Introduction
Osteoporosis is a systemic disease characterized by
reduction of bone tissue content in a unit volume and degeneration
of bone microstructure, which increases the risk of fragility
fractures (1). Osteoporosis has
become a serious social problem as the aging population increases
(2). Among the patients affected by
osteoporosis, post-menopausal women are disproportionately
affected, accounting for >70% of the overall patients (3). The imbalance between bone formation and
bone resorption has become an important factor for the formation of
osteoporosis (4). Therefore, it is
necessary to identify an effective therapeutic agent for the
treatment of osteoporosis.
The application of traditional Chinese medicine in
China dates back thousands of years and therefore has a high degree
of recognition among the population (5). Puerarin is a major bioactive component
extracted from the root of Pueraria Labata (Willd.) Ohwi,
also known as Kudzu, and is one of the earliest medicinal plants to
be used in China. (6). Puerarin
displays a series of properties that attenuate osteoporosis,
inflammation and liver injury (7).
In osteoporosis studies, puerarin has been demonstrated to reduce
bone reabsorption, promoting bone formation and increasing bone
mineral density (8,9). Therefore, it is important to explore
the therapeutic effects and underlying mechanisms of puerarin on
osteoporosis.
MicroRNAs (miRNAs or miRs) are endogenous,
non-coding, single-stranded small RNAs with a length of ~22
nucleotides, which are widespread in eukaryotic genomes (10). miRNAs function in the
post-transcriptional regulation of gene expression by binding to
the 3′-untranslated regions (UTRs) of target mRNAs (10,11),
which leads to translation inhibition or degradation of mRNA
(12). miRNAs are known to have a
significant role in biological processes, including cell
proliferation (13,14), apoptosis (15,16),
differentiation (17,18) and tumor formation (19). Recent studies suggest that miRNAs may
also be associated with bone formation and osteoblast
differentiation (20–22). For example, Wang et al
(23) identified that microRNA-106b
inhibited osteolysis by targeting receptor activator of nuclear
factor-κB ligand (RANKL) in giant cell tumors in the bone. However,
whether miR-106b participates in the anti-osteoporotic effects of
puerarin is currently unknown.
The aim of the present study was to explore the
effects and related mechanisms of puerarin on the prevention of
osteoporosis in MC3T3-E1 cells. Based on the important role of
miR-106b in osteoporosis, it was investigated whether miR-106b is
associated with the anti-osteoporotic effect of puerarin.
Materials and methods
Cell lines, chemicals and biochemical
reagents
The MC3T3-E1 cell line, which is an osteoblast-like
cell line from C57BL/6 mouse calvaria, was obtained from American
Type Culture Collection (CRL-2593; Manassas, VA, USA) and cultured
in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% heat
inactivated fetal bovine serum (Sigma-Aldrich, Merck KGaA;
Darmstadt, Germany) 100 U/ml penicillin and 100 µg/ml streptomycin
and incubated at 37°C in an atmosphere containing 5%
CO2. Puerarin was purchased from the National Institutes
for Food and Drug Control (Beijing, China). The assay kit for
alkaline phosphatase (ALP; cat. no. A059-2) was obtained from
Nanjing Jiancheng Biotechnology Institute Co., Ltd. (Nanjing,
China). ELISA kits for type 1 collagen (COL I; cat. no. DY6220-05)
and osteocalcin (OCN; cat. no. QC137) assays were purchased from
R&D Systems, Inc. (Minneapolis, MN, USA).
MTT assay
Cell proliferation and growth was determined using
an MTT assay according to the manufacturer's instructions. A total
of ~2×104 cells/well were seeded in a 96-well culture
plate for 24 h at 37°C and treated with different concentrations
(0, 5, 10, 20 or 40 µM) of puerarin for 1, 2, 3, 4 and 5 days at
37°C in a humidified incubator with 5% CO2. Non-treated
cells served as the control. Subsequently, 0.5 mg/ml MTT solution
(Sigma-Aldrich; Merck KGaA) was added to each well and incubated at
37°C for 3 h. Following the removal of the culture medium, the
cells were washed twice with PBS and 100 µl of 0.01%
HCl-isopropanol solution was added to dissolve the formazan
crystals. Absorbance was recorded at 490 nm using a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
COL I and OCN content and ALP
activity
COL I and OCN content of MC3T3-E1 cells were
measured as described previously (24,25). A
total of ~1.0×105 cells in 1 ml DMEM supplemented with
10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin were added
to each well at 37°C for 24 h following the addition of diluted
puerarin (5, 10, 20 or 40 µM). After 7 and 14 days, the COL I and
OCN contents were determined using the ELISA assay kit. Absorbance
was recorded at 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc.). The supernatant was used for ALP activity
determination after 3, 5 and 7 days of incubation with ALP assay
kits. Untreated MC3T3-E1 cells served as controls.
