Introduction
Congenital vascular lumps were previously generally
referred to as hemangioma; however, these vascular abnormalities
were classified in detail in 1982 by Mulliken and Glowacki in
Harvard University (1). Based on
different biological characteristics of endothelial cells and
histopathological features, as well as clinical manifestations,
vascular diseases are divided into two distinct categories:
Hemangiomas and vascular malformations, and both may cause
Kasabach-Merritt syndrome (KMS) (2–4). KMS was
firstly reported in 1940 by Kasabach and Merritt (5), and is a relatively rare disease
accounting for only 1% of all hemangiomas (6). Hemangiomas was responsible for platelet
trapping, which, by abnormally proliferating endothelium within the
hemangioma, can result in the activation of platelets with
secondary activation of coagulation cascades, eventually leading to
consumption of various clotting factors (7). An immunohistochemical study using
monoclonal antibody against CD61, a marker of platelets, and
isotope studies using 111indium-labeled platelets and 51Cr-labeled
platelets support the possible role of platelet trapping in the
development of KMS (8). Patients
with KMS develop local tumor intravascular coagulation, which leads
to severe thrombocytopenia, coagulation disorders, anemia and
systemic inflammatory responses (9).
Clinical manifestations of KMS, including local or systemic
subcutaneous and visceral bleeding, high flow heart failure and red
or purple vascular tumors, could be combined with laboratory blood
tests, imaging or pathological examinations for diagnosis of this
disease (10).
The key to ensure effective treatment of KMS is to
remove or shrink the vascular tumors. Improving the condition of
disseminated intravascular coagulation and thrombocytopenia
combined with glucocorticoid therapy remains the first-line
treatment course of KMS (11). For
patients who are insensitive to glucocorticoid therapy, other
therapeutic methods, including interferon therapy, β-blockers,
chemical treatment, anti-platelet drugs, radiotherapy, supportive
care or combination therapy of glucocorticoids, could be given as
alternative treatments. The International Association for the Study
of Vascular Anomalies (ISSVA) classification method for vascular
diseases may also be adapted to KMS, and the majority of
pathological types of vascular tumors associated with KMS are
acquired tufted angioma (ATA) or Kaposi hemangioendothelioma (KHE)
(12). KHE, possessing features of
hemangioma and Kasposi's sarcoma, is a kind of locally aggressive
or borderline vascular tumor, whereas ATA is considered as a benign
vascular tumor (13).
Hypercalcemia (HC) refers to abnormal serum calcium
increment and occurs when the amount of calcium absorbed into
extracellular fluid (predominantly through intestine and bone) is
much more than that discharged through intestinum crassum and
kidney (14). Clinical
manifestations of HC vary greatly from asymptomatic phenotypes to
hypercalcemic crisis, and the latter is life-threatening (13,15). As
the calcium balance is the combined effect of regulation through
parathyroid hormone (PTH), calcitonin and active vitamin D3
[1,25-(OH)2D3] upon various organs, including intestine, kidney and
bones, abnormalities in any segments of this regulatory process may
lead to calcium metabolic disturbance (16). Typical causes of HC include
hyperparathyroidism (primary, secondary or atopic), vitamin D
metabolic disorder, PTH-related protein secretion from tumors and
many other pathogenesis, including sarcoidosis, granulomatous, milk
alkaline syndrome and adrenal insufficiency (17).
The most common reason of HC syndrome is primary
hyperparathyroidism or malignancy-associated HC (18), whereas cases of KMS combined with HC
are extremely rare and have not been reported previously. The
present report described the diagnosis and treatment of an infant
with KMS combined with HC.
Case report
Patient data
In September 2015, a 35-day-old male infant with
swelling on the upper right arm for >1 month and
thrombocytopenia for 1 day was admitted to Hunan Provincial
People's Hospital (Changsha, China) for the first time. The infant
developed a dark purple lump in his upper right arm with a size of
15×4×8 cm since birth. The guardians (parents of the patient)
provided written informed consent for the publication of the
clinical information of the patient.
