Introduction
Pancreatic cancer (PC) is associated with high rates
of morbidity and mortality. In the United States, the incidence and
mortality rates of PC ranked 8 and 4th of all tumors, respectively
(1,2). In China, the incidence of PC has been
increasing yearly, and its incidence and mortality rates ranked 7
and 6th, respectively (3).
Therefore, PC is a common tumor of the digestive system.
At present there is no widely accepted screening
method for the early detection of PC. Surgical treatment is
currently used to prolong the survival rate of patients with PC;
however, the 5-year survival rate of patients typically remains low
(15–25%) following radical resection of the tumor (4). Biomarkers are considered to be
important in predicting the prognosis of PC (5–7), and
numerous biological markers associated with PC have been identified
by previous studies (8,9). In particular, transcriptional
coactivator with PDZ-binding motif (TAZ), as a type of 14-3-3
binding protein, was identified in 2000 (10). TAZ is a costimulatory protein
transcription factor that contains a WW domain, and is involved in
the proliferation, migration and metastasis of tumors through
modulation of its target genes, including ankyrin repeat
domain-containing protein (ANKRD), cysteine-rich 61 (CYR61) and
connective tissue growth factor (11–14). The
oncogenic role of TAZ in PC has been verified (15,16).
More recently, studies into microRNAs (miRNAs) have demonstrated
that upregulation of numerous miRNAs may suppress the development,
proliferation and metastasis of tumors by regulating the expression
of TAZ (17,18). However, to the best of our knowledge,
no experimental or clinical studies have documented the potential
roles of miRNA (miR)-185 in PC.
The present study investigated the expression of TAZ
at the mRNA and protein levels in the tumor tissues, blood and
pancreatic fluid of PC patients and controls using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blot analysis and ELISA. The miRNAs targeting TAZ were also
predicted by bioinformatics methods, and TAZ was confirmed as a
target of miR-185 by a dual-luciferase reporter assay. Furthermore,
the expression of miR-185 was measured in the samples obtained from
PC patients.
Materials and methods
PC tissue specimens
A total of 46 patients with PC who were diagnosed
and received surgery between June 2012 and December 2015 at the
Affiliated Hospital of Qingdao University (Qingdao, China) were
enrolled. A total of 35 patients with chronic pancreatitis with
non-neoplastic diseases were included as a control group. Among the
46 PC patients, there were 28 male and 18 female patients, and the
median age was 56 years (ranging between 31 and 75 years). Among
the 35 controls, there were 20 male and 15 female patients, and the
median age of patients was 58 years (ranging between 25 and 77
years). All subjects were diagnosed and had no history of hormone
medicine, radiotherapy and chemotherapy prior to surgery. The Tumor
Node Metastasis (TNM) clinical stages of the 46 cases of PC were as
follows: Stage I: 2 cases; stage II: 1 case; stage III: 12 cases
and stage IV: 31 cases (19).
Clinical information and medical records of patients were also
collected. Prior written informed consent was obtained from all
patients and the study was approved by the Ethics Review Board of
the Affiliated Hospital of Qingdao University.
Sample collection
Paired human PC tissues and their matched
peritumoral non-cancerous tissues were collected during surgery and
stored in liquid nitrogen (−196°C) until further analysis. Serum
was collected from the peripheral blood of PC and chronic
pancreatitis patients. Briefly, 10–15 ml peripheral blood was kept
at 4°C for 1–2 h, and serum in the upper layer was aspirated and
centrifuged for 10 min at 400 × g and 4°C. The serum was then
aliquoted and stored at −80°C. A total of 10 ml pancreatic juice
was also collected from PC and chronic pancreatitis patients during
endoscopic retrograde cholangiopancreatography examination and
stored at −80°C.
Plasmid construction
The coding sequence (CDS) of the taz gene
(10,20) was cloned into a PCDNA 3.1 plasmid
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
using a ClonExpress II One Step Cloning kit (C112-01; Vazyme
Biotech Co., Ltd., Nanjing, China) according to the manufacturer's
protocol. The primers used for the clone were as follows:
Taz CDS forward,
5′-CTAGCGTTTAAACTTAAGCTTATGCCTCTGCACGTGAAGTGG-3′ and reverse,
5′-CCACACTGGACTAGTGGATCCCTATCTCCCAGGCTGGAGGTG-3′, and the
Taz CDS sequence was confirmed by sequencing (Sangon Biotech
Co., Ltd., Shanghai, China) (21).
Cell culture and transfection
The human PC cell lines HPAC and PANC-1, as well as
293T cells, were purchased from cell bank of Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in
F12/Dulbecco's modified Eagle medium (DMEM) or RPMI 1640 medium
(Gibco; Thermo Fisher Scientific, Inc.), respectively, supplemented
with 10% heat inactivated fetal bovine serum (FBS; Sangon Biotech
Co., Ltd.) and 1% penicillin-streptomycin. Cells were incubated in
a humidified atmosphere with 5% CO2 at 37°C. One day
before transfection, 3×105 cells were inoculated into
24-well plates with F12/DMEM or RPMI-1640 medium supplemented with
10% FBS. When cell confluence reached 70% at 37°C, cells were
transfected with TAZ small interfering RNA (siRNA), agomiR-185
(5′-UGGAGAGAAAGGCAGUUCCUGA-3′) or, scramble RNA
(5′-AAGACUAGGGAGUUUAGGACCG-3′; negative control, NC; RiboBio Co.,
Ltd., Guangzhou, China) using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Untransfected cells were used as a control. For a
rescue assay, cells were cotransfected with agomiR-185 and
Taz CDS-expressing plasmid or with agomiR-185 and the empty
plasmid (control). Cells transfected with scramble RNA and empty
plasmid were used as a NC. Cells were collected at 48 h
post-transfection and stored at 37°C for further experiments.
Sangon Biotech Co., Ltd. provided three TAZ siRNA sequences, and
the first siRNA sequence with the highest knockdown efficiency was
selected. The sequences of the three siRNA sequences were as below.
No. 1, sense: 5′-GGGAAAGUGAACAUGAGUUdTdT-3′; antisense:
5′-AACUCAUGUUCACUUUCCCdTdT-3′; No. 2, sense:
5′-UGACGUCCUUCCUAACAGUdTdT-3′; antisense:
5′-ACUGUUAGGAAGGACGUCAdTdT-3′; No. 3, sense:
5′-GAGGAAUUCCAGCAUCUGAdTdT-3′; antisense:
5′-UCAGAUGCUGGAAUUCCUCdTdT-3′.
