Introduction
Peripheral nerve regeneration is slow and
incomplete, and this leads to the development of denervated muscle
atrophy, which is the main reason for hypokinesia following
peripheral nerve injury (1). The
clinical consequences of muscle atrophy seriously affects the
quality of life of the patients. Muscle wasting is an independent
index of mortality and morbidity. There is currently no reliable
pharmacological treatment to prevent muscle atrophy, so effective
therapies to treat muscle atrophy are required (2). Neural stem cells (NSCs) are used as a
novel therapeutic agent for repairing nerve injuries (3,4). NSCs
are undifferentiated cells that are widely distributed in the
nervous system and when transplanted into peripheral nerves, they
differentiate into neurons and form functional motor endplate
nerve-muscle connections with the denervated muscle (5).
Neurotrophic factors are used by nerve tissues and
previous studies have demonstrated that neurotrophin-3 (NT-3)
serves a regulatory role in denervated muscle atrophy (6–8). NSCs
differentiate into motor neurons and form functional nerve-muscle
connections, thereby increasing the number and length of
regenerated axons. However, the endogenous secretion of NT-3 is low
and exogenous NT-3 lacks sufficient time to accumulate due to its
short half-life (9). Thus, it was
hypothesized that the transfection of NT-3 into NSCs in order to
produce endogenous NT-3 may adequately supply nerves with NT-3 and
promote the differentiation of NSCs into motor neurons.
A previous study demonstrated that transfection
mediated by ultrasound with microbubbles (MBs) with the appropriate
parameters exhibits low toxicity and noninvasion (10). In addition, this transfection
technique enhances the effect of stem cell transplantation
(11,12). However, the effects of using
ultrasound with MBs to transfect NT-3 into NSCs remain unknown.
Thus, the current study investigated the feasibility of
transfecting NT-3 into NSCs using ultrasound with MBs and the
subsequent transplantation of NSCs in vivo to treat
denervated muscle atrophy.
Materials and methods
Animal model
A total of 32 male Sprague-Dawley rats (n=8 per
group, 200–220 g, 8–10 weeks old) purchased from Guangdong Medical
Laboratory Animal Center (Foshan, China) were used in the current
study. These rats were individually housed under a constant
temperature (23±2°C) and maintained in a 12-h light/dark cycle with
free access to food and water. Rats were anesthetized
intraperitoneally with 300 mg/kg chloral hydrate (TargetMol,
Boston, MA, USA) prior to transplantation of NSCs. The hair on the
thighs of the rats was removed and each animal was placed in the
prostrate position on an operating table. A 2 cm skin incision
parallel to the femur and inferior at 1 cm was made on the right
thigh of the rat to expose the sciatic nerve. The nerve was cut off
at 1.5 cm, piercing the piriformis, and a neurological defect of 1
cm was made. The proximal nerve was ligated using a 7-0 noninvasive
micro stitch and the distal end was placed aside. The wound was
washed with 0.1% povidone-iodine and sutured. All animal
experiments were conducted in accordance with the guidelines
developed by the National Institutes of Health (13) and approved by the Institutional
Animal Care and Use Committee of Peking University Shenzhen
Hospital (permit no. 09–215).
Ultrasound equipment
The ultrasound system used in the current study
included an arbitrary AFG3102 waveform generator (Tektronix, Inc.,
Beaverton, OR, USA), an AR150A100B RF power amplifier (AR, Inc.,
Souderton, PA, USA) and a 1.0 MHz unfocused single-element
transducer (Panametrics, Inc., Waltham, MA, USA). The parameters of
ultrasonic intensity and repetition frequency were optional.
NSC culture
Hippocampi from 7 embryonic day (E)14 Sprague-Dawley
rats purchased from Guangdong Medical Laboratory Animal Center
(Foshan, China; permit no. SCXK2013-0002) were isolated in a
biological safety cabinet and placed in a flask for primary NSC
culture. Hippocampal tissues were sheared into 1 mm3
sized tissue blocks. 0.25% trypsin (EMD Millipore, Billerica, MA,
USA; 1:250) was used to digest the tissue block at room temperature
for 20 min. The digested tissue solution was collected and placed
in a 15 ml centrifuge tube, and then Dulbecco's modified Eagle's
medium (DMEM)/F12 (1:1 v/v, Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) was added to stop the digestion.
Thereafter, the solution was centrifuged at 450 × g at room
temperature for 5 min. Then, cells were harvested and cultured in
DMEM/F12 (1:1 v/v) containing 2% B27 (Gibco; Thermo Fisher
Scientific, Inc.) and 1% N2 (Gibco; Thermo Fisher Scientific, Inc.)
supplements, 0.5 mM L-glutamine (Hyclone; GE Healthcare, Logan, UT,
USA), 0.5 mM non-essential amino acids, 20 ng/ml basic fibroblast
growth factor (Promega Corp., Madison, WI, USA), 50 IU/ml
penicillin (Hyclone; GE Healthcare) and 50 µg/ml streptomycin
(Hyclone; GE Healthcare). The cells were cultured in an incubator
containing 5% CO2 at 37°C. The culture medium was
replaced half every 3 days, and the cells were passaged after 7
days of culture. Cells were observed and used at day 20.
Transfection of NT-3
A total of 200 µl MBs (3×108/ml; Bracco
Spa, Milan, Italy) with a mean size of 2–5 µm were mixed with 20 µl
pEGFP-NT-3 recombinant plasmid (1 µg/µl) (Corning Incorporated,
Corning, NY, USA) in 24-well plates at room temperature for 30 min.