Mineralization assay (Alizarin Red-S
staining)
MC3T3-E1 cells (1×105 cells/well) were
seeded into a 12-well plate. After 14 days of puerarin treatment
(5–40 µM) at 37°C, the supernatant was removed and the cells were
fixed with 4% neutral formaldehyde in PBS at room temperature for
10 min and the cells were washed twice with distilled water.
Subsequently, cells were stained with 0.1% (w/v) Alizarin Red-S (pH
4.2; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min (26). Following staining, the cultures were
washed thoroughly with deionized water and the absorbance was
recorded on a microplate reader (Bio-Rad Laboratories, Inc.) at 562
nm.
Transfection
The miR-106b mimics (5′-TAAAGTGCTGACAGTGCAGAT-3′),
mimics negative control (mimics NC; 5′-UUGUACUACACAAAAGUACUG-3′),
miR-106b inhibitors (5′-ATCTGCACTGTCAGCACTTTA-3′) and inhibitor NC
(5′-TTCTCCGAACGTGTCACGT-3′) were purchased from RiboBio Co., Ltd.
(Guangzhou, China). MC3T3-E1 cells were transfected with
oligonucleotides (50 pmol/ml) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Cell lysates were harvested at 48 h after transfection. A
total of 50 nM miR-106b inhibitors or inhibitor NC were transfected
into MC3T3-E1 cells then induced with peurarin for 3, 5, 7 or 14
days. Following induction, the supernatant was used for COL I, OCN
and ALP analyses (on 7 and 14 days) and the cells (on days 3, 5, 7)
were used for ALP activity determination using ALP assay kits.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from MC3T3-E1 cells was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
cDNA was synthesized from 2 µg of total RNA using PrimeScript RT
Reagent kit (cat. no. DRR037A; Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. The
reverse transcription step consisted of an incubation at 50°C for 5
min. qPCR anaylsis of miR-106b was performed using the TaqMan miRNA
assay RT-PCR kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The specific
primers were synthesized commercially from Sangon Biotech Co., Ltd.
(Shanghai, China) as follows: miR-106b, forward
5′-TAAAGTGCTGACAGTGCAGATAGTG-3′, and reverse
5′-CAAGTACCCACAGTGCGGT-3′, and U6, forward 5′-CTCGCTTCGGCAGCACA-3′,
and reverse 5′-AACGCTTCACGAATTTGCGT-3′. The PCR conditions were as
follows: Initial denaturation at 95°C for 10 min followed by 40
cycles consisting of 95°C for 5 sec, 60°C for 30 sec and 72°C for
10 sec. The fluorescence signal was monitored at 585 nm during each
extension. Data were analyzed using 7500 software version 2.0.1
(Applied Biosystems; Thermo Fisher Scientific, Inc.), and
calculated using the 2−ΔΔCq method (27), with U6 small nuclear RNA as the
endogenous control. PCR reactions were performed using QuantiTect
SYBR Green PCR Master mix (Qiagen, Inc., Valencia, CA, USA) in an
Applied Biosystems 7500 instrument according to the manufacturer's
protocol.
Luciferase reporter assay
The 3′-UTR of RANKL with wild-type (WT) or mutant
(Mut) binding sites for miR-106b was amplified and subcloned into
the pGL3 vector (Promega Corporation, Madison, WI, USA) to generate
the plasmid pGL3-WT-RANKL-3′-UTR or pGL3-Mut-RANKL-3′-UTR,
respectively. The HEK293 cells were seeded in 24-well plate in
duplicate (5×104 cell/well), when the cells reached 70%
confluence they were co-transfected with 1–2 µg/ml of either
pGL3-WT-RANKL-3′-UTR or pGL3-Mut-RANKL-3′-UTR plasmids and 25 nM
miR-106b mimics and miR-106b inhibitor using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
Mimics and inhibitors NC were transfected using the same procedure.
The pRL-TK plasmid (Promega Corporation) was used as a normalizing
control. After 48 h of incubation, luciferase activity was analyzed
using the Dual-Luciferase® Reporter Assay System
(Promega Corporation).
Western blot analysis
MC3T3-E1 cells were lysed on ice with RIPA lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China) and
protein concentrations were determined using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). A total of
40 µg/lane protein was separated by 10% SDS-PAGE (Bio-Rad
Laboratories, Inc.) and transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA), which was
incubated with 5% fat-free skim milk in TBS containing 0.05%
Tween-20 for 1 h at room temperature. The blots were then incubated
overnight at 4°C with anti-RANKL (Rabbit monoclonal; 1:1,000; cat.
no. ab45039) and anti-β-actin (Rabbit monoclonal; 1:1,000; cat. no.
ab32572) primary antibodies (both Abcam, Cambridge, MA, USA). The
blots were subsequently incubated with horseradish
peroxidase-conjugated secondary antibodies (monoclonal; 1:10,000;
cat. no. ab99702; Abcam) for 1 h at room temperature. An enhanced
chemiluminescence substrate (EMD Millipore) was used to visualize
signals. β-actin was used as an endogenous protein for
normalization. Relative band intensities were determined by
densitometry using Quantity One 4.6.2 software (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA). Data are presented as the mean ± standard deviation as
indicated. One-way analysis of variance followed by a Tukey's post
hoc test or two-tailed Student's t-test was used for comparisons
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
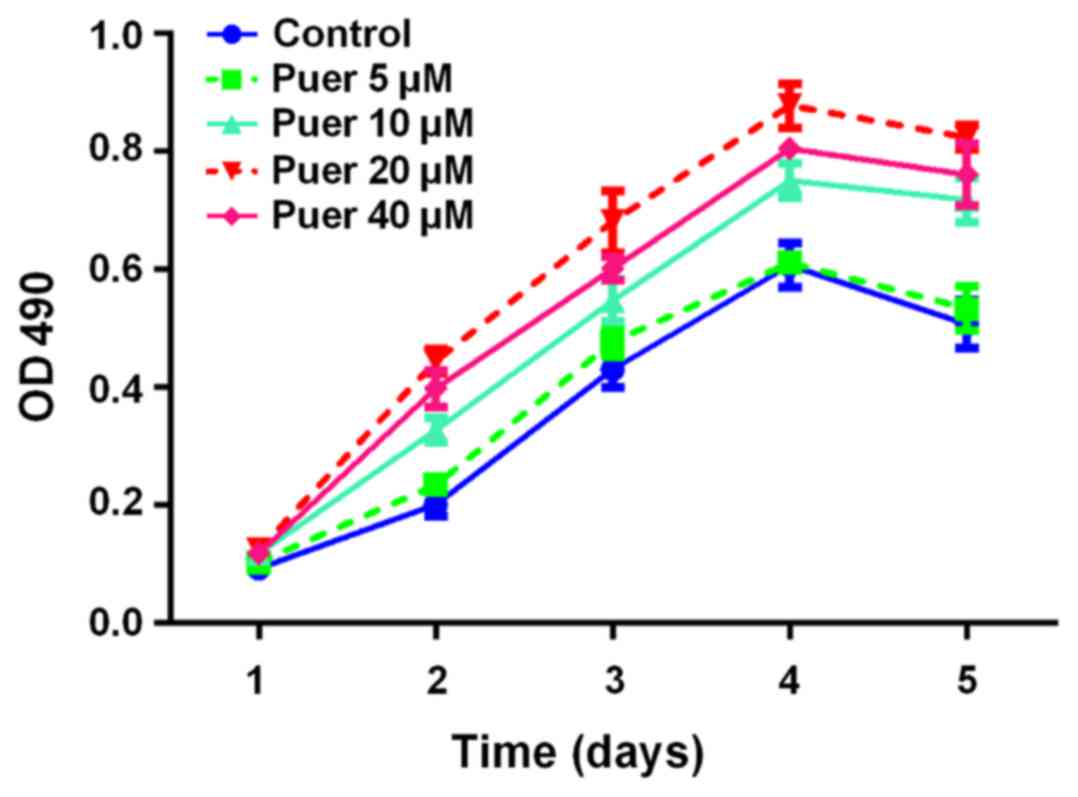
Growth promotion of MC3T3-E1 cells
induced by puerarin
To investigate the effect of puerarin on the growth
of MC3T3-E1 cells, the MTT assay was performed to measure cell
growth of MC3T3-E1 cells following treatment with various
concentrations (0–40 µM) of puerarin for 1, 2, 3, 4 and 5 days. As
indicated in Fig. 1, the
proliferation activity of MC3T3-E1 cells was markedly promoted by
puerarin in a time- and dose-dependent manner, with the peak level
at 20 µM. Based on the dose dependent curves, 20 µM was chosen as
the optimal dosage for consequent experiments.
Effect of puerarin on osteoblast
differentiation
To clarify the role of puerarin in osteoblast
differentiation, MC3T3-E1 cells were incubated with different
concentrations of puerarin. As shown in Fig. 2A, ALP activity was increased by
puerarin treatment in a time-dependent manner up to a concentration
of 20 µM, after which the activity was reduced. ALP activity was
significantly increased in the 20 and 40 µM puerarin treatment
groups at 3 (P<0.01 and P<0.05, respectively), 5 (P<0.01)
and 7 (P<0.01) days compared with the control group, and 10 µM
puerarin treatment at 7 days also exhibited a significant increase
in ALP activity compared with the control (P<0.05). COL I and
OCN content was significantly increased in a dose-dependent manner
at 7 and 14 days of culture compared with the control group with 20
and 40 µM puerarin treatment (P<0.01), although both were
markedly reduced following 40 µM treatment in comparison with 20 µM
(Fig. 2B and C). Furthermore,
mineralization was significantly increased after 14 days of culture
with 10 (P<0.05), 20 (P<0.01) or 40 µM (P<0.01) puerarin
treatment (Fig. 2D). In addition,
these results indicated that the optimal concentration of puerarin
effect on cell differentiation was 20 µM. Furthermore, these data
indicated that puerarin may promote osteoblast differentiation.
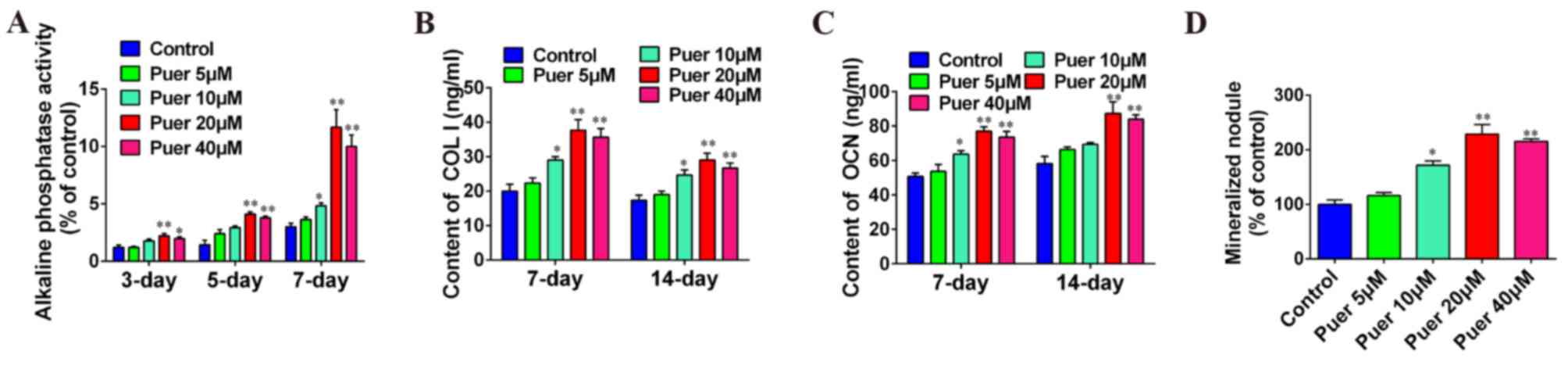 | Figure 2.Puerarin promoted cell differentiation
in MC3T3-E1 cells. MC3T3-E1 cells were incubated with various
concentrations (0, 5, 10, 20 or 40 µM) of puerarin for 3, 5, 7 or
14 days. Medium was collected for COL I and OCN content (at 7 and
14 days) and ALP activity (at 3, 5 and 7 days) determination using
ELISA kits. Medium was collected for Alizarin red staining at 14
days. (A) Effect of puerarin on alkaline phosphatase activity, (B)
COL I secretion, and (C) OCN secretion of MC3T3-E1 cells, and (D)
bone mineralization of MC3T3-E1 cells. Data are presented as the
mean ± standard deviation of three independent experiments.
*P<0.05 and **P<0.01 vs. control. Puer, puerarin; COL I,
collagen type 1; OCN, osteocalcin. |
Effect of puerarin on the expression
of miR-106b
It has been recently reported that miR-106b may
inhibit osteoclastogenesis and osteolysis in giant cell tumors of
bone (20). Therefore, it was
assumed that puerarin may induce the differentiation of MC3T3-E1
cells by upregulating the expression of miR-106b. The expression
levels of miR-106b were determined by RT-qPCR following treatment
with puerarin in MC3T3-E1 cells for 48 h. Fig. 3A showed that 10, 20 and 40 µM
puerarin treatment significantly increased the expression levels of
miR-106b in MC3T3-E1 cells compared with the control (P<0.05,
P<0.01 and P<0.01, respectively).
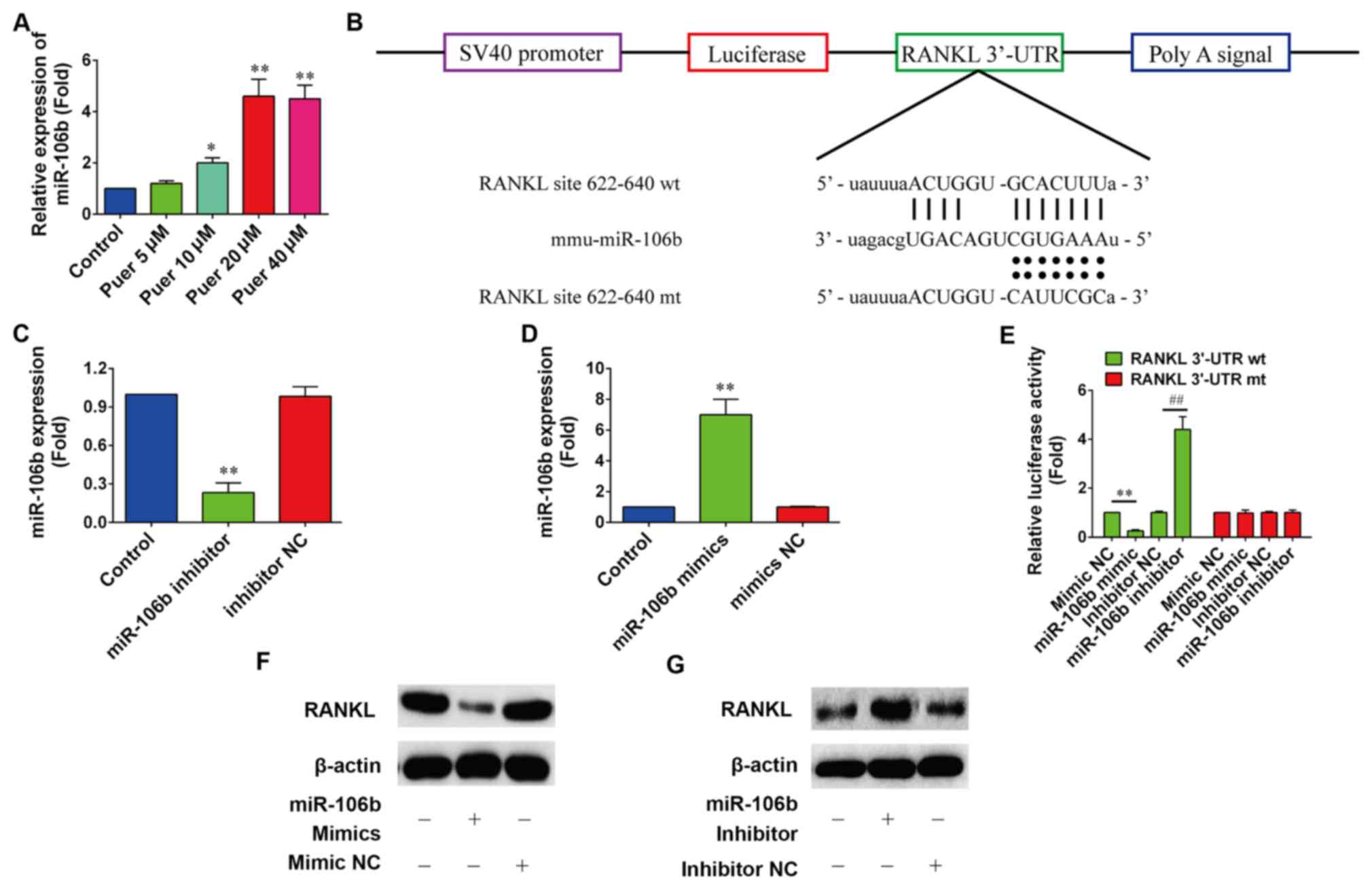 | Figure 3.Puerarin promoted the expression of
miR-106b. (A) Expression levels of miR-106b were detected by
reverse transcription-quantitative polymerase chain reaction in
MC3T3-E1 cells following puerarin treatment for 72 h. (B) Schema of
the firefly luciferase reporter constructs for RANKL, which
indicated the interaction sites between miR-106b and the 3′-UTRs of
the RANKL. (C and D) Expression levels of miR-106b following
treatment with miR-106b mimic or miR-106b inhibitor were determined
(n=6). *P<0.05 and **P<0.01 vs. control. (E) Dual-luciferase
