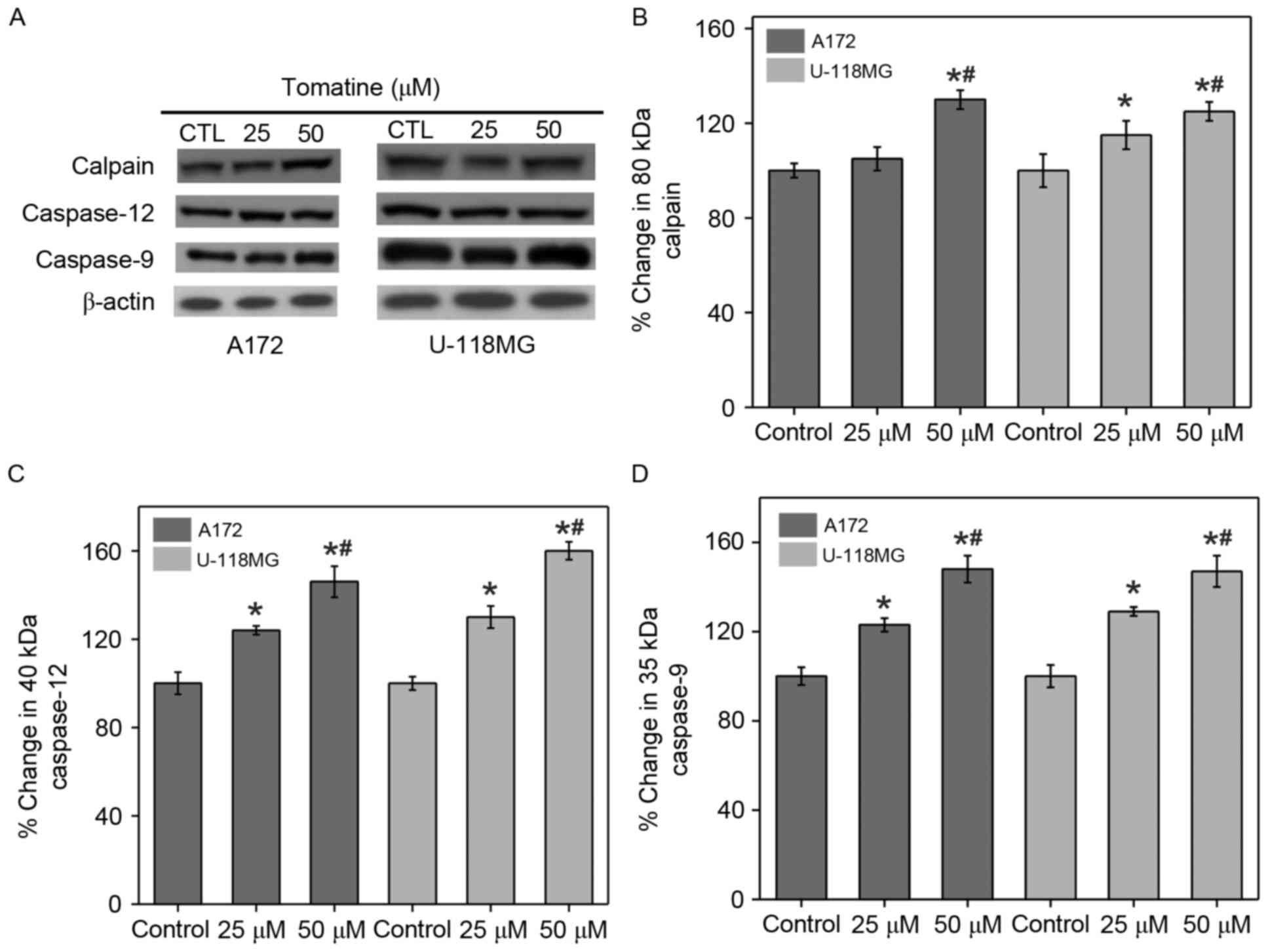
Calpain expression and activation of
caspases
α-Tomatine mediated ER stress can increase
[Ca2+]i and trigger Ca2+-dependent
calpain expression for activating caspase. Western blot experiments
were performed to study the induction of calpain expression and
caspase activation in A172 and U-118 MG cells upon treatment with
α-tomatine (Fig. 4A). The results
indicated that treatment with 25 and 50 µM α-tomatine caused an
increase in calpain protein expression of 7% (non-significant) and
36% (P<0.01) in A172 respectively, and of 15% (P<0.05) and
26% (P<0.01) in U-118 MG cell lines, respectively, as compared
with the control (Fig. 4B). In
addition, α-tomatine (25 and 50 µM) significantly increased the
protein levels of caspase-12 (40 kDa) in A172 cell lines, by 24%
(P<0.05) and 46% (P<0.01) respectively and by 28% (P<0.05)
and 56% (P<0.01) respectively in U-118 MG cell lines (Fig. 4C). Similarly, the protein levels of
caspase-9 (35 kDa) were significantly upregulated upon
administration with α-tomatine (25 µM, P<0.05; 50 µM, P<0.01)
in A172 and U-118 MG cell lines (Fig.
4D). These results revealed the participation of caspase
activation in the apoptosis induction.