The treatments used in each group were as follows: i) In the
control group 500 µl NSCs (3×105/ml) were incubated in
complete medium (mentioned above) with no supplements; ii) in the
NSC+MB+pEGFP-NT-3 group the number of NSCs following trypsin
digestion were counted (500 µl; 3×105/ml) and mixed with
20 µl pEGFP-NT-3 recombinant plasmid (1 µg/µl) and 200 µl MBs; iii)
in NSC+MB group the number of NSCs following trypsin digestion were
counted (500 µl; 3×105/ml) and mixed with 200 µl MBs;
and iv) in the NSC+MB+NT-3 group the number of NSCs following
trypsin digestion were counted (500 µl; 3×105/ml) and
mixed with 200 µl MBs and NT-3 protein (PeproTech, Inc., Rocky
Hill, NJ, USA) (100 µl; 50 ng/ml) in place of the plasmid. The
ultrasound probe was then placed below each well to sonicate the
mixture for the transfection of NT-3 using the following
parameters: Ultrasonic intensity, 1.5 W/cm2; sonication
time, 60 sec; duty cycle, 25%; and MB concentration,
3×108/ml. Cells were then incubated with 5%
CO2 at 37°C for 48 h.
Transplantation of NSCs
NSCs were collected and centrifuged at 450 × g at
room temperature for 5 min following sonication. A microsyringe at
a depth of 4 mm was then used to vertically inject 100 µl NSC
(1×107/ml) suspension into the upper third of the right
tibialis anterior muscle in the belly of the rats (n=8 in each
group). The needle was retained in the muscle for 10 min and
200,000 units penicillin was then intramuscularly injected into the
left thigh.
Tibialis anterior muscle weight
measurement
After being sacrificed, the bilateral tibialis
anterior muscle was dissected completely from the origin to the
insertion and weighed immediately with an electron scale with 0.001
g precision. The muscle wet weight ratio of
affected-side/health-side was compared between groups to exclude
the individual differences in the weight of the tibialis anterior
muscle.
Tissue preparation and histological
examination
All rats were sacrificed 6 weeks following
transplantation for histological evaluation. The entirety of the
bilateral tibialis anterior muscle with 2 mm deep peroneal nerve
muscular branches of the anterior tibial muscle were immediately
removed and samples were weighed using an analytical balance
(accuracy to 0.001 g). The muscle from the right thigh was then
divided into four parts: The tissue block on the right side of the
nerve entering point otherwise known as muscle hilus; the tissue
block on the left side of the muscle hilus; and two tissue blocks
(1×1×2 mm) around the muscle hilus.
Hematoxylin and eosin (H&E)
staining
The tissue blocks on the right side of the muscle
hilus were fixed in 10% buffered neutral formalin at room
temperature for 12 hand a series of sections (5 µm) were cut for
H&E staining at room temperature for 15 min. Three fields of
view of each section were randomly captured at a magnification of
×200 (Olympus BX53, Olympus Corporation, Tokyo, Japan) and analyzed
using ImageJ software (version 1.48, National Institutes of Health,
Bethesda, MD, USA). Four muscle fibers were randomly selected and
their cross-sectional areas were measured.
Acetylcholinesterase (AChE)
staining
AChE staining was used to assess the number of motor
endplates in all groups. The tissue blocks on the left side of the
muscle hilus were embedded in optimum cutting temperature compound
for cryosection at −20°C for 10 min. The serial sections were
frozen, repeatedly washed with PBS, and incubated in AChE
incubation solution (Beijing Leagene Biotech Co., Ltd., Beijing,
China) at 4°C for 12 h and washed again with PBS. The washed
sections were then mounted with neutral gum. Three sections were
randomly selected from each rat. Two fields of view of each section
were randomly selected and observed using an Olympus BX53
microscope at a magnification of ×100. The number of motor
endplates was counted.
Gold chloride staining
Gold chloride staining was used to estimate the
shape and number of motor endplates. The tissue blocks around the
muscle hilus (1×1×2 mm) were fixed in 20% formic acid solution at
room temperature for 5 h and then stained with 1% gold chloride
until the color changed to a brown-yellow shade at room temperature
for 1 h. The tissue blocks were then washed three times with
distilled water and restored in 20% formic acid solution until the
color changed to a chocolate brown. The tissue was then washed
twice with distilled water and stored in glycerin until it
softened. Following this, the tissue samples were mounted on glass
slides. Two fields of view of each section were randomly selected
and observed using an Olympus BX53 microscope at a magnification of
×100.