Routine clinical examination
Following hospitalization, a series of routine
examinations, including blood routine examination, liver function
test, marrow cytology inspection and antiplatelet antibody test,
were performed. General lung computed tomography (CT) scans and
enhanced CT scanning were also undertaken. In addition, magnetic
resonance imaging (MRI) scans of the infant's upper right arm
followed by enhanced MRI and magnetic sensitive as well as magnetic
resonance venography (MRV) were conducted. Intracranial CT and
abdominal ultrasonography were also undertaken when the infant was
hospitalized again at 90 days old.
General condition
The patient was born full-term, with normal delivery
and precipitate labor. Physical examination indicated that the
patient had mild-moderate jaundice of the systemic skin, and dark
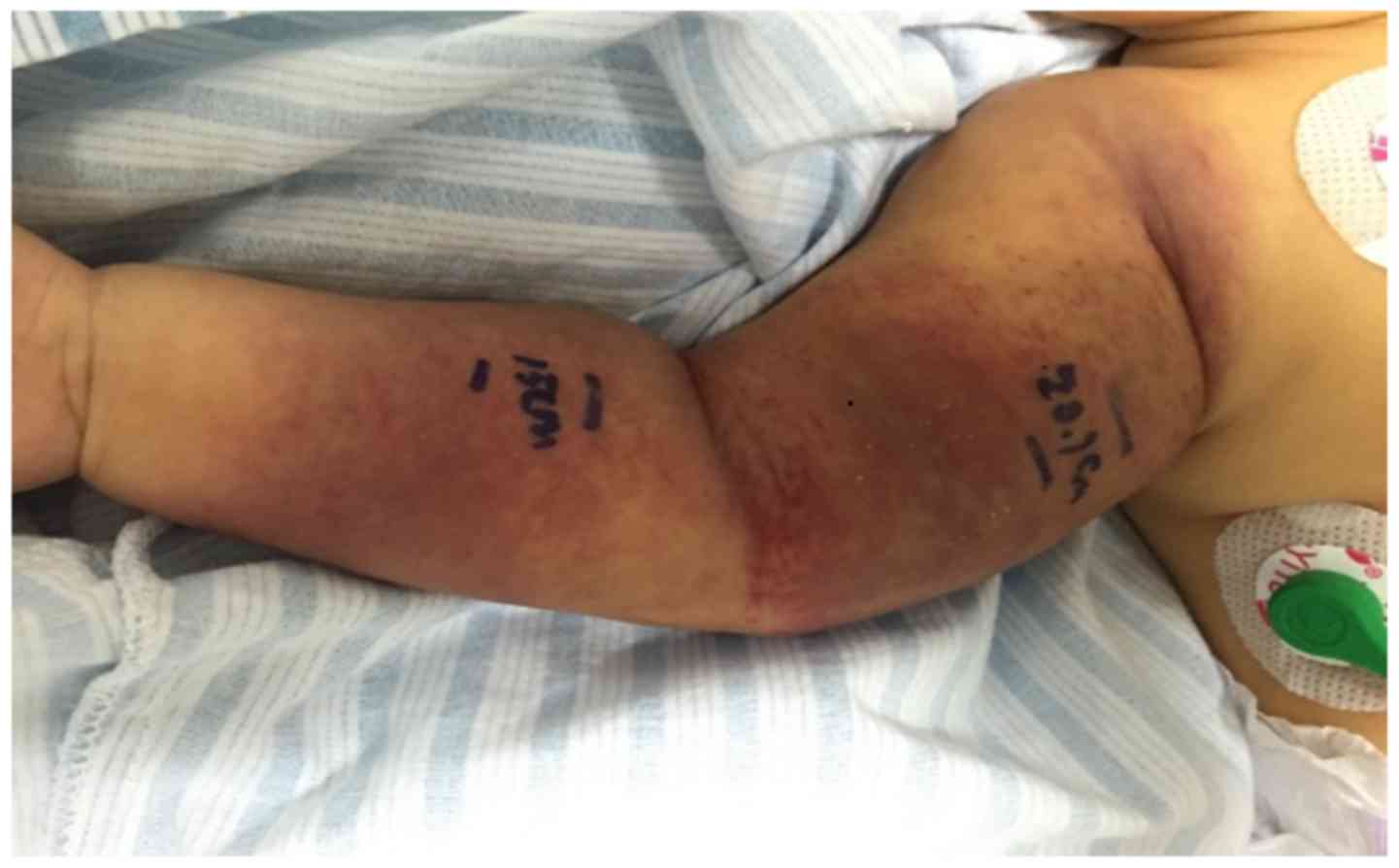
purple lumps with a length of ~15 cm were observed surrounding his
right arm (Fig. 1). The surface of
these lumps was shiny, swollen, and slightly hard and hot to the
touch together with few desquamations.