RNA extraction and RT-qPCR
Total RNA was isolated from HPAC/PANC-1 cells and
patient samples using TRIzol® isolation reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. RNA was quantified using Nanodrop 2000 (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). mRNA (1 µg) was reverse
transcribed using a TIANScriptII cDNA first strand cDNA synthesis
kit (Tiangen Biotech Co., Ltd., Beijing, China) into cDNA. miRNA
was reverse transcribed using a miRcute miRNA cDNA synthesis kit
(Tiangen Biotech Co., Ltd.). qPCR was performed using a SuperReal
PreMix (SYBR Green; Tiangen Biotech Co., Ltd.) for mRNA, or a
miRcute miRNA qPCR detection kit (Tiangen Biotech Co., Ltd.) for
miRNA. For mRNA amplification, the reaction mixture was incubated
for 1 cycle at 95°C for 30 sec, followed by 40 cycles at 95°C for
10 sec and 62°C for 40 sec. For miRNA, the reaction mixture was
incubated for 1 cycle at 95°C for 3 min, followed by 40 cycles at
95°C for 12 sec, 62°C for 35 sec and 72°C for 15 sec. For mRNA
amplification, the primers used were as follows: For TAZ, forward,
5′-CTTGGATGTAGCCATGACCTT-3′ and reverse,
5′-TCAATCAAAACCAGGCAATG-3′; for β-actin, forward,
5′-CGGGAAATCGTGCGTGAC-3′ and reverse, 5′-CAGGAAGGAAGGCTGGAAG-3′;
for ANKRD, forward, 5′-AGTAGAGGAACTGGTCACTGG-3′ and reverse,
5′-TGGGCTAGAAGTGTCTTCAGAT-3′; for CYR61, forward,
5′-CCTTGTGGACAGCCAGTGTA-3′ and reverse, 5′-ACTTGGGCCGGTATTTCTTC-3′;
and for GAPDH, forward, 5′-CTCCTGCACCACCAACTGCT-3′ and reverse,
5′-GGGCCATCCACAGTCTTCTG-3′. For miRNA, the primers used were as
follows: For miR-185, forward, 5′-TGGAGAGAAAGGCAGTTCCTGA-3′ and
reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; and for small nuclear U6,
forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The relative expression levels were
calculated using the 2−ΔΔCq method (22). The expressions of β-actin, GAPDH and
U6 were used to normalize that of TAZ mRNA, ANKRD and CYR61 mRNA
and miR-185, respectively. Each experiment was replicated three
times.
MTT assay
PC cells were seeded into 96-well plates at a
concentration of 2,000 cells/well in triplicate. After culturing by
DMEM high glucose medium (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) and FBS (Sangon Biotech Co., Ltd.) for 24, 48 and
72 h, 20 µl MTT reagent (5 mg/ml: Beyotime Institute of
Biotechnology, Beijing, China) was added, and cells were incubated
for another 4 h. On the last day of incubation, the culture
supernatant was removed and 150 µl dimethyl sulfoxide was added and
incubated at 37°C for 4 h until a purple precipitate was visible.
The absorbance was measured at 490 nm on a microplate reader. Cell
growth curves were generated based on the absorbance values.
Western blot analysis
Proteins were extracted from HPAC/PANC-1 cells and
patient samples by incubation with radioimmunoprecipitation assay
buffer and the protease inhibitor phenylmethylsulfonyl fluoride
(cat. no. KGP250; KeyGene Biotech, Jiangsu, China) according to the
manufacturer's protocol. Protein concentration was determined using
a bicinchoninic acid assay kit [RTP7102; Real-Times (Beijing)
Biotechnology Co., Ltd., Beijing, China]. Next, 20 µg total protein
per lane was separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. Following blocking by 5%
skimmed milk for 2 h at room temperature, the membranes were probed
with the following primary antibodies: Rabbit anti-TAZ (1:1,000;
ab84927) or rabbit anti-β-actin (1:5,000; ab129348; both from
Abcam, Cambridge, MA, USA) at 4°C overnight. For detection,
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibodies (1:3,000; ab6721; Abcam) were used at room temperature
for 1 h. Signal detection was conducted using an enhanced
chemiluminescence reaction (ab65623; Abcam). The acquired images
were analyzed using Image lab 3.0 software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and relative protein expression was
expressed as the densitometric value ratio of TAZ band density to
β-actin band density. Each experiment was replicated 3 times.
ELISA
Levels of TAZ protein in the serum and pancreatic
fluid of patients were measured using a TAZ ELISA kit (FS-Ea-05614;
Feng Shou Shi Ye Biotechnology Co., Ltd., Shanghai, China)
according to the kit protocol. Briefly, 1:4 dilutions of serum
samples and eight serial dilutions of standard substrate at a final
volume of 50 µl were incubated overnight at 4°C. Following
incubation with HRP-conjugated secondary antibody for 1 h, samples
were washed 5 times and the chromogenic substrate solution was
added. The reaction was stopped with H2SO4
and read at 450 nm by a Multiskan FC microplate reader (Thermo
Fisher Scientific, Inc.).
Bioinformatics analysis
To verify the miRNAs that may regulate the
expression of TAZ, bioinformatics software (23–27),
namely miRanda (http://www.microma.org/rnicroma/home.do), TargetScan
(www.targetscan.org), PiTa (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
and PicTar (http://pictar.mdc-berlin.de/) were used for a reliable
prediction of the miRNAs that may target taz mRNA.
Dual-luciferase reporter gene
assay
According to results of the bioinformatics
prediction, a conservative miR-185 binding sequence of the
3′untranslated region (UTR) of taz mRNA (wild-type;
5′-CAGAUGUUCUCUCC-3′) or a mutant sequence (mutant;
5′-CAGAUGAAGAGAGG-3′) was cloned as previously described (28). Luciferase reporter plasmids were
generated as previously described (28) by insertion of the wild-type or mutant
taz sequences into the multiple cloning site (SpeI,
5′-AAGCTT-3′ and HindIII, 5′-ACTAGT-3′) of a pMIR-REPORT™
Luciferase plasmid (Thermo Fisher Scientific, Inc.) downstream of
the luciferase reporter gene. 293T cells (1×105 per
well) were transfected with 0.8 µg of the luciferase constructs and
100 nM agomiR-185 or NC RNA using Lipofectamine. A total of 10 ng
pMIR-REPORT™ β-gal control plasmid (AM5795; Ambion; Thermo Fisher
Scientific, Inc.) was transfected as an internal control to
evaluate transfection efficiency. Luminescence was measured 24 h
after transfection at 37°C using a Dual-Luciferase®
Reporter Assay System (Promega Corporation, Madison, WI, USA)
according to the manufacturer's instructions. Measurements of
luminescence were conducted on a Glomax 20/20 Luminometer (Promega
Corporation).
Statistical analysis
Data analysis was performed using SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA) and expressed as the mean ± standard
deviation. Normality tests were performed for all data. Differences
between groups were evaluated for significance using one-way
analysis of variance. Least significant difference or
Student-Newman-Keuls tests were used when variances were equal, and
Tamhane's T2 or Dunnett's T3 tests were used when variances were
not equal. P<0.05 was considered to indicate a statistically
significant difference.