reporter assay detected the interaction between miR-106b and the
3′-UTR of RANKL. miR-106b mimic, mimic NC, miR-106b inhibitor or
inhibitor NC were co-transfected with pGL-RANKL reporter vectors
into HEK293 cells for 48 h. (n=6). **P<0.01 vs. mimics NC,
##P<0.01 vs. inhibitor NC. (F and G) Protein
expression levels of RANKL following treatment with miR-106b mimic
or miR-185 inhibitor were indicated (n=6). All data are presented
as the mean ± standard deviation results of three independent
experiments. miR-106b, microRNA-106b; RANKL, receptor activator of
nuclear factor-κB ligand; UTR, untranslated region; NC, negative
control; mt, mutant; wt, wild-type. |
A recent study demonstrated that miR-106b inhibited
osteoclastogenesis and osteolysis by suppressing the expression of
RANKL (28). However, whether
puerarin was able to promote the proliferation and differentiation
of MC3T3-E1 cells through miR-106b by targeting RANKL is unknown.
To determine whether miR-106b directly targets RANKL, the direct
binding of miR-106b to RANKL mRNA 3′-UTR was investigated using the
luciferase report assay (Fig. 3B).
According to RT-qPCR analysis, a significant decrease in miR-106b
expression following transfection of miR-106b inhibitor was
indicated compared with the control (P<0.01; Fig. 3C), and a 6.5-fold significant
increase of mature miR-106b expression was revealed in MC3T3-E1
cells at 24-h post-transfection of miR-106b mimics compared with
the control (P<0.01; Fig. 3D).
The luciferase results showed that overexpression of miR-106b
significantly decreased (P<0.01) the luciferase activity in
pGL3-RANKL 3′-UTR wild-type transfected cells, whereas it had no
significant effect on pGL3 RANKL 3′-UTR mutant cells (Fig. 3E). Furthermore, western blot analysis
revealed that miR-106b overexpression markedly decreased the
protein expression level of RANKL (Fig.
3F), whereas miR-106b inhibition markedly increased the protein
expression of RANKL (Fig. 3G).
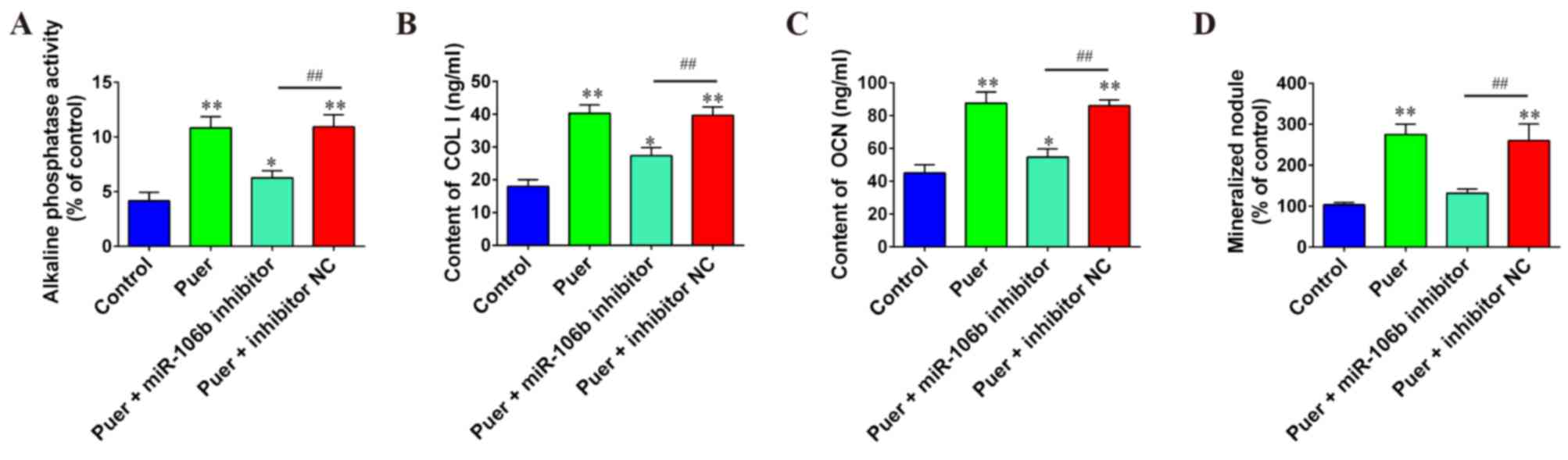
Effect of miR-106b on osteogenic
differentiation induced by puerarin
To explore the role of miR-106b during osteogenic
differentiation induced by puerarin, miR-106b inhibitor and
respective NC were co-transfected into MC3T3-E1 cells, following
puerarin treatment. Results indicated that knockdown of miR-106b
significantly decreased differentiation and mineralization of
MC3T3-E1 pre-osteoblastic cells caused by puerarin as evidenced by
the decreased contents or activities of major markers COL I
(P<0.01), OCN (P<0.01), ALP (P<0.01) and mineral nodule
formation (P<0.01) compared to the puer+inhibitor NC group
(Fig. 4). These results indicated
that the elevation of miR-106b may be associated with the process
of puerarin-induced differentiation in MC3T3-E1 cells.
Discussion
In the present study, it was indicated that puerarin
promoted the growth of MC3T3-E1 cells in a time- and dose-dependent
manner up to a concentration of 20 µM. Furthermore, these findings
have provided evidence that puerarin may promote the
differentiation of MC3T3-E1 cells through miR-106b by targeting
RANKL. The present work revealed the anti-osteoporotic effects of
puerarin in MC3T3-E1 cells, suggested the participation of miR-106b
in this process and thus indicated that puerarin may be a novel
agent for treatment of osteoporosis.
Several reports have demonstrated that puerarin is
able to promote osteoblast proliferation and differentiation
(29,30). A study from Wong and Rabie (31) showed that puerarin treatment may
prevent bone loss in a dose-dependent manner. Similarly, Urasopon
et al (32) showed that in
cultures of newborn Wistar rat osteoblasts, puerarin was also
effective in stimulating osteoblastic bone formation. Thus, it is
important to understand the underlying mechanisms of this effect.
Sheu et al (33) previously
discovered that puerarin-mediated osteoblast proliferation is
likely to be mediated by bone morphogenetic proteins and nitric
oxide (NO) pathways in adult female mouse osteoblasts. In agreement
with these studies, the present data reinforced that puerarin
affects the induction of osteoblast proliferation and
differentiation.
Previous studies have reported that miRNAs were
associated with osteogenesis (34,35). For
example, in human adipose tissue-derived stem cells, mouse
mesenchymal ST2 stem cells and mouse premyogenic C2C12 cells,
several miRNAs (miR-26a, −125b, −133 and −135) have been reported
to regulate osteoblast cell growth or differentiation (36). A study performed by Shi et al
(37) demonstrated that in C2C12
cells under osteogenic differentiation, miR-214 has an important
role as a suppressor by targeting osterix. However, it is unknown
whether miRNAs mediate the anti-osteoporotic effect of
puerarin.
A variety of studies have demonstrated that miR-106b
participates in the progression of osteoporosis (38,39). For
example, miR-106b expression levels may enhance osteoclast
differentiation and bone resorption by targeting RANKL,
twist-related protein (TWIST) and matrix metallopeptidase (MMP)2,
which may partly elucidate the role of miR-106b downregulation in
giant cell tumors and bone metastasis (23). Previous studies have suggested that
various target genes of miR-106b, including interleukin-8 (40), MMP2 (41) and TWIST (42), have been reported to induce cell
proliferation and invasion in cancers. Zheng et al (43) identified that miR-106b induced
epithelial-mesenchymal transition by targeting paired related
homeobox 1 in colorectal cancer. The present data indicated that
puerarin promoted the expression of miR-106b, which mediated the
anti-osteoporotic effect of puerarin in MC3T3-E1 cells via
regulating RANKL expression levels.
In conclusion, the present study investigated the
potential functions of puerarin in cell growth and differentiation
in MC3T3-E1 cells. The present findings suggested that puerarin
positively affected osteogenic differentiation through the
upregulation of miR-106b by directly targeting RANKL. Therefore,
the present results indicate a novel mechanism for puerarin-induced
promotion of bone formation activity, which may be used as a
therapeutic target for the treatment of osteoporosis.
References
|
1
|
Wirries A, Schubert AK, Zimmermann R,
Jabari S, Ruchholtz S and El-Najjar N: Thymoquinone accelerates
osteoblast differentiation and activates bone morphogenetic
protein-2 and ERK pathway. Int Immunopharmacol. 15:381–386. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnell O and Kanis JA: An estimate of the
worldwide prevalence and disability associated with osteoporotic
fractures. Osteoporos Int. 17:1726–1733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Liu JP and Xia Y: Chinese herbal
medicines for treating osteoporosis. Cochrane Database Syst Rev:
CD005467. 2014.
|
|
6
|
Keung WM, Lazo O, Kunze L and Vallee BL:
Potentiation of the bioavailability of daidzin by an extract of
Radix puerariae. Proc Natl Acad Sci USA. 93:pp. 4284–4288. 1996;
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maji AK, Pandit S, Banerji P and Banerjee
D: Pueraria tuberosa: A review on its phytochemical and therapeutic
potential. Nat Prod Res. 28:2111–2127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Persson I, Weiderpass E, Bergkvist L,
Bergström R and Schairer C: Risks of breast and endometrial cancer
after estrogen and estrogen-progestin replacement. Cancer Causes
Control. 10:253–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orija IB and Mehta A: Hormone replacement
therapy: Current controversies. Clin Endocrinol (Oxf). 59:6572003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erson AE and Petty EM: MicroRNAs in
development and disease. Clin Genet. 74:296–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laneve P, Di Marcotullio L, Gioia U, Fiori
ME, Ferretti E, Gulino A, Bozzoni I and Caffarelli E: The interplay
between microRNAs and the neurotrophin receptor tropomyosin-related
kinase C controls proliferation of human neuroblastoma cells. Proc
Natl Acad Sci USA. 104:pp. 7957–7962. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song G, Zhang Y and Wang L: MicroRNA-206
targets notch3, activates apoptosis, and inhibits tumor cell
migration and focus formation. J Biol Chem. 284:31921–31927. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson BJ and Cohen SM: The Hippo
pathway regulates the bantam microRNA to control cell proliferation
and apoptosis in Drosophila. Cell. 126:767–774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X and Carthew RW: A microRNA mediates
EGF receptor signaling and promotes photoreceptor differentiation
in the Drosophila eye. Cell. 123:1267–1277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawasaki H and Taira K: Retraction: Hes1
is a target of microRNA-23 during retinoic-acid-induced neuronal
differentiation of NT2 cells. Nature. 426:1002003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foekens JA, Sieuwerts AM, Smid M, Look MP,
de Weerd V, Boersma AW, Klijn JG, Wiemer EA and Martens JW: Four
miRNAs associated with aggressiveness of lymph node-negative,
estrogen receptor-positive human breast cancer. Proc Natl Acad Sci
USA. 105:pp. 13021–13026. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arfat Y, Xiao WZ, Ahmad M, Zhao F, Li DJ,
Sun YL, Hu L, Zhihao C, Zhang G, Iftikhar S, et al: Role of
microRNAs in osteoblasts differentiation and bone disorders. Curr
Med Chem. 22:748–758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papaioannou G, Mirzamohammadi F and
Kobayashi T: MicroRNAs involved in bone formation. Cell Mol Life
Sci. 71:4747–4761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pi C, Li YP, Zhou X and Gao B: The
expression and function of microRNAs in bone homeostasis. Front
Biosci. 20:119–138. 2015. View
Article : Google Scholar
|
|
23
|
Wang T, Yin H, Wang J, Li Z, Wei H, Liu Z,
Wu Z, Yan W, Liu T, Song D, et al: MicroRNA-106b inhibits
osteoclastogenesis and osteolysis by targeting RANKL in giant cell
tumor of bone. Oncotarget. 6:18980–18996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horiguchi Y, Nakai T and Kume K: Effects
of Bordetella bronchiseptica dermonecrotic toxin on the structure
and function of osteoblastic clone MC3T3-e1 cells. Infect Immun.