Routine clinical examination
results
To understand the patient's condition, routine
clinical examination, including physical check, blood routine
examination, coagulation function test and liver function
examination, were performed. Blood routine examination demonstrated
that the hemoglobin level was 81 g/l (normal range, 113–151 g/l),
hematocrit level was 24.3% (normal range, 33–45%), platelet count
was 7×109 platelets/l and mean platelet volume was 8.9
fl (normal range, 6–14 fl). The coagulation function test indicated
that the fibrin degradation product was >100 µg/ml (normal
range, 0–5 µg/ml) and D-dimer (DDI) was >10 mg/ml (normal range,
0–0.55 mg/l), with a prothrombin time of 14.3 sec (normal range,
10.0–15.5 sec), prothrombin time activity of 85.6% (normal range,
80–140%), prothrombin time-international normalized ratio of 1.246
(normal range, 0.8–1.5), fibrinogen of 0.482 g/l (normal range, 2–4
g/l), activated partial thromboplastin time of 43.3 sec (normal
range, 24–38.6 sec), thrombin time of 25.4 sec (normal range, 14–21
sec) and antithrombin-III of 63.0% (normal range, 82–132%). Liver
function examination demonstrated that alanine aminotransferase and
aspartate aminotransferase were normal with total bilirubin, direct
bilirubin and indirect bilirubin levels of 145.9, 13.2 and 132.70
µmol/l, respectively. Bone marrow cytology results revealed that
the patient developed proliferative anemia bone marrow, while
laboratory tests presented normal antiplatelet antibodies. General
lung CT scans and enhanced CT scanning indicated diffuse lesions in
both lungs, therefore, the patient was suspected to have bronchial
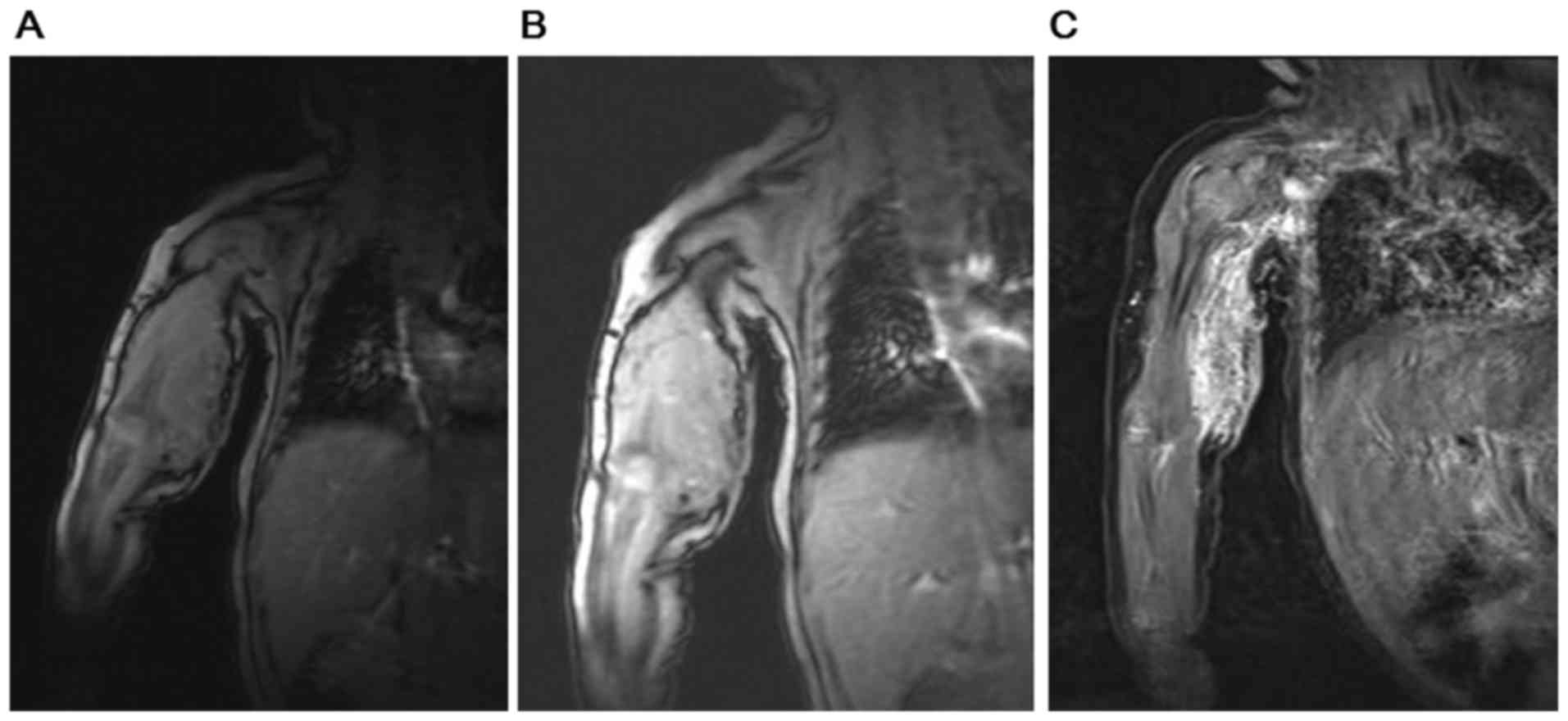
pneumonia or pulmonary hemorrhage. As illustrated in Fig. 2, the results from MRI scans of the
infant's upper arm combined with enhanced MRI, magnetic sensitive
and MRV demonstrated occupancy lesions in the right armpit, upper
right arm as well as the right elbow, which were potential
hemangiomas. The result of blood gas analysis indicated that the pH