Results
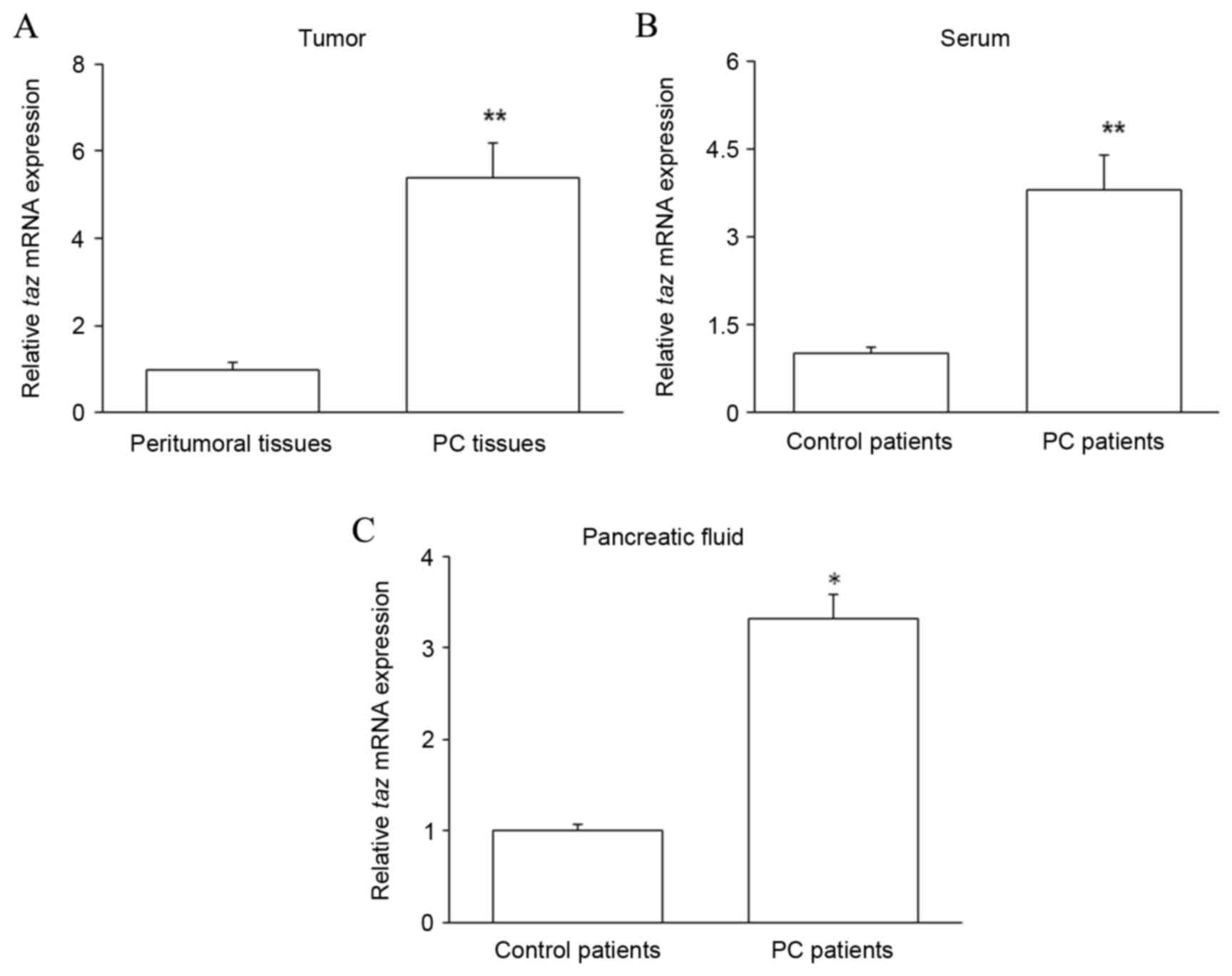
Taz mRNA is upregulated in the PC
tissues, pancreatic juice and serum of PC patients
PC tissues and matched peritumoral tissues were
collected from patients with PC. Pancreatic juice and serum were
collected from PC patients and control patients with chronic
pancreatitis. The expression of taz mRNA was assessed by
RT-qPCR in the patient samples. The level of taz mRNA was
significantly increased in PC tissues compared with that in
peritumoral tissues (P<0.01; Fig.
1A). Furthermore, relative to that in control patients,
taz mRNA expression was significantly increased in the serum
(P<0.01; Fig. 1B) and pancreatic
juice (P<0.05; Fig. 1C) of PC
patients. These results suggest that high levels of TAZ expression
are correlated with PC.
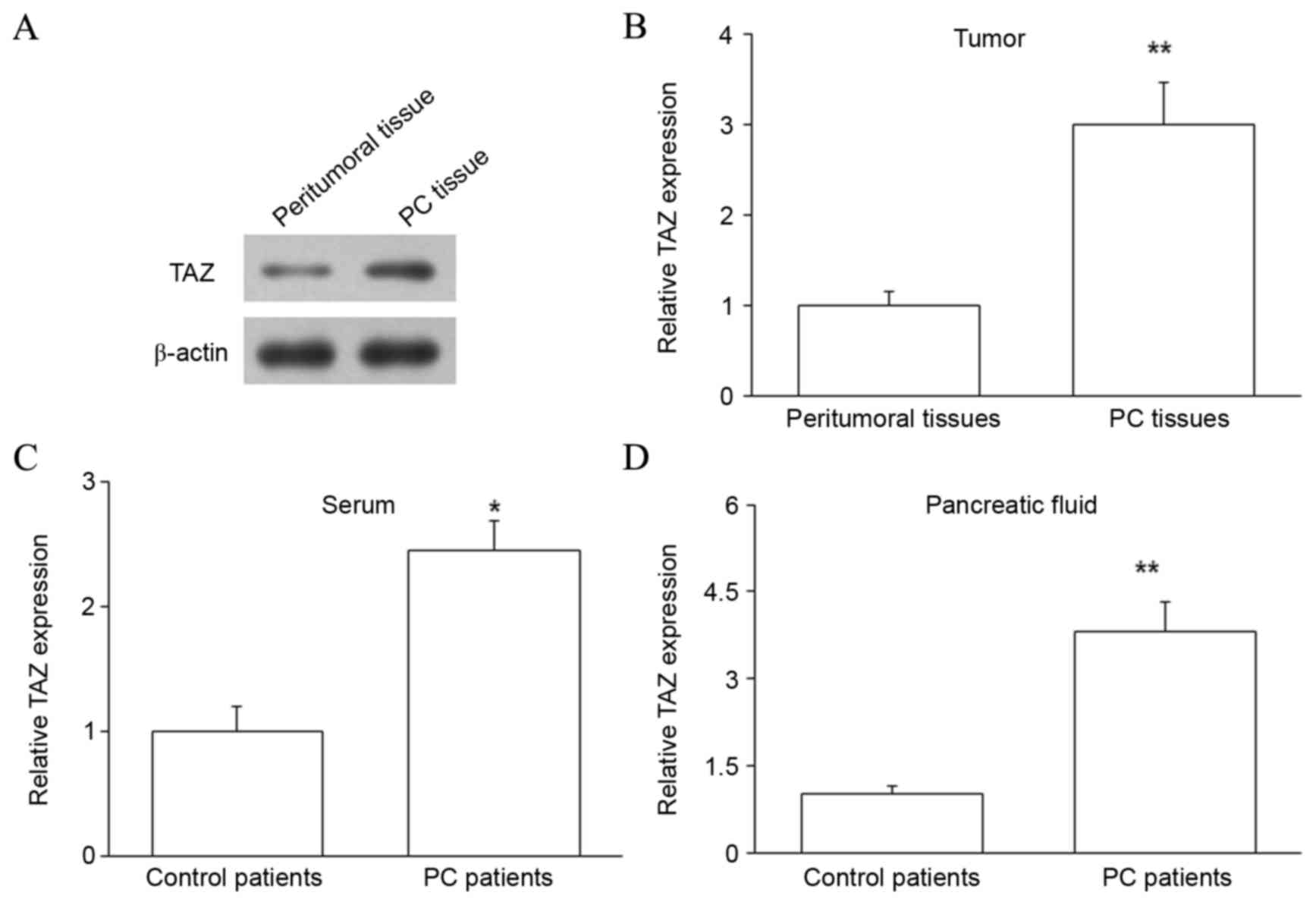
TAZ protein is upregulated in the PC
tissues, pancreatic juice and serum of PC patients
The expression of TAZ protein was subsequently
assessed in PC tissues and matched peritumoral tissues by western
blot analysis. The level of TAZ protein was upregulated in PC
tissues compared with peritumoral tissues (P<0.01; Fig. 2A and B). Thus, TAZ mRNA and protein
were upregulated in PC, indicating that increased TAZ expression
may regulate the development of PC. The expression of TAZ protein
in the pancreatic juice and serum of PC and control patients was
also investigated by ELISA, and revealed that samples from PC
patients contained higher levels of TAZ protein (P<0.05 for
serum and P<0.01 for pancreatic juice; Fig. 2C and D). As blood is a common channel
for tumor metastasis, upregulated expression of TAZ protein in the
blood may influence the metastasis of PC. Furthermore, upregulated
TAZ protein in the pancreatic juice may be due to the release of
large quantities of DNA and proteins from the pancreas into the
pancreatic juice.