Transmission electron microscopy
(TEM)
TEM was used to evaluate the ultrastructural basis
of the attenuation of denervated muscle atrophy. The tissue blocks
around the muscle hilus (1×1×2 mm) were fixed by perfusion using
2.5% paraformaldehyde and 1.5% glutaraldehyde in 0.1 M phosphate
buffer (pH 7.2) at room temperature for 24 h and then post-fixed in
a solution of 1% osmium tetroxide and 1.5% potassium ferrocyanine
(1 h, 4°C). The tissues were then dehydrated with increasing
ethanol (25, 50, 70, 90 and 100%) for 5 min each. The samples were
sectioned (50 nm) and observed using a LIBRA 200 FE microscope
(Zeiss GmbH, Jena, Germany).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The experiments were repeated three times. One-way
analysis of variance followed by Tukey's post hoc test was used to
compare the means between groups using SPSS (version 17.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of the tibialis anterior
muscle wet weight between groups
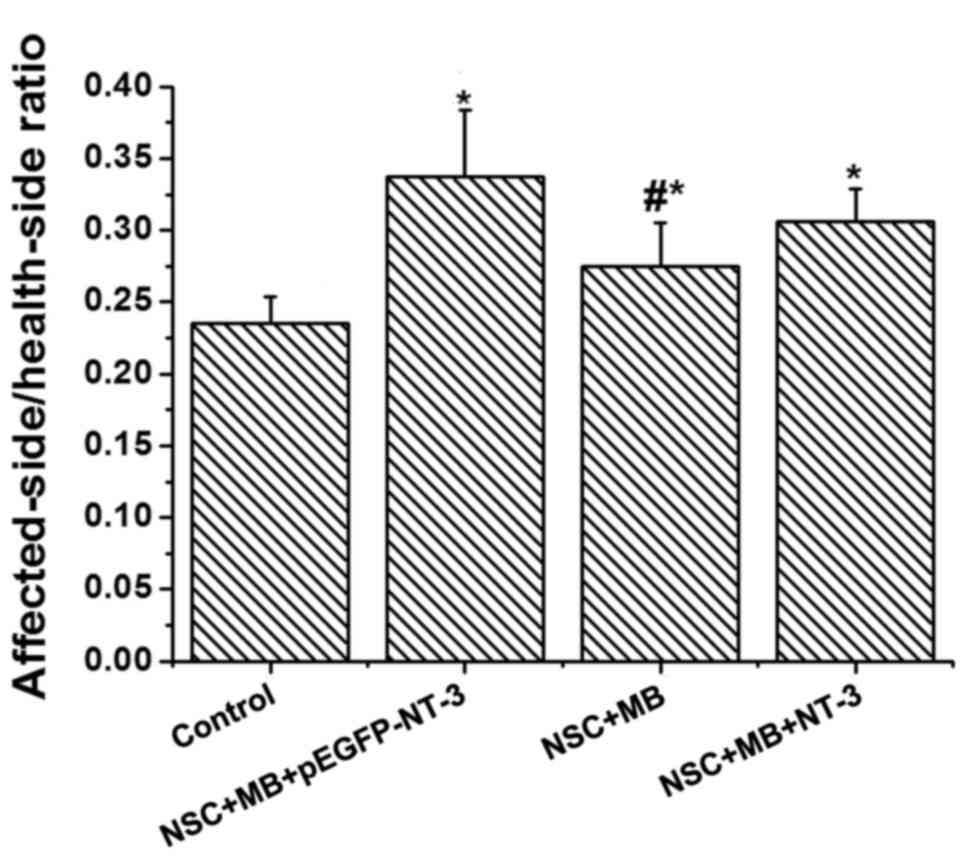
The affected-side/health-side ratio of the muscle
wet weight was compared between groups to exclude the individual
differences in the weight of the tibialis anterior muscle (Fig. 1). The NSC+MB+pEGFP-NT-3 group had the
highest ratio, whereas the control group had the lowest. The ratios
in the NSC+MB+pEGFP-NT-3, NSC+MB and NSC+MB+NT-3 groups were
significantly increased compared with the control (P<0.05). A
pairwise comparison also identified that the
affected-side/health-side ratio of the muscle wet weight was
significantly increased in the NSC+MB+pEGFP-NT-3 group compared
with the NSC+MB group (P<0.05).
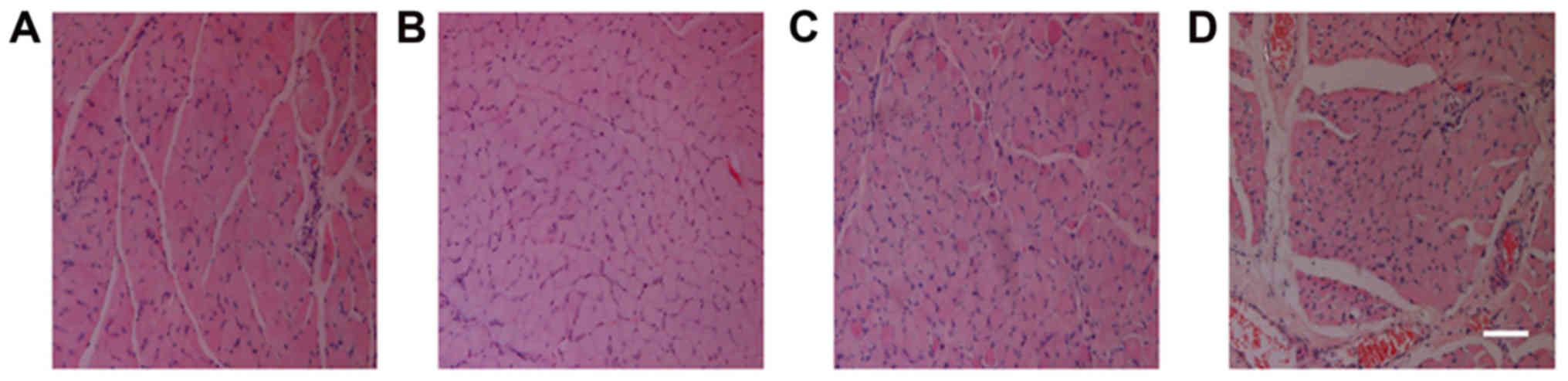
Comparison of muscle fiber H&E
staining and cross-sectional area measurements between groups
Following H&E staining of the tibialis anterior
muscle, visual observation of the sections demonstrated that the
control group had the smallest cross-sectional area of muscle
fibers, followed by the NSC+MB and NSC+MB+NT-3 groups, while the
NSC+MB+pEGFP-NT-3 group had the largest cross-sectional area
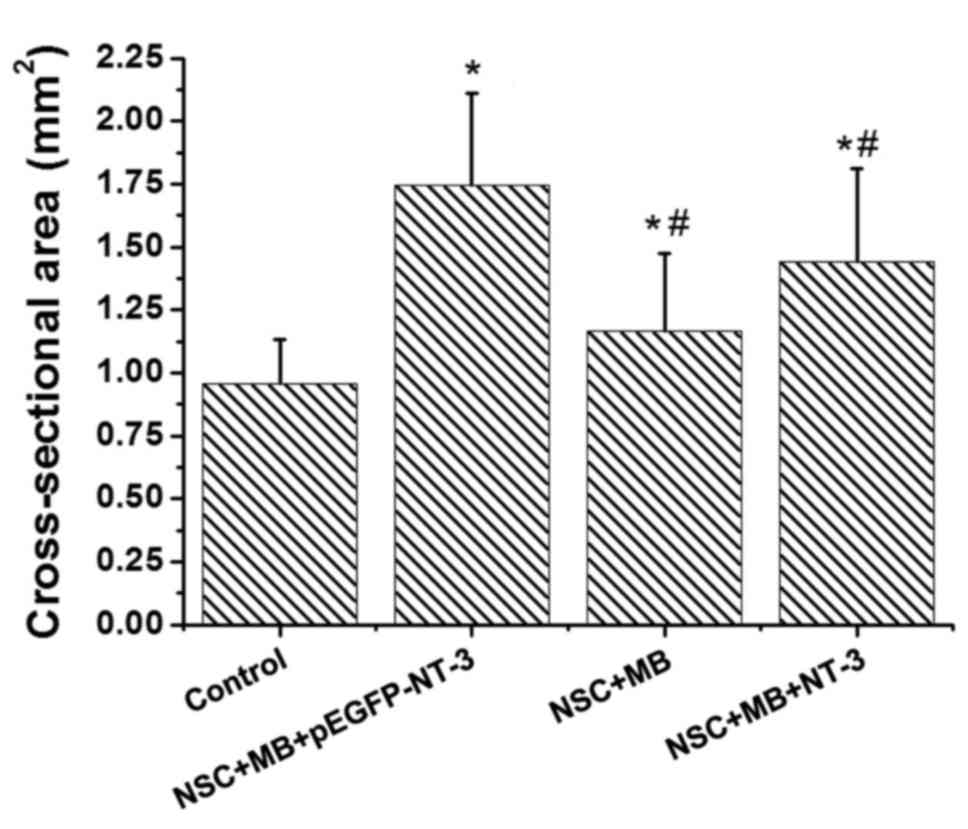
(Fig. 2). The comparison made using
ImageJ software revealed significant differences in the
cross-sectional area of the muscle fiber among the groups (Fig. 3). Pairwise comparison demonstrated
that the cross sectional area of the muscle fibers in the
NSC+MB+pEGFP-NT-3, NSC+MB and NSC+MB+NT-3 groups was significantly
increased compared with the control (P<0.05). In addition, the
cross sectional area of the muscles fibers in the NSC+MB and
NSC+MB+NT-3 groups was significantly decreased compared with the
NSC+MB+pEGFP-NT-3 group (P<0.05). However, the difference in the
cross-sectional area of the muscle fibers between the NSC+MB and
NSC+MB+NT-3 groups was not significant.
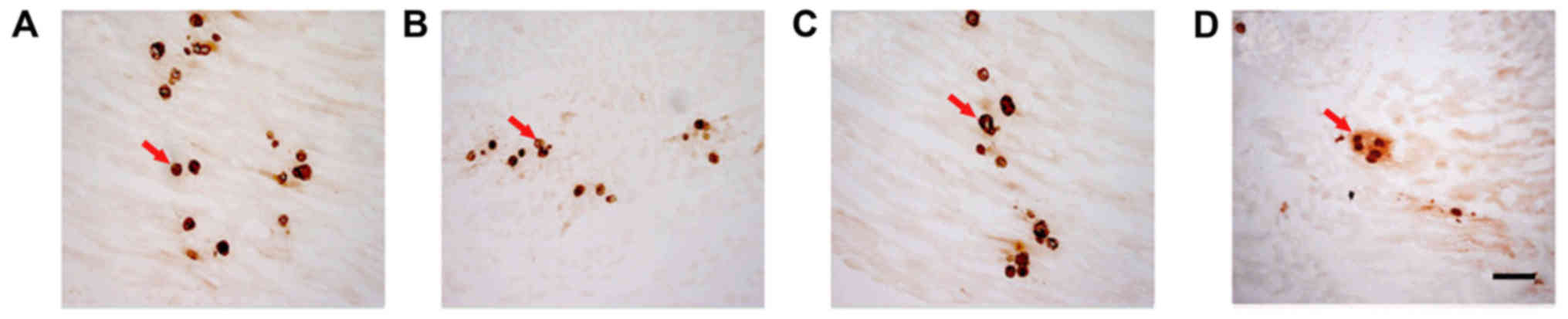