value was 7.46, which was slightly higher than the normal range
(7.35–7.45).
Treatment course
In line with all the examinations, the infant was
diagnosed as KMS with manifestations of giant hemangioma and
thrombocytopenia (19). The patient
also developed severe anemia with hyperbilirubinemia and
disseminated intravascular coagulation. A series of treatments,
including heparin anticoagulant, platelet transfusions as well as
heparinized cryoprecipitate and fibrinogen supplement were
implemented. Methylprednisolone sodium succinate (China National
Pharmaceutical Group Corportation, Beijing, China) injection was
also conducted, with a dose of 15 mg/kg per day for 3 days followed
by a gradual reduction of dosage to 2 mg/kg per day for 9 days and
sustained for a further 2 months. γ-globulin antiplatelet
antibodies (Nanyue Biological Pharmaceutical Co., Ltd., Hengyang,
China) were administered once. Propranolol (Jiangsu Yabang Epson
Pharmaceutical Co. Ltd., Changzhou, China) was also prescribed (0.5
mg/kg per day and then the amount gradually increased to 2 mg/kg
per day) and stopped before surgery. Subsequently, the patient's
condition improved and the patient was discharged with continued
oral prednisone (Zhejiang Xianju Pharmaceutical Co. Ltd., Taizhou,
China; 2 mg/kg per day).
The infant was readmitted to our hospital at
90-days-old with repeatedly regurgitating milk associated with
cough for 10 days, as well as reduced activity and poor response
for 2 days. Physical examination indicated that the infant was 8 KG
and presented special features including round face, rubefaction
and poor spirit. The infant's right arm was swollen with moderate
hardness and normal strength, whereas both his left arm and right
leg demonstrated slightly reduced myodynamia. Joints in the overall
body were normal without swelling and the pathological meningeal
irritation result was negative. Head CT scans indicated no
abnormalities, nor did the cerebrospinal fluid routine or
biochemical examinations.