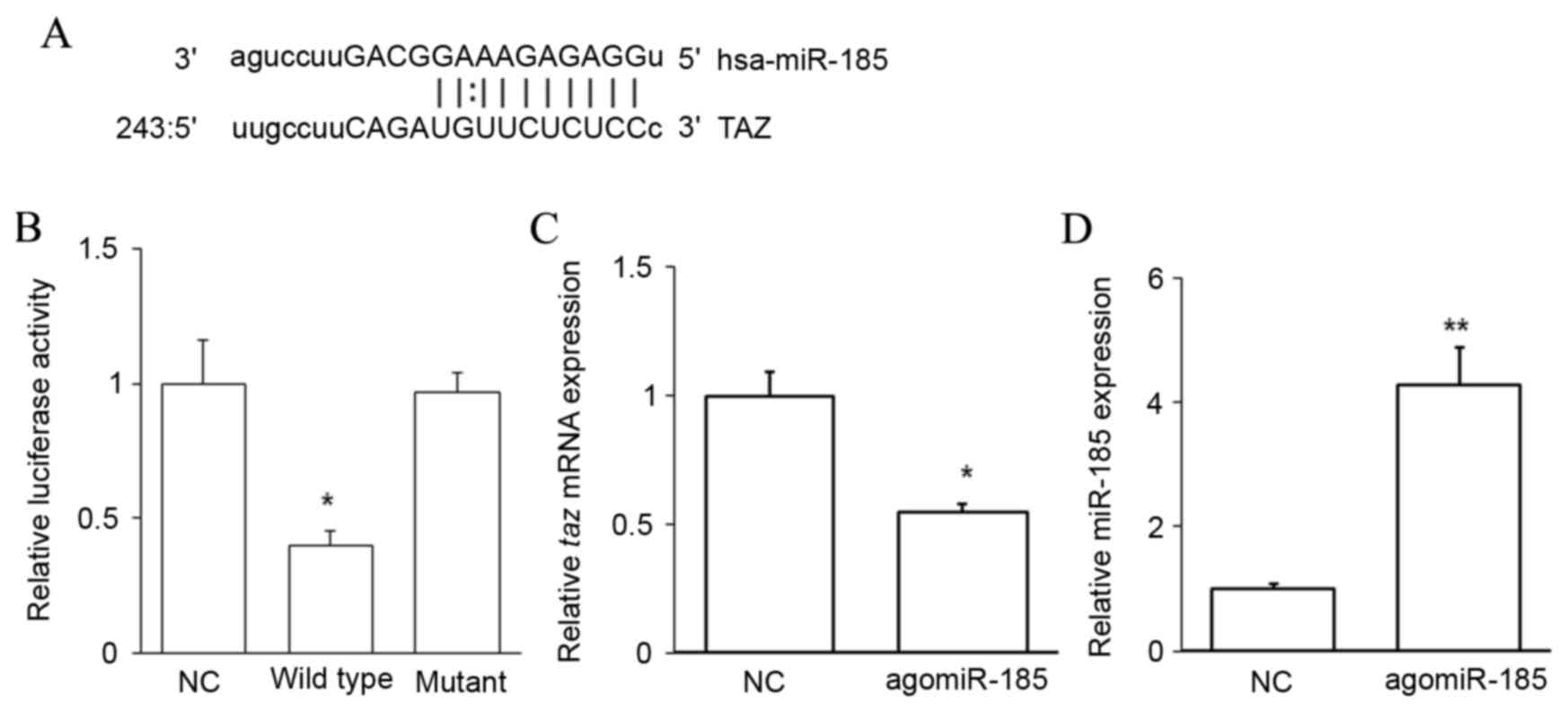
TAZ is a target of miR-185
To evaluate the upstream regulator of TAZ, miRNAs
complementary to the 3′UTR of TAZ were predicted using
bioinformatics methods. An miR-185 binding site was identified in
the 3′UTR of taz mRNA (Fig.
3A). A dual-luciferase reporter assay was performed to confirm
this prediction. Cotransfection with agomiR-185 and a wild-type
pMIR-REPORT-TAZ construct significantly decreased luciferase
activity when compared with cells cotransfected with NC RNA and the
wild-type pMIR-REPORT-TAZ construct (P<0.05). However,
cotransfection with agomiR-185 and a mutant pMIR-REPORT-TAZ
construct did not reduce luciferase activity (Fig. 3B). It was also observed that
agomiR-185 transfection significantly downregulated the level of
taz mRNA in HPACs (P<0.05 vs. NC; Fig. 3C) when miR-185 was successfully
overexpressed (P<0.01 vs. NC; Fig.
3D). These results indicate that TAZ is a target of miR-185,
and that miR-185 may regulate the expression of TAZ by binding to
its 3′UTR and downregulating its expression at the mRNA level.