Comparison of AChE staining between
groups
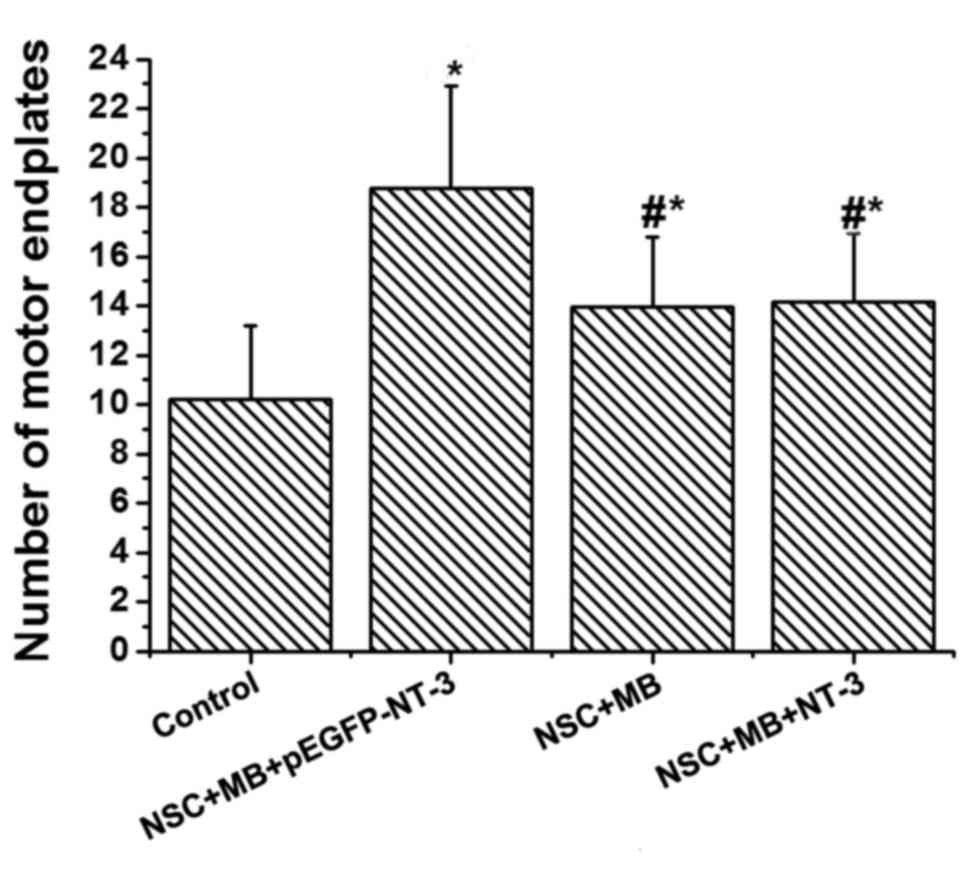
AChE staining was used to determine the number of
motor endplates in each group (Fig.
4). The lowest number was recorded in the control. Pairwise
comparison demonstrated that the number of motor endplates in the
NSC+MB+pEGFP-NT-3 group was significantly increased compared with
the NSC+MB group, and the number in the control was significantly
decreased compared with the NSC+MB and NSC+MB+NT-3 groups (all
P<0.05; Fig. 5). However, the
difference in the number of motor endplates between the NSC+MB and
NSC+MB+NT-3 groups was not significantly different (Fig. 5).
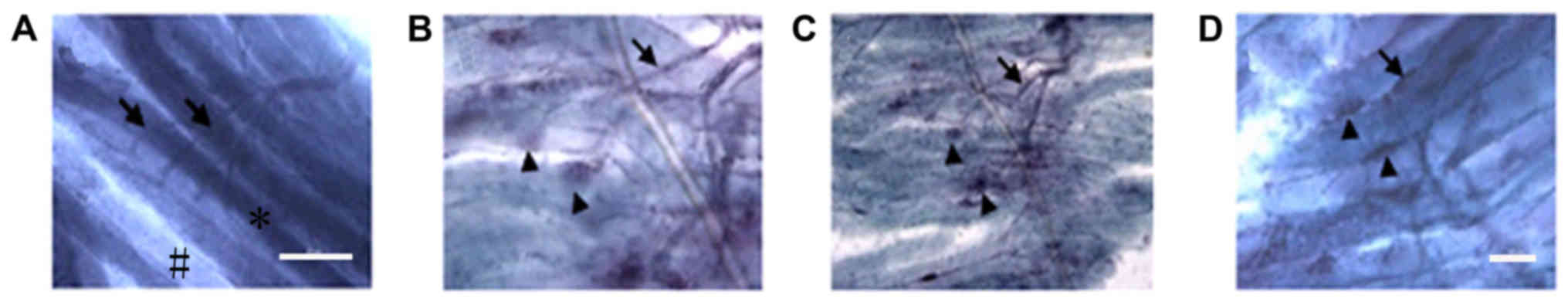
Comparison of gold chloride staining
between groups
Normal (control group) motor endplates stained with
gold chloride exhibited a horseshoe or oval shape and clear color,
whereas normal muscle fibers were irregularly shaped (Fig. 6). The motor endplates in the
experimental groups (NSC+MB and NSC+MB+NT-3 groups) were fewer,
smaller, unevenly dyed and irregular. In addition, atrophy
manifestations and fibrous connective tissue increased to different
levels. The motor endplates in the control, NSC+MB and NSC+MB+NT-3
groups exhibited unclearly stained areas with an irregular shape
and were markedly decreased compared with the NSC+MB+pEGFP-NT-3
group, in which motor endplates exhibited a more regular shape.
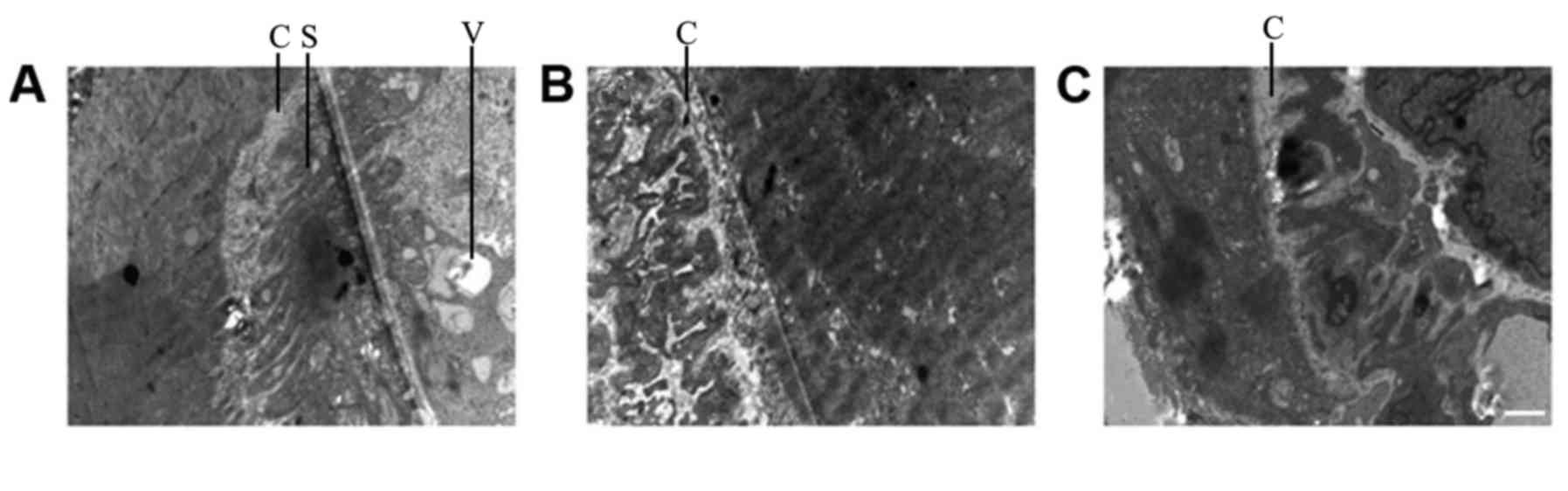
Comparison of TEM results between
groups
The muscle fibers in the NSC+MB+pEGFP-NT-3 group
differed slightly compared with the normal (control group)
ultrastructure (Fig. 7). The Z
lines, dark band and M line were clear, and their shapes were
normal. In addition, muscle fiber atrophy and a slightly loose
arrangement were observed, and the mitochondrial cristas were
shorter. In the NSC+MB+NT-3 and NSC+MB groups, the Z line was
slightly blurred, the dark bands and M lines were not clear, the
muscle fibers were not neatly arranged. In the muscle fibers of the
control group severely abnormal morphology was observed, the Z line
was blurred and discontinuous, and the dark bands and M lines were
unclear. In the NSC+MB and MSC+MB+NT-3 groups, the motor endplate
synaptic cleft was shortened and the secondary folds became shallow
or disappeared (Fig. 8). In the
NSC+MB+pEGFP-NT-3 group, the secondary folds became slightly
shallow and the number of synaptic vesicles in the motor endplates
was increased. There were no clear motor endplates present in the
control group.
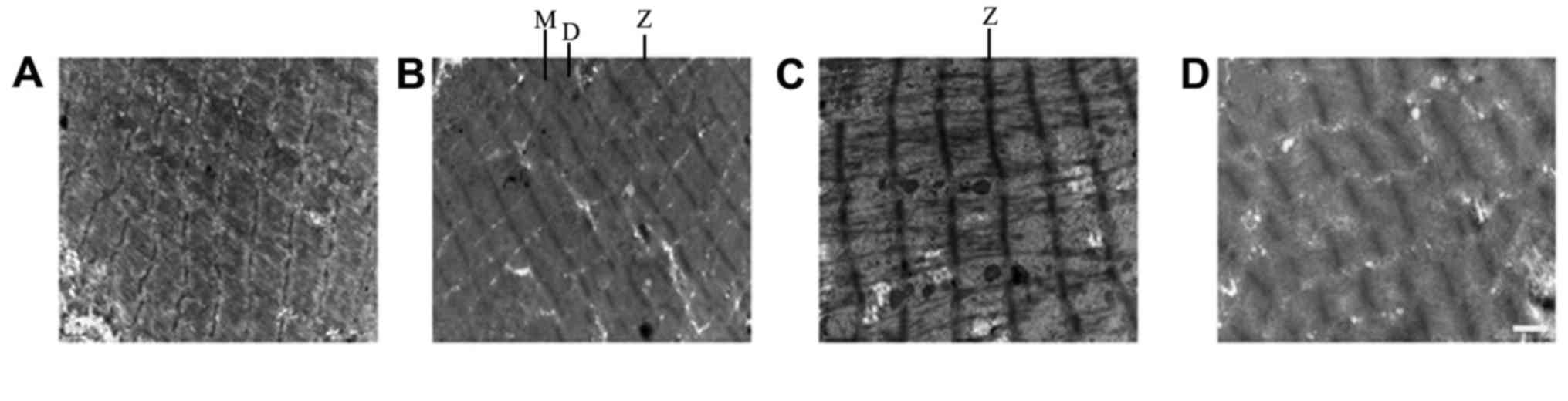 | Figure 7.TEM images of muscle fibers. TEM
images of the (A) control, (B) NSC+MB+pEGFP-NT-3, (C) NSC+MB and
(D) NSC+MB+NT-3 groups. Scale bar, 1 µm. TEM, transmission electron
microscopy; NSC, neural stem cell; MB, microbubble; NT-3,
neurotrophin-3; Z, Z line; M, M line; D, dark bands. |
Discussion
Preventing the atrophy of denervated muscle
following peripheral nerve injury is challenging; however, it has
been demonstrated that stem cell transplantation may be used as a
treatment (14). Kubo et al
(5) demonstrated that the
co-incubation of myotubules and motor neurons differentiated from
NSCs lead to new nerve-muscle connections forming in vitro.
In addition, it has been demonstrated using animal experiments that
local intramuscular injection of motor neurons may relieve muscle
denervation atrophy (4). The study
demonstrated that locally transplanted NSCs are able to survive in
the muscle and form new nerve-muscle connections to attenuate
denervated muscle atrophy (4).
NT-3 is an early signaling factor that induces NSC
differentiation by inhibiting the basic fibroblast growth factor
during the proliferation of embryonic NSCs. Lim et al
(7) conducted an experiment on mice,
which demonstrated that by binding to neurotrophic tyrosine kinase
receptor type 3 on the cell membrane, NT-3 amplifies the
phosphoinositide-3-kinase/protein kinase B and extracellular
signal-regulated kinase signaling pathways to induce NSC
differentiation into neurons, and induces synaptic growth by the
specific phosphorylation of mitogen-activated protein kinase 14. It
was also demonstrated that NT-3 sustains the survival of muscle
spindles, tendons and skin afferent sensory neurons, promotes motor
neuron regeneration and nerve-muscle connection maturation, and
increases the number and length of regenerative axons and NSCs,
thereby preventing muscle atrophy following nerve injury. NT-3 has
a continuous and long-term effect on muscle cells, but exogenous
neurotrophic factors have low biological activity, limited sources
and a short half-life, which restricts their function (9).
The current focus of research into treatments for
denervated muscle atrophy is on resolving the shortcomings of NT-3
and its application. It has previously been demonstrated that gene
transfer is a reliable method of inducing stable and sustained
expression of NT-3 in vivo (15). Common methods of transfection include
viral and liposomal transfection. However, the biosecurity of viral
transfection poses a challenge to its clinical application
(16). During liposomal transfection
cationic liposomes may move from the serum and accumulate in the
lung tissues, inducing a strong anti-inflammatory response, thus
limiting its clinical application (17,18).
Micron-sized MB contrast agents for ultrasonography have been
widely used in clinical settings (19,20).
Ultrasound with MBs is a recognized technology for enhancing the
efficiency of transfection (21). It
has been considered that the mechanism by which ultrasound with MBs
enhances transfection is based on the cavitation effect, where at a
certain ultrasound frequency, the MBs vibrate rapidly and collapse
into pieces, producing shock waves, jet streams and other strong
localized effects that increase cell membrane permeability and gene
delivery (22). Ultrasound with MBs
significantly enhances the efficiency of transfection and
expression of the gene transfected, due to its low toxicity and
immunogenicity. Thus, ultrasound with MBs has become the primary
focus of numerous studies worldwide.
To the best of our knowledge, NSC transfection using
ultrasound with MBs was applied for the first time in the present
study. At 6 weeks following nerve injury, the rats in the
NSC+MB+pEGFP-NT-3 group exhibited the highest
affected-side/health-side ratio of muscle wet weight in the
cross-sectional area. Furthermore, TEM demonstrated that the muscle
fibers in the NSC+MB+pEGFP-NT-3 group differed compared with the
control, and the Z lines, dark band and M line were clear, and
their shapes were normal. NSC+MB+pEGFP-NT-3 group could better
maintain muscle weight compared with other groups, and had the
heaviest muscle fibers among all groups. TEM further revealed
differences in the motor endplates of the NSC+MB+pEGFP-NT-3 group,
and gold chloride staining demonstrated that an increased number of
motor endplates in the NSC+MB+pEGFP-NT-3 group retained regular
shapes compared with the other three groups. AChE staining
demonstrated that the NSC+MB+pEGFP-NT-3 group had the highest
number of motor endplates among the groups. These results suggest
that the NSC+MB+pEGFP-NT-3 group had the lowest rate of motor
endplate degeneration, and that the transfection of NT-3 into NSCs
using ultrasound with MBs and the subsequent transplantation of
NSCs in vivo attenuates denervated muscle atrophy. The
difference of histological and electron microscopic observation
between the NSC+MB and MSC+MB+NT-3 groups was not marked, possibly
due to the NT-3 protein being metabolized in the MSC+MB+NT-3 group,
which limited it from exerting stable neurotrophic effects.
In conclusion, the results of the present study
suggest that the transfection of NT-3 into NSCs using ultrasound
with MBs, and the subsequent transplantation of the cells in
vivo, is a novel, safe and effective strategy for preventing
and treating denervated muscle atrophy and promoting the functional
recovery of peripheral nerves in clinical settings. The future
development of novel types of MBs is required to improve the
efficiency of transfection. This development may reduce the
morbidity of patients with peripheral nerve injury, thereby
providing social and economic benefits.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. U1204810), Guangdong
Natural Science Foundation (grant nos. 2014A030313709,
2014A030313710 and 2015A030313889), Shenzhen Science and Technology
Planning Project (grant nos. ZDSYS201504301045406,
JCYJ20170413100222613, JCYJ20170306154931588,
JCYJ20150403110829621, JCYJ20140415162338855, JCYJ20140415162542975
JCYJ20140415162338774 and JCYJ20140828163634004) and the Guangdong
Bureau of Traditional Chinese Medicine Project (grant no.
20171228).