59:1112–1116. 1991.PubMed/NCBI
|
|
25
|
James AW: Review of signaling pathways
governing MSC osteogenic and adipogenic differentiation.
Scientifica (Cairo). 2013:6847362013.PubMed/NCBI
|
|
26
|
Tang X, Lin J, Wang G and Lu J:
MicroRNA-433-3p promotes osteoblast differentiation through
targeting DKK1 expression. PLoS One. 12:e01798602017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Yang C, Xie WL, Zhao YW, Li ZM,
Sun WJ and Li LZ: Puerarin concurrently stimulates osteoprotegerin
and inhibits receptor activator of NF-κB ligand (RANKL) and
interleukin-6 production in human osteoblastic MG-63 cells.
Phytomedicine. 21:1032–1036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lv H, Che T, Tang X, Liu L and Cheng J:
Puerarin enhances proliferation and osteoblastic differentiation of
human bone marrow stromal cells via a nitric oxide/cyclic guanosine
monophosphate signaling pathway. Mol Med Rep. 12:2283–2290. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang C, Meng MX, Tang XL, Chen KM, Zhang
L, Liu WN and Zhao YY: The proliferation, differentiation, and
mineralization effects of puerarin on osteoblasts in vitro. Chin J
Nat Med. 12:436–442. 2014.PubMed/NCBI
|
|
31
|
Wong R and Rabie B: Effect of puerarin on
bone formation. Osteoarthritis Cartilage. 15:894–899. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Urasopon N, Hamada Y, Cherdshewasart W and
Malaivijitnond S: Preventive effects of Pueraria mirifica on bone
loss in ovariectomized rats. Maturitas. 59:137–148. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sheu SY, Tsai CC, Sun JS, Chen MH, Liu MH
and Sun MG: Stimulatory effect of puerarin on bone formation
through co-activation of nitric oxide and bone morphogenetic
protein-2/mitogen-activated protein kinases pathways in mice. Chin
Med J (Engl). 125:3646–3653. 2012.PubMed/NCBI
|
|
34
|
Li Z, Hassan MQ, Volinia S, van Wijnen AJ,
Stein JL, Croce CM, Lian JB and Stein GS: A microRNA signature for
a BMP2-induced osteoblast lineage commitment program. Proc Natl
Acad Sci USA. 105:pp. 13906–13911. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kapinas K, Kessler C, Ricks T, Gronowicz G
and Delany AM: miR-29 modulates Wnt signaling in human osteoblasts
through a positive feedback loop. J Biol Chem. 285:25221–25231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Itoh T, Nozawa Y and Akao Y: MicroRNA-141
and −200a are involved in bone morphogenetic protein-2-induced
mouse pre-osteoblast differentiation by targeting distal-less
homeobox 5. J Biol Chem. 284:19272–19279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi K, Lu J, Zhao Y, Wang L, Li J, Qi B,
Li H and Ma C: MicroRNA-214 suppresses osteogenic differentiation
of C2C12 myoblast cells by targeting Osterix. Bone. 55:487–494.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Collison J: Bone: miR-106b promotes
osteoporosis in mice. Nat Rev Rheumatol. 13:1302017. View Article : Google Scholar
|
|
39
|
Liu K, Jing Y, Zhang W, Fu X, Zhao H, Zhou
X, Tao Y, Yang H, Zhang Y, Zen K, et al: Silencing miR-106b
accelerates osteogenesis of mesenchymal stem cells and rescues
against glucocorticoid-induced osteoporosis by targeting BMP2.
Bone. 97:130–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chuang TD, Luo X, Panda H and Chegini N:
miR-93/106b and their host gene, MCM7, are differentially expressed
in leiomyomas and functionally target F3 and IL-8. Mol Endocrinol.
26:1028–1042. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ni X, Xia T, Zhao Y, Zhou W, Wu N, Liu X,
Ding Q, Zha X, Sha J and Wang S: Downregulation of miR-106b induced
breast cancer cell invasion and motility in association with
overexpression of matrix metalloproteinase 2. Cancer Sci.
105:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dong P, Kaneuchi M, Watari H, Sudo S and
Sakuragi N: MicroRNA-106b modulates epithelial-mesenchymal
transition by targeting TWIST1 in invasive endometrial cancer cell
lines. Mol Carcinog. 53:349–359. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng L, Zhang Y, Lin S, Sun A, Chen R and
Ding Y and Ding Y: Down-regualtion of miR-106b induces
epithelial-mesenchymal transition but suppresses metastatic
colonization by targeting Prrx1 in colorectal cancer. Int J Clin
Exp Pathol. 8:10534–10544. 2015.PubMed/NCBI
|