Introduction
Tacrolimus is used as an immunosuppressive drug in
patients following transplant and has a narrow therapeutic window,
and is primarily metabolized by cytochrome P450 (CYP) 3A4 and
CYP3A5 (1,2). CYP3A5 has been demonstrated to serve a
key role in the pharmacokinetics of tacrolimus, specifically in
organ transplant patients, and it has been documented that the
blood concentration of tacrolimus in patients with a CYP3A5 *1/*1
or *1/*3 genotype (expressors, EX) was lower than that of patients
with a *3/*3 genotype (non-expressors, NEX) (3–5). In
Japan, tacrolimus has previously been administered to patients with
autoimmune diseases, including ulcerative colitis, myasthenia
gravis, lupus nephritis and rheumatoid arthritis (6). However, it remains unclear whether the
CYP3A5 genotype impacts the pharmacokinetics of tacrolimus in
patients with autoimmune diseases in addition to organ transplant
recipients.
To determine the optimal dose of tacrolimus in organ
transplant patients, physicians typically make dose adjustments
based on results obtained from monitoring blood concentration
levels of the drug (5). By contrast,
the approved dose for patients with autoimmune diseases is fixed,
such that 3 mg/day is the dose for myasthenia gravis patients
(7). Among these patients with
autoimmune diseases, those that present with a CYP3A5 expressor
genotype may not exhibit the anticipated effect of the drug, due to
a lowered blood concentration of tacrolimus when compared with
non-expressors (5). By contrast, the
tacrolimus concentration of non-expressors may unpredictably
increase and lead to adverse effects, including renal dysfunction
and/or infection complications (5).
The ImmuKnow (IMK) assay, which was approved by the
Food and Drug Administration (Silver Spring, MD, USA.) in 2002,
monitors the function of cluster of differentiation (CD)
4+ T cells by measuring the intracellular concentration
of adenosine triphosphate (ATP) (8).
The IMK assay has previously been used to identify transplant
patients at risk of infection (patients with low IMK ATP levels:
<225 ng/ml) or rejection (patients with high IMK ATP levels:
>525 ng/ml) (1,2,9).
However, it has been argued that the IMK assay is not a useful
indicator of infection or rejection risk (3,4), and for
patients with autoimmune diseases, the efficacy of the IMK assay in
monitoring immunological aspects remains unclear.
The present study evaluated the association between
CYP3A5 genotype and the pharmacokinetics of tacrolimus in patients
with autoimmune diseases. Furthermore, the efficacy of the IMK
assay in monitoring immunological aspects in patients with
autoimmune diseases was investigated.
Materials and methods
Study design
A total of 25 randomly selected autoimmune disease
patients who underwent treatment with tacrolimus at the Mie
University Hospital (Mie, Japan) between October 2013 and July 2014
were enrolled in the current study. Patients were assessed using
IMK and tacrolimus concentration assays following the collection of
signed informed consent. Patients were administered tacrolimus with
the dose approved by the Japanese Ministry of Health, Labour and
Welfare (Tokyo, Japan) as a prescription drug for the treatment of
rheumatoid arthritis (3 mg/day), lupus nephritis (3 mg/day),
myasthenia gravis (3 mg/day) and ulcerative colitis (0.025 mg/kg
twice a day) (6,7,10). The
physicians in charge of patients prospectively evaluated the
incidence of insufficient effect. Any adverse effects of tacrolimus
in each patient were also retrospectively assessed. The Clinical
Ethics Review Board of Mie University Hospital approved the present
study (No. 2605).
Patients
A total of 25 patients with a median age of 57
(range, 28–88) and 5/20 male: female ratio with autoimmune diseases
(rheumatoid arthritis: n=15, lupus nephritis: n=6, myasthenia
gravis: n=2 and ulcerative colitis: n=2) who were administered with
tacrolimus at the Mie University Hospital between October 2013 and
July 2014, were enrolled in the present study. CYP3A5 genotype,
peripheral blood CD4+ ATP activity, tacrolimus
concentration and clinical effects were evaluated in all patients.
Patients who did not take tacrolimus were excluded from this
study.
ImmuKnow (IMK) assay
Peripheral blood samples were collected in sodium
heparin tubes on admission at the ward or the outpatient clinic,
and the intracellular ATP level was measured using an ImmuKnow
assay kit (Cylex, Inc., Columbia, MD, USA). Blood samples were
processed on the day of sample collection. Briefly, 250 µl
anti-coagulated whole blood was diluted with the kit diluent to
make a final volume of 1,000 µl. In accordance with the
manufacturers instructions, samples were added to wells of a
96-well plate with phytohemagglutinin (Medical & Biological
Laboratories, Co., Ltd.) and incubated for 15–18 h with at 37°C and
5% CO2 atmosphere. After enrichment for CD4+
T cells by the addition of magnetic particles coated with an
anti-human CD4 monoclonal antibody (cat. no. 4402329; dilution as
provided in the kit; Dynabeads; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), cells were washed with PBS and
bovine serum albumin (concentration as provided in the kit; Cyclex,
Inc., Columbia, MO, USA) and lysed to release intracellular ATP.
