Introduction
Sleep apnea is the brief, involuntary cessation of
breathing during sleep. Sufferers often experience loud, sudden
snoring and sudden body movements as a result. Sleep apnea is
predominantly often caused by obstruction of the upper airway and
obstructive sleep apnea hypopnea syndrome (OSAHS) refers to a
collection of physio-pathological syndromes associated with sleep
apnea, including body hypoxemia and hypercapnia. Primary clinical
manifestations of OSAHS are apnea and hypopnea, snoring, sleep
structure disorder, and lethargy during waking hour (1). The morbidity rate of OSAHS among adult
males is ~4%, whereas the morbidity rate among adult females is
~2%. The morbidity rate among people aged 60 and above may be as
high as 20–40% (2).
Obesity, age, and muscular flaccidity have been
demonstrated to be risk factors for OSAHS, which is thought to have
genetic origins in genes associated with obesity, metabolic
syndrome, craniofacial structural abnormalities, defects in the
muscular control of ventilation and the upper airway, and sleep and
circadian rhythm disorders (3). In
particular, abnormalities in blood lipid distribution genes, such
as leptin, insulin-like growth factor-1, glucokinase, adenine
nucleotide deaminase, melatonin-3 receptor, glucose-regulating
protein, TNF-α and β, adrenergic receptor, orexin, and sugar
regulating proteins, have been associated with OSAHS, as well as
receptor protein tyrosine kinase, nerve growth factor, endothelin-1
(ET-1) and −3, nitric oxide synthase, and angiotensin converting
enzyme (4). OSAHS has also been
found to be associated with hypertension, insulin resistance, type
2 diabetes, arrhythmia, heart failure, cerebrovascular disease,
ocular lesions, hypertension, coronary heart disease, and cerebral
stroke (5). Chronic intermittent
hypoxia brought on by OSAHS can lead to reperfusion injuries to the
heart and cause oxidative stress reactions, lipid metabolism
disorders, abnormal sympathetic nerve activation, and vascular
endothelial functional disorder (6).
Abnormal circulating levels of inflammatory factors
resulting from OSAHS are thought to contribute to the development
of atherosclerosis (7), thus the
prevention of OSAHS-associated inflammatory reactions is of notable
clinical interest. High expression of an atherosclerosis-related
protein, endothelin-1 (ET-1), has been detected in the peripheral
blood of patients with OSAHS (8).
ET-1, which is a potent vasoconstrictor, has been demonstrated to
be correlated with the severity of OSAHS and continuous positive
airway pressure (CPAP) treatment has been shown to be effective in
lowering circulating ET-1 (9).
Nitric oxide (NO) has an important role in blood
pressure and immune system regulation and its dysregulation is
associated with cardiac failure, hypertension, coronary heart
disease, diabetes, and hyperlipidemia (10). Patients with OSAHS have decreased
circulating NO levels, suggesting that NO may also be involved with
OSAHS-related cardiovascular and cerebrovascular diseases (10). Effective CPAP treatment has been
demonstrated to reduce the occurrence of OSAHS-related
cardiovascular distress by improving NO levels (11). Patients with OSAHS also have a high
expression of TNF-α in peripheral blood and the independent
application of CPAP and postoperative application of CPAP
sequential treatment have been shown to lower the concentration of
TNF-α and other indicators of hypoxemia (12,13).
Patients with OSAHS often experience abnormal blood
pressure variations, which are thought to be a result of nocturnal
hypoxemia (14). After controlling
for age, body mass index (BMI), and blood pressure, the
apnea-hypopnea index (AHI) of patients with OSAHS is positively
correlated with 24-h systolic and diastolic pressure variability,
daytime systolic and diastolic pressure variability, and nocturnal
systolic and diastolic pressure variability (15). OSAHS can further aggravate blood
pressure elevation in patients with cardiovascular and
cerebrovascular diseases and destroy the circadian rhythm of blood
pressure (16). Effective CPAP,
along with drug therapy, has been shown to improve the circadian
rhythm of blood pressure and lower the category and dosage of
combined blood pressure lowering agents, improving prognosis
(17,18).
Surgical treatment to correct OSAHS is highly
invasive and has poor long-term efficacy. CPAP has the advantage of
being non-invasive and is simple to operate, making it the
preferred treatment for OSAHS (19).
The present study aimed to assess the correlation between OSAHS,
carotid atherosclerosis, and blood pressure variability (BPV), and
to evaluate the therapeutic effects of CPAP in order to inform
ongoing efforts to improve CPAP treatments and reduce the risk of
cardiovascular disease in patients with OSAHS.
Materials and methods
Study participants
In total, 120 patients with OSAHS that visited the
Sleep Center of the First People's Hospital of Yancheng City
between June 2013 and June 2015 were evaluated for participation in
the present study. All patients conformed to the diagnostic
criteria presented in the Guideline for OSAHS Diagnosis and
Treatment (Modified Version) formulated by the Sleep Breathing
Disorder Study Group of China, Society of Respiratory Disease Study
of Chinese Medical Association (20), and diagnosis was confirmed by
polysomnogram. None of the patients received surgical or mechanical
ventilation treatment before inclusion in the study. Patients with
heart disease, malignant tumors, serious hepatic or renal
functional lesions, or chronic inflammatory diseases were excluded,
along with any patients with a history of serious lung diseases,
hyperlipemia, diabetes, hypertension, a history of serious trauma
within 2 weeks of enrollment, or long-term application of
anti-inflammatory drugs, anticoagulant drugs, or drugs that may
influence the observation indices used in the present study. Of the
patients included in the study, 105 were men and 15 were women,
ranging in age from 24 to 67 years with a mean age of 46.4±10.8
years. Mean BMI was 28.2±4.3 kg/m2.
Patients were assigned into groups according to
their AHI: a mild group (15 times/h>AHI≥5 times/h; 28 patients),
moderate group (30 times/h>AHI≥15 times/h; 48 patients), and
severe group (AHI>30 times/h; 44 patients). A total of 40
healthy volunteers made up the control group and were matched with
the patients in the case group in terms of age, sex, and BMI
(Table I). OSAHS was ruled out for
the control group after clinical examination, and the same
exclusion criteria were applied. The study protocol was approved by
the Medical Ethics Committee of the First People's Hospital of
Yancheng City (Yancheng, China), and all subjects provided written
informed consent.
 | Table I.Baseline characteristics of the study
participants. |
Table I.
Baseline characteristics of the study
participants.
| Indices | Mild group
(n=28) | Moderate group
(n=48) | Severe group
(n=44) | Control group
(n=40) |
|---|
| AHI (time/h) | 9.18±2.57 | 21.29±4.02 | 39.52±6.41 | 2.13±1.26 |
| Age (years) | 46.1±9.5 | 46.5±10.9 | 47.1±11.2 | 46.5±12.3 |
| Sex
(male/female) | 25/3 | 41/7 | 39/5 | 35/5 |
| BMI
(kg/m2) | 27.8±4.6 | 28.8±5.1 | 28.6±5.5 | 27.5±6.2 |
Experimental treatment
Patients in the moderate and severe OSAHS groups
underwent 6 months of CPAP treatment. On the first night of
treatment, a breathing machine was used to perform CPAP pressure
titration with the initial pressure set as 4 cm H2O. The
pressure was subsequently adjusted according to the patient's
apnea-hypopnea status every 5 min until apnea disappeared during
each sleep period in all body positions and the percentage of
arterial oxygen saturation (SaO2) was >90%. At this
point, the CPAP pressure was determined to be the optimal
ventilation pressure and CPAP treatment was administered at this
pressure (8–15 cm H2O) continuously for 5 to 8 h whilst
patients slept each evening for 6 months.
Cardiovascular health indicators and
detection methods
All indicators were determined at enrollment. A
multi-lead sleep monitor was used to determine AHI and lowest
SaO2 (LSaO2) values. A color Doppler was used
to determine carotid intima-media thickness (IMT) by a senior
physician in the Ultrasound Department. With the probe frequency
set at 8 MHz, the posterior wall at the distal end close to the
furcation of the common carotid artery and the site 1 cm above the
initial part of internal carotid artery were measured three times
and the mean was calculated. Morning fasting peripheral vein blood
draws were collected and, following anticoagulation, plasma was
stored at −80°C for subsequent testing. ELISA was used to detect NO
(cat. no. 191426; BioAssay Systems LLC; Hayward, CA, USA) and TNF-α
(cat. no. EK0526; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) according to the manufacturer's protocol and plasma ET-1 was
detected by radioimmunoassay. Low frequency blood pressure
variability (BPV LF) was measured via lectrocardiogram (ECG) 5 min
before and 5 min after sleeping.