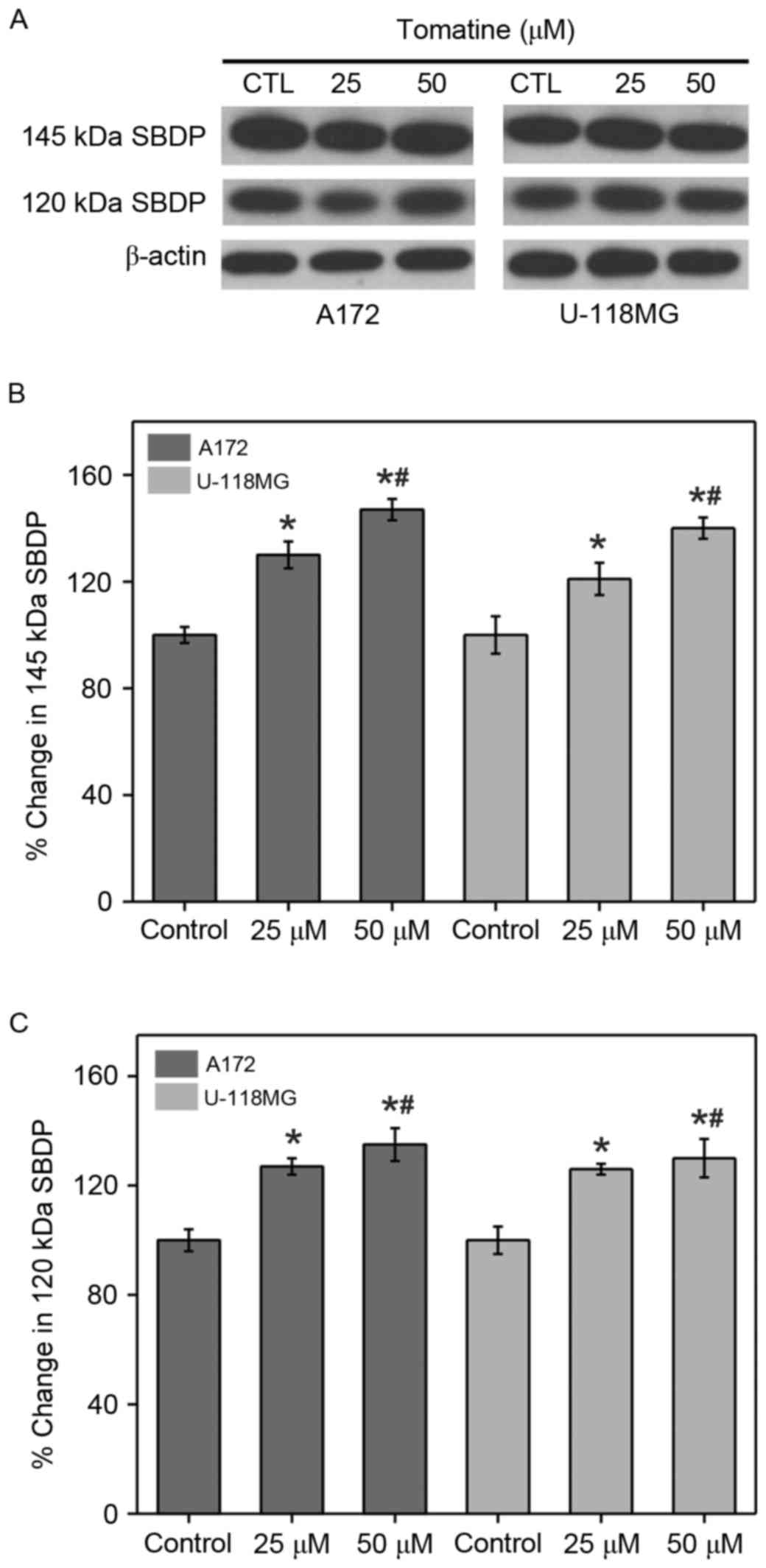
Calpain and caspase-3 activities
The enhancement in calpain and caspase-3 activities
from the generation of αII-spectrin breakdown products (SBDP) (145
and 120 kDa, respectively) were analyzed (Fig. 5A). The results indicated that the
protein levels of SBDP (145 and 120 kDa subunits) were
significantly upregulated upon addition of α-tomatine (25 µM,
P<0.05; 50 µM, P<0.01) in A172 and U-118 MG cell lines
(Fig. 5B and C). The above results
are in concordance with the results of caspase-3 in A172 and U-118
MG cell lines.
