Introduction
Hepatocellular carcinoma (HCC) is one of the most
common causes of cancer-related mortality worldwide (1). It is estimated that of >1,000,000
cases of patients diagnosed with HCC, <5% survive beyond five
years (2). Treatment of HCC
primarily consists of liver transplantation, surgical resection and
chemotherapy. In addition, ionizing radiation is a well-established
and widely used therapeutic modality for patients with HCC
(3). However, the majority of the
available natural or synthetic compounds are not successful
clinically due to their toxicity and side effects. Therefore, novel
and effective therapeutics are urgently required for HCC
treatment.
The fungi, Ganoderma lucidum (Leyss ex fr)
Karst (Lingzhi) has been used in China to treat various human
diseases such as hepatitis, bronchitis and tumorigenic diseases
(4). Polysaccharides, one of the
major categories of the bioactive ingredients of ganoderma
lucidum, exhibit multiple biological properties including the
prevention of oxidative damage and protection of the liver
(5). Previous studies have
demonstrated that Ganoderma lucidum polysaccharide (GLP) has
antitumor effects, mediated by boosting the activity of host immune
cells (6). Furthermore, accumulating
evidence supports the hypothesis that GLP exhibits non-toxic and
reduced toxicity-related adverse effects compared with chemotherapy
or radiotherapy (7). Collectively,
these studies suggest that GLP may potentially serve as a
chemotherapeutic agent for cancer treatment. In the present study,
the effects of GLP on the radiosensitivity of HCC were assessed in
addition to its underlying mechanism.
Materials and methods
Cell culture and treatment
The human HCC cell line HepG2 was obtained from the
Chinese Academy of Sciences (Beijing, China). Cells were grown in
Dulbecco's Modified Eagle Medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 100
U/ml penicillin and 100 mg/ml streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.). Cells were cultured in in a humidified
incubator containing 5% CO2. For irradiation, HepG2
cells were incubated with GLP (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C for 72 h. Then, cells were exposed to
radiation using an X-RAD 320 Irradiator (Precision X-Ray, Inc.,
North Branford, CT, USA) at 6 Gy for 30 min and collected for the
subsequent experiments.
Immunofluorescence
HepG2 cells were fixed with 4% formaldehyde for 15
min at room temperature and permeabilized with 0.04% Triton X-100.
Then cells were incubated with γ-H2AX antibodies (1:1,000; ab2893;
Abcam, Cambridge, MA, USA) overnight at 4°C, washed in PBST for 10
min three times and incubated with fluorescein isothiocyanate
(FITC)-conjugated secondary antibodies (1:5,000; ab97050; Abcam) at
37°C for 2 h. The nuclei were visualized by staining with
4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich; Merck KGaA).
Images were captured using fluorescence confocal microscopy
(VF1000; Olympus Corporation, Tokyo, Japan).
Apoptosis assay
HepG2 cells were exposed to radiation, GLP, or GLP
combined with Akt inhibitor A-674563 at 37°C for 72 h and then
collected by centrifugation at 3,000 × g at room temperature for 15
min. Cell apoptosis was detected by using the Annexin V-FITC
apoptosis detection kit (cat. no. 556547; BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer's protocol. Apoptosis
was determined by calculating the percentage of apoptotic cells
relative to the total number of cells.
Western blot analysis
HepG2 cells were incubated with different doses of
GLP (0, 10, and 100 µM) alone or in combination with Akt inhibitor
A-674563 (50 µM). Afterwards, HepG2 cells were harvested, washed
twice with PBS for 10 min, and lysed in radioimmunoprecipitation
assay lysis buffer (Sigma-Aldrich; Merck KGaA). The cell lysates
were centrifuged at 12,000 × g for 5 min at 4°C. Protein
concentration was quantified using a Bradford Protein kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Samples with 20 µg of
protein were separated by 12% SDS-PAGE, and then the proteins were
transferred onto a nitrocellulose membrane. The membrane was
blocked with 5% not-fat milk in tris-buffered saline and Tween-20
(TBST) and incubated with primary antibodies against pDNA-PK (1:200
dilution; sc-101664; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), DNA-PK (1:2,000 dilution; sc-390698; Santa Cruz
Biotechnology, Inc.), pATM (1:200 dilution; sc-47739; Santa Cruz
Biotechnology, Inc.) and ATM (1:200 dilution; sc-7230; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The membrane was washed with
TBST three times and incubated with a goat anti-mouse IgG secondary
antibody that was conjugated with horseradish peroxidase (1:5,000
dilution; sc-5362; Santa Cruz Biotechnology, Inc.) for 1 h. The
proteins were detected using an enhanced chemiluminescence
detection kit (cat. no. 5384; Roche Diagnostics, Basel,
Switzerland). Three replicate blots were analyzed using Quantity
One 4.2.1 software (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Data were expressed as mean ± standard deviation and
statistical comparisons were performed using one-way analysis of
variance. P<0.05 was considered to represent statistically
significant differences.
Results
Effects of GLP on radiation-induced
growth inhibition of HepG2 cells
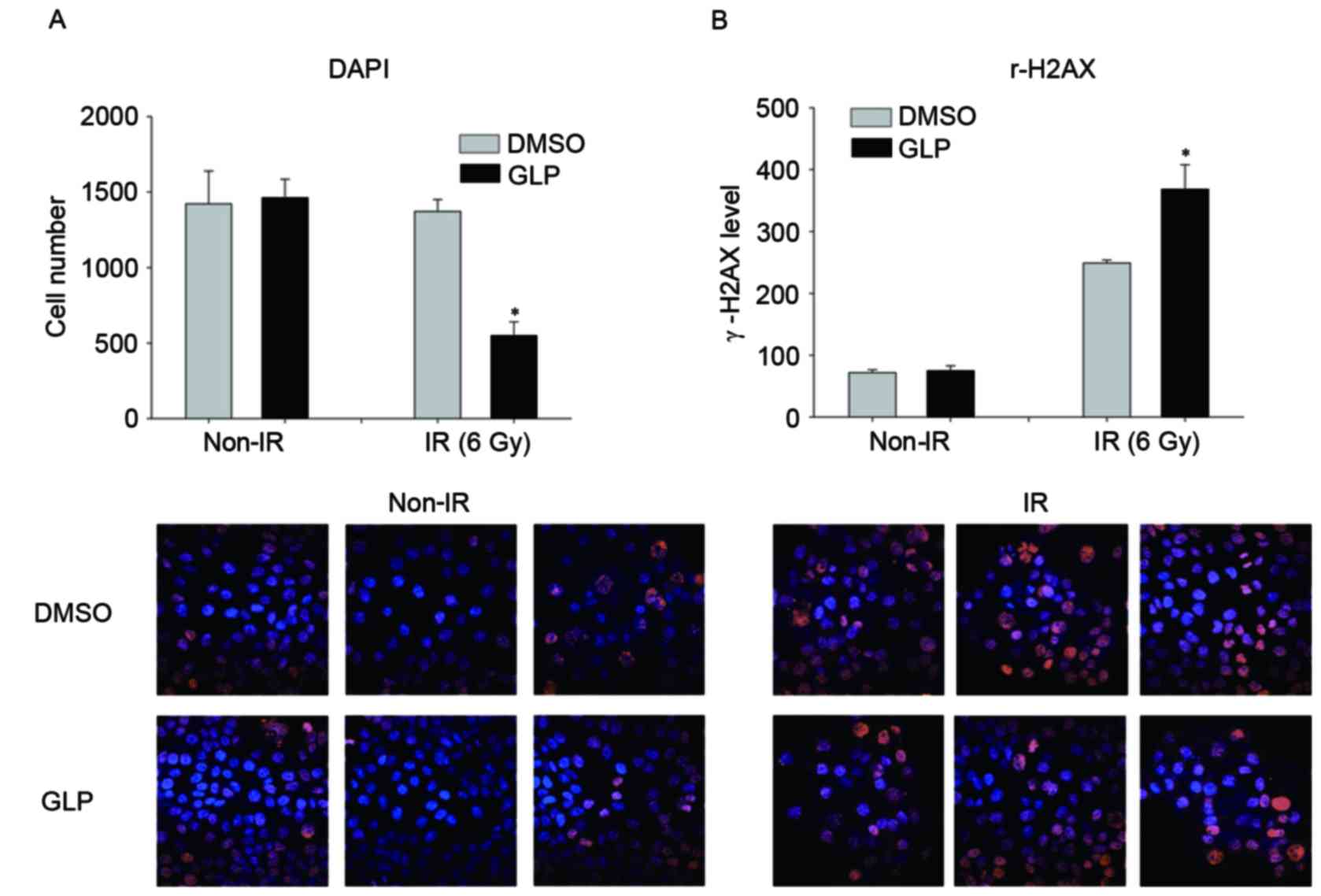
HepG2 cells treated with DMSO or GLP were exposed to
radiation at 6 Gy. DAPI staining indicated that administration of
GLP had no significant effect on cell number without radiation
exposure. However, GLP in combination with radiation significantly
reduced the HepG2 cell number compared with the control (P<0.05;
Fig. 1A). In addition,
immunofluorescence staining for γ-H2AX foci revealed that tumor
cells treated with GLP exhibited an increased proportion of nuclei
under the radiation condition (P<0.05; Fig. 1B). These data suggest that GLP
treatment enhances radiation-induced growth inhibition of HCC
cells.
Effects of GLP on radiation-induced
apoptosis of HepG2 cells
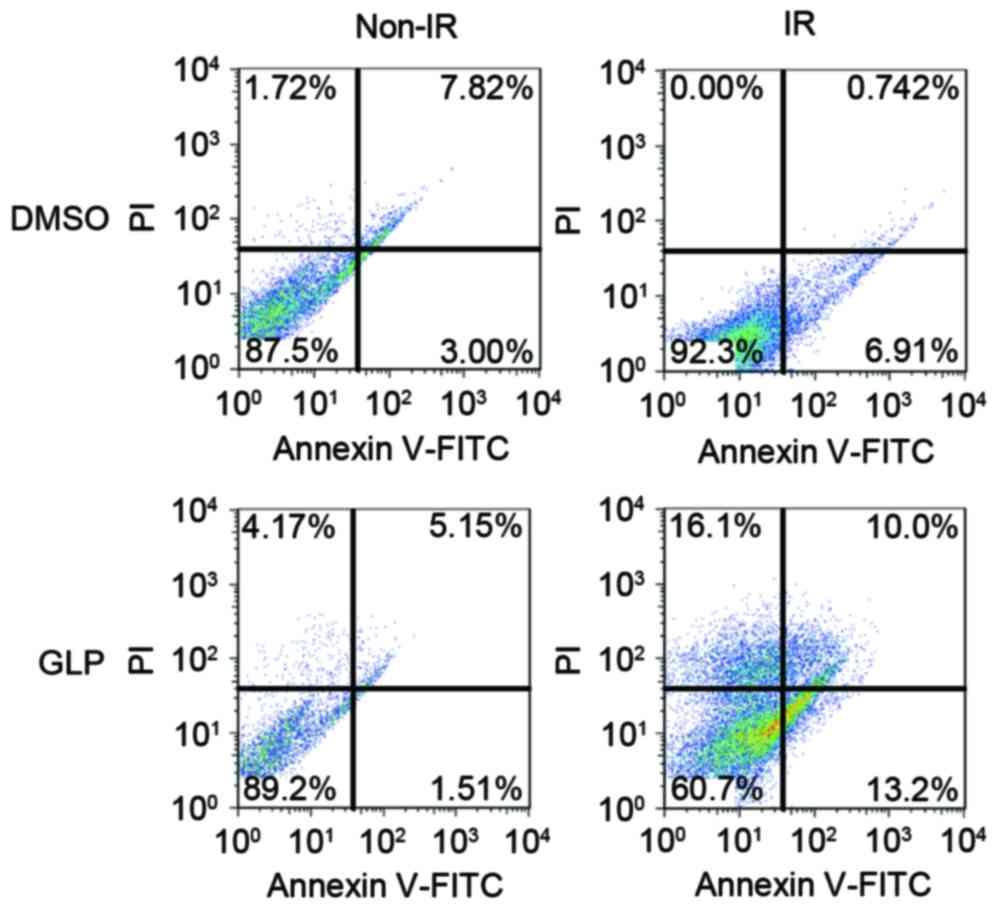
It was demonstrated that radiation treatment alone
(6 Gy) does not significantly affect the apoptosis rate of HepG2
cells. However, in combination with GLP, radiation treatment
significantly promoted HepG2 apoptosis death (23.2%) compared with
radiation (7.7%) or GLP (6.7%) alone (P<0.05; Fig. 2). These results demonstrate that GLP
co-administration with radiation promotes HCC cell apoptosis under
the radiation condition.
Effects of GLP on DNA repair
associated proteins expression
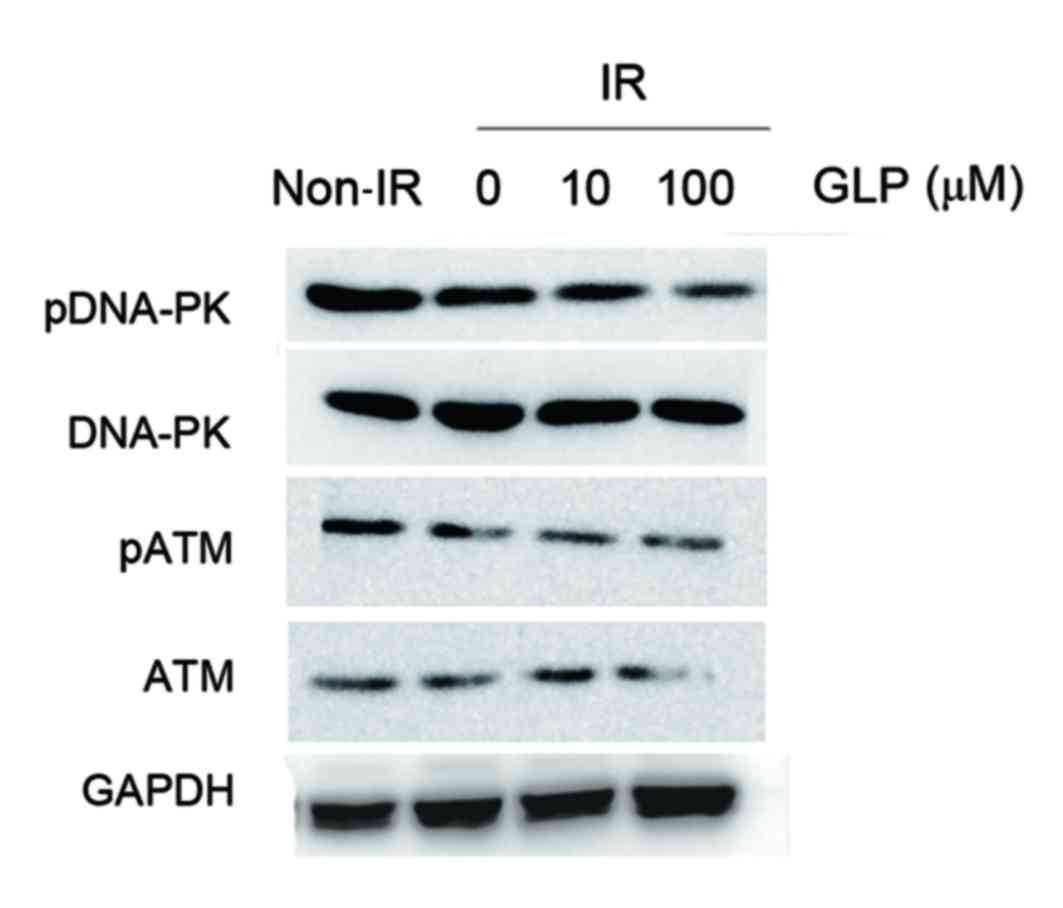
In order to investigate the role of GLP in DNA
damage upon radiation, the expression of DNA repair associated
proteins such as ataxia-telangiectasia mutated (ATM) and DNA
dependent-protein kinase (DNA-PK) was assessed. HepG2 cells were
incubated with different doses of GLP and then exposed to radiation
at 6 Gy. Western blot analysis demonstrated that the
phosphorylation of DNA-PK was markedly reduced in GLP-treated HepG2
cells.
In addition, the co-administration of GLP and
radiation also inhibited the ATM activities compared with HepG2
cells treated with radiation alone (Fig.
3). These data suggest that GLP may suppress the activity of
DNA repair associated proteins in liver cancer cells under the
radiation condition.
Akt is associated with the
GLP-regulated radiosensitivity in HepG2 cells exposed to
radiation
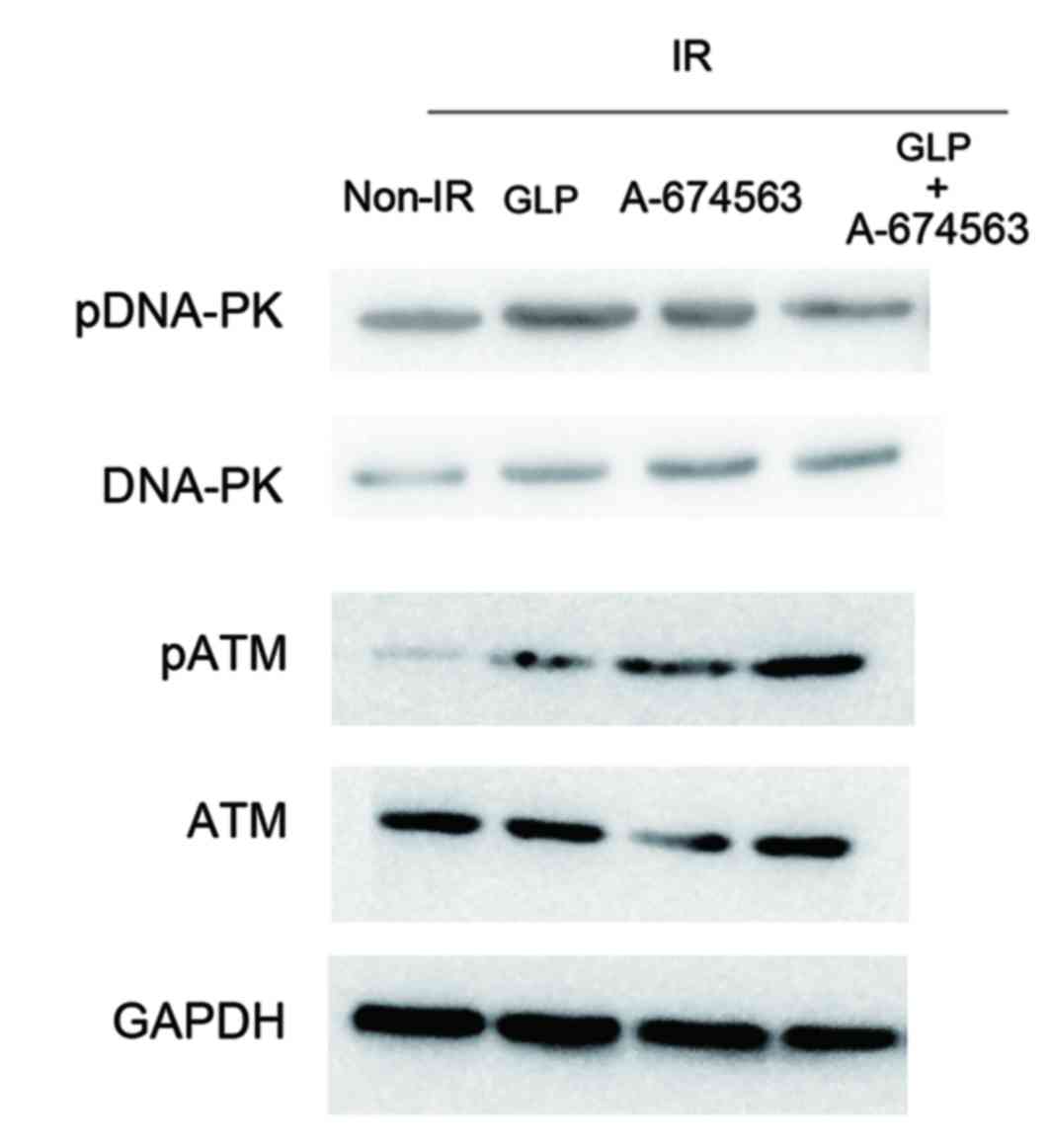
It was then assessed whether Akt was associated with
GLP-mediated ATM activity in HepG2 cells exposed to radiation.
HepG2 cells were incubated with Akt inhibitor A-674563 alone or in
combination with GLP. Western blot analysis indicated that the
phosphorylation level of DNA-PK and ATM was elevated following
treatment with A-674563 (Fig. 4). In
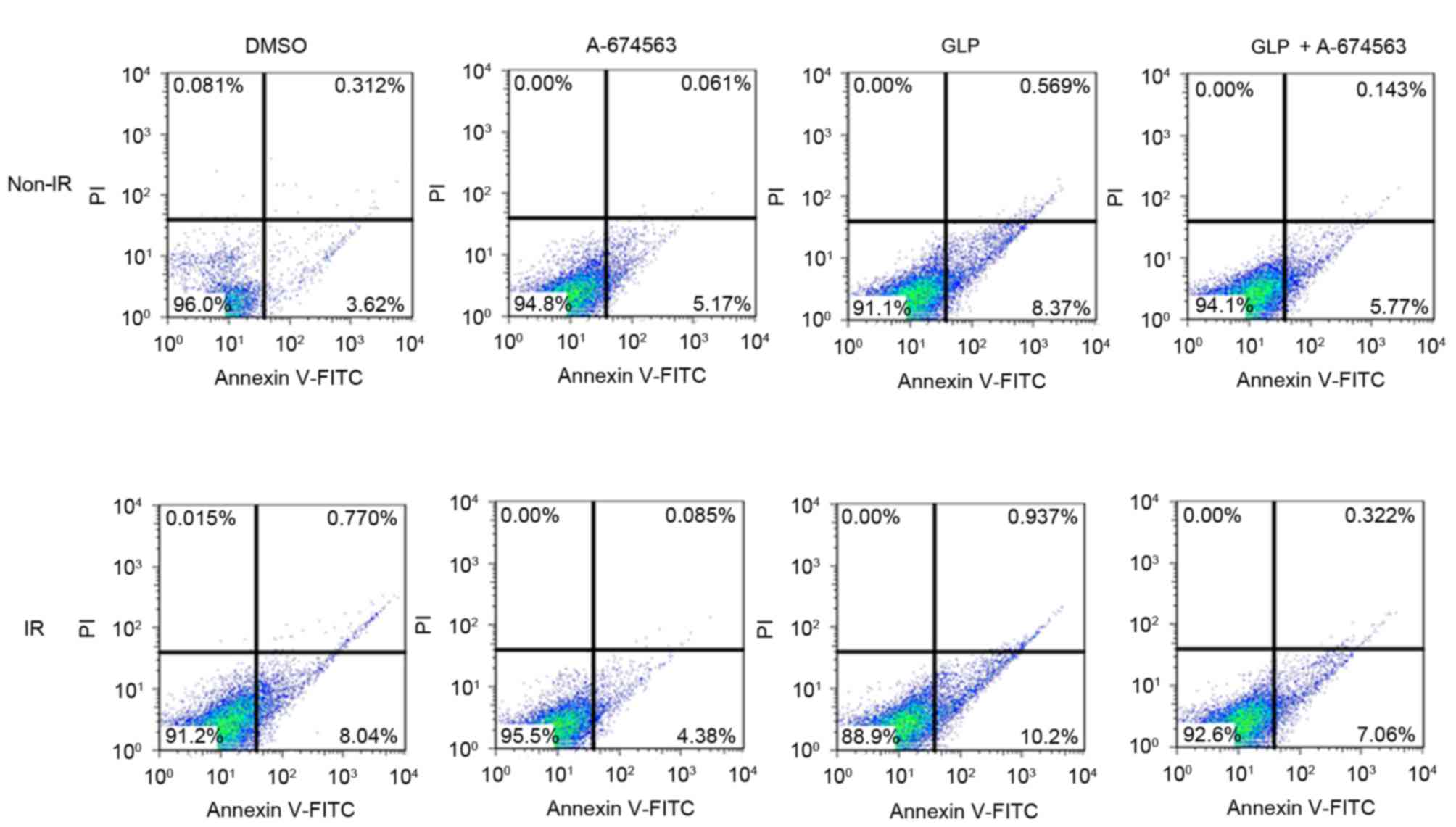
addition, it was demonstrated that GLP treatment enhanced the
apoptotic death of HepG2 cells under radiation compared with
DMSO-treated cells (11.13 vs. 8.81%). However, addition of the Akt
inhibitor suppressed the GLP-induced HepG2 cell injury under
radiation condition (7.38 vs. 11.13%; Fig. 5). These results suggest that Akt
participates in GLP-mediated radiosensitivity of HepG2 cells
exposed to radiation.
Discussion
Over the past 30 years, a wide range of traditional
Chinese herbs and botanical formulations have been identified as
radiosensitizers in cancer treatment (8–10). A
number of these even have undergone clinical trials and exhibited
favorable therapeutic effects with safety and lower toxicity
(8). GLP, an extract of a
basidiomycete fungus, possesses various pharmacologic properties
including antitumor effects (5,11,12). In
the current study, treatment with GLP was demonstrated to
significantly promote the apoptosis of HepG2 cells under radiation
condition, which may be mediated by the Akt signaling pathway.
These findings suggest that GLP has a potential radiosensitization
effect on HepG2 cells exposed to radiation.
Polysaccharides widely exist in microorganisms,
algae, plants and animals. Together with polynucleotides and
proteins, they are essential biomacromoleules in the biological
processes and serve critical roles in cell growth, adhesion, and
apoptosis (13). Previously,
polysaccharides extracted from natural sources have attracted
attention in the field of cancer therapy due to their antitumor
properties (14). In the present
study, GLP treatment was identified to promote radiation-induced
growth inhibition and apoptotic death of HCC cells.
DNA damage response (DDR) serves a critical role in
the maintenance of genome integrity (15). During cancer therapy, DDR attenuates
the efficiency of radio- or chemotherapeutic agents and thus
induces drug resistance of tumor cells to genotoxic stress
(16). Therefore, inhibition of DNA
repair-associated enzymes may be a promising strategy to increase
the sensitivity of ionizing radiation on cancer cells. Cancer cells
exposed to radiation exhibit DNA lesions and double strand breaks,
which are repaired by ATM and DNA-PK (17). In order to examine the effects of GLP
on DDR in HCC cells, the protein expression of ATM and DNA-PK in
HepG2 cells exposed to radiation was measured. Results indicated
that the phosphorylation of ATM and DNA-PK was markedly suppressed
following the administration of GLP, suggesting that GLP may
inhibit radiation-induced DNA repair.
The Akt signaling pathway has been demonstrated to
regulate multiple biological activities, including cell
proliferation, apoptosis and differentiation (18,19).
Upregulation of Akt is observed in numerous types of cancer, and it
may be associated with uncontrolled cell growth (20). In addition, the Akt signaling pathway
has been implicated in a number of processes associated with cell
cycle regulation, including DNA replication and damage repair
(21). As demonstrated in Figs. 4 and 5, the addition of Akt inhibitor was
demonstrated to elevate the activities of DNA-PK and ATM and
suppress the GLP-induced HepG2 cell injury under radiation
condition. These results indicate that Akt signaling regulated the
GLP-mediated radiosensitivity in HepG2 cells exposed to
radiation.
In conclusion, the present study demonstrated that
GLP treatment may enhance the radiosensitivity of HCC cells via
regulation of the Akt signaling pathway. These findings suggest
that GLP may be developed into a potential radiation sensitizer to
improve the therapeutic efficacy of HCC.
References
|
1
|
McGlynn KA, Petrick JL and London WT:
Global epidemiology of hepatocellular carcinoma: An emphasis on
demographic and regional variability. Clin Liver Dis. 19:223–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lau WY and Lai EC: Hepatocellular
carcinoma: Current management and recent advances. Hepatobiliary
Pancreat Dis Int. 7:237–257. 2008.PubMed/NCBI
|
|
4
|
Xu Z, Chen X, Zhong Z, Chen L and Wang Y:
Ganoderma lucidum polysaccharides: Immunomodulation and
potential anti-tumor activities. Am J Chin Med. 39:15–27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo L, Xie J, Ruan Y, Zhou L, Zhu H, Yun
X, Jiang Y, Lü L, Chen K, Min Z, et al: Characterization and
immunostimulatory activity of a polysaccharide from the spores of
Ganoderma lucidum. Int Immunopharmacol. 9:1175–1182. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin ZB and Zhang HN: Anti-tumor and
immunoregulatory activities of Ganoderma lucidum and its
possible mechanisms. Acta pharmacologica Sinica. 25:1387–1395.
2004.PubMed/NCBI
|
|
7
|
Zhou X, Lin J, Yin Y, Zhao J, Sun X and
Tang K: Ganodermataceae: Natural products and their related
pharmacological functions. Am J Chin Med. 35:559–574. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ZL, Zhu WR, Zhou WC, Ying HF, Zheng L,
Guo YB, Chen JX and Shen XH: Traditional Chinese medicinal herbs
combined with epidermal growth factor receptor tyrosine kinase
inhibitor for advanced non-small cell lung cancer: A systematic
review and meta-analysis. J Integr Med. 12:346–358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia L, Ma S, Hou X, Wang X, Qased AB, Sun
X, Liang N, Li H, Yi H, Kong D, et al: The synergistic effects of
traditional Chinese herbs and radiotherapy for cancer treatment.
Oncol Lett. 5:1439–1447. 2013.PubMed/NCBI
|
|
10
|
Yang L, Wei DD, Chen Z, Wang JS and Kong
LY: Reversal effects of traditional Chinese herbs on multidrug
resistance in cancer cells. Nat Prod Res. 25:1885–1889. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Z, Huang K, Fu X, Zhou Z, Cui Y and Li
H: A chemically sulfated polysaccharide derived from Ganoderma
lucidum induces mitochondrial-mediated apoptosis in human
osteosarcoma MG63 cells. Tumour Biol. 35:9919–9926. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tie L, Yang HQ, An Y, Liu SQ, Han J, Xu Y,
Hu M, Li WD, Chen AF, Lin ZB and Li XJ: Ganoderma lucidum
polysaccharide accelerates refractory wound healing by inhibition
of mitochondrial oxidative stress in type 1 diabetes. Cell Physiol
Biochem. 29:583–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pillai TG, Maurya DK, Salvi VP,
Janardhanan KK and Nair CK: Fungal beta glucan protects radiation
induced DNA damage in human lymphocytes. Ann Transl Med.
2:132014.PubMed/NCBI
|
|
14
|
Zeng G, Shen H, Tang G, Cai X, Bi L, Sun
B, Yang Y and Xun W: A polysaccharide from the alkaline extract of
Glycyrrhiza inflata induces apoptosis of human oral cancer SCC-25
cells via mitochondrial pathway. Tumour Biol. 36:6781–6788. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korwek Z, Sewastianik T, Bielak-Zmijewska
A, Mosieniak G, Alster O, Moreno-Villanueva M, Burkle A and Sikora
E: Inhibition of ATM blocks the etoposide-induced DNA damage
response and apoptosis of resting human T cells. DNA Repair (Amst).
11:864–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Moraes Nestal G, Bella L, Zona S,
Burton MJ and Lam EW: Insights into a Critical Role of the
FOXO3a-FOXM1 Axis in DNA Damage Response and Genotoxic Drug
Resistance. Curr Drug Targets. 17:164–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu S, Opiyo SO, Manthey K, Glanzer JG,
Ashley AK, Amerin C, Troksa K, Shrivastav M, Nickoloff JA and
Oakley GG: Distinct roles for DNA-PK, ATM and ATR in RPA
phosphorylation and checkpoint activation in response to
replication stress. Nucleic Acids Res. 40:10780–10794. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ourique F, Kviecinski MR, Felipe KB,
Correia JF, Farias MS, Castro LS, Grinevicius VM, Valderrama J,
Rios D, Benites J, et al: DNA damage and inhibition of akt pathway
in mcf-7 cells and ehrlich tumor in mice treated with
1,4-naphthoquinones in combination with ascorbate. Oxid Med Cell
Longev. 2015:4953052015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davis WJ, Lehmann PZ and Li W: Nuclear
PI3K signaling in cell growth and tumorigenesis. Front Cell Dev
Biol. 3:242015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Zhu G, Getzenberg RH and Veltri RW:
The upregulation of PI3K/Akt and MAP kinase pathways is associated
with resistance of microtubule-targeting drugs in prostate cancer.
J Cell Biochem. 116:1341–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King SM, Quartuccio SM, Vanderhyden BC and
Burdette JE: Early transformative changes in normal ovarian surface
epithelium induced by oxidative stress require Akt upregulation,
DNA damage and epithelial-stromal interaction. Carcinogenesis.
34:1125–1133. 2013. View Article : Google Scholar : PubMed/NCBI
|