Released ATP was measured with a luciferin/luciferase assay in a
luminometer (Berthold Technologies, LLC, Midway, TN, USA) according
to the manufacturers protocol. The patients level of immune
response was expressed as the amount of ATP (ng/ml).
According to a previous study (3), the subjects were divided into 3 groups,
using group boundaries previously established in transplant
patients. The present study defined the ATP low-level group as
<225 ng/ml, in which patients exhibited an over-immunosuppressed
state, the ATP high-level group as >525 ng/ml, in which patients
exhibited an under-immunosuppressed state, and the ATP middle-level
group as 226–525 ng/ml, in which patients were considered to be
within the target immunological response zone.
Administration of tacrolimus
For patients with rheumatoid arthritis, lupus
nephritis and myasthenia gravis, the approved maximum dose of
tacrolimus administered orally is 3.0 mg/day after food from the
day of patient admission. The trough level of tacrolimus in these
diseases remains unclear; however, 3.0 mg/day is recommended as
this induces minimal renal dysfunction (11). For ulcerative colitis, the
recommended dose of tacrolimus is 0.025 mg/kg twice a day orally
from the day of admission. The target whole-blood trough level for
tacrolimus was 10–15 ng/ml during the first 2 weeks and 5–10 ng/ml
after 2 weeks.
Evaluation of tacrolimus blood
concentration and concentration/dose (C/D) ratio
The tacrolimus blood concentration was measured
using a chemiluminescent immunoassay (ARCHITECT® i2000
tacrolimus Abott 1L77-25; Abbott Laboratories S.A., Shanghai,
China) according to the manufacturers protocol. The daily dose of
tacrolimus was adjusted based on tacrolimus blood concentration
measurements and its weight-adjusted dose (mg/kg per day) was
calculated. The measured blood tacrolimus concentration was then
normalized to the corresponding dose per body weight 24 h prior to
blood sampling to obtain the concentration/dose (C/D) ratio.
Genotyping of CYP450 3A5
According to a previous study (2), the CYP3A5 A6986G (rs776746)
polymorphism was analyzed to detect the *3 allele, as CYP3A5*3 is
the major defective allele (2).
Furthermore, other functional exonic single-nucleotide
polymorphisms (SNPs) are rare in the Japanese population (12). Based on the CYP3A5 genotype, patients
were allocated into 2 groups: CYP3A5 *1/*1 or CYP3A5 *1/*3 (EX,
n=6) and CYP3A5 *3/*3 (NEX, n=19).
Definitions of insufficient and
adverse effects
Insufficient effect was defined as a worsening or
lack of improvement in the patients clinical condition. If
worsening of the patients clinical condition was observed, an
increased dosage of tacrolimus was administered or the treatment
was changed to other drugs as cyclosporine, or additional
immunosuppressants (e.g. Mizoribine, Endoxan) were administered
(Table I). Adverse effects were
defined as a reduction or loss in the effects of tacrolimus, and
when patients required treatment for renal dysfunction,
hyperkalemia, tremor, headaches, and/or hyperuricemia.
 | Table I.Characteristics of patients exhibiting
an insufficient effect following TAC therapy. |
Table I.
Characteristics of patients exhibiting
an insufficient effect following TAC therapy.
| Patient no. | Genotype | Gender | Age | Adaptation
disease | Months after TAC
therapy | TAC dose, mg/day | TAC conc, ng/ml | IMK, ng/ml | Clinical
condition | Concurrent
medication | Additional
therapy |
|---|
| 1 | EX | F | 29 | LN | 33.2 | 3.0 | 4.5 | 535.5 | NI | PSL 10.0 mg/day | None |
| 2 | EX | F | 47 | LN | 9.7 | 3.0 | 1.9 | 422.5 | W | PSL 7.5 mg/day | Change to CYA |
|
|
|
|
|
|
|
|
|
|
|
| 4 mg/kg |
| 3 | EX | M | 64 | RA | 11.9 | 2.0 | 3.1 | 704.0 | W | PSL 5.0 mg/day | Increase of TAC |
|
|
|
|
|
|
|
|
|
|
| Iguratimod 25.0
mg/day | 3.0 mg/day |
|
|
|
|
|
|
|
|
|
|
| Etanercept 25.0
mg/week |
|
| 4 | NEX | F | 66 | LN | 0.1 | 2.0 | 9.9 | 574.0 | W | PSL 30.0 mg/day | Addition of
mizoribine |
|
|
|
|
|
|
|
|
|
|
|
| 150 mg/day |
| 5 | NEX | F | 49 | LN | 0.5 | 2.0 | 4.7 | 1,007.0 | NI | PSL 15.0 mg/day | None |
| 6 | NEX | F | 43 | LN | 0.6 | 2.0 | 2.9 | 360.5 | W | PSL 45.0 mg/day | PE Pulse of CPA |
|
|
|
|
|
|
|
|
|
|
|
| 20 mg/kg/3 weeks |
| 7 | NEX | F | 72 | RA | 14.4 | 1.5 | 7.2 | 357.5 | W | MTX 4.0 mg/week | Increase of MTX |
|
|
|
|
|
|
|
|
|
|
|
| 6.0 mg/week |
Statistical analysis
All values were expressed as the median (min-max) as
appropriate. Fishers exact tests were used for categorical factors.