In order to determine the blood compound contents,
serum electrolyte level was detected. The results demonstrated that
total calcium was 3.47 mmol/l, which was notably higher than the
normal range (2.1–2.9 mmol/l), whereas sodium was 128.0 mmol/l,
which was markedly reduced compared to the normal range (normal
range, 137–147 mmol/l). The levels of potassium (normal range,
3.5–5.3 mmol/l) and chloridion (normal range, 99–110 mmol/l) were
4.85 and 92.1 mmol/l, respectively. Thyroid function was normal and
bone alkaline phosphatase was 200 U/l (normal range, ≤200 U/l). PTH
level was 2.24 pg/ml while 25-hydroxy-vitamin D level was normal
(20.0 ng/ml). Urine analysis indicated that the calcium level was
2.78 mmol/l (normal range: 2.5–7.5 mmol/l) whereas the ratio of
calcium to creatinine was 1.717, which was notably higher than the
normal range (<0.21). Abdominal ultrasonography demonstrated
enhanced renal vertebrae echo with radial arrangement. Treatments
including calcium reducing and potassium supplement were performed
to correct electrolyte turbulence. Fluid infusion was administered
to the infant accompanied with diuretic therapy by furosemide
(Shanghai Zhaohui Pharmaceutical Co. Ltd., Shanghai, China) and
calcium reducing treatment by alendronate sodium (Hangzhou MSD
Pharmaceutical Co. Ltd., Hangzhou, China). Prednisone was taken
continuously to increase the potassium level; however, the effect
was limited. The blood calcium remained high with fluctuation
between 2.8 and 3.37 mmol/l. From all of the above, it was
considered that the patient developed cancer-related HC and
surgical treatment was advised. Eventually, the hemangioma in the
upper right arm of the patient was embolized by microcatheter
(Progreat 1.8F, Terumo Corporation, Tokyo, Japan) under general
anesthesia on day 22 after the patient was readmitted, according to
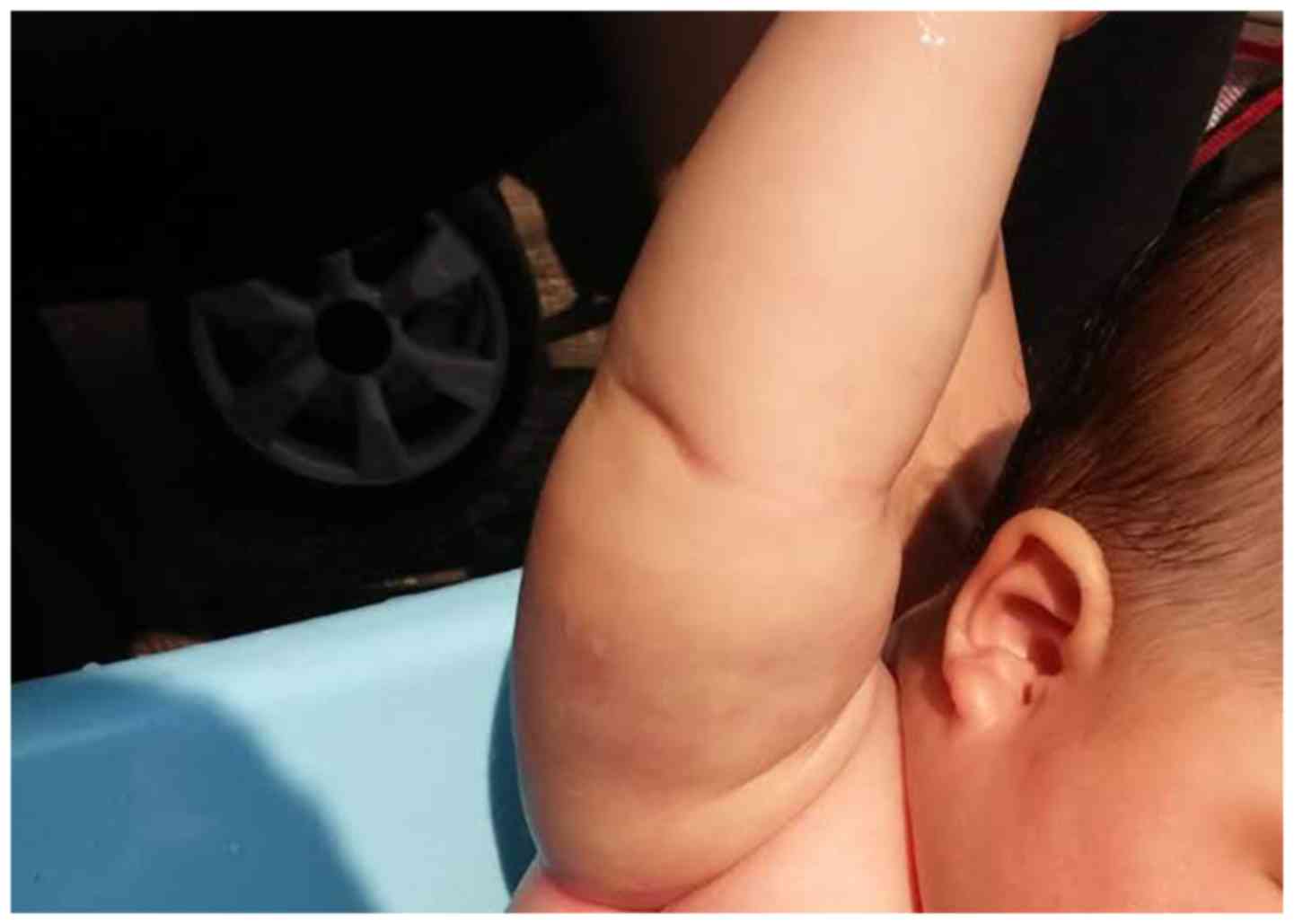
the manufacturer's protocol. As demonstrated in Fig. 3, the lump markedly reduced in size
following surgery. Follow-up at 4 months after surgery indicated
that the patient was in good condition.
Discussion
Infant hemangiomas are the most common benign
vascular tumor in infancy (20).
Generally, infant hemangiomas may be divided into three phases:
Proliferative phase, stabilized phase and involuting phase
(21). The majority of hemangiomas
are self-limiting, while 10–20% require additional treatment
because of complication generation (22). Several local complications may need
to be treated in time as hemangiomas may sometimes develop
collapse, necrosis or hemorrhage, while vascular tumor growth in
particular parts of the body, such as the upper eyelid, could lead
to ametropia, amblyopia or astigmatism (23). Vascular tumors growing near vital
organs, including the upper respiratory tract and liver, should be
taken into account seriously, since those hemangiomas could cause
severe diseases, including acute respiratory failure or congestive
heart failure (24). Vascular tumors
may also be combined with systemic diseases, and the infant in the
present report is a case of hemangiomas associated with
thrombocytopenia and coagulopathy.
In 1940, Kasabach and Merritt (5) reported a case of a 1-week-old male
infant demonstrating widespread purpura and swelling in the left
thigh. Biochemical examinations revealed thrombocytopenia and
coagulation disorders and results from tissue biopsy diagnosis
indicated that the infant had capillary hemangioma. Following this,
symptoms manifested as a huge capillary hemangioma combined with
platelet reduction in the infant, and this was termed KMS (5).
The etiology and pathogenesis of KMS is currently
unclear; however, increasing evidence has demonstrated that
hemangiomas combined with thrombocytopenia and coagulation
disorders are not common vascular tumors (25). The majority of pathological types are
KHE and ATA (25–28). ISSVA classified ATA into benign
vascular tumors in 2014, while KHE was categorized as locally
aggressive or borderline vascular tumors (10).
In the present report, the infant was born with
lumps in the upper right arm and the lumps increased suddenly,
associated with thrombocytopenia and bleeding tendency. Combined
with the confirmation of vascular tumor from radiological
technology examination, the infant was diagnosed with KMS. Since
KHE and ATA are the most common types of KMS and the use of
propranolol during hospitalization had slowed the infant's heart
rate to 80 bpm, oral prednisone treatment was given to the patient
consecutively after his discharge from hospital. Cushing's
syndrome-like features were evident in the patient and the
hemangioma shrank during the prednisone treatment. At the same
time, the patient did not develop thrombocytopenia and coagulation
abnormalities.
However, the patient was readmitted again 2 months
later due to HC. Multiple calcium reducing treatments were
undertaken but the condition did not improve. Results from PTH and
vitamin D examination indicated that the patient had neither
primary hyperparathyroidism nor vitamin D metabolic disturbance.
Since KHE was a kind of borderline tumor, and cases of borderline
tumors combined with HC had also been reported previously (29,30), it
was speculated whether HC generation was closely related to
PTH-related protein (PTHrp) produced by hemangioma. PTHrp is the
homologous protein of PTH and shares the same receptor (PTH1
receptor) with PTH to implement similar biological functions
(31,32). PTH/PTHrp serves a dual role in bone
metabolism and promotes bone formation and resorption, which are
predominantly mediated by the protein kinase (PK) A and PKC
pathways and dependent on functional regulations of osteoblasts and
osteoclasts (33). Although the
level of PTHrp was not measured in the present report due to
detection limitation, surgical treatment of the hemangioma was
recommended for the patient. Due to the large mass of the vascular
tumor, resection was not suitable. Therefore, the hemangioma was
removed through pipeline arteriosclerosis embolization.
Pathological examination was not performed due to the high risk to
take pathological specimens. Serum calcium returned to normal range
quickly following surgical treatment and the patient was in a good
condition at a 4-month follow-up evaluation.
Acknowledgements
The present study was supported by the Planed
Projects of Hunan Provincial Science and Technology Department
(grant no. 2013FJ6028) and the Scientific Fund of Health and Family
Planning Commission of Hunan Province (grant no. C2013-023).
References
|
1
|
Mulliken JB and Glowacki J: Hemangiomas
and vascular malformations in infants and children: A
classification based on endothelial characteristics. Plast Reconstr
Surg. 69:412–422. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trenor CC III and Chaudry G: Complex
lymphatic anomalies. Semin Pediatr Surg. 23:186–190. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Massarweh S, Munis A, Karabakhtsian R,
Romond E and Moss J: Metastatic angiosarcoma and kasabach-merritt
syndrome. Rare Tumors. 6:53662014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Croteau SE, Kozakewich HP, Perez-Atayde
AR, Fishman SJ, Alomari AI, Chaudry G, Mulliken JB and Trenor CC
III: Kaposiform lymphangiomatosis: A distinct aggressive lymphatic
anomaly. J Pediatr. 164:383–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasabach H and Merritt K: Capillary
hemangioma with extensive purpura-Report of a case. Am J Dis
Children. 59:1063–1070. 1940. View Article : Google Scholar
|
|
6
|
Thomson H: Cutaneous hemangiomas and
lymphangiomas. Clin Plast Surg. 14:341–356. 1987.PubMed/NCBI
|
|
7
|
Kim T, Roh MR, Cho S and Chung KY:
Kasabach-Merritt syndrome arising from tufted angioma successfully
treated with systemic corticosteroid. Ann Dermatol. 22:426–430.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hall GW: Kasabach-Merritt syndrome:
Pathogenesis and management. Br J Haematol. 112:851–862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia H, He Z, Zhu Z, Zhu X, Zhu X, Xie W
and Ouyang T: Clinical analysis of 10 cases of Kasabach-Merritt
syndrome. Chin J Prac Pediat. 26:125–127. 2011.(In Chinese).
|
|
10
|
Rodriguez V, Lee A, Witman PM and Anderson
PA: Kasabach-merritt phenomenon: Case series and retrospective
review of the mayo clinic experience. J Pediatr Hematol Oncol.
31:522–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maguiness S and Guenther L:
Kasabach-merritt syndrome. J Cutan Med Surg. 6:335–339. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dasgupta R and Fishman SJ: ISSVA
classification. Semin Pediatr Surg. 23:158–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zukerberg LR, Nickoloff BJ and Weiss SW:
Kaposiform hemangioendothelioma of infancy and childhood. An
aggressive neoplasm associated with Kasabach-Merritt syndrome and
lymphangiomatosis. Am J Surg Pathol. 17:321–328. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thosani S and Hu MI: Denosumab: A new
agent in the management of hypercalcemia of malignancy. Future
Oncol. 11:2865–2871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dellay B and Groth M: Emergency management
of malignancy-associated hypercalcemia. Adv Emerg Nurs J. 38:15–25.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Komisarenko IuI: Mineral metabolism and
metabolic markers in patients with concomitant endocrine disorders
and vitamin D3 deficiency. Lik Sprava. 51–55. 2013.(In Ukrainian).
PubMed/NCBI
|
|
17
|
Žofková I: Hypercalcemia.
Pathophysiological aspects. Physiol Res. 65:1–10. 2016.PubMed/NCBI
|
|
18
|
Edelson GW and Kleerekoper M:
Hypercalcemic crisis. Med Clin North Am. 79:79–92. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao Q: Basic and Clinical of Pediatric
Hematology. Beijing People's Medical Publishing House; Beijing: pp.
702–703. 2001
|
|
20
|
Mulliken JB, Fishman SJ and Burrows PE:
Vascular anomalies. Curr Probl Surg. 37:517–584. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hohenleutner U, Landthaler M, Hamm H and
Sebastian G: Hemangiomas of infancy and childhood. J Dtsch Dermatol
Ges. 5:334–338. 2007.(In English, German). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng CE and Friedlander SF: Infantile
hemangiomas, complications and treatments. Semin Cutan Med Surg.
35:108–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ranchod TM, Frieden IJ and Fredrick DR:
Corticosteroid treatment of periorbital haemangioma of infancy: A
review of the evidence. Br J Ophthalmol. 89:1134–1138. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masoomi H, Nguyen B, Smith BR, Stamos MJ
and Nguyen NT: Predictive factors of acute respiratory failure in
esophagectomy for esophageal malignancy. Am Surg. 78:1024–1028.
2012.PubMed/NCBI
|
|
25
|
Kelly M: Kasabach-Merritt phenomenon,
Pediatr Clin North Am. 57:1085–1089. 2010. View Article : Google Scholar
|
|
26
|
Sarkar M, Mulliken JB, Kozakewich HP,
Robertson RL and Burrows PE: Thrombocytopenic coagulopathy
(Kasabach-Merritt phenomenon) is associated with Kaposiform
hemangioendothelioma and not with common infantile hemangioma.
Plast Reconstr Surg. 100:1377–1386. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Enjolras O, Wassef M, Mazoyer E, Frieden
IJ, Rieu PN, Drouet L, Taïeb A, Stalder JF and Escande JP: Infants
with Kasabach-Merritt syndrome do not have ‘true’ hemangiomas. J
Pediatr. 130:631–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiu YE, Drolet BA, Blei F, Carcao M,
Fangusaro J, Kelly ME, Krol A, Lofgren S, Mancini AJ, Metry DW, et
al: Variable response to propranolol treatment of kaposiform
hemangioendothelioma, tufted angioma, and Kasabach-Merritt
phenomenon. Pediatr Blood Cancer. 59:934–938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boukhris I, Azzabi S, Kéchaou I, Chérif E,
Kooli C, Romdhane KB, Omar S and Khalfallah N: Hypercalcemia
related to PTH-rP revealing malignant hepatic epithelioid
hemangioendothelioma. Ann Biol Clin (Paris). 74:98–102.
2016.PubMed/NCBI
|
|
30
|
Donovan PJ, Achong N, Griffin K, Galligan
J, Pretorius CJ and McLeod DS: PTHrP-mediated hypercalcemia: Causes
and survival in 138 patients. J Clin Endocrinol Metab.
100:2024–2029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okazaki M, Ferrandon S, Vilardaga JP,
Bouxsein ML, Potts JT Jr and Gardella TJ: Prolonged signaling at
the parathyroid hormone receptor by peptide ligands targeted to a
specific receptor conformation. Proc Natl Acad Sci USA. 105:pp.
16525–16530. 2008, View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong MH, Stockler MR and Pavlakis N:
Bisphosphonates and other bone agents for breast cancer. Cochrane
Database Syst Rev: CD003474. 2012. View Article : Google Scholar
|
|
33
|
Niizuma H, Fujii K, Sato A, Fujiwara I,
Takeyama J and Imaizumi M: PTHrP-independent hypercalcemia with
increased proinflammatory cytokines and bone resorption in two
children with CD-negative precursor B acute lymphoblastic leukemia.
Pediatr B1ood Cancer. 49:990–993. 2007. View Article : Google Scholar
|