Statistical analysis
The SPSS 20.0 statistical package (IBM Corp, Armonk,
NY, USA) was used to establish a database from which statistical
analysis was performed. Measurement data were expressed as the mean
± standard deviation. Single factor analysis of variance was used
to assess differences between multiple groups while the least
significant difference method was used for pair-wise comparison and
the paired Student's t-test was used to assess differences within
the same group before and after treatment. Pearson's correlation
and multiple linear regression analysis were used for correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
AHI, IMT, ET-1, TNF-α,
LSaO2, and NO are affected by OSAHS
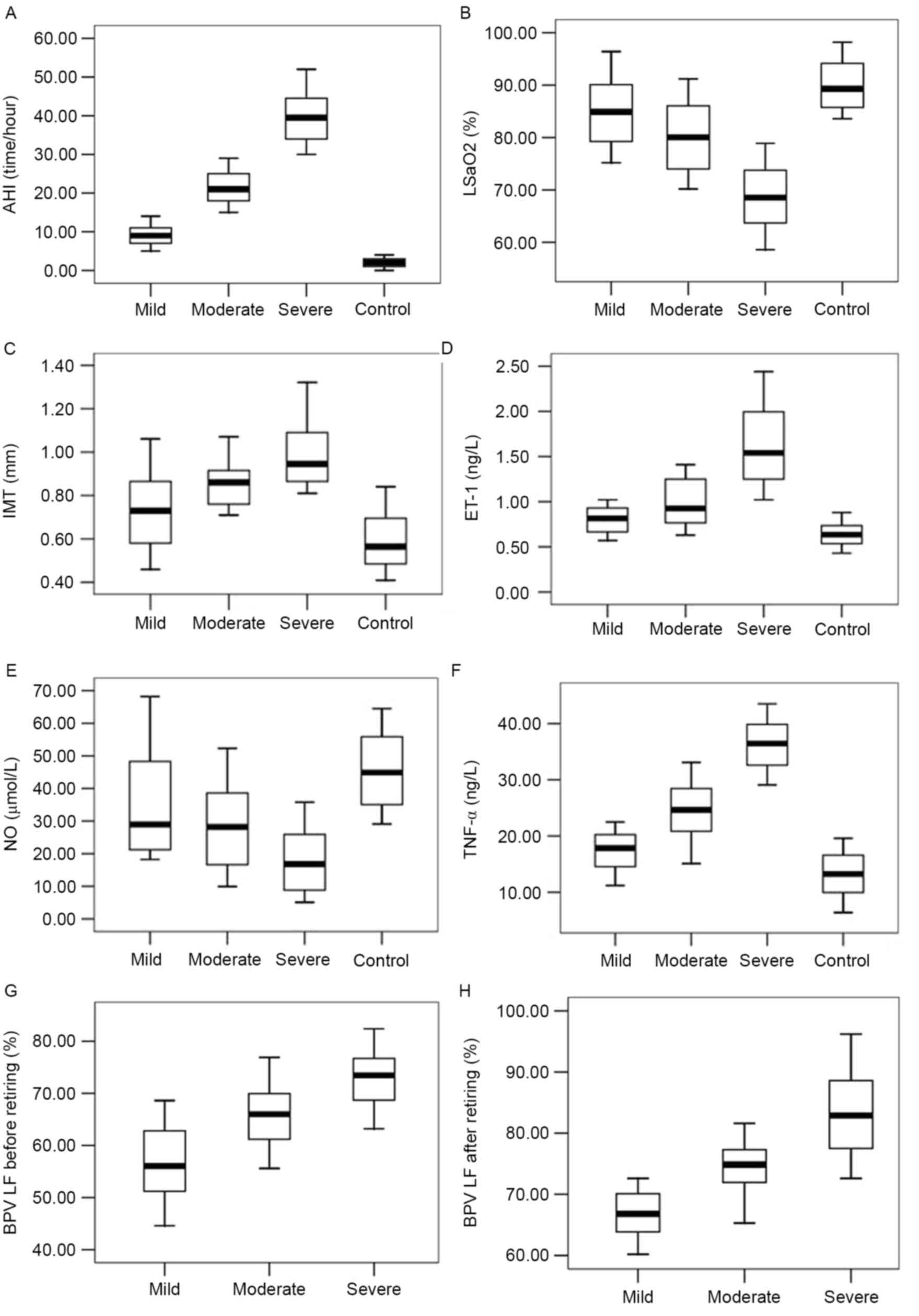
Numerous physiological parameters were significantly
altered in patients with OSAHS, compared with healthy controls
prior to the start of CPAP treatment. AHI, IMT, plasma ET-1, and
plasma TNF-α levels of the patients with OSAHS were significantly
higher than those in control patients (P<0.05), while
LSaO2 and plasma NO levels were significantly lower in
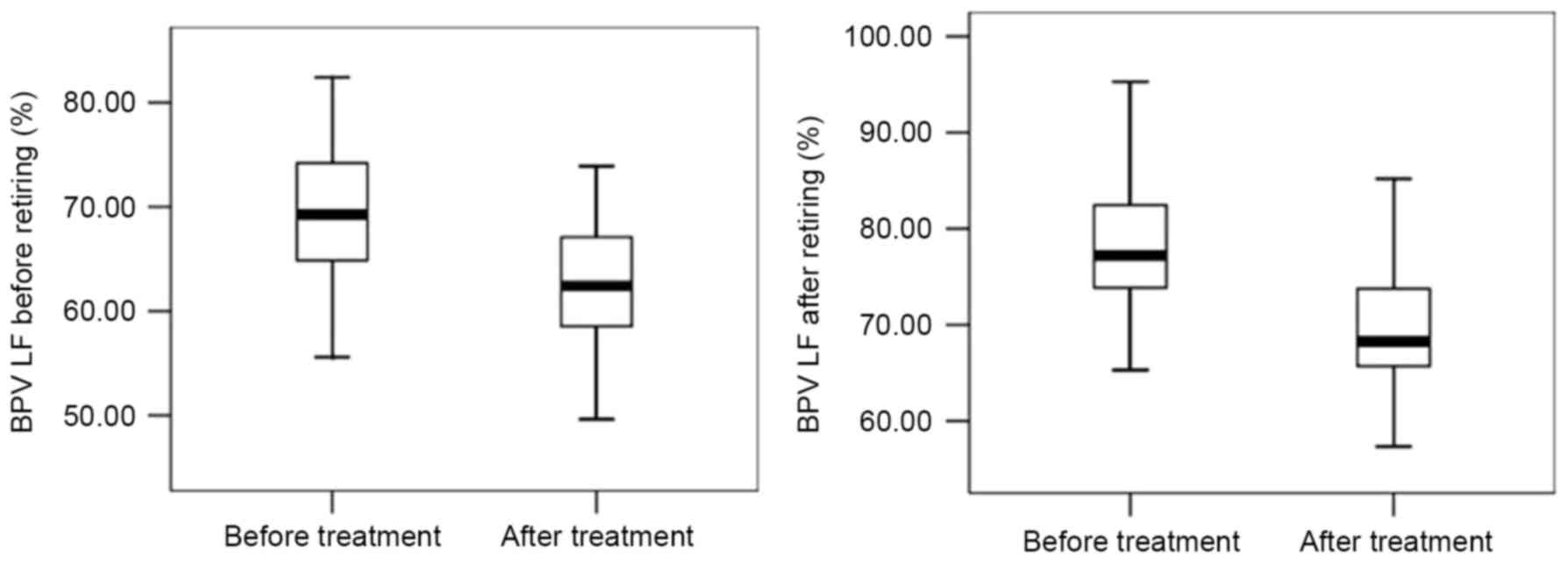
OSAHS patients than those in the control group (P<0.05; Fig. 1). All apnea patients' BPV LF
significantly increased after sleep (P<0.05). The degree to
which these parameters were affected was associated with the
severity of the patients' condition.
Carotid arteriosclerosis is correlated
with multiple physiological parameters in patients with OSAHS
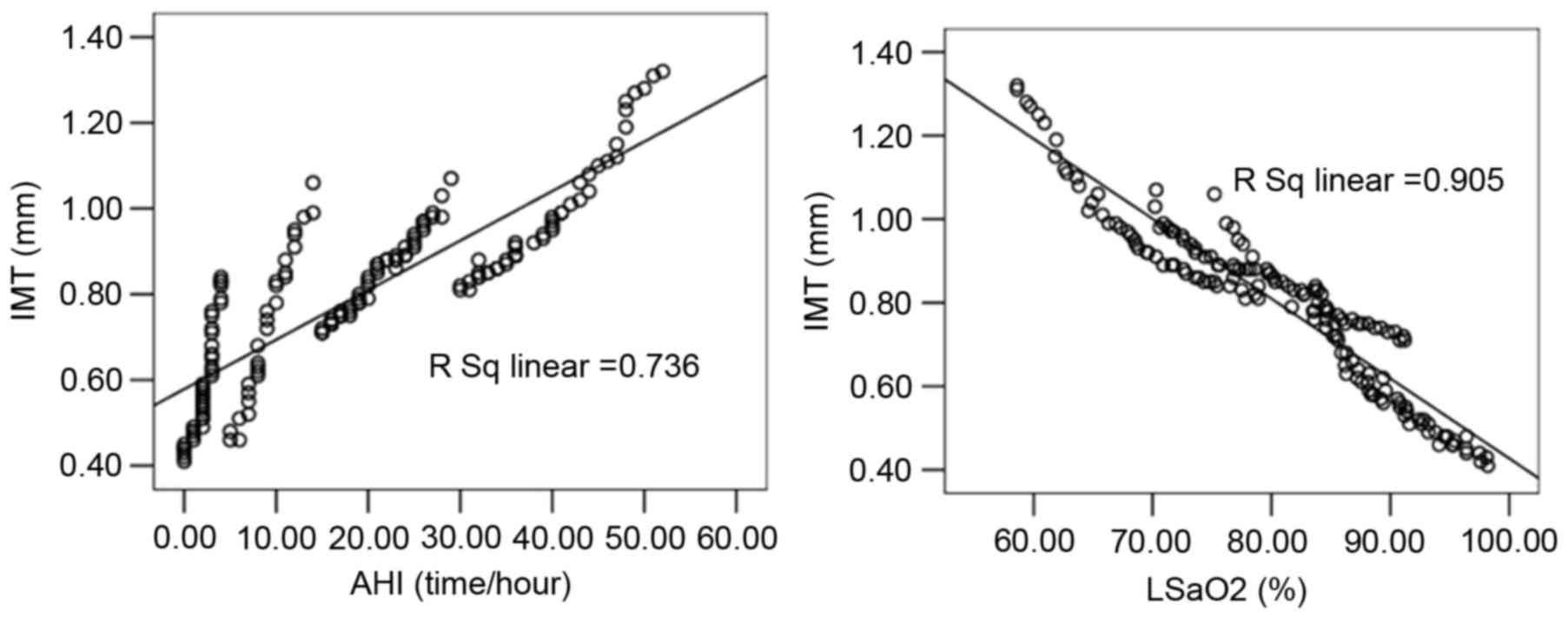
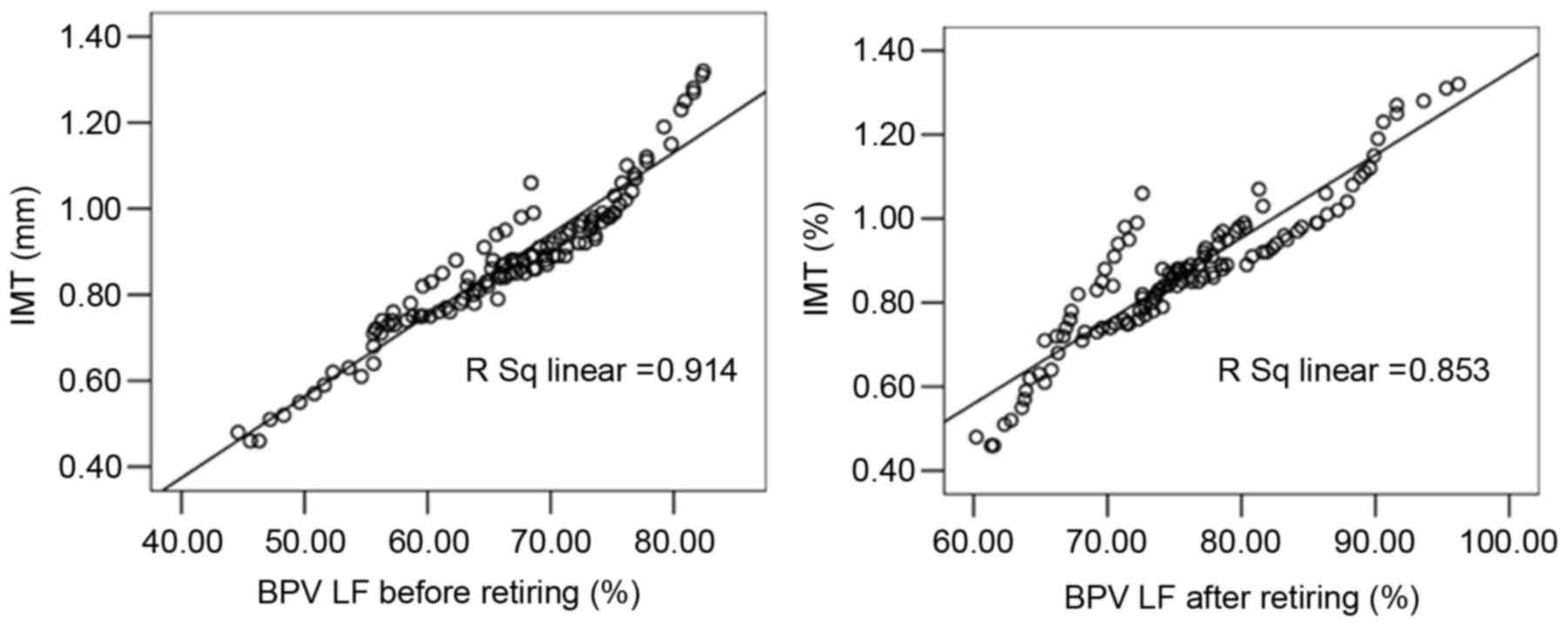
Linear correlation analysis indicated that the
carotid IMT of patients in the case groups was significantly
correlated with AHI (r=0.858), LSaO2 (r=−0.952), plasma
ET-1 (r=0.901), plasma NO (r=−0.944), plasma TNF-α (r=0.918), BPV
LF before sleep (r=0.956), and BPV LF after sleep (r=0.924;
Figs. 2–4). Multiple linear regression analysis with
the patient's carotid IMT as the dependent variable and the
physiological parameters as independent variables demonstrated that
IMT was significantly correlated with AHI (standardized regression
coefficient, 0.700), LSaO2 (standardized regression
coefficient, −0.536), plasma ET-1 (standardized regression
coefficient, 0.489), plasma NO (standardized regression
coefficient, −1.673), and BPV LV before sleep (standardized
regression coefficient, 1.213; P<0.05), as shown in Table II. No significant correlation was
detected between IMT and TNF-α or BPV LF after sleep.
 | Table II.Multiple linear regression analysis of
the influence of carotid IMT on OSAHS patients. |
Table II.
Multiple linear regression analysis of
the influence of carotid IMT on OSAHS patients.
|
| Unstandardized
coefficients | Standardized
coefficients |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | B | SE | Beta | t | P-value |
|---|
| (Constant) | 0.999 | 0.473 |
| 2.112 | 0.037 |
| AHI | 0.009 | 0.003 | 0.700 | 3.490 | 0.001 |
| LSaO2 | −0.010 | 0.005 | −0.536 | −2.180 | 0.031 |
| ET-1 | 0.175 | 0.051 | 0.489 | 3.454 | 0.001 |
| NO | −0.033 | 0.005 | −1.673 | −6.734 | 0.000 |
| TNF-α | 0.000 | 0.001 | 0.036 | 0.350 | 0.727 |
| BPV LF before
retiring | 0.024 | 0.003 | 1.213 | 7.202 | <0.001 |
| BPV LF after
retiring | 0.007 | 0.004 | 0.305 | 1.747 | 0.083 |
CPAP treatment improves indicators of
cardiovascular health in patients with moderate and severe
OSAHS
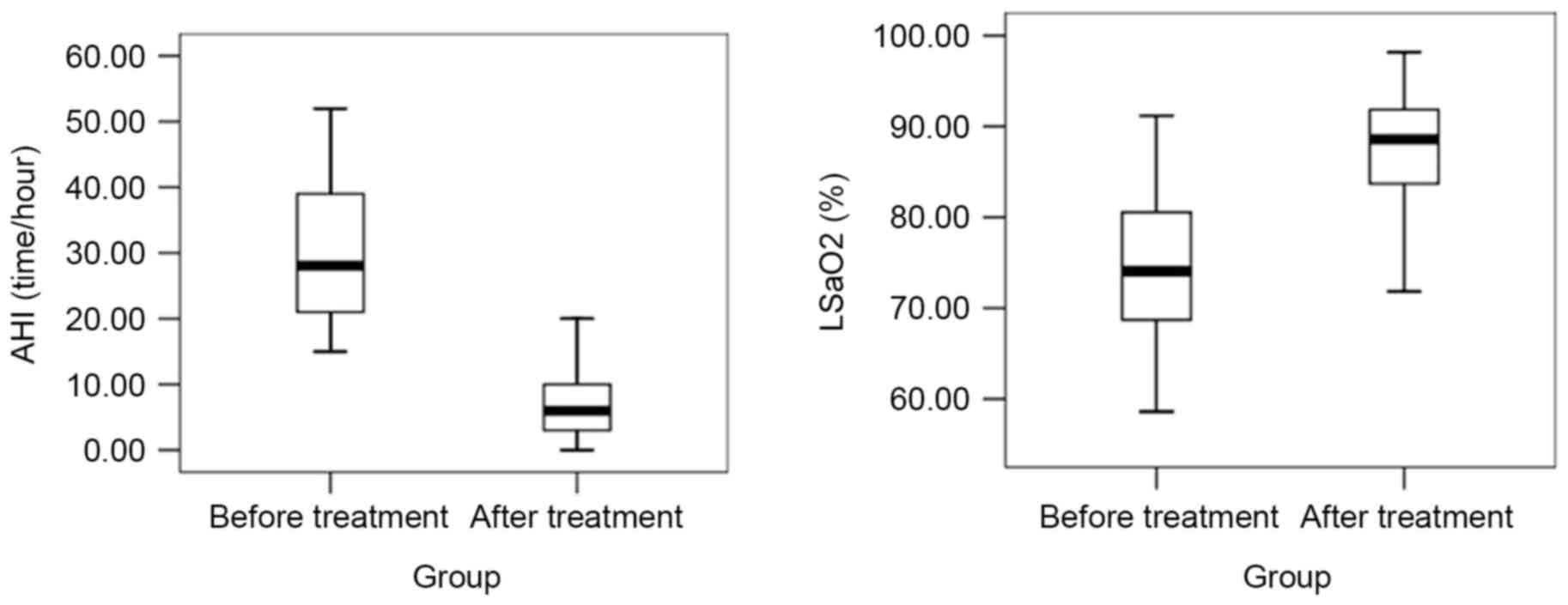
After six months of CPAP treatment, the levels of
AHI, IMT, ET-1, TNF-α, BPV LF before and after retiring were
decreased, while those of LSaO2 and NO were increased
(P<0.05; Figs. 5–7). In brief, all 92 patients following 6
months of CPAP treatment exhibited improvements in the measurements
of the physiological parameters observed in this study.
Discussion
The results of the present study demonstrated that
AHI, IMT, plasma ET-1, plasma TNF-α levels, and blood pressure
measurements of patients with OSAHS were higher than those of the
control group, whereas LSaO2 and plasma NO levels were
lower in the control group. These differences became more
pronounced as the severity of OSAHS increased. Furthermore, carotid
IMT of patients with OSAHS was significantly correlated with AHI,
LSaO2, plasma ET-1, plasma NO, plasma TNF-α, BPV LF
before sleep, and BPV LF after sleep. These findings are consistent
with those of previous studies that noted the correlation between
sleep apnea and various physiological and plasma markers of
cardiovascular function (4,7–10,14–16).
Following 6 months of CPAP treatment, all of the
physiological variables observed in our study were significantly
improved among the patients with OSAHS. The results corroborate the
findings of earlier studies, which reported benefits to these
variables separately following CPAP treatment (9,11–13,17,18).
Therefore, the present findings support the hypothesis that CPAP
treatment as an effective strategy for improving the pathological
status of patients with moderate and severe OSAHS, whilst lowering
the risk of cardiovascular disease.
The present study has some notable strengths. The
inclusion of a follow-up period enabled assessment of the
short-term benefits to treatment. Furthermore, the study included a
sufficient sample size to stratify the patient population by
severity. However, this study also has some limitations. Although
the sample size was able to detect statistical differences,
randomized controlled trials with larger samples are required to
validate the utility of CPAP in treating OSAHS and reducing the
risk of cardiovascular disease. A longer follow-up period would
also enable an improved understanding of the benefits of treatment
for chronic OSAHS in improving cardiovascular risks. A more
comprehensive measure of cardiovascular health markers may help
develop a clear picture of the effect of CPAP treatment on
cardiovascular health, as well as guiding future work to understand
the underlying mechanisms.
References
|
1
|
Chen X, Sun J, Yuan W, Yuan W and Li J:
OSAHS obstructive plane localization: Comparative study between
ag200 and friedman classification. Int J Clin Exp Med. 8:2240–2246.
2015.PubMed/NCBI
|
|
2
|
Lombardi C, Musicco E, Bettoncelli G,
Milanese M, Senna G, Braido F and Canonica GW: The perception of
Obstructive Sleep Apnoea/Hypopnoea Syndrome (OSAHS) among Italian
general practitioners. Clin Mol Allergy. 13:42015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Li Y, Jiang Q, Xu X, Zhang X,
Simayi Z and Ye H: Analysis of early kidney injury-related factors
in patients with hypertension and Obstructive Sleep Apnea Hypopnea
Syndrome (OSAHS). Arch Iran Med. 18:827–833. 2015.PubMed/NCBI
|
|
4
|
Chen H, Hu K, Zhu J, Xianyu Y, Cao X, Kang
J, He J, Zhao P and Mei Y: Polymorphisms of the 5-hydroxytryptamine
2A/2C receptor genes and 5-hydroxytryptamine transporter gene in
Chinese patients with OSAHS. Sleep Breath. 17:1241–1248. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vijayan VK: Morbidities associated with
obstructive sleep apnea. Expert Rev Respir Med. 6:557–566. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen S, Shi S, Xia Y, Liu F, Chen D, Zhu
M, Li M and Zheng H: Changes in sleep characteristics and airway
obstruction in OSAHS patients with multi-level obstruction
following simple UPPP, UPPP-GA, or UPPP-TBA: A prospective,
single-center, parallel group study. ORL J Otorhinolaryngol Relat
Spec. 76:179–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi S, Xia Y, Zhu M, Wei K, Liu F, Chen D,
Chen S and Zheng H: Characterization of upper airway obstruction by
fiber-optic nasolaryngoscopy and MRI in preoperative OSAHS
patients. ORL J Otorhinolaryngol Relat Spec. 76:321–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chalghoum A, Noichri Y, Dandana A, Azaiez
S, Baudin B, Jeridi G, Miled A and Ferchichi S: Relationship
between the A(8002)G intronic polymorphism of pre-pro-endothelin-1
gene and the endothelin-1 concentration among Tunisian coronary
patients. BMC Cardiovasc Disord. 15:1522015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anunciato IF, Lobo RR, Coelho EB, Verri WA
Jr, Eckeli AL, Evora PR, Nobre F, Moriguti JC, Ferriolli E and Lima
NK: Big endothelin-1 and nitric oxide in hypertensive elderly
patients with and without obstructive sleep apnea-hypopnea
syndrome. Arq Bras Cardiol. 101:344–351. 2013.PubMed/NCBI
|
|
10
|
Sukhovershin RA, Yepuri G and Ghebremariam
YT: Endothelium-derived nitric oxide as an antiatherogenic
mechanism: Implications for therapy. Methodist Debakey Cardiovasc
J. 11:166–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reddy YS, Kiranmayi VS, Bitla AR, Krishna
GS, Rao PV and Sivakumar V: Nitric oxide status in patients with
chronic kidney disease. Indian J Nephrol. 25:287–291. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong A, Xiong X, Xu H and Shi M: An
updated meta-analysis of the association between tumor necrosis
factor-α −308G/A polymorphism and obstructive sleep apnea-hypopnea
syndrome. PLoS One. 9:e1062702014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Almpanidou P, Hadjigeorgiou G,
Gourgoulianis K and Papadimitriou A: Association of tumor necrosis
factor-α gene polymorphism (−308) and obstructive sleep
apnea-hypopnea syndrome. Hippokratia. 16:217–220. 2012.PubMed/NCBI
|
|
14
|
Chenniappan M: Blood pressure variability:
Assessment, prognostic significance and management. J Assoc
Physicians India. 63:47–53. 2015.PubMed/NCBI
|
|
15
|
An S, Bao M, Wang Y, Li Z, Zhang W, Chen
S, Li J, Yang X, Wu S and Cai J: Relationship between
cardiovascular health score and year-to-year blood pressure
variability in China: A prospective cohort study. BMJ Open.
5:e0087302015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brockmann PE: Cardiovascular consequences
in children with obstructive sleep apnea: Is it possible to predict
them? Sleep. 38:1343–1344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ewen S, Dörr O, Ukena C, Linz D, Cremers
B, Laufs U, Hamm C, Nef H, Bauer A, Mancia G, et al: Blood pressure
variability after catheter-based renal sympathetic denervation in
patients with resistant hypertension. J Hypertens. 33:2512–2518.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Chen X and Sun J: Is the grading
system of the severity of the OSAHS used presently rational or not?
From the view of incidence of hypertension in different severity
groups. Eur Arch Otorhinolaryngol. 271:2561–2564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ben Saad H, Ben Hassen I, Ghannouchi I,
Latiri I, Rouatbi S, Escourrou P, Ben Salem H, Benzarti M and
Abdelghani A: 6-Min walk-test data in severe
obstructive-sleep-apnea-hypopnea-syndrome (OSAHS) under continuous-
positive- airway- pressure (CPAP) treatment. Respir Med.
109:642–655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chinese Medical Association Respiratory
Diseases Branch: OSAHS Diagnosis and Treatment (Modified Version).
Chinese J Tuberculosis Respiratory Dis. 35:9–12. 2012.(In
Chinese).
|