A Mann-Whitney test was used to compare the results of two groups
and a Kruskal-Wallis test was used to compare the results of three
groups. The data were analyzed using GraphPad Prism 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA), and P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The characteristics of patients are presented in
Table II. There were 6 patients in
the EX group and 19 in the NEX group. The two groups exhibited a
similar sex ratio, age range, body weight, primary disease and
laboratory data during the period that tacrolimus concentration was
measured, and no significant differences were observed in the
patient variables. However, the duration of tacrolimus therapy (in
months after initiation of tacrolimus treatment) was significantly
shorter in the NEX group when compared with the EX group (P=0.0416;
Table II).
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Characteristic | CYP3A5 EX
(n=6) | CYP3A5 NEX
(n=19) | P-value |
|---|
| Sex,
male/female | 1/5 | 4/15 | 1.0000 |
| Age, years | 55.5
(29.0–76.0) | 57.0
(28.0–88.0) | 0.7927 |
| Body weight,
kg | 50.8
(34.9–99.1) | 51.5
(38.2–91.5) | 0.6781 |
| Primary
disease |
|
|
|
|
Rheumatoid arthritis | 4 | 11 | 0.6575 |
| Lupus
nephritis | 2 | 4 |
|
|
Ulcerative colitis | 0 | 2 |
|
|
Myasthenia gravis | 0 | 2 |
|
| Months after
starting tacrolimus therapy | 46.6
(9.7–88.6) | 13.7
(0.1–87.5) | 0.0416a |
| Laboratory
data |
|
|
|
| BUN,
mg/dl | 14.5
(7.0–20.0) | 16.0
(9.0–32.0) | 0.2795 |
| CRE,
mg/dl | 0.7 (0.6–0.8) | 0.6 (0.5–1.3) | 0.6990 |
| K,
mEq/l | 3.9 (3.6–4.2) | 4.1 (3.2–5.1) | 0.0746 |
| AST
(U/l) | 23.5
(13.0–30.0) | 20.0
(6.0–39.0) | 0.3396 |
| ALT
(U/l) | 16.5
(7.0–48.0) | 13.0
(4.0–40.0) | 0.8160 |
| WBC,
mm−3 | 7,460.0
(3,750.0–9,920.0) | 6,150.0
(3,150.0–14,650.0) | 0.4367 |
Impact of CYP3A5 genotype on
tacrolimus pharmacokinetics and clinical response
The tacrolimus dose did not differ significantly
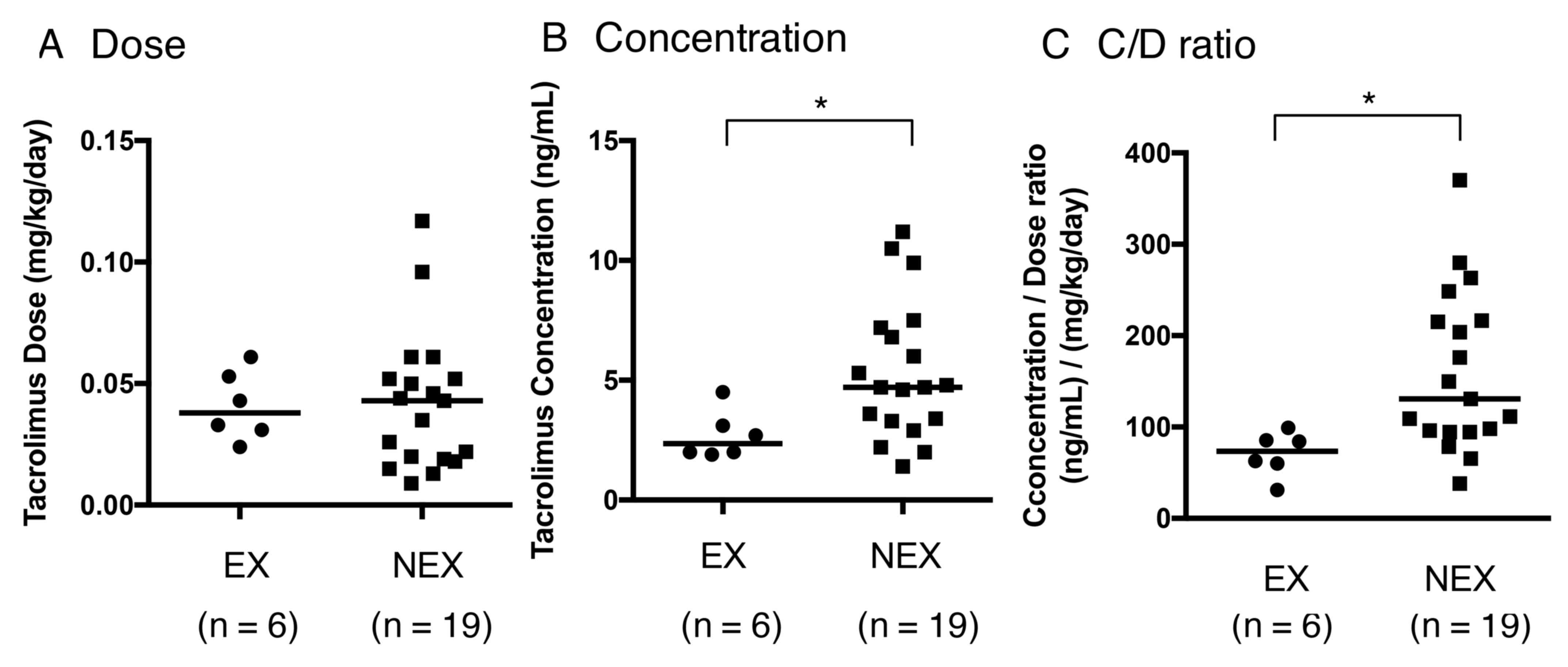
between the EX and NEX groups (P=0.6980; Fig. 1A). However, the EX group exhibited
significantly lower tacrolimus concentrations and C/D ratios when
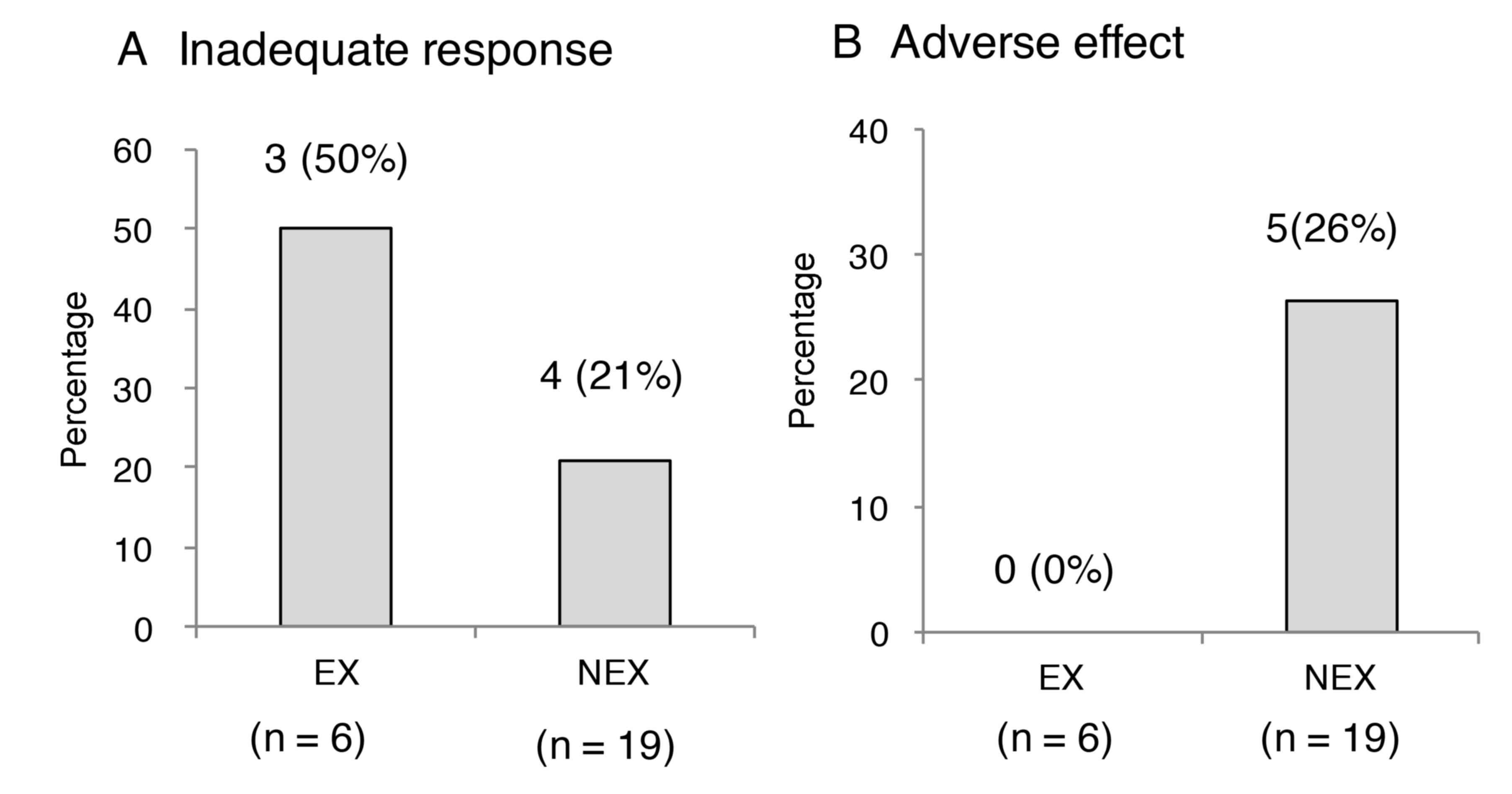
compared with the NEX group (P=0.0108; Fig. 1B and P=0.0056; Fig. 1C, respectively). A total of 3
patients presented with insufficient effect (50%) in the EX group
and 4 cases (21%) of insufficient effect were observed in the NEX