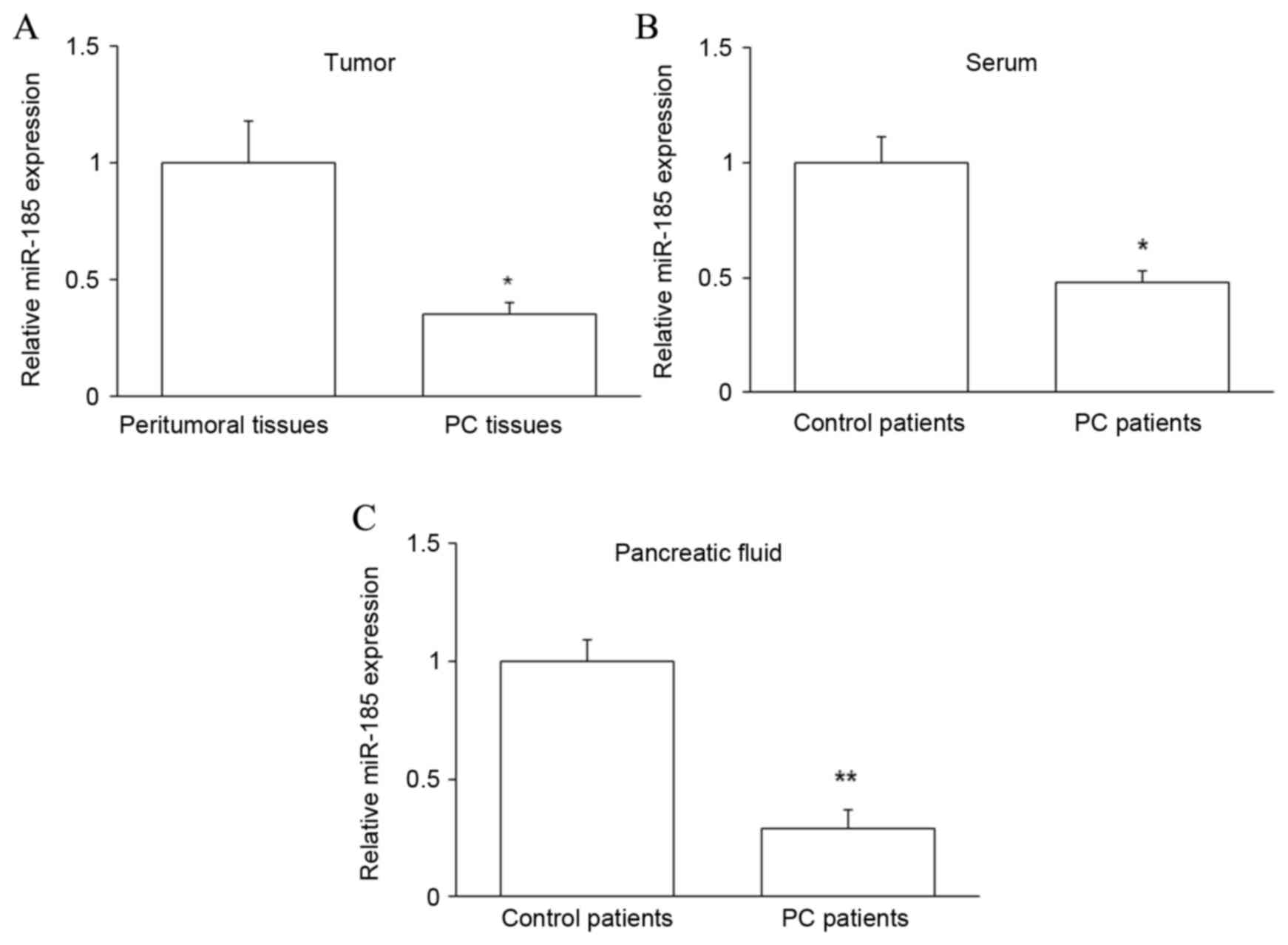
Expression of miR-185 in PC
patients
To further investigate the relationship between TAZ
and miR-185 in PC, the expression of miR-185 was assessed by
RT-qPCR in PC tissues and peritumoral tissues, and in the
pancreatic juice and serum of PC and control patients. It was
observed that miR-185 expression was significantly downregulated in
PC tissues and samples from PC patients (P<0.05 for tissue and
serum, P<0.01 for pancreatic juice; Fig. 4). Collectively, these data suggest
that miR-185 may contribute to the regulation of PC by targeting
taz mRNA and subsequently modulating the level of TAZ
protein.
Suppression of TAZ or agomiR-185
transfection inhibits the proliferation of PC cells
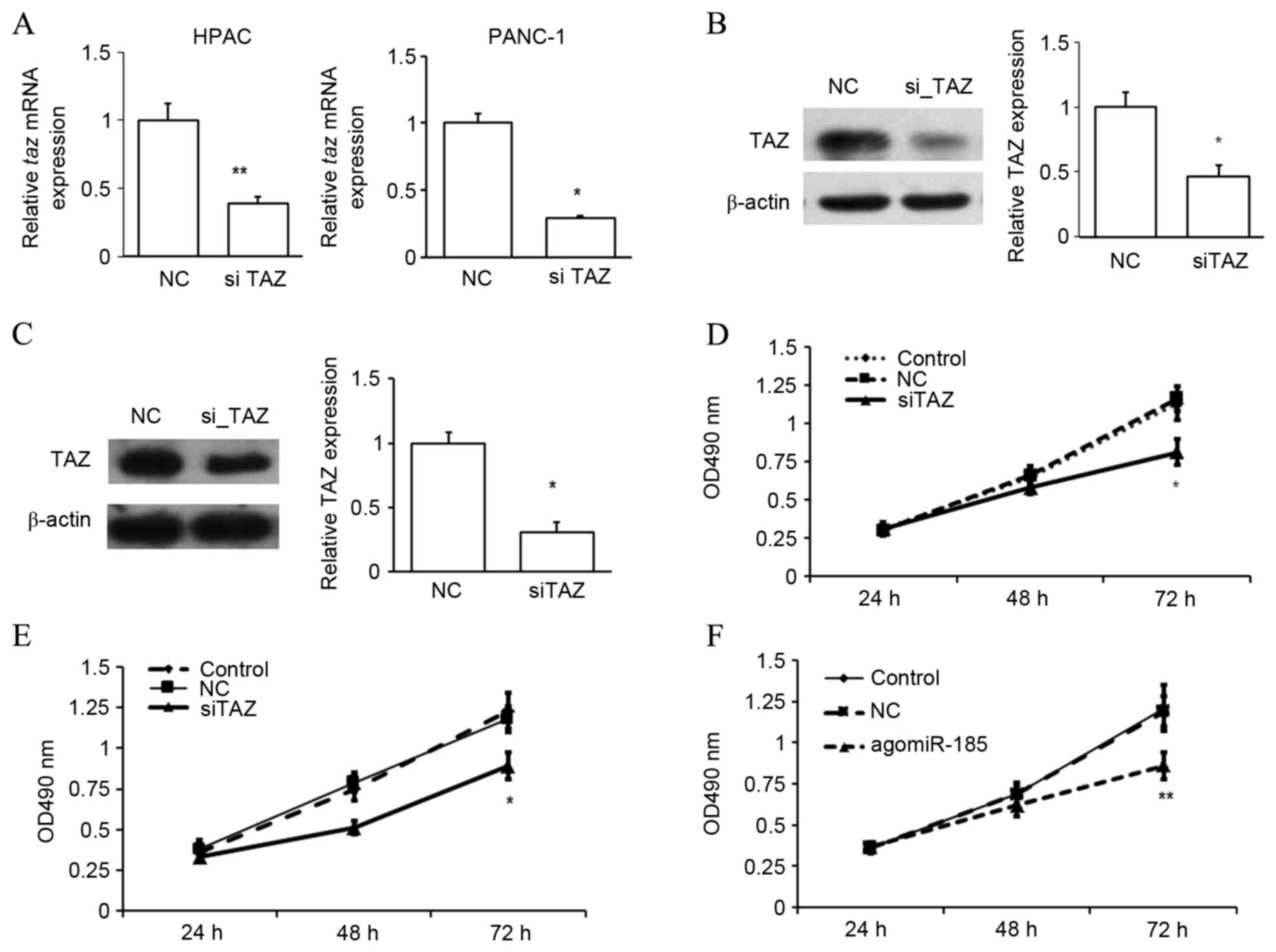
To determine whether TAZ regulated the proliferation
of PC cells, TAZ expression was suppressed in HPACs and PANC-1
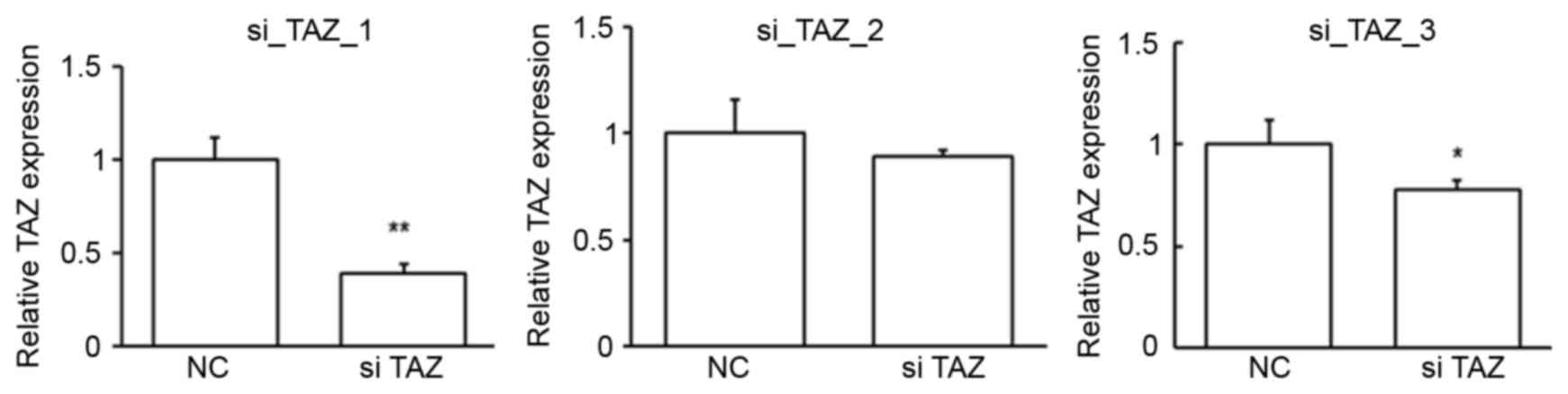
cells. To obtain the best suppression efficiency, the first siRNA
was chosen for the following studies (Fig. 5). The repression of TAZ expression
was confirmed by RT-qPCR (Fig. 6A)
and western blot analysis (Fig. 6B and
C). In an MTT assay, it was observed that repression of TAZ
significantly inhibited the proliferation rate of HPACs (Fig. 6D) and PANC-1 cells (Fig. 6E) after 72 h (both P<0.05 vs. NC),
suggesting a possible role of TAZ as an oncogene in the development
of PC. As miR-185 was found to bind to the 3′UTR of taz
mRNA, the potential role of miR-185 in regulating the development
of PC was investigated. Similar to TAZ inhibition, it was observed
that overexpression of miR-185 in HPACs significantly inhibited the
rate of cell proliferation after 72 h, as determined by an MTT
assay (P<0.01 vs. NC; Fig. 6F).
These data indicate that miR-185 and TAZ may serve opposing roles
in the proliferation of pancreatic cells.
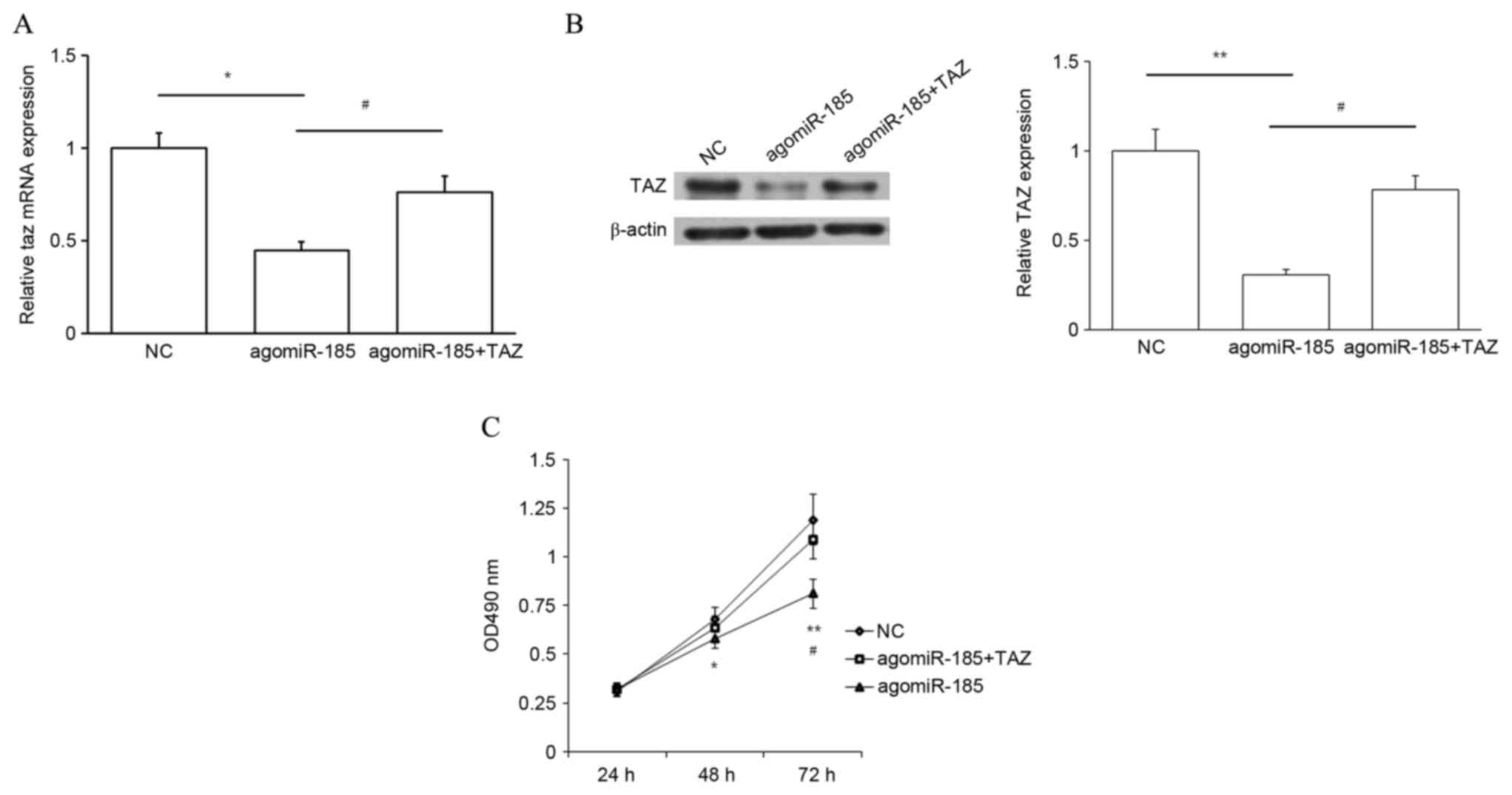
TAZ works downstream of miR-185 to
regulate cell proliferation
The aforementioned results indicate that miR-185
inhibits cell proliferation while TAZ promotes cell proliferation.
In addition, miR-185 potentially targets TAZ and downregulates its
expression. Therefore, whether miR-185 regulated cell growth
through TAZ was subsequently investigated. HPACs were cotransfected
with agomiR-185 and TAZ CDS-expressing plasmid, or with agomiR-185
and empty plasmid. Cells cotransfected with NC and empty plasmid
were used as a NC. Transfection with agomiR-185 significantly
decreased TAZ expression at the mRNA (P<0.05 vs. NC) and protein
(P<0.01 vs. NC) levels, and this repression was rescued by
cotransfection with TAZ expression plasmid (P<0.05; Fig. 7A and B). Results of an MTT assay also
demonstrated that overexpression of miR-185 repressed cell
proliferation at 48 and 72 h (P<0.05 and P<0.01,
respectively, vs. NC), and upregulation of TAZ expression
significantly rescued cell proliferation at 72 h (P<0.05;
Fig. 7C). Thus, targeting of TAZ by
miR-185 may inhibit the proliferation of PC cells.
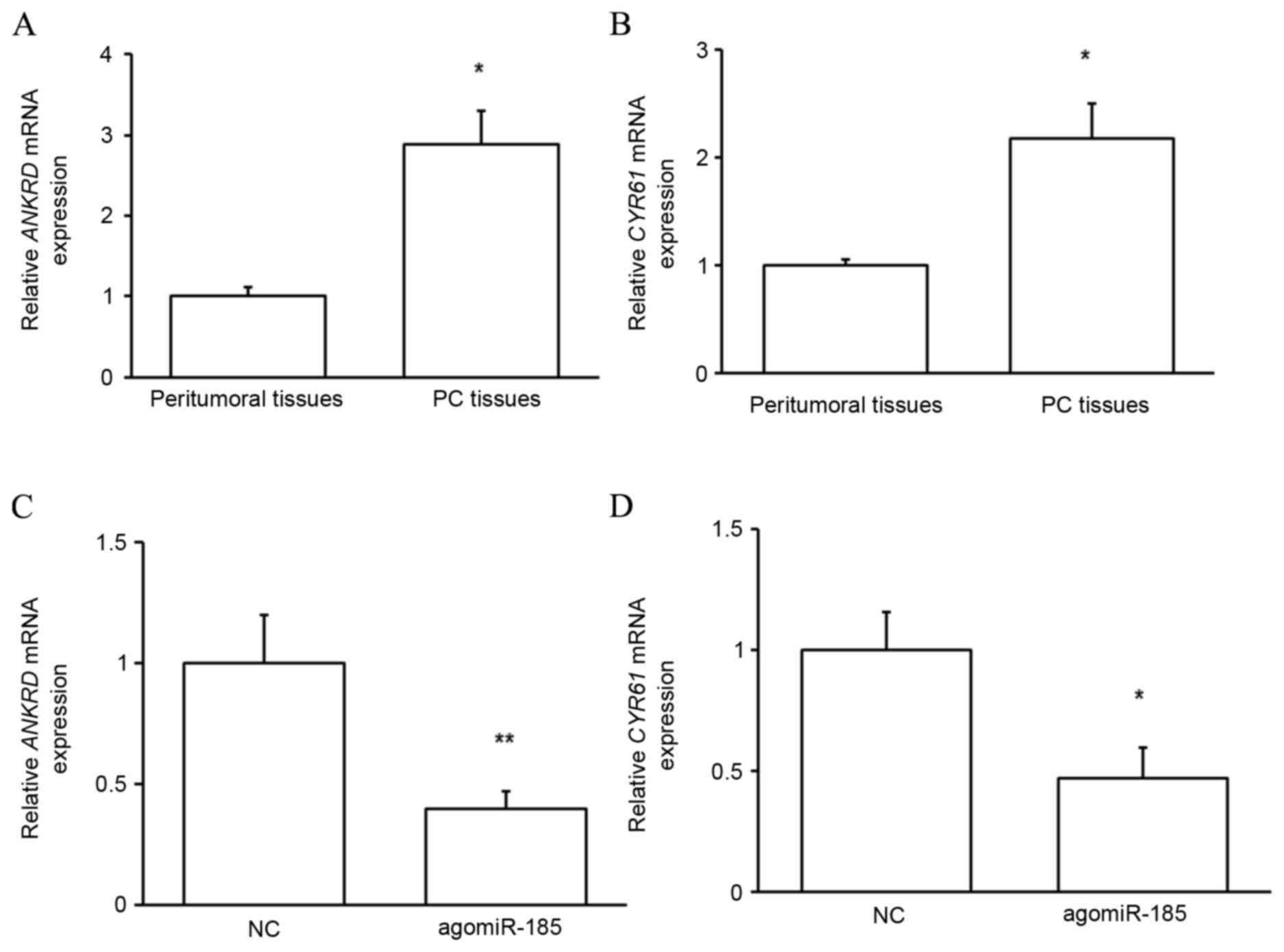
TAZ function in PC is dependent on its
target proteins
To determine whether upregulated TAZ in PC tissues
served functional roles, the expressions of two reported targets of
TAZ, ANKRD and CYR61 (14), were
evaluated. As predicted, the mRNA expression of the target proteins
was significantly upregulated in PC tissues compared with that in
peritumoral tissues (P<0.05; Fig. 8A
and B), indicating that upregulated TAZ mediates the
development of PC through its target proteins. It was subsequently
determined whether miR-185 regulated the function of TAZ. As
depicted in Fig. 8C and D,
transfection of HPACs with agomiR-185 significantly reduced the
expressions of ANKRD (P<0.01) and CYR61 (P<0.05) at the mRNA
level. These results indicate that miR-185 indirectly regulates the
growth of pancreatic cells by targeting TAZ expression and
downstream protein function.
Discussion
In the present study, the expression of TAZ at the
mRNA and protein levels was analyzed in PC tissues, pancreatic
juice and serum samples. Expression of miR-185, as the upstream
regulator of TAZ, was also analyzed in these samples. In addition,
the biological functions of TAZ and miR-185 in the development of
PC were discussed.
The development of PC is a complex, multi-step
process that involves multiple genes and epigenetic changes
(29). With developments in
biological therapy, it is useful to clarify the pathogenesis of PC
and to search for novel genes as potential therapeutic targets. The
hippo signaling pathway, first identified in the 1990s through
genetic screens in overgrown Drosophila, is a conserved
signaling pathway that serves essential roles in the regulation of
organ growth, stem cell function, regeneration and tumor
suppression (30,31). The transcriptional coactivator Yes
associated protein (YAP) and TAZ interact with transcriptional
enhancer associate domain 14 in the nucleus to promote biological
functions downstream of the hippo signaling pathway (32,33).
Previous studies of TAZ/YAP demonstrated that overexpression of
TAZ/YAP induced the epithelial-mesenchymal transition, inhibited
apoptosis and promoted proliferation of tumor cells (34,35).
Furthermore, Xie et al (36)
documented that TAZ was expressed in 66.8% of patients with
non-small cell lung cancer, and its expression was significantly
associated with poorer differentiation and prognosis. Similarly,
Yue et al (37) identified
the expression of TAZ protein in 77.4% of gastric cancer samples,
and high-level expression of TAZ was observed in a higher
percentage of gastric cancer samples with a histology of signet
ring cell carcinoma rather than adenocarcinoma. A study by Wang and
Tang (38) also demonstrated that
YAP/TAZ expression was significantly higher in liver cancer tissues
than that in adjacent normal tissues, and their expression was
associated with Committee on Cancer Staging stage and alpha
fetoprotein expression. In the present study, significantly higher
levels of TAZ mRNA and protein were detected in PC tissues compared
with matched peritumoral tissues, which was in accordance with
former reports (15,16), indicating that TAZ is involved in the
occurrence and development of PC. Thus, ectopic expression of TAZ
may be a critical factor for the occurrence of PC. Indeed, the
present in vitro assays in HPACs revealed that cell
proliferation was inhibited after TAZ siRNA transfection.
To elucidate the regulatory mechanism of TAZ
expression, a bioinformatics analysis was performed. MiR-185 is
considered to be closely associated with TAZ, as miR-185 is a
potential upstream regulator of TAZ. Previous results have
demonstrated that miR-185 is a potential target in the genetic
prevention, diagnosis and treatment of multiple diseases (39–41). For
example, Kim et al (39)
demonstrated that miR-185 effectively blocked cardiac hypertrophy
signaling through multiple targets.. Furthermore, a study by Ma
et al (40) revealed that
miR-185 may inhibit cell proliferation and induce cell apoptosis by
downregulating vascular endothelial growth factor A (VEGF-A) in Von
Hippel-Lindau-inactivated clear cell renal cell carcinomas. Bao
et al (41) also reported a
role of miR-185 in the regulation of insulin secretion and β-cell
growth in diabetes through its potential targeting of suppressor of
cytokine signaling 3.
MiR-185 has also been implicated in the inhibition
of breast cancer cell proliferation through its potential
regulatory effects on the expression of c-Met and/or VEGF-A
(42,43). In the present study, TAZ was
identified as a target of miR-185 in a dual-luciferase reporter
assay, and the interrelationship between miR-185 and TAZ expression
was further evaluated. In the PC tissues, pancreatic juice and
serum of PC patients, miR-185 and TAZ were inversely expressed. In
addition, following agomiR-185 transfection in PC cells, the
expression of taz mRNA was significantly decreased. Rescue
assays also demonstrated that coexpression of TAZ with agomiR-185
alleviated the suppressive effects of agomiR-185 on cell
proliferation, indicating that TAZ functions downstream of miR-185.
Collectively, these results suggest that TAZ is a target of
miR-185.
To verify that upregulated TAZ in PC patients served
functional roles in the development of PC, the expression of two
TAZ target proteins associated with cancer, namely ANKRD and CYR61
(14), was investigated. The
expression of ANKRD is typically induced in injured tissues,
myometrial hypertrophy and denervated tissues, and is important in
muscle metabolism and the maintenance of muscle tissue function
(44). A previous study demonstrated
that its functions mainly involve the regulation of transcription
levels, cell cycling and apoptosis, cytoskeletal stability and
endocytosis activities (45). CYR61
has been associated with cardiac cancer and is a biomarker of tumor
metastasis (46). In the process of
tumor formation, CYR61 promotes angiogenesis and enhances cell
proliferation (47). Therefore,
activation of ANKRD and CYR61 by upregulated TAZ in PC, as observed
in the present study, may be a possible mechanism for the
development of PC.
In conclusion, miR-185 regulated the development of
PC by targeting taz mRNA, and the downregulation of TAZ
protein expression resulted in the inhibition of cell
proliferation. These functions of miR-185 and TAZ may have been
achieved through the downstream effector proteins of TAZ. In future
studies, it would be useful to detect the subcellular location and
cell-specific expression of TAZ, and to specify the correlation of
TAZ with clinicopathological indices of PC (age, gender, duke
stages and TNM stages amongst others). In addition, further
investigation into the underlying molecular mechanisms of the
TAZ/miR-185 axis in PC may provide a theoretical basis for the
prevention, diagnosis and treatment of PC.
Acknowledgements
The present study was supported by projects of the
Medical and Health Technology Development Program in Shandong,
China (grant no. 2013WS0270).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Zeng H and Zhang S: The
updated incidences and mortalities of major cancers in China, 2011.
Chin J Can. 34:502–507. 2015.
|
|
4
|
Tempero MA, Arnoletti JP, Behrman S,
Ben-Josef E, Benson AB III, Berlin JD, Cameron JL, Casper ES, Cohen
SJ, Duff M, et al: Pancreatic adenocarcinoma. J Natl Compr Canc
Netw. 8:972–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le N, Sund M and Vinci A; GEMS
collaborating group of Pancreas, : 2000.Prognostic and predictive
markers in pancreatic adenocarcinoma. Dig Liver Dis. 48:223–230,
2016. View Article : Google Scholar
|
|
6
|
Arnachellum RP, Cariou M, Nousbaum JB,
Jezequel J, Le Reste JY and Robaszkiewicz M: Pancreatic
Adenocarcinoma in the Finistère Area, france, between 2002 and 2011
(1002 Cases): Population characteristics, treatment and survival.
Pancreas. 45:953–960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho JH, Ryu JK, Song SY, Hwang JH, Lee DK,
Woo SM, Joo YE, Jeong S, Lee SO, Park BK, et al: Prognostic
validity of the American joint committee on cancer and the European
neuroendocrine tumors staging classifications for pancreatic
neuroendocrine tumors: A retrospective nationwide multicenter study
in South Korea. Pancreas. 45:941–946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L, Xu HX, Wang WQ, Wu CT, Xiang JF,
Liu C, Long J, Xu J, Fu de L, Ni QX, et al: Serum CA125 is a novel
predictive marker for pancreatic cancer metastasis and correlates
with the metastasis-associated burden. Oncotarget. 7:5943–5956.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alsubai J, Matters GL, McGovern CO, Liao
J, Gilius EL and Smith JP: Germline mutation of the CCK receptor: A
novel biomarker for pancreas cancer. Clin Transl Gastroenterol.
7:e1342016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeon YH, Park YH, Lee JH, Hong JH and Kim
IY: Selenoprotein W enhances skeletal muscle differentiation by
inhibiting TAZ binding to 14-3-3 protein. Biochim Biophys Acta.
1843:1356–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan SW, Lim CJ, Guo K, Ng CP, Lee I,
Hunziker W, Zeng Q and Hong W: A role for TAZ in migration,
invasion, and tumorigenesis of breast cancer cells. Cancer Res.
68:2592–2598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F,
Pei XH, Zhao S, Xiong Y and Guan KