Introduction
Vitreoretinopathy is one of the most common
retinopathies and is one of the complications of rhegmatogenous
retinal detachment retinal detachment surgery (1). There are many clinical manifestations
of vitreoretinopathy, such as glass brown granules and grey cells
existing in vitreous body, hyperplasia of proliferative vitreous
retinopathy, retinal stiffness and wrinkles, subretinal membranes
and tractional detachment of retina (2,3).
Researches have showed that retinal detachment with avascularity of
the peripheral retina typically is associated with familial
exudative vitreoretinopathy, which can cause by mutations of KIF11
and lead to microcephaly, lymphedema, chorioretinal dysplasia,
microcephaly, chorioretinal dysplasia and mental retardation
(4). Currently, surgeries and drug
treatments are efficient way to cure vitreoretinopathy, while
vascular active vitreoretinopathy (VAVR) frequently remains one of
the most severe complications of rhegmatogenous retinal detachment
(RD) with an incidence of 5–11% (5).
VAVR also presents one of the most frequent causes of surgical
failure for patients with VAVR (6).
Previous report has showed that pegaptanib sodium
(PGSD) treatment is efficient for VAVR by decreasing subretinal
exudation and leakage determined by fluorescein angiography
(7). PGSD is selective vascular
endothelial growth factor (VEGF) inhibitor that can decrease the
formation of new blood vessels in the choroid and reduce the
leakage of pathological changes of the blood vessels (8,9). An
exploratory analysis has indicated the efficacy of PGSD for early
treatment of nonvascular age-related macular degeneration (10). Interestingly, the therapeutic effects
of PGSD for ocular vascular disease also have been reviewed
(11). However, the importance of
PGSD treatment for patients with VAVR has not been well
investigated.
In this study, we investigated the efficacy of PGSD
for clinical nursing of VAVR patients. We evaluated the
ameliorative effects of PGSD for VAVR patients after surgical
treatment. Our investigations suggest that PGSD injection is a
potential agent for the treatment of patients with VAVR.
Materials and methods
Study design, subjects and
sampling
A total of 82 patients with VAVR were recruited in
this retrospective study. All patients were confirmed VAVR by
pathophysiology reported previously (12). The age of patient's was 48.8±12.6
years. Subjects include 42 female patients and 40 male patients.
Inclusion criteria for individuals with VAVR were diagnosed by
fluorescence fundus angiography. The Institutional Review Board
approval was obtained for this study. The study protocol was
approved by the Central Ethics Committee (Ethics Committee of
Center of Tianjin Medical University; Approval number:
TJMU20140311EX). Inclusion criteria include patients with
rhegmatogenous retinal detachment retinal detachment surgery.
Exclusion criteria include patients with no other metabolic disease
(such as diabetes mellitus and scurvy). All patients were required
to write informed consent with signature.
Drugs administration
In total, 82 patients were enrolled in this study
and were randomized into two groups based on age and gender match.
All of the patients completed the study in 12 months follow-up
period. The indicated dosage of ophthalmic solutions was PGSD (0.3
mg, Macugen; Eyetech Pharmaceuticals, New York, NY) or placebo
(same amount of normal sodium, Harbin pharmaceutical group, China)
injection was used to treat VAVR patients. Patients with VAVR were
given 0.3 mg and patients in the placebo group received 0.3 mg
sodium solution via intravitreal injection once every six week.
ELISA
Serum levels of IL-1β (MBS700340, Thermo Fisher
Scientific) and TNFα (MBS6080, Thermo Fisher Scientific) were
analyzed in patients with VAVR after received PGSD intravitreal
injection or placebo using ELISA kit according to the
manufacturer's instrument. The serum concentration levels of IL-1β
and TNFα were measured by an enzyme micro-plate reader at 450
nm.
Inflammation severity score and
chamber flare
Criteria for evaluation were the reduction in
anterior chamber flare and inflammation severity score (primary
efficacy criteria) as well as different secondary efficacy and
safety evaluation criteria. Mean inflammation severity score were
evaluated according to previous report (13).
Intraocular pressure measurement
Corneal surface intraocular pressure in each patient
with VAVR was measured using a Tono-Pen AVIA®
Applanation Tonometer (Reichert Technologies, USA). To minimize
circadian oscillation, intraocular pressure measurement
measurements were measured once every 7 days at 12:30 pm in all
patients during 12-months follow-up. The intraocular pressure was
sorted out by call visits.
Clinical Assessments
All measurements were performed by the same
technician in two groups during the 12-month follow-up, on day 0
after surgery, 4th, and 12th months. The efficacy of PGSD on
aqueous flare, subretinal exudation and leakage, visual acuity and
vitreoretinal traction was using methods reported previously
(14–16). Each result of clinical assessments
was determined based on the mean of five measurements.
Statistical analysis
Continuous variables were shown as mean ± SD and
analyzed by students t test. All data were analyzed using SPSS
Statistics 19.0 (version 19.0; SPSS Inc., Chicago, IL, USA) and
Graphpad Prism version 5.0 with the help of Microsoft Excel.
Unpaired data was determined by Student's t test and comparisons of
data between multiple groups were analyzed by variance (ANOVA). A
P-value of ≤0.05 was considered statistically significant.
Results
Characteristics of patients with
VAVR
A total of 82 patients with FEVR were enrolled and
to analyze the efficacy of PGSD injection treatment. Mean age of
patients were 48.8±12.6 years and 42 patients were female and 40
patients were male. The average inflammation score was 2.5±1.0 and
intra-ocular pressure 12.4±3.6 mm Hg. Patients were randomly
divided into two groups and received a single intravitreal
injection of PGSD (10 mg/day) or placebo. The characteristics of
patients with VAVR were shown in Table
I.
 | Table I.Clinical characteristic of patients
with VAVR. |
Table I.
Clinical characteristic of patients
with VAVR.
| Characteristic | Placebo | PGSD | P-value |
|---|
| Number | 38 | 44 | >0.05 |
| Gender
(male/female) | 18/20 | 23/21 | >0.05 |
| Age (years) | 36.2–60.4 | 36.5–61.4 | >0.05 |
| Corneal thickness
(µm) | 527.3±53.7 | 526.8±58.5 | >0.05 |
| Inflammation
severity | 3.4±0.7 | 3.3±0.8 | >0.05 |
| Intraocular pressure
(mm Hg) | 15.2±3.5 | 15.4±3.2 | >0.05 |
| Aqueous flare
(p/msec) | 9.1±1.8 | 9.2±2.0 | >0.05 |
The efficacy treatment of PGSD
intravitreal injection on subretinal exudation and leakage in
patients with VAVR
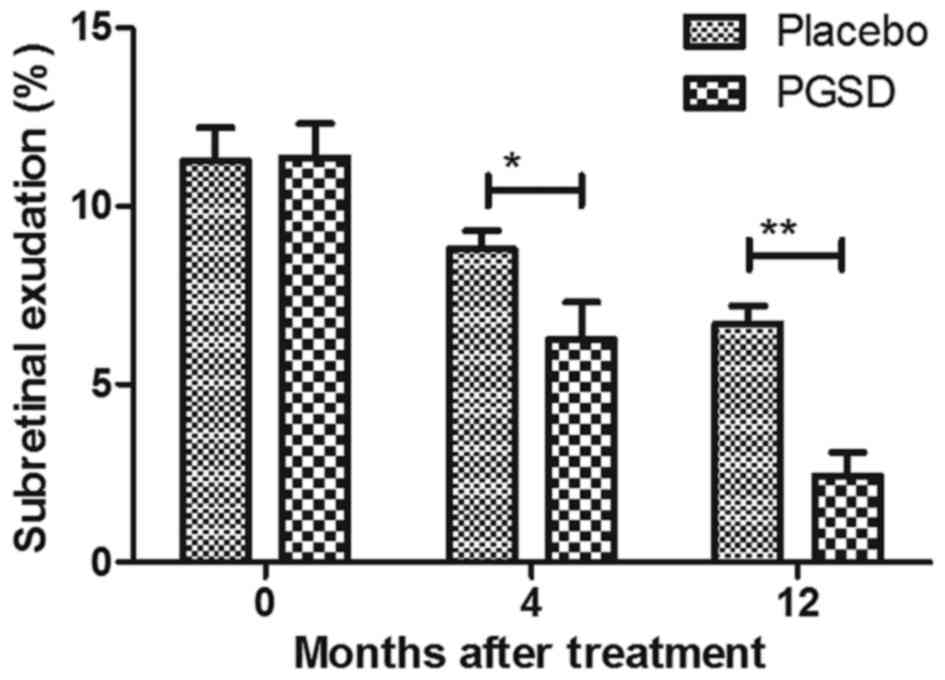
The efficacies of PGSD injection in on subretinal
exudation and leakage were investigated in patients with VAVR after
4 and 12 month treatment. Outcomes presented a significantly
reduction of subretinal exudation in patients after receiving
treatment of intravitreal injection of PGSD (Fig. 1). We observed intravitreal injection
of PGSD markedly decreased leakage by fluorescein angiography in
patients with VAVR (Fig. 2). These
outcomes suggest that PGSD intravitreal injection were
significantly improved subretinal exudation and leakage in patients
with VAVR after 12-month treatment, which presented enough benefits
compared to placebo and 0- and 4-month PGSD treatment.
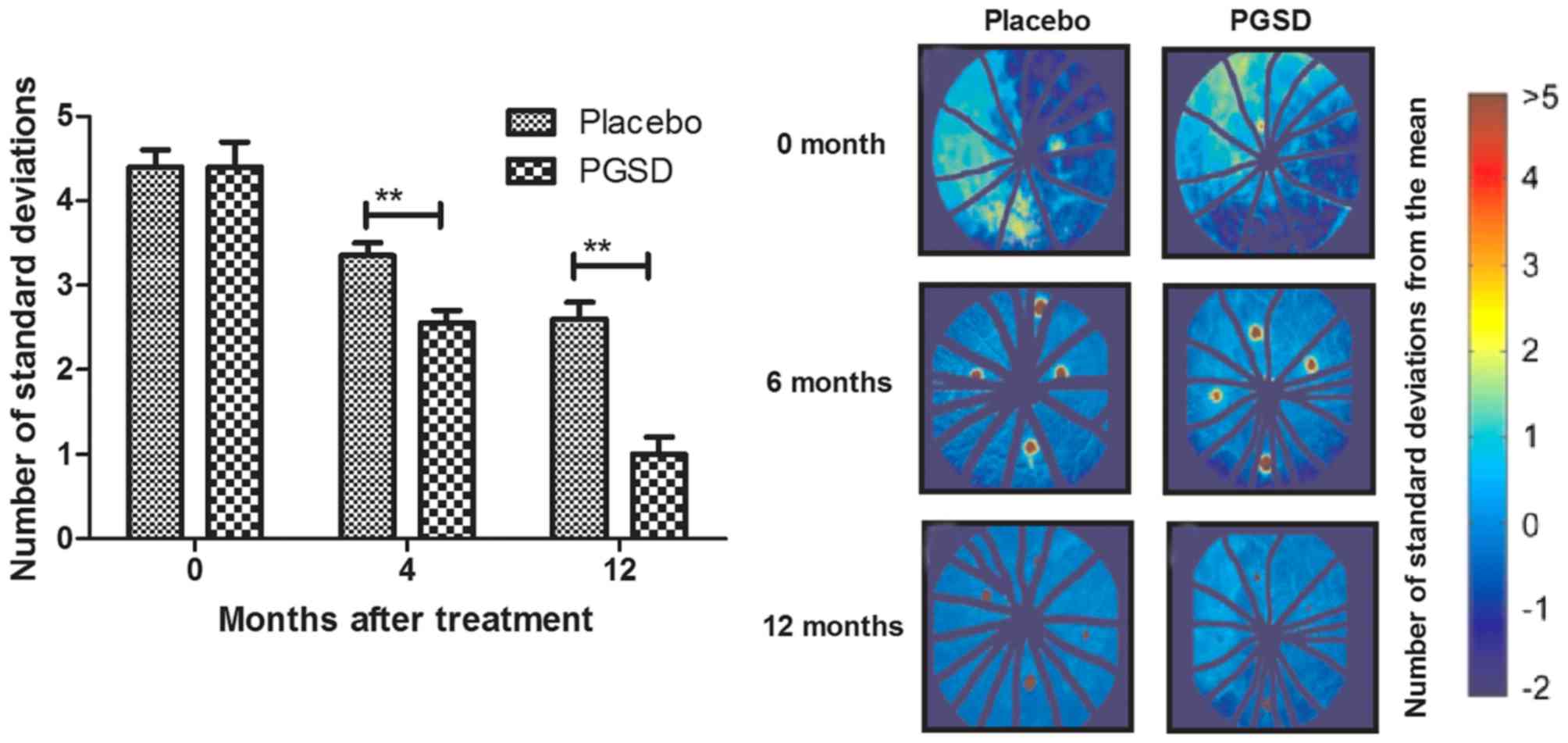
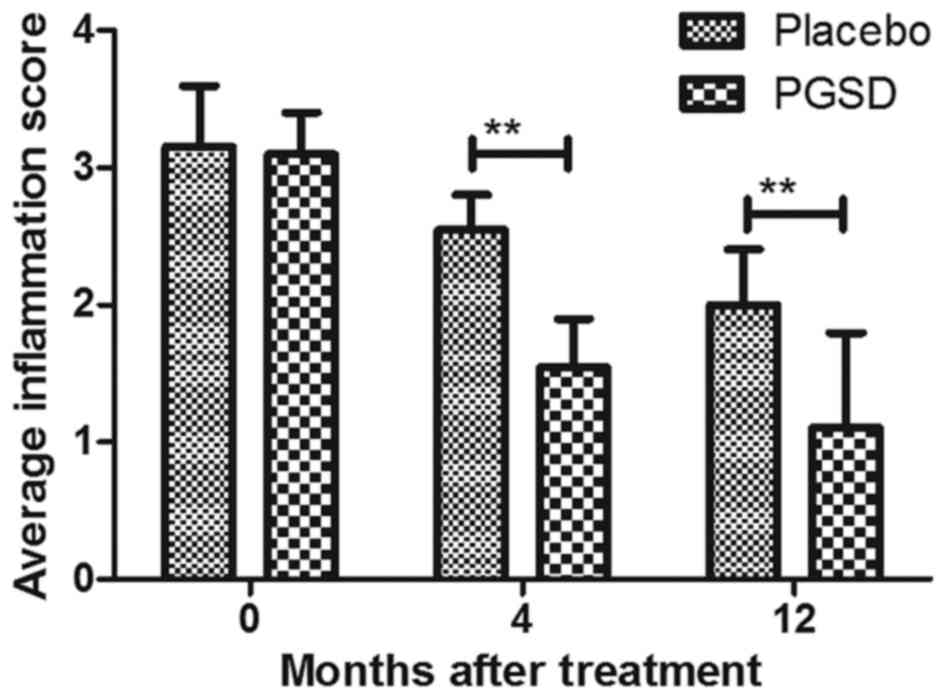
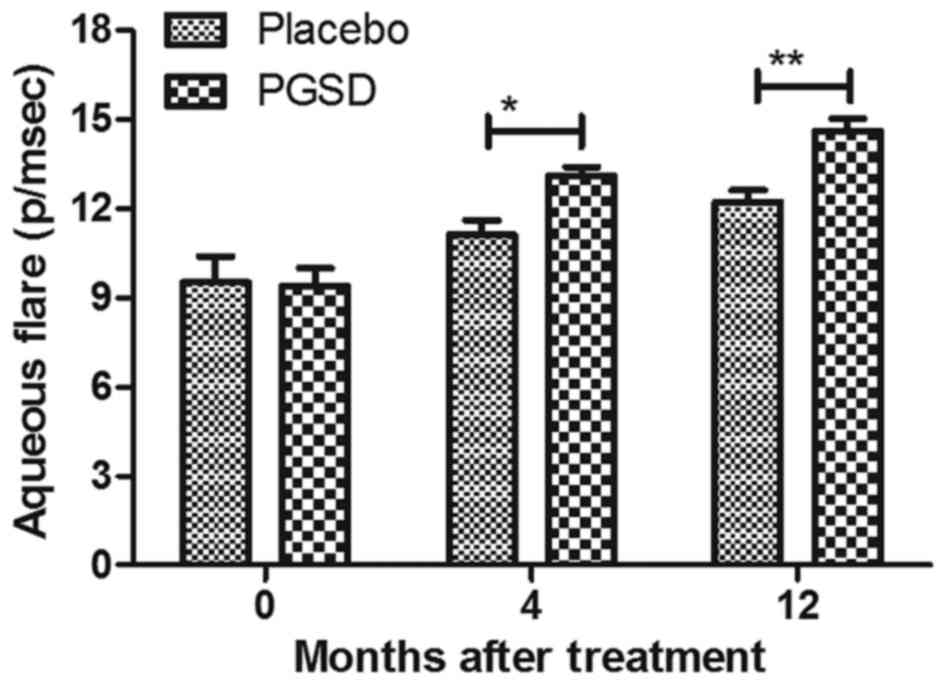
The efficacy of treatment of PGSD
intravitreal injection on inflammation and intra-ocular pressure in
patients with VAVR
We evaluated the ameliorative effects of treatment
of PGSD intravitreal injection on inflammation and intra-ocular
pressure in patients with VAVR. Outcomes demonstrated that average
inflammation score was lower in PGSD treatment group (1.1±0.7) than
in placebo (1.9±0.7) (P<0.05, Fig.
3) after 12-month observations. Aqueous flare examination also
showed that PGSD significantly improved aqueous for patients with
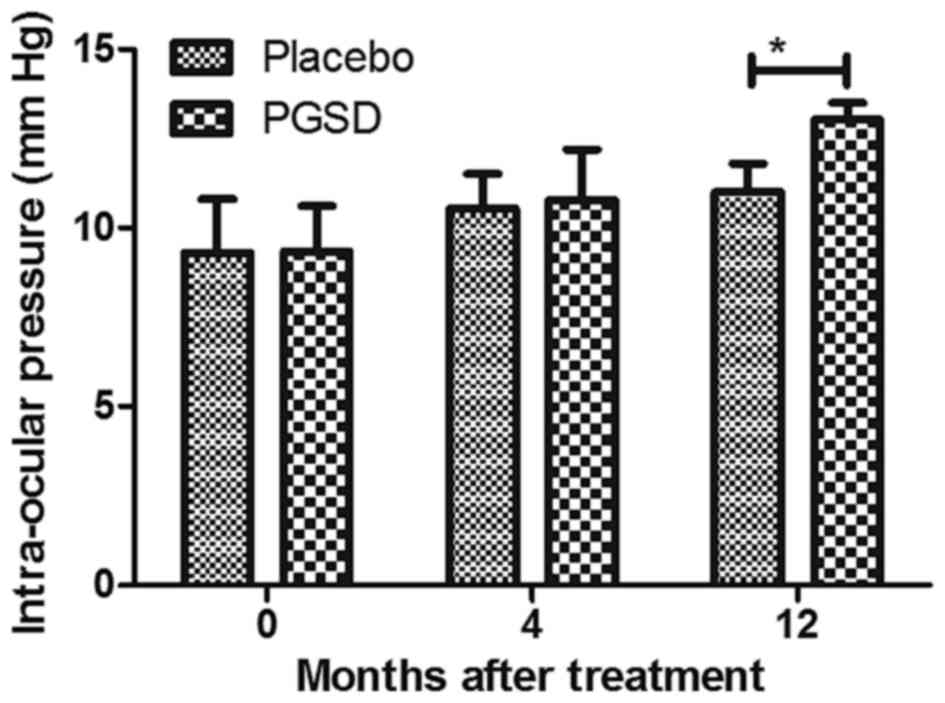
VAVR (Fig. 4). Intra-ocular pressure
analysis showed that PGSD intravitreal injection (13.6±3.8)
improved intra-ocular pressure compared to placebo (10.2±4.1 mmHg)
(Fig. 5). Serum levels of
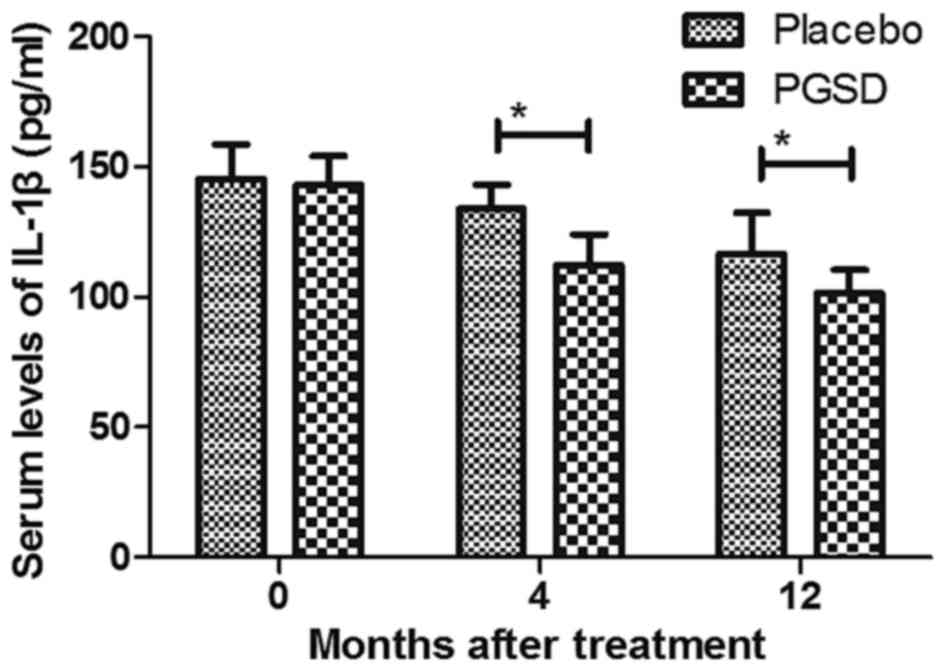
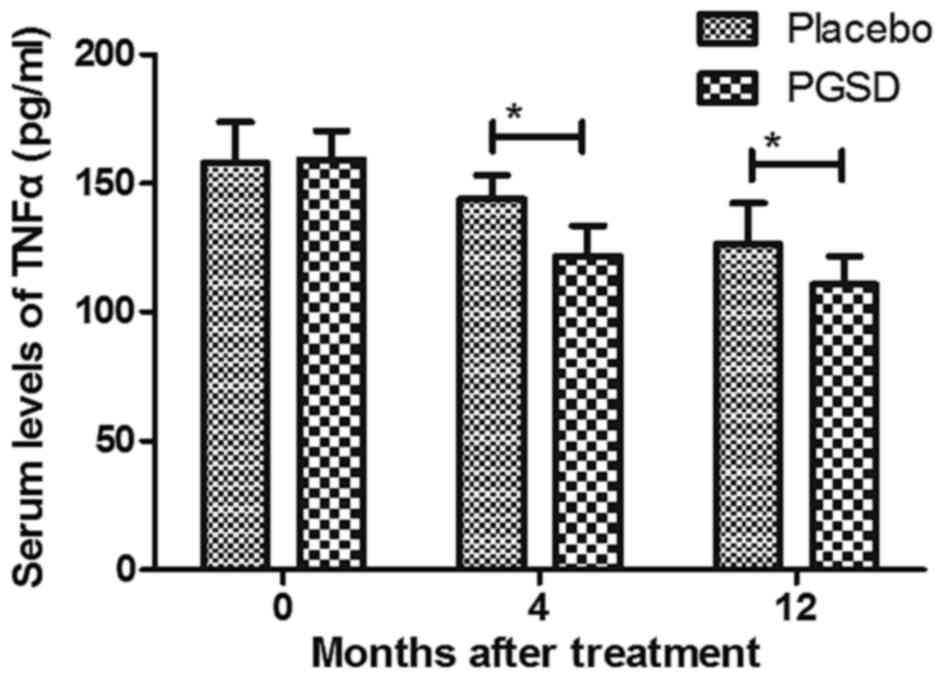
inflammatory cytokines IL-1β and TNFα were decreased in PGSD
intravitreal injection-treated patients with VAVR (Figs. 6 and 7). We demonstrated that 12-month PGSD
intravitreal injection presented enough benefits on inhibition of
inflammation and improvements of intra-ocular pressure compared to
placebo and 0- and 4-month PGSD treatment. These results suggest
that PGSD intravitreal injection treatment plays ameliorative role
in inflammation and intra-ocular pressure in patients with
VAVR.
The efficacy of PGSD intravitreal
injection treatment on visual acuity and vitreoretinal traction in
patients with VAVR
The efficacy of PGSD intravitreal injection on
visual acuity and vitreoretinal traction was analyzed in patients
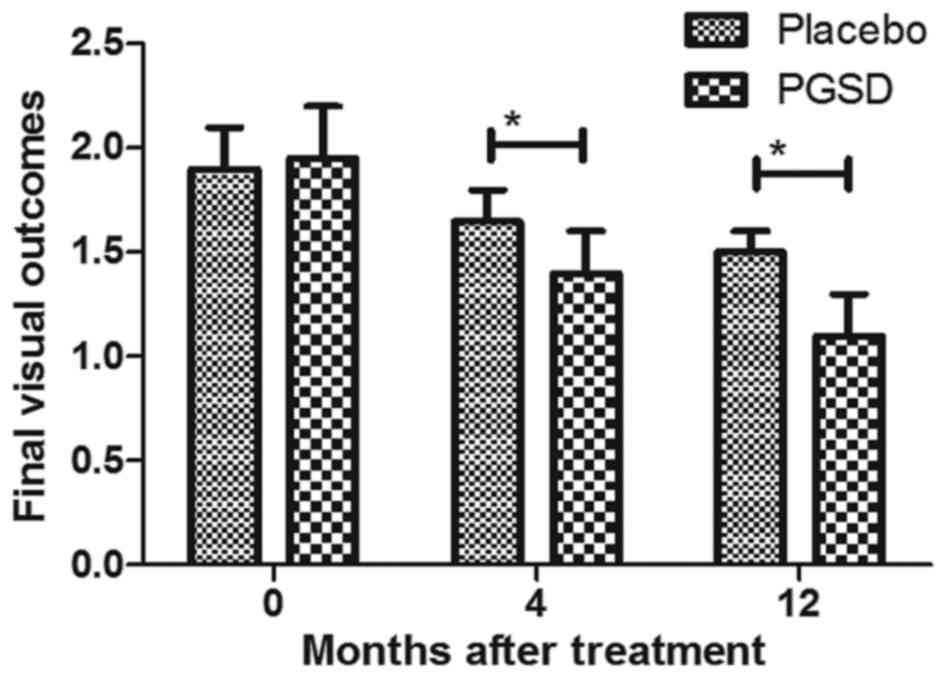
with VAVR. As shown in Fig. 8, PGSD
treatment significantly improved final visual outcomes compared to
placebo group. Results demonstrated mean best-corrected visual
acuity significantly improved from 0.30 at baseline to 0.11 in PGSD
group, while it was from 0.32 at baseline to 0.15 in placebo group.
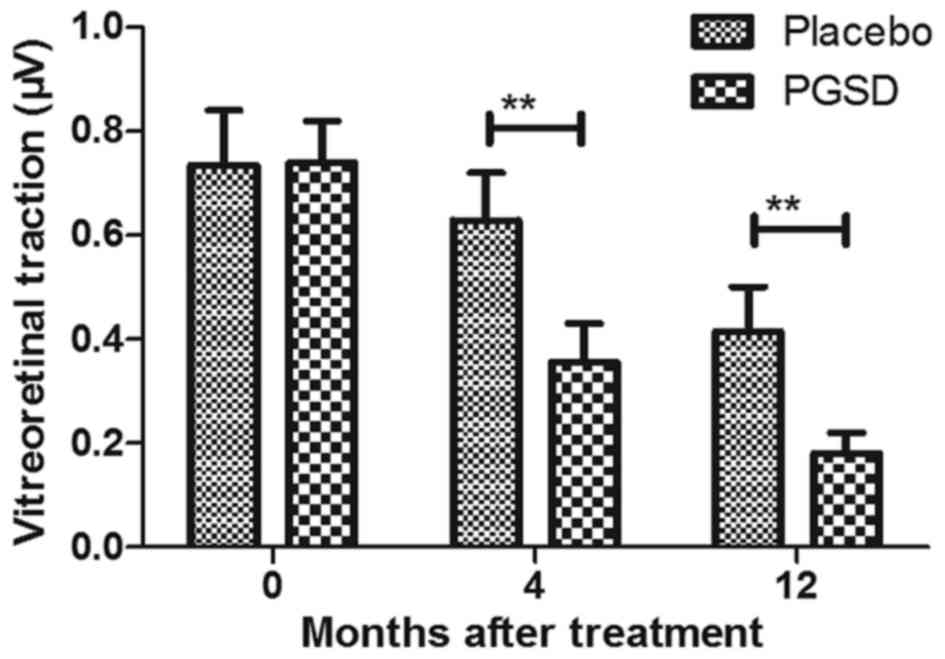
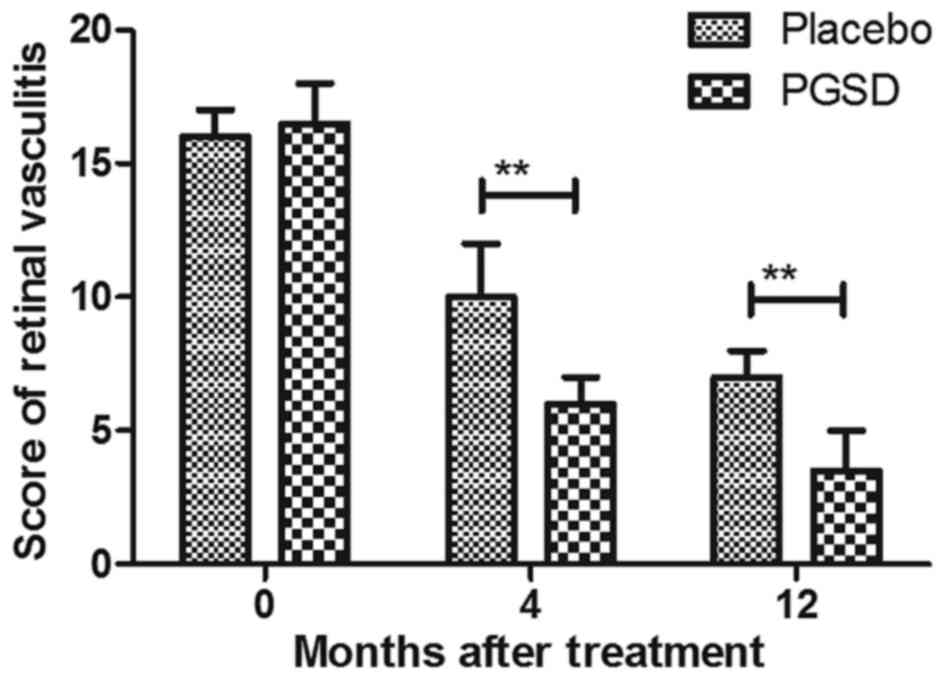
Vitreoretinal traction was improved and further meliorated retinal
vasculitis for patients after treatment with PGSD intravitreal
injection (Figs. 9 and 10). These results suggest that 12-month
PGSD intravitreal injection presented more efficacies compared to
placebo and 0- and 4-month PGSD treatment on visual acuity and
vitreoretinal traction in patients with VAVR.
Discussion
Vitreoretinopathy is a serious ophthalmic disease
and shows various complications of rhegmatogenous retinal
detachment retinal detachment surgery (17). Despite remarkable advances in
vitreoretinal surgery, VAVR still remains a common cause of severe
ophthalmic complications and even visual loss (18). Evidences have suggested that clinical
drug nursing of PGSD treatment presents more advantages for
age-related macular degeneration and vitreoretinopathy since VEGF
plays essential role in the epidemiology and the symptoms of the
development ophthalmic disease (19,20). The
purpose of the current study systematically analyzed the role of
PGSD treatment in patients with VAVR. Previous study presented that
11.2 months follow-up period of PGSD intravitreal injection
significantly improved visual acuity in patients with VAVR
(7). Although we observed that there
were improvements a certain extent over time in patients receive 4-
and 12-month treatment with placebo due to autoregulation, the
efficacy of autogenous repairing is limited for patients with VAVR.
Outcomes indicated that 12-month PGSD intravitreal injection
markedly improved vitreoretinal traction, retinal vasculitis and
visual acuity compared to placebo and 0-, 4- and 12-month treatment
with PGSD for patients with VAVR.
Tractional retinal detachment induced by the
formation of contractile preretinal fibrous membranes is the main
reason vitreoretinopathy-induced eye syndrome or blindness
(21). Results in this study
indicated that PGSD intravitreal injection improves tractional
retinal and intra-ocular pressure in patients with VAVR. Rinaldi, M
et al have suggested that intravitreal PGSD (Macugen) is
efficient for treatment of myopic choroidal neovascularization in a
morphologic and functional study (22). Patients with VAVR receiving
intravitreal injection of PGSD treatment significantly decreased
subretinal exudation and leakage by fluorescein angiography
compared to placebo in a 12-months follow-up. Notably, maintenance
therapy with PGSD for nonvascular age-related macular degeneration
is an effective and well-tolerated option (23). We reported that 12-month PGSD
intravitreal injection markedly improved subretinal exudation and
leakage compared to placebo and 0- and 4-month PGSD treatment.
Report also indicated that intravitreous injection of PGSD resulted
in significant clinical benefit for ocular vascular diseases by
targeting of Anti-VEGF aptamer (24). Outcome showed that the pathological
changes in vascular activity, amount of exudation, and visual
acuity were significantly improved by intravitreal injection of
PGSD treatment.
Currently, the efficacy of PGSD in improving visual
acuity was identified in the 11.2-month mean follow-up period
(7). In this study, we found that
PGSD intravitreal injection not only improved visual acuity, but
also deducted subretinal exudation and leakage in patients with
VAVR. We further reported that PGSD relieved visual acuity via
inhibiting inflammatory cytokines IL-1β and TNFα for patients with
VAVR. Inflammation is associated with the pathogenesis of
vitreoretinopathy (25). A
retrospective analysis has analyzed the safety of PGSD in the
treatment of age-related macular degeneration in subjects with or
without diabetes mellitus, which primary showed the efficacy of
PGSD for inflammation (26).
Tikhonovich et al have investigated the role of inflammation
in the development of proliferative vitreoretinopathy (27). Rojas et al have indicated that
TNFα implicated for understanding the mechanisms of VAVR and
provided evidences that increased TNFα may a potential new
therapeutic target for Proliferative vitreoretinopathy prophylaxis
(28). In addition, Keane-Myers
et al have showed that IL-1 receptor antagonist
down-modulated the recruitment of eosinophils and other
inflammatory cells essential for the immunopathogenesis of ocular
atopy by targeting IL-1-mediated inflammatory signal pathway
(29). Our results showed that PGSD
intravitreal injection treatment decreased inflammatory score and
inhibited inflammatory cytokines IL-1β and TNFα for patients with
VAVR. Findings demonstrated that 12-month PGSD intravitreal
injection significantly inhibited inflammation and improved of
intra-ocular pressure compared to placebo and 0- and 4-month PGSD
treatment.
In conclusion, VAVR is still a major cause of
failure of rhegmatogenous retinal detachment surgery (30–32).
Intravitreal injection of PGSD treatment can improve subretinal
exudation, leakage, inflammation, intra-ocular pressure, visual
acuity and vitreoretinal traction for patients with VAVR, which may
be a potential drug for treatment of patients with VAVR in clinic.
However, further studies should be performed in a large number of
populations and long-term observation in future clinic trial.
References
|
1
|
Gandhi JK, Tollefson TT and Telander DG:
Falciform macular folds and chromosome 22q11.2: Evidence in support
of a locus for familial exudative vitreoretinopathy (FEVR).
Ophthalmic Genet. 35:112–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennock S, Haddock LJ, Mukai S and
Kazlauskas A: Vascular endothelial growth factor acts primarily via
platelet-derived growth factor receptor α to promote proliferative
vitreoretinopathy. Am J Pathol. 184:3052–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Radke NV, Panakanti TK, Radke SN and
Ravikoti R: Comment on ‘Intrasilicone oil injection of bevacizumab
at the end of retinal reattachment surgery for severe proliferative
vitreoretinopathy’. Eye (Lond). 28:15252014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robitaille JM, Gillett RM, LeBlanc MA,
Gaston D, Nightingale M, Mackley MP, Parkash S, Hathaway J, Thomas
A, Ells A, et al: Phenotypic overlap between familial exudative
vitreoretinopathy and microcephaly, lymphedema, and chorioretinal
dysplasia caused by KIF11 mutations. JAMA Ophthalmol.
132:1393–1399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi H, Guo T, Liu PC, Wang QY, Du YR, Liu
QY, He MM, Liu JL and Yu J: Steroids as an adjunct for reducing the
incidence of proliferative vitreoretinopathy after rhegmatogenous
retinal detachment surgery: A systematic review and meta-analysis.
Drug Des Devel Ther. 9:1393–1400. 2015.PubMed/NCBI
|
|
6
|
Chiquet C and Rouberol F: Proliferative
vitreoretinopathy: Curative treatment. J Fr Ophtalmol. 37:653–659.
2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quiram PA, Drenser KA, Lai MM, Capone A Jr
and Trese MT: Treatment of vascularly active familial exudative
vitreoretinopathy with pegaptanib sodium (Macugen). Retina. 28 3
Suppl:S8–S12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hussar DA: New drugs: Entecavir,
ibandronate sodium, and pegaptanib sodium. J Am Pharm Assoc (2003).
45:412–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moshfeghi AA and Puliafito CA: Pegaptanib
sodium for the treatment of neovascular age-related macular
degeneration. Expert Opin Investig Drugs. 14:671–682. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzales CR: VEGF Inhibition Study in
Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group:
Enhanced efficacy associated with early treatment of neovascular
age-related macular degeneration with pegaptanib sodium: An
exploratory analysis. Retina. 25:815–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shukla D, Namperumalsamy P, Goldbaum M and
Cunningham ET Jr: Pegaptanib sodium for ocular vascular disease.
Indian J Ophthalmol. 55:427–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rouberol F and Chiquet C: Proliferative
vitreoretinopathy: Pathophysiology and clinical diagnosis. J Fr
Ophtalmol. 37:557–565. 2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lane SS and Holland EJ: Loteprednol
etabonate 0.5% versus prednisolone acetate 1.0% for the treatment
of inflammation after cataract surgery. J Cataract Refract Surg.
39:168–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolfe JD and Hubbard GB III: Spontaneous
regression of subretinal exudate in coats disease. Arch Ophthalmol.
124:1208–1209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodriguez A, Rodriguez FJ, Valencia M and
Castaño C: Late development of a lamellar macular hole after the
spontaneous separation of vitreoretinal traction: Case report. Eur
J Ophthalmol. 26:e168–e170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodriguez A, Valencia M and Gomez FE:
Vitreoretinal traction and lamellar macular holes associated with
cicatricial toxoplasmic retinochoroiditis: Case series report. Eur
J Ophthalmol. 26:e128–e133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feist RM Jr, King JL, Morris R,
Witherspoon CD and Guidry C: Myofibroblast and extracellular matrix
origins in proliferative vitreoretinopathy. Graefes Arch Clin Exp
Ophthalmol. 252:347–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tosi GM, Marigliani D, Romeo N and Toti P:
Disease pathways in proliferative vitreoretinopathy: An ongoing
challenge. J Cell Physiol. 229:1577–1583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lipski A, Bornfeld N and Jurklies B:
Multifocal electroretinography in patients with exudative amd and
intravitreal treatment with pegaptanib sodium. Retina. 27:864–872.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ulinska M: Pegaptanib sodium in treatment
of wet AMD. Klin Oczna. 108:482–488. 2006.(In Polish). PubMed/NCBI
|
|
21
|
Foy JW, Rittenhouse K, Modi M and Patel M:
Local tolerance and systemic safety of pegaptanib sodium in the dog
and rabbit. J Ocul Pharmacol Ther. 23:452–466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rinaldi M, Chiosi F, Dell'Omo R, Romano
MR, Parmeggiani F, Semeraro F, Menzione M and Costagliola C:
Intravitreal pegaptanib sodium (Macugen) for treatment of myopic
choroidal neovascularization: A morphologic and functional study.
Retina. 33:397–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishibashi T: LEVEL-J Study Group:
Maintenance therapy with pegaptanib sodium for neovascular
age-related macular degeneration: An exploratory study in Japanese
patients (LEVEL-J study). Jpn J Ophthalmol. 57:417–423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ng EW and Adamis AP: Anti-VEGF aptamer
(pegaptanib) therapy for ocular vascular diseases. Ann N Y Acad
Sci. 1082:151–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moysidis SN, Thanos A and Vavvas DG:
Mechanisms of inflammation in proliferative vitreoretinopathy: From
bench to bedside. Mediators Inflamm. 2012:8159372012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dombi T, Kwok KK and Sultan MB: A
retrospective, pooled data analysis of the safety of pegaptanib
sodium in the treatment of age-related macular degeneration in
subjects with or without diabetes mellitus. BMC Ophthalmol.
12:372012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tikhonovich MV, Iojleva EJ and Gavrilova
SA: The role of inflammation in the development of proliferative
vitreoretinopathy. Klin Med (Mosk). 93:14–20. 2015.(In Russian).
PubMed/NCBI
|
|
28
|
Rojas J, Fernandez I, Pastor JC, Maclaren
RE, Ramkissoon Y, Harsum S, Charteris DG, Van Meurs JC, Amarakoon
S, Ruiz-Moreno JM, et al: A genetic case-control study confirms the
implication of SMAD7 and TNF locus in the development of
proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci.
54:1665–1678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keane-Myers AM, Miyazaki D, Liu G, Dekaris
I, Ono S and Dana MR: Prevention of allergic eye disease by
treatment with IL-1 receptor antagonist. Invest Ophthalmol Vis Sci.
40:3041–3046. 1999.PubMed/NCBI
|
|
30
|
Hocaoglu M, Karacorlu M, Sayman Muslubas
I, Ersoz MG and Arf S: Anatomical and functional outcomes following
vitrectomy for advanced familial exudative vitreoretinopathy: A
single surgeon's experience. Br J Ophthalmol. 101:946–950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tikhonovich M, Lyskin P, Ioyleva E and
Gavrilova S: Expression of cyclooxygenases and trophic and growth
factors in epiretinal membranes at late stages of proliferative
vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 254:2277–2279.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sadaka A, Sisk RA, Osher JM, Toygar O,
Duncan MK and Riemann CD: Intravitreal methotrexate infusion for
proliferative vitreoretinopathy. Clin Ophthalmol. 10:1811–1817.
2016. View Article : Google Scholar : PubMed/NCBI
|