References
|
1
|
Armstrong RJ, Harrower TP, Hurelbrink CB,
McLaughin M, Ratcliffe EL, Tyers P, Richards A, Dunnett SB, Rosser
AE and Barker RA: Porcine neural xenografts in the immunocompetent
rat: Immune response following grafting of expanded neural
precursor cells. Neuroscience. 106:201–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su Z, Hu L, Cheng J, Klein JD, Hassounah
F, Cai H, Li M, Wang H and Wang XH: Acupuncture plus low-frequency
electrical stimulation (Acu-LFES) attenuates denervation-induced
muscle atrophy. J Appl Physiol (1985). 120:426–436. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bost F, Caron L, Marchetti I, Dani C, Le
Marchand-Brustel Y and Binétruy B: Retinoic acid activation of the
ERK pathway is required for embryonic stem cell commitment into the
adipocyte lineage. Biochem J. 361:621–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dooley D, Vidal P and Hendrix S:
Immunopharmacological intervention for successful neural stem cell
therapy: New perspectives in CNS neurogenesis and repair. Pharmacol
Ther. 141:21–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kubo T, Randolph MA, Groger A and Winograd
JM: Embryonic stem cell-derived motor neurons form neuromuscular
junctions in vitro and enhance motor functional recovery in vivo.
Plast Reconstr Surg. 123(2 Suppl): 139S–148S. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lawrie A, Brisken AF, Francis SE,
Cumberland DC, Crossman DC and Newman CM: Microbubble-enhanced
ultrasound for vascular gene delivery. Gene Ther. 7:2023–2027.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim MS, Nam SH, Kim SJ, Kang SY, Lee YS
and Kang KS: Signaling pathways of the early differentiation of
neural stem cells by neurotrophin-3. Biochem Biophys Res Commun.
357:903–909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin S, Xu J, Hu S, Xu L, Zhang C, Wang Y
and Gu Y: Combined application of neutrophin-3 gene and neural stem
cells is ameliorative to delay of denervated skeletal muscular
atrophy after tibial nerve transection in rats. Cell Transplant.
20:381–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sahenk Z, Galloway G, Clark KR, Malik V,
Rodino-Klapac LR, Kaspar BK, Chen L, Braganza C, Montgomery C and
Mendell JR: AAV1.NT-3 gene therapy for charcot-marie-tooth
neuropathy. Mol Ther. 22:511–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raju BI, Leyvi E, Seip R, Sethuraman S,
Luo X, Bird A, Li S and Koeberl D: Enhanced gene expression of
systemically administered plasmid DNA in the liver with therapeutic
ultrasound and microbubbles. IEEE Trans Ultrason Ferroelectr Freq
Control. 60:88–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin WH, Fan CH, Ting CY, Liu HL and Yeh
CK: Dynamic perfusion assessment by contrast-enhanced ultrasound in
blood-brain barrier disruption. Conf Proc IEEE Eng Med Biol Soc.
2013:pp. 1152–1155. 2013; PubMed/NCBI
|
|
12
|
Ménard C, Hein P, Paquin A, Savelson A,
Yang XM, Lederfein D, Barnabé-Heider F, Mir AA, Sterneck E,
Peterson AC, et al: An essential role for a MEK-C/EBP pathway
during growth factor-regulated cortical neurogenesis. Neuron.
36:597–610. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo YX, Xue YX, Liu JF, Shi HS, Jian M,
Han Y, Zhu WL, Bao YP, Wu P, Ding ZB, et al: A novel UCS memory
retrieval-extinction procedure to inhibit relapse to drug seeking.
Nat Commun. 6:76752015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Modo M, Rezaie P, Heuschling P, Patel S,
Male DK and Hodges H: Transplantation of neural stem cells in a rat
model of stroke: Assessment of short-term graft survival and acute
host immunological response. Brain Res. 958:70–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nomikou N, Feichtinger GA, Redl H and
McHale AP: Ultrasound-mediated gene transfer (sonoporation) in
fibrin-based matrices: Potential for use in tissue regeneration. J
Tissue Eng Regen Med. 10:29–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan Z, Kumon RE and Deng CX: Mechanisms of
microbubble-facilitated sonoporation for drug and gene delivery.
Ther Deliv. 5:467–486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nomikou N and McHale AP: Exploiting
ultrasound-mediated effects in delivering targeted, site-specific
cancer therapy. Cancer Lett. 296:133–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ochi M, Kwong WH, Kimori K, Takemoto S,
Chow SP and Ikuta Y: Delay of the denervation process in skeletal
muscle by sensory ganglion graft and its clinical application.
Plast Reconstr Surg. 97:577–586. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phillips LC, Klibanov AL, Wamhoff BR and
Hossack JA: Targeted gene transfection from microbubbles into
vascular smooth muscle cells using focused, ultrasound-mediated
delivery. Ultrasound Med Biol. 36:1470–1480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shih R, Bardin D, Martz TD, Sheeran PS,
Dayton PA and Lee AP: Flow-focusing regimes for accelerated
production of monodisperse drug-loadable microbubbles toward
clinical-scale applications. Lab Chip. 13:4816–4826. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J and Nyborg WL: Ultrasound, cavitation
bubbles and their interaction with cells. Adv Drug Deliv Rev.
60:1103–1116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yohn DC, Miles GB, Rafuse VF and
Brownstone RM: Transplanted mouse embryonic stem-cell-derived
motoneurons form functional motor units and reduce muscle atrophy.
J Neurosci. 28:12409–12418. 2008. View Article : Google Scholar : PubMed/NCBI
|