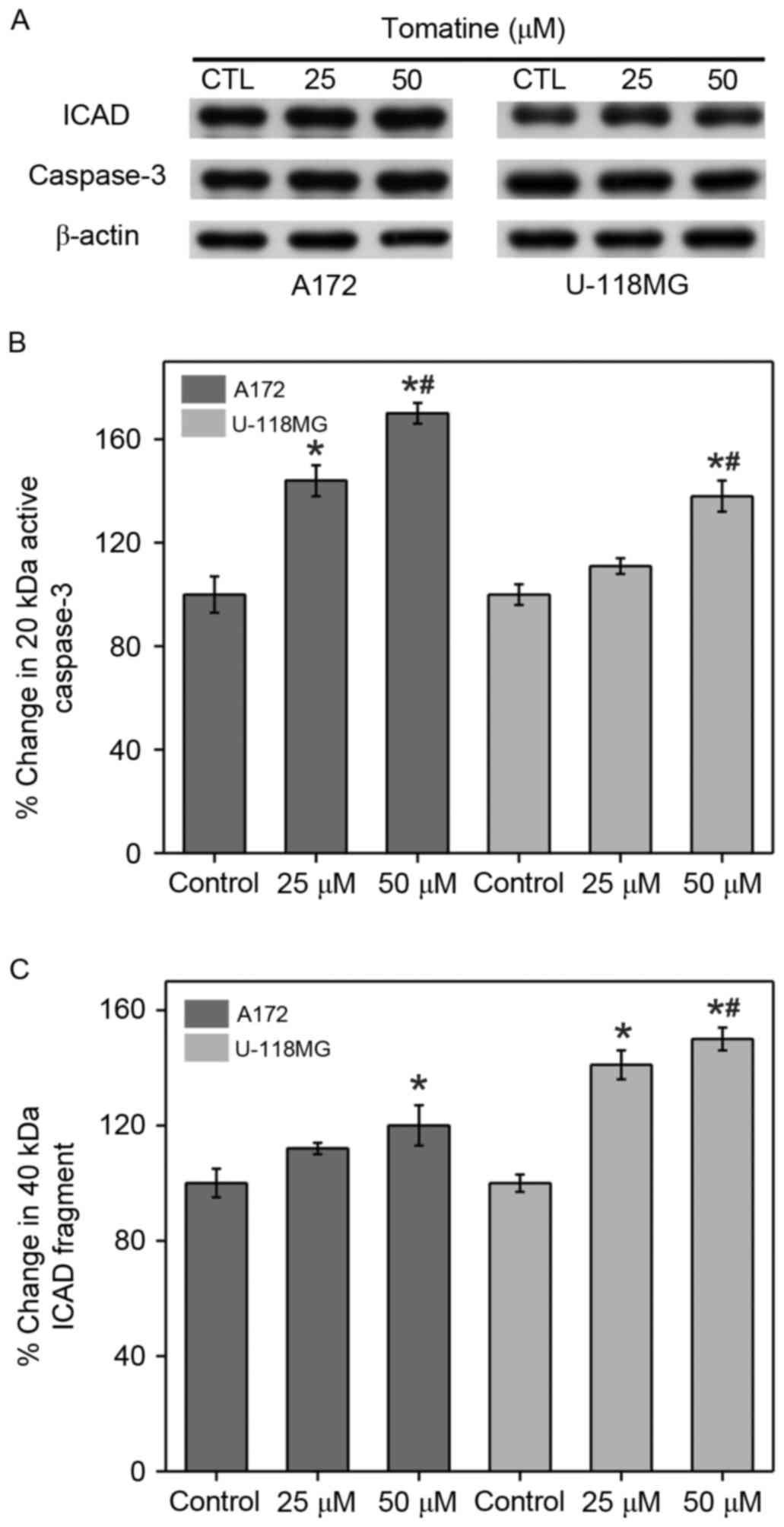
Caspase-3 activation and inhibition of
ICAD cleavage
The current study also investigated the activation
of caspase-3 and ICAD cleavage, which is capable of translocating
DNase activated by caspase to the nucleus for DNA fragmentation
(Fig. 6). In A172 and U-118 MG cell
lines, the protein expression of caspase-3 fragments (20 kDa) and
ICAD (40 kDa) was significantly enhanced after treatment with
α-tomatine (25 and 50 µM). Treatment with 25 and 50 µM α-tomatine
resulted in an increase in the protein expression of caspase-3
fragments (20 kDa) by 43% (P<0.05) and 71% (P<0.01) in A172
respectively, and by 10% (non-significant) and 38% (P<0.01) in
U-118 MG cell lines, respectively, as compared with the control
(Fig. 6B). Treatment with 25 and 50
µM α-tomatine increased the protein expression of ICAD (40 kDa) by
13% (non-significant) and 21% (P<0.05) in A172 respectively, and
by 40% (P<0.05) and 48% (P<0.01) in U-118 MG cell lines,
respectively, as compared with the control (Fig. 6C).
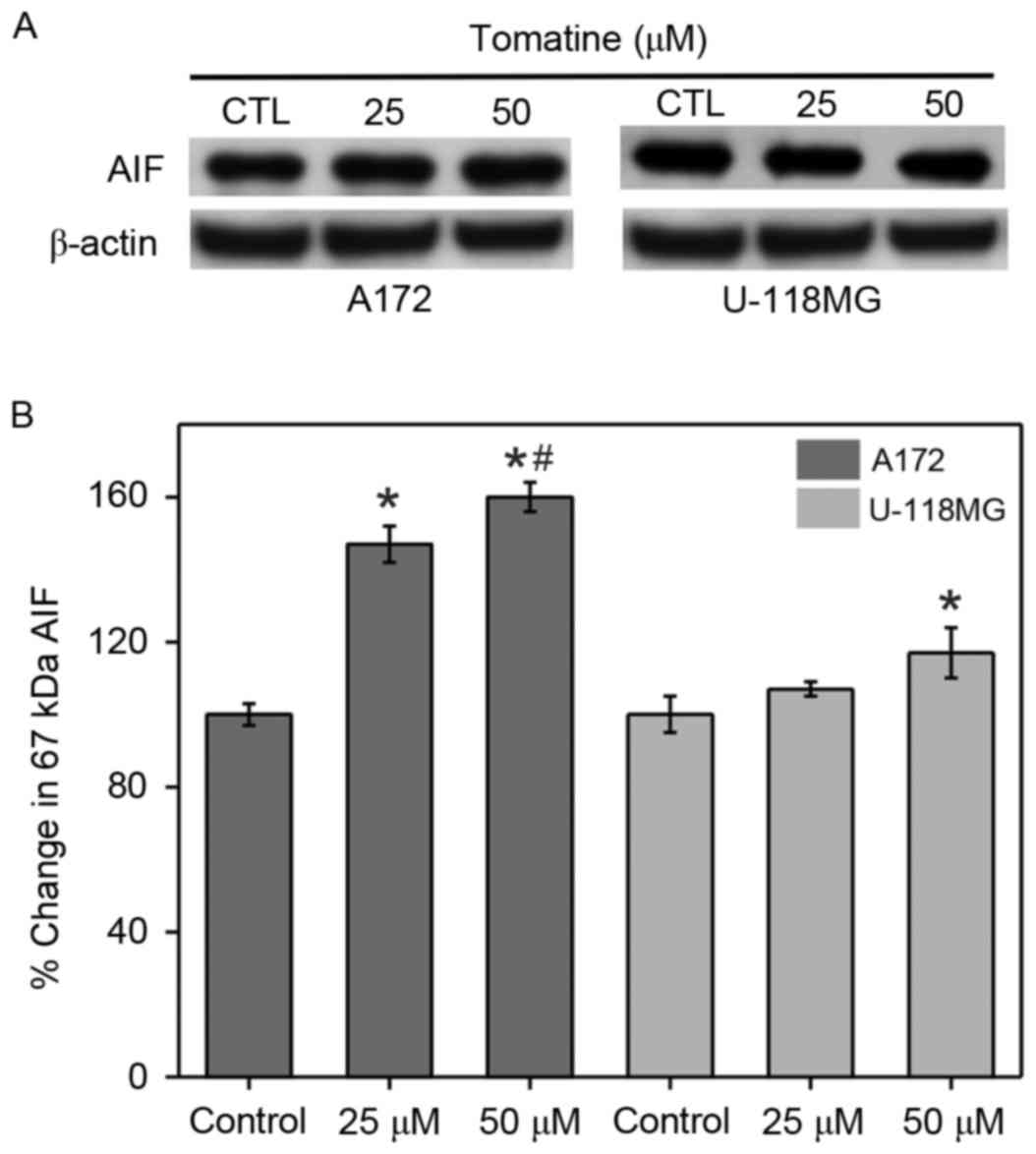
Enhancement in AIF cytosolic
levels
Elevation of the permeability of the mitochondrial
membrane can cause AIF release into the cytoplasm. Thus, AIF levels
in cytosolic fractions were determined in α-tomatine-treated A172
and U-118 MG cells (Fig. 7). The
protein expression level of AIF in the two cell lines was found to
be increased by α-tomatine treatment. Treatment with 25 µM
α-tomatine was observed to significantly increase AIF expression in
A172 cells compared with control cells (P<0.05), whereas this
increase was non-significant in U-118 MG cells. Treatment with 50
µM α-tomatine in the A172 and U-118 MG cell lines produced a
significant increase in the cytosolic AIF levels of 60% (P<0.01)
and 17% (P<0.05), respectively. These results suggested the
involvement of a caspase-independent pathway of apoptosis.
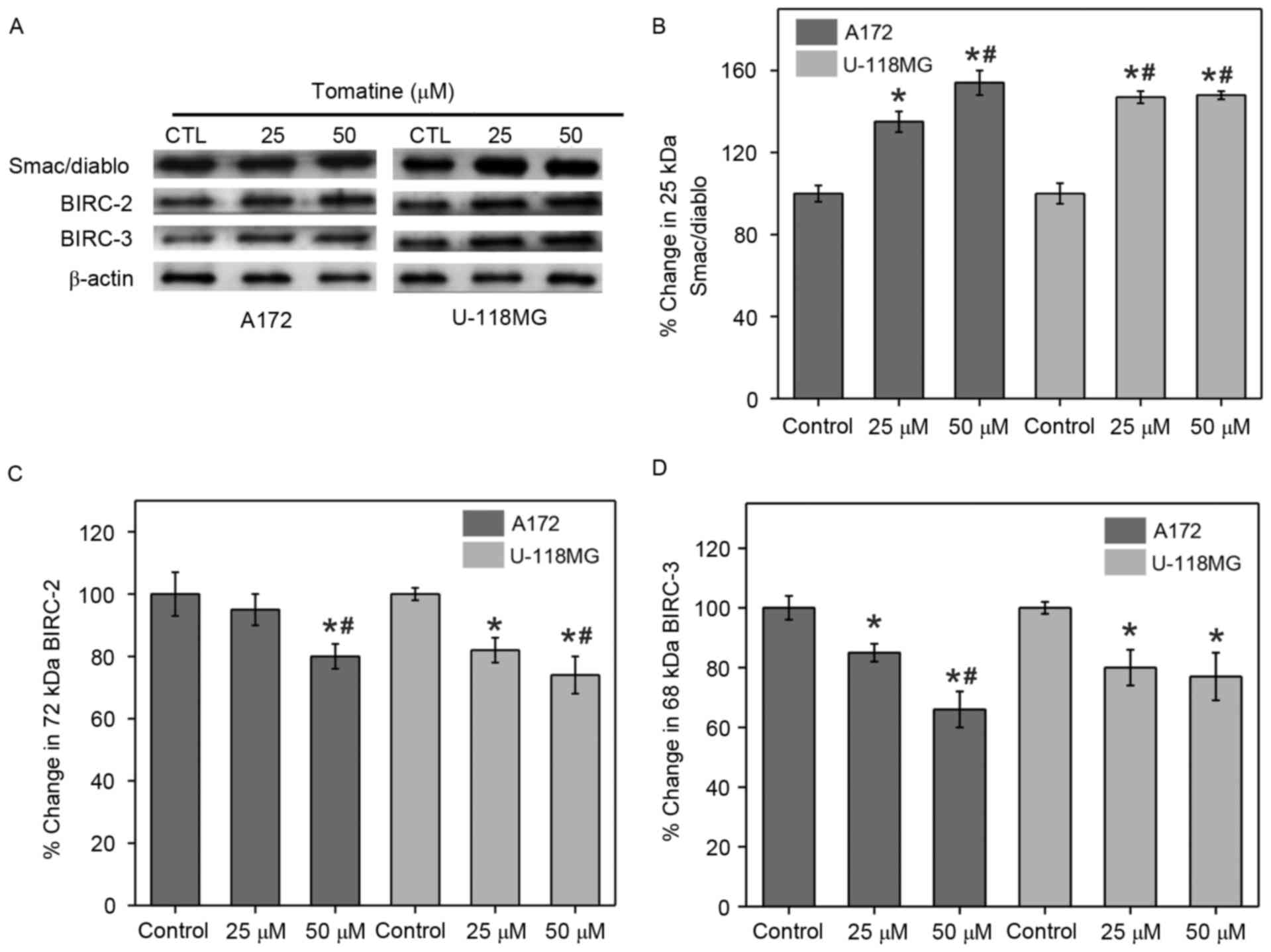
Changes in Smac/Diablo and inhibitor
of apoptosis proteins (IAPs)
The release of mitochondrial Smac/Diablo proteins
into the cytoplasm and the quantity of IAPs in the cytosol serve
major functions in apoptosis. Therefore, the present study
determined these levels with the help of western blotting
experiments using α-tomatine-treated A172 and U-118 MG cells.
Treatment with 25 µM α-tomatine resulted in a 35% (P<0.05) and
47% (P<0.01) increase in Smac/Diablo protein expression in A172
and U-118 MG cells, respectively (Fig.
8A and B). Also, treatment with 50 µM α-tomatine signficantly
increased the Smac/Diablo cytosolic levels in both A172 (54%;
P<0.01) and U-118 MG (48%; P<0.01) cells.
The current study also monitored the expression
levels of two IAPs, namely c-IAP1 and c-IAP2 that are also known as
Baculoviral IAP repeat-containing-2 (BIRC-2) and BIRC-3,
respectively, using western blot experiments. Treatment with 25 µM
α-tomatine did not show any significant difference on BIRC-2
expression in A172 cells (7%), while it caused a significant
reduction in U-118 MG cells (27%; P<0.05) as compared with the
control. However, 50 µM α-tomatine treatment significantly reduced
the protein expression of BIRC-2 in A172 (20%; P<0.01) and U-118
MG (29%; P<0.01) cells as compared with control (Fig. 8C). Furthermore, treatment with 25 µM
α-tomatine significantly downregulated the protein expression of
BIRC-3 in A172 (17%; P<0.05) and U-118 MG (20%; P<0.05) cell
lines as compared with control. Treatment with 50 µM α-tomatine
significantly reduced the expression of BIRC-3 in A172 (37%;
P<0.01) and U-118 MG (23%; P<0.05) cells as compared with
control (Fig. 8D). These reduced
expression levels of BIRC-2 and BIRC-3 are favorable for the
apoptosis process.
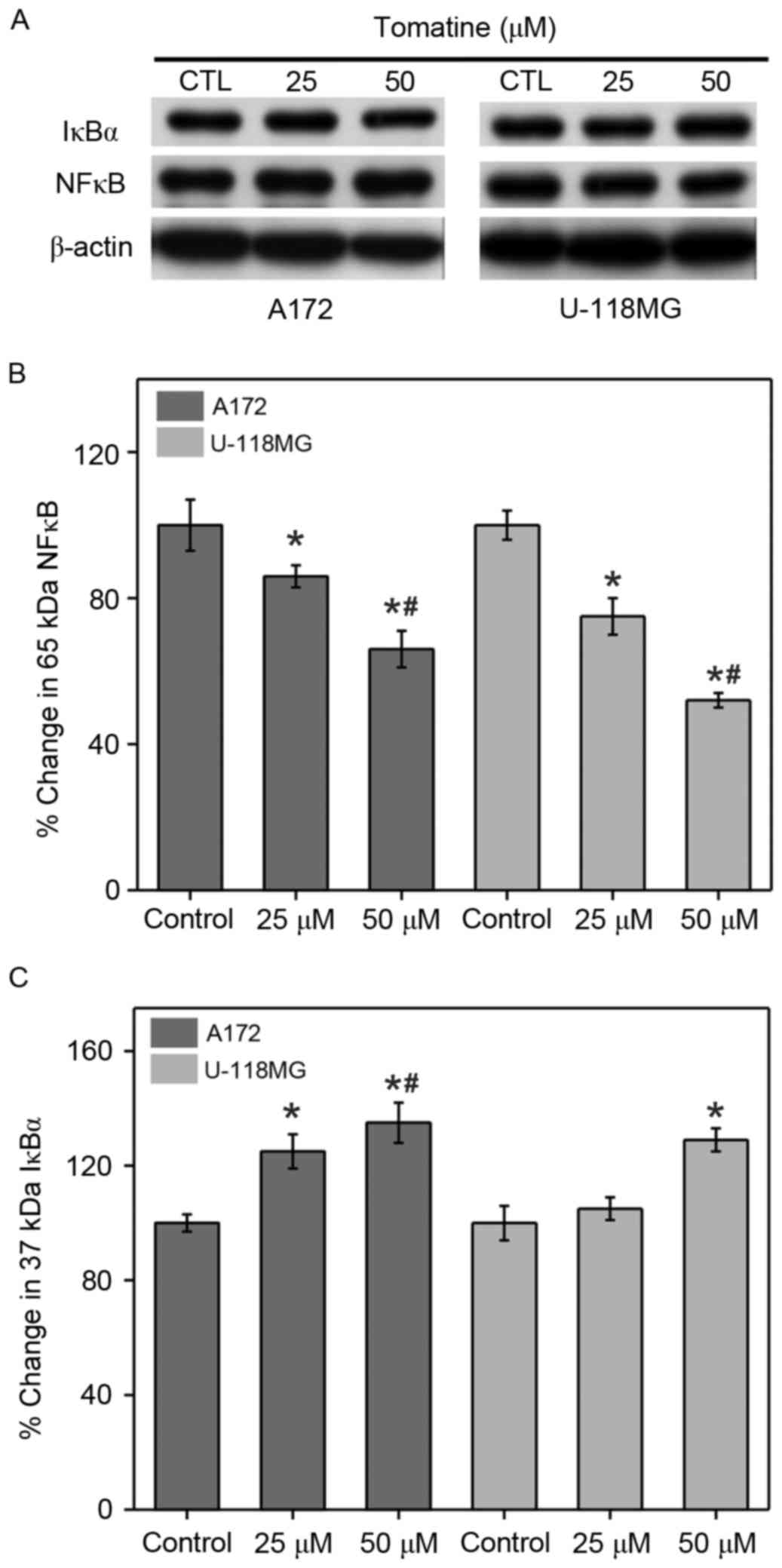
Effect on NF-κB expression
Cancer cells generally increase NF-κB and reduce the
inhibitor of NF-κB (IκBα) expression levels in order to promote IAP
expression for cell survival. Therefore, western blotting
experiments were conducted in the present study to assess the NF-κB
and IκBα levels in A172 and U-118 MG cells (Fig. 9). Treatment with 25 µM α-tomatine
generated a significant ~16% (P<0.05) decrease in NF-κB
expression in A172 cells, and a significant decrease of ~25%
(P<0.05) in U-118 MG cells as compared with the control.
Treatment with 50 µM α-tomatine significantly decreased NF-κB
expression in A172 (~34%; P<0.01) and U-118 MG (~48%; P<0.01)
cells (Fig. 9B). Furthermore,
treatment with 25 µM α-tomatine significantly increased the protein
expression of IκBα in A172 (26%; P<0.05), whereas no significant
increase was observed in U-118 MG cells. However, 50 µM α-tomatine
treatment significantly increased IκBα expression in both A172
(35%; P<0.01) and U-118 MG (28%; P<0.05) cell lines (Fig. 9C). These results suggest that the
alterations in the expression levels of NF-κB and IκBα were due to
α-tomatine-induced apoptosis.
Discussion
In the present study, α-tomatine-induced cell death
in A172 and U-118 MG human glioblastoma cell lines was reported for
the first time. Based on the obtained results from these
experiments, it was demonstrated that apoptosis was induced by
α-tomatine treatment and the occurrence of multiple molecular
mechanisms was observed. Apoptosis was investigated by observing
the morphological and biochemical characteristics of A172 and U-118
MG cells. For instance, α-tomatine triggered an enhancement in
[Ca2+]i that can induce cell death. The obtained results
revealed a connection between [Ca2+]i elevation and
calpain activity in the induction of cell death. Bax and Bcl-2
levels were also examined in the cell lines, and the ratio of
Bax:Bcl-2 was found to be raised following α-tomatine treatment,
which may assist in the induction of apoptosis. Overexpression of
Bax is known to alter the mitochondrial membrane permeability to
release cytochrome c, and thus the current study further
investigated the changes in the cytochrome c levels in both
cytosolic and mitochondrial fragments. The results indicated that
the cytosolic fraction levels were raised and mitochondrial levels
were decreased upon α-tomatine addition, strongly indicating the
participation of mitochondrial cytochrome c release in
apoptosis induction.
The present study also examined the
α-tomatine-induced cell death along with caspase-9, caspase-12 and
calpain activation in A172 and U-118 MG cells. The results
indicated that ER stress was caused by α-tomatine treatment for
apoptosis induction in human glioblastoma cell lines. The current
study results are comparable with similar apoptosis models shown by
other researchers (24,27,28).
Earlier studies demonstrated that ER stress can induce caspase-12
activation, which is directly associated with caspase-9 activation
(29,30). Furthermore, the present study
measured the activities of calpain and caspase-3 activation,
according to previous findings stating that the degradation of
α-spectrin to 145 and 120 kDa SBDP is responsible for caplain and
caspase-3 activation, respectively (31,32).
From the results obtained, it was proven that elevated calpain and
caspase-3 levels served a crucial function in α-tomatine-triggered
apoptosis in A172 and U-118 MG cell lines. A previous relevant
report also supported the association of calpain and caspase-3
activation with apoptosis (33).
Furthermore, α-tomatine was observed to trigger the
activation of caspase-3 as identified from the 20 kDa fragment of
caspase-3 and ICAD cleavage in the present study. These results
demonstrated that α-tomatine induced caspase-3 activation, which is
able to cleave ICAD to permit translocation of CAD into the nucleus
for caspase-dependent DNA nuclear fragmentation, and this finding
is consistent with the observations of a previous study (34).
Caspase-independent apoptosis is known to also be
induced by mitochondria upon the release of AIF (35); thus, the present study examined the
changes in AIF cytosolic levels to determine the feasibility of
caspase-independent apoptosis following α-tomatine treatment in the
glioblastoma cell lines. The data strongly indicated the
enhancement of AIF cytosolic levels after α-tomatine treatment,
proving that α-tomatine induced AIF mitochondrial efflux into the
cytosol. This translocation may produce caspase-independent
apoptosis and DNA nuclear fragmentation (36). According to the aforementioned
results, it can be demonstrated that both caspase-dependent and
independent pathways participated in the α-tomatine-directed
apoptosis in A172 and U-118 MG glioblastoma cell lines.
The Smac/Diablo release from mitochondria upon
α-tomatine treatment was also investigated. Since IAPs, such as
BIRC-2 and BIRC-3, can be downregulated by Smac/Diablo
pro-apoptotic molecules in the cytosol, the BIRC-2 and BIRC-3
cytosolic levels were investigated. α-Tomatine treatment was
observed to cause reduction in the expression levels of these two
genes, which are considered as feasible oncogenes (37). Thus, this suggests that the apoptosis
of α-tomatine may be due to the oncoprotein downregulation. In
addition, collective results indicated that α-tomatine treatment
induced apoptosis by elevating Smac/Diablo and reducing IAP levels,
as well as by caspase activation.
In cell survival, NF-κB activation serves a crucial
role (38), and thus, the
downregulation of NF-κB by compounds extracted from plant may be a
promising approach for preventing cancer development (39). In the experiments of the present
study, α-tomatine treatment triggered IκBα elevation and IAP
downregulation in the two cell lines, suggesting that other than
the apoptosis induction, the mechanisms of α-tomatine action
include IAP and NF-κB inhibition for the prevention of cell
survival signals. The α-tomatine-induced inhibition of NF-κB was
due to its decreased binding with DNA. Furthermore, the apoptosis
induction in A172 and U-118 MG by α-tomatine treatment was shown to
be p53 independent (40).
In conclusion, the present study explored the
multiple molecular mechanisms involved in the apoptosis induction
following α-tomatine treatment in A172 and U-118 MG cell lines.
α-Tomatine exhibited its proapoptotic potentials at 25 and 50 µM
levels within the physiological dosage. Xenografted/allografted
animal models will be used in our future studies for further
confirmation of these findings.
Acknowledgements
The authors would like to thank The People's
Hospital of Zoucheng for providing financial support to perform
this study.
References
|
1
|
Eitel K, Wagenknecht B and Weller M:
Inhibition of drug-induced DNA fragmentation, but not cell death,
of glioma cells by non-caspase protease inhibitors. Cancer Lett.
142:11–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Castro MG, Cowen R, Smith-Arica J,
Williams J, Ali S, Windeatt S, Gonzalez-Nicolini V, Maleniak T and
Lowenstein PR: Gene therapy strategies for intracranial tumours:
Glioma and pituitary adenomas. Histol Histopathol. 15:1233–1252.
2000.PubMed/NCBI
|
|
3
|
Yang QY, Shen D, Sai K, Jiang XB, Ke C,
Zhang XH, Mou YG and Chen ZP: Survival of newly diagnosed malignant
glioma patients on combined modality therapy. Zhonghua yi xue za
zhi. 93:8–10. 2013.(In Chinese). PubMed/NCBI
|
|
4
|
Takahashi H and Teramoto A: Trial of
targeting therapy against malignant glioma using monoclonal
antibody. J Nippon Med Sch. 71:2–3. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jamal M, Rath BH, Tsang PS, Camphausen K
and Tofilon PJ: The brain microenvironment preferentially enhances
the radioresistance of CD133(+) glioblastoma stem-like cells.
Neoplasia. 14:150–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan W, Lu J, Huang M, Li Y, Chen M, Wu G,
Gong J, Zhong Z, Xu Z, Dang Y, et al: Anti-cancer natural products
isolated from Chinese medicinal herbs. Chin Med. 6:272011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meiyanto E, Hermawan A and Anindyajati:
Natural products for cancer targeted therapy: Citrus flavonoids as
potent chemopreventive agents. Asian Pac J Cancer Prev. 13:427–436.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong JB, Seo EW and Jeong HJ: Effect of
extracts from pine needle against oxidative DNA damage and
apoptosis induced by hydroxyl radical via antioxidant activity.
Food Chem Toxicol. 47:2135–2141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh BN, Singh BR, Singh RL, Prakash D,
Dhakarey R, Upadhyay G and Singh HB: Oxidative DNA damage
protective activity, antioxidant and anti-quorum sensing potentials
of Moringa oleifera. Food Chem Toxicol. 47:1109–1116. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blankemeyer JT, White JB, Stringer BK and
Friedman M: Effect of alpha-tomatine and tomatidine on membrane
potential of frog embryos and active transport of ions in frog
skin. Food Chem Toxicol. 35:639–646. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roddick JG: The steroidal glycoalkaloid
α-tomatine. Phytochemistry. 13:9–25. 1974. View Article : Google Scholar
|
|
13
|
Chiu FL and Lin JK: Tomatidine inhibits
iNOS and COX-2 through suppression of NF-kappaB and JNK pathways in
LPS-stimulated mouse macrophages. FEBS Lett. 582:2407–2412. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih YW, Shieh JM, Wu PF, Lee YC, Chen YZ
and Chiang TA: Alpha-tomatine inactivates PI3K/Akt and ERK
signaling pathways in human lung adenocarcinoma A549 cells: Effect
on metastasis. Food Chem Toxicol. 47:1985–1995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friedman M and Levin CE: alpha-Tomatine
content in tomato and tomato products determined by HPLC with
pulsed amperometric detection. J Agric Food Chem. 43:1507–1511.
1995. View Article : Google Scholar
|
|
16
|
Lee KR, Kozukue N, Han JS, Park JH, Chang
EY, Baek EJ, Chang JS and Friedman M: Glycoalkaloids and
metabolites inhibit the growth of human colon (HT29) and liver
(HepG2) cancer cells. J Agric Food Chem. 52:2832–2839. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shieh JM, Cheng TH, Shi MD, Wu PF, Chen Y,
Ko SC and Shih YW: α-Tomatine suppresses invasion and migration of
human non-small cell lung cancer NCI-H460 cells through
inactivating FAK/PI3K/Akt signaling pathway and reducing binding
activity of NF-κB. Cell Biochem Biophys. 60:279–310. 2011.
View Article : Google Scholar
|
|
18
|
Lee ST, Wong PF, Cheah SC and Mustafa MR:
Alpha-tomatine induces apoptosis and inhibits nuclear factor-kappa
B activation on human prostatic adenocarcinoma PC-3 cells. PLoS
One. 6:e189152011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kúdelová J, Seifrtová M, Suchá L, Tomšík
P, Havelek R and Řezáčová M: Alpha-tomatine activates cell cycle
checkpoints in the absence of DNA damage in human leukemic MOLT-4
cells. J Appl Biomed. 11:93–103. 2013. View Article : Google Scholar
|
|
20
|
Shi MD, Shih YW, Lee YS, Cheng YF and Tsai
LY: Suppression of 12-O-tetradecanoylphorbol-13-acetate-induced
MCF-7 breast adenocarcinoma cells invasion/migration by α-tomatine
through activating PKCα/ERK/NF-κB-dependent MMP-2/MMP-9
expressions. Cell Biochem Biophys. 66:161–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang YW, Wu CA and Morrow WJ: The
apoptotic and necrotic effects of tomatine adjuvant. Vaccine.
22:2316–2327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ray SK, Wilford GG, Crosby CV, Hogan EL
and Banik NL: Diverse stimuli induce calpain overexpression and
apoptosis in C6 glioma cells. Brain Res. 829:18–27. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ray SK, Karmakar S, Nowak MW and Banik NL:
Inhibition of calpain and caspase-3 prevented apoptosis and
preserved electrophysiological properties of voltage-gated and
ligand-gated ion channels in rat primary cortical neurons exposed
to glutamate. Neuroscience. 139:577–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das A, Sribnick EA, Wingrave JM, Del Re
AM, Woodward JJ, Appel SH, Banik NL and Ray SK: Calpain activation
in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons
exposed to glutamate: Calpain inhibition provides functional
neuroprotection. J Neurosci Res. 81:551–562. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grynkiewicz G, Poenie M and Tsien RY: A
new generation of Ca2+ indicators with greatly improved
fluorescence properties. J Biol Chem. 260:3440–3450.
1985.PubMed/NCBI
|
|
26
|
Hansen CA, Monck JR and Williamson JR:
Measurement of intracellular free calcium to investigate
receptor-mediated calcium signaling. Methods Enzymol. 191:691–706.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sribnick EA, Ray SK and Banik NL: Estrogen
prevents glutamate-induced apoptosis in C6 glioma cells by a
receptor-mediated mechanism. Neuroscience. 137:197–209. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sribnick EA, Ray SK, Nowak MW, Li L and
Banik NL: 17beta-estradiol attenuates glutamate-induced apoptosis
and preserves electrophysiologic function in primary cortical
neurons. J Neurosci Res. 76:688–696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rao RV, Castro-Obregon S, Frankowski H,
Schuler M, Stoka V, del Rio G, Bredesen DE and Ellerby HM: Coupling
endoplasmic reticulum stress to the cell death program. An
Apaf-1-independent intrinsic pathway. J Biol Chem. 277:21836–21842.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent activation
of caspase-9 by caspase-12. J Biol Chem. 277:34287–34294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nath R, Raser KJ, Stafford D,
Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P,
Gilbertsen RB and Wang KK: Non-erythroid alpha-spectrin breakdown
by calpain and interleukin 1 beta-converting-enzyme-like
protease(s) in apoptotic cells: Contributory roles of both protease
families in neuronal apoptosis. Biochem J. 319:683–690. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang KK, Posmantur R, Nath R, McGinnis K,
Whitton M, Talanian RV, Glantz SB and Morrow JS: Simultaneous
degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in
apoptotic cells. J Biol Chem. 273:22490–22497. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neumar RW, Xu YA, Gada H, Guttmann RP and
Siman R: Cross-talk between calpain and caspase proteolytic systems
during neuronal apoptosis. J Biol Chem. 278:14162–14167. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jayanthi S, Deng X, Noailles PA, Ladenheim
B and Cadet JL: Methamphetamine induces neuronal apoptosis via
cross-talks between endoplasmic reticulum and
mitochondria-dependent death cascades. FASEB J. 18:238–251. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cregan SP, Dawson VL and Slack RS: Role of
AIF in caspase-dependent and caspase-independent cell death.
Oncogene. 23:2785–2796. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Susin SA, Daugas E, Ravagnan L, Samejima
K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T,
Prévost MC, et al: Two distinct pathways leading to nuclear
apoptosis. J Exp Med. 192:571–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Imoto I, Yang ZQ, Pimkhaokham A, Tsuda H,
Shimada Y, Imamura M, Ohki M and Inazawa J: Identification of cIAP1
as a candidate target gene within an amplicon at 11q22 in
esophageal squamous cell carcinomas. Cancer Res. 61:6629–6634.
2001.PubMed/NCBI
|
|
38
|
Lee R and Collins T: Nuclear factor-kappaB
and cell survival: IAPs call for support. Circ Res. 88:262–264.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dorai T and Aggarwal BB: Role of
chemopreventive agents in cancer therapy. Cancer Lett. 215:129–140.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fimognari C, Sangiorgi L, Capponcelli S,
Nüsse M, Fontanesi S, Berti F, Soddu S, Cantelli-Forti G and Hrelia
P: A mutated p53 status did not prevent the induction of apoptosis
by sulforaphane, a promising anti-cancer drug. Invest New Drugs.
23:195–203. 2005. View Article : Google Scholar : PubMed/NCBI
|