group, and no significant difference was determined between the two
groups (P=0.562; Fig. 2A). Adverse
effects developed in 5 cases (26%; renal dysfunction: n=3,
hyperkalemia: n=3, hyperuricemia: n=3, with overlap) of the NEX
group, and no adverse effects were identified in the EX group
during the treatment period. Thus, all incidences of adverse effect
were observed in the NEX group (P=0.289; Fig. 2B). Table
I presents the characteristics of patients that exhibited
insufficient effect following tacrolimus therapy (rheumatoid
arthritis in 2 and lupus nephritis in 5 cases). Clinical conditions
worsened in 5 cases (rheumatoid arthritis: n=2, lupus nephritis:
n=3) and did not improve in 2 cases (lupus nephritis: n=2; Table I).
Association between IMK ATP level and
tacrolimus pharmacokinetics, clinical response and CYP3A5
genotype
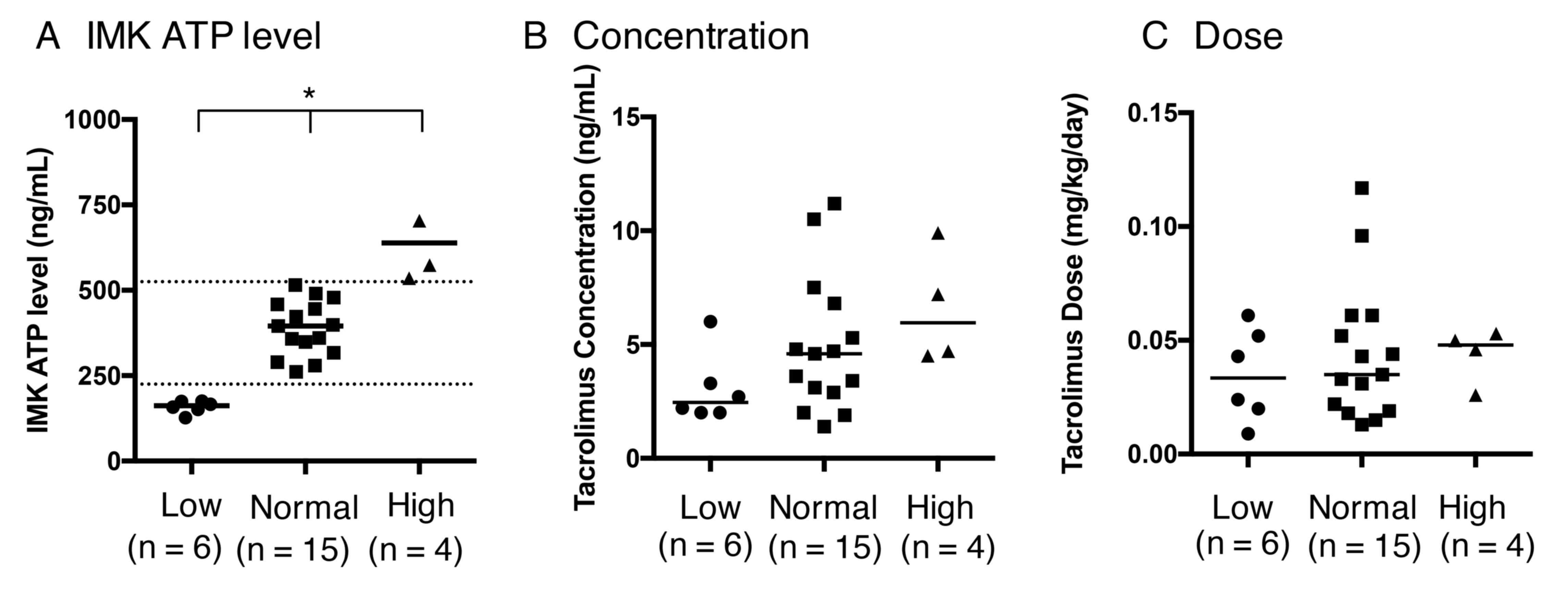
Patients were categorized into three different
categories according to IMK ATP levels (low, normal and high), as
previously established in transplant patients (3) (Fig. 3A;
P=0.0001). The concentration and dose of tacrolimus did not differ
significantly between the low, normal and high ATP groups (Fig. 3B and C; P=0.1092 and 0.6999,
respectively). The incidences of insufficient effect were 100%
(4/4) in the high ATP group, 20% (3/15) in the normal group and 0%
(0/6) in the low group, which were deemed to be significantly
different (Fig. 4A; P=0.0014). The
incidences of adverse effects due to tacrolimus did not differ
significantly among the three groups (Fig. 4B; P=0.9492), and no significant
difference was observed in the number of CYP3A5 expressors among
the three groups (Fig. 4C;
P=0.8105).
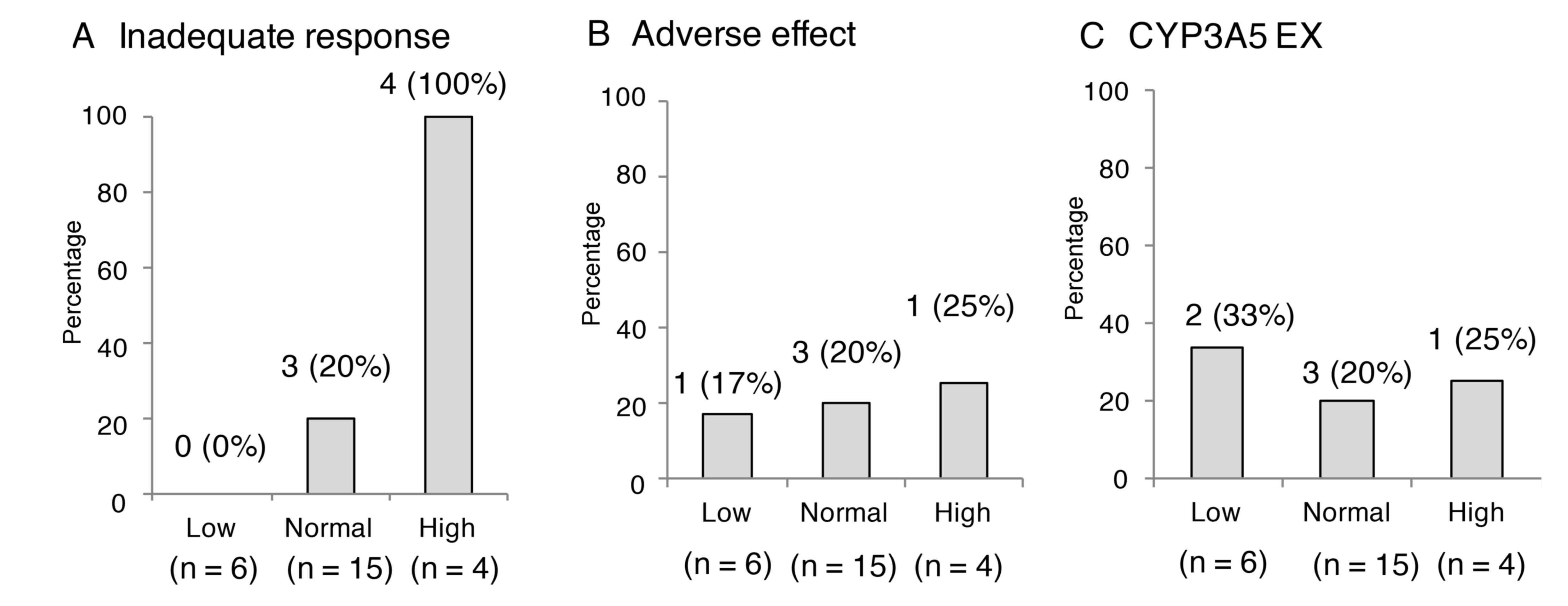 | Figure 4.Occurrence rate of insufficient
effect, adverse effect and CYP3A5 genotype at different IMK ATP
levels. (A) The incidences of insufficient effect were 100% (4/4)
in the High ATP group, 20% (3/15) in the Normal group and 0% (0/6)
in the Low group, which were deemed to be significantly different
(P=0.0014). (B) The incidences of adverse effects due to tacrolimus
did not differ significantly among the three groups (P=0.9492). (C)
No significant difference was observed in the number of CYP3A5
expressors among the three groups (P=0.8105). Patients were
categorized into three established zones based on IMK ATP level:
Low, <225 ng/ml, Normal, 225–525 ng/ml and High, >525 ng/ml.
CYP3A5, cytochrome P450 3A5; IMK, ImmuKnow assay; ATP, adenosine
triphosphate. |
Discussion
For patients that have undergone organ
transplantation, previous results have suggested that the
metabolism of tacrolimus is affected by CYP3A5 genotype (5). However, it is unclear whether CYP3A5
genotype affects patients with autoimmune diseases. Results of the
present study suggested that the pharmacokinetics of tacrolimus in
patients with autoimmune diseases were influenced by CYP3A5 in a
similar way to that in transplant patients. Notably, the range of
the IMK assay, as defined in organ transplant patients, may be a
useful indicator of clinical response in autoimmune patients.
Tacrolimus is characterized by high inter-individual
variation in its pharmacokinetics, which makes it difficult to
establish an optimal dose regimen of the drug in transplant
patients (13). A factor that
contributes to the pharmacokinetic variability of tacrolimus is
considered to be SNPs of CYP3A5 (14). The use of tacrolimus has been
approved for the treatment of autoimmune diseases in Japan, and an
approved dosage has been fixed, such that 3 mg/day is the dose for
myasthenia gravis patients (7). One
reason for this is that the approved tacrolimus dosage may be
regulated by primarily focusing on adverse events and not clinical
effects.
In the present study, the CYP3A5 genotype
significantly influenced the clearance of tacrolimus in patients
with autoimmune diseases, though the dose did not differ. While not
significantly different, the incidences of insufficient effect were
notably higher in the EX group when compared with the NEX group,
and adverse effects developed only in the NEX group, indicating an
inadequate dosage for treatment. There are ethnic differences in
the distribution of CYP3A5 SNPs, and the frequency of the expressor
genotype has been identified in ~40% of the Japanese population
(5,15). Therefore, CYP3A5 genotype should be
identified in patients eligible for tacrolimus treatment,
specifically in Japan.
The IMK assay is considered to be a useful tool for
monitoring immune activity in transplant recipients (1,3).
However, the benefits of the IMK assay in the monitoring of
immunological aspects in patients with autoimmune diseases remain
unclear. Kowalski et al (1)
reported that the range of IMK is a useful tool for predicting the
immune state of patients following liver transplantation.
Therefore, the present subjects were divided into three groups
using group boundaries previously established in transplant
patients (3). In the current study,
the IMK ATP level was not associated with the concentration or dose
of tacrolimus, as reported previously (16). By contrast, the IMK ATP level was
associated with clinical response. No cases developed infectious
complications due to over-immunosuppression in the present study,
indicating that the approved dose for autoimmune patients may be
set and fixed based on the safety of drug use. In addition,
clinical response was associated with IMK ATP level and not
tacrolimus dose. The present results indicate that the IMK ATP
level is reflected in the clinical response of patients with
autoimmune diseases, which may be a useful indicator for
determining the regimen of tacrolimus in autoimmune patients.
However, the present study had a number of
limitations that should be considered. Firstly, it was difficult to
exclude the potential effects of other unknown cofounders in the
current single-institution retrospective study. Secondly, the
results remain a matter of speculation due to the small number of
cases assessed for each disease. Thirdly, the current study was not
able to evaluate chronological change in each patient. Further
multi-study analyses conducted in a prospective randomized
controlled fashion and with a greater number of patients are now
required.
In conclusion, the clearance of tacrolimus in
patients with autoimmune diseases was affected by CYP3A5 genotype,
as previously reported for patients who had undergone organ
transplantation. The IMK ATP level may be a useful indicator of
clinical response, irrespective of tacrolimus concentration, in
patients with autoimmune diseases in addition to organ transplant
patients.
Acknowledgements
The present study was supported by the Mie
University Hospital Seed Grant Program 2013 (no grant number).
References
|
1
|
Kowalski R, Post D, Schneider MC, Britz J,
Thomas J, Deierhoi M, Lobashevsky A, Redfield R, Schweitzer E,
Heredia A, et al: Immune cell function testing: An adjunct to
therapeutic drug monitoring in transplant patient management. Clin
Transplant. 17:77–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizuno S, Hamada T, Nakatani K, Kishiwada
M, Usui M, Sakurai H, Tabata M, Sakamoto Y, Nishioka J, Muraki Y,
et al: Monitoring peripheral blood CD4+ adenosine triphosphate
activity after living donor liver transplantation: Impact of
combination assays of immune function and CYP3A5 genotype. J
Hepatobiliary Pancreat Sci. 18:226–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kowalski RJ, Post DR, Mannon RB, Sebastian
A, Wright HI, Sigle G, Burdick J, Elmagd KA, Zeevi A, Lopez-Cepero
M, et al: Assessing relative risks of infection and rejection: A
meta-analysis using an immune function assay. Transplantation.
82:663–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Israeli M, Yussim A, Mor E, Sredni B and
Klein T: Preceeding the rejection: In search for a comprehensive
post-transplant immune monitoring platform. Transpl Immunol.
18:7–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muraki Y, Usui M, Isaji S, Mizuno S,
Nakatani K, Yamada T, Iwamoto T, Uemoto S, Nobori T and Okuda M:
Impact of CYP3A5 genotype of recipients as well as donors on the
tacrolimus pharmacokinetics and infectious complications after
living-donor liver transplantation for Japanese adult recipients.
Ann Transplant. 16:55–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawakami K, Inoue T, Murano M, Narabayashi
K, Nouda S, Ishida K, Abe Y, Nogami K, Hida N, Yamagami H, et al:
Effects of oral tacrolimus as a rapid induction therapy in
ulcerative colitis. World J Gastroenterol. 21:1880–1886. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagaishi A, Yukitake M and Kuroda Y:
Long-term treatment of steroid-dependent myasthenia gravis patients
with low-dose tacrolimus. Intern Med. 47:731–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
The US Food and Drug Administration, .
Class II Special Controls Guidance Document: cyclosporine and
tacrolimus assays; guidance for industry and FDA. 2002, http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm092778.htm
|
|
9
|
Nobuoka Y, Mizuno S, Nishikawa K, Nakatani
K, Muraki Y, Yamada T, Okuda M, Nobori T, Sugimura Y and Isaji S:
Immune response following liver transplantation compared to kidney
transplantation: Usefulness of monitoring peripheral blood CD4+
adenosine triphosphate activity and cytochrome P450 3A5 genotype
assay. Clin Dev Immunol. 2013:9360632013. View Article : Google Scholar
|
|
10
|
Tanaka H, Tsuruga K, Aizawa-Yashiro T,
Watanabe S and Imaizumi T: Treatment of young patients with lupus
nephritis using calcineurin inhibitors. World J Nephrol. 1:177–183.
2012. View Article : Google Scholar
|
|
11
|
Asamiya Y, Uchida K, Otsubo S, Takei T and
Nitta K: Clinical assessment of tacrolimus therapy in lupus
nephritis: One-year follow-up study in a single center. Nephron
Clin Pract. 113:c330–c336. 2009. View Article : Google Scholar
|
|
12
|
Fukuen S, Fukuda T, Maune H, Ikenaga Y,
Yamamoto I, Inaba T and Azuma J: Novel detection assay by PCR-RFLP
and frequency of the CYP3A5 SNPs, CYP3A5*3 and *6, in a Japanese
population. Pharmacogenetics. 12:331–334. 2002. View Article : Google Scholar
|
|
13
|
Denton MD, Magee CC and Sayegh MH:
Immunosuppressive strategies in transplantation. Lancet.
353:1083–1091. 1999. View Article : Google Scholar
|
|
14
|
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M,
Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, et al:
Sequence diversity in CYP3A promoters and characterization of the
genetic basis of polymorphic CYP3A5 expression. Nat Genet.
27:383–391. 2001. View
Article : Google Scholar
|
|
15
|
Uesugi M, Masuda S, Katsura T, Oike F,
Takada Y and Inui K: Effect of intestinal CYP3A5 on postoperative
tacrolimus trough levels in living-donor liver transplant
recipients. Pharmacogenet Genomics. 16:119–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizuno S, Muraki Y, Nakatani K, Tanemura
A, Kuriyama N, Ohsawa I, Azumi Y, Kishiwada M, Usui M, Sakurai H,
et al: Immunological aspects in late phase of living donor liver
transplant patients: Usefulness of monitoring peripheral blood CD4+
adenosine triphosphate activity. Clin Dev Immunol. 2013:9821632013.
View Article : Google Scholar : PubMed/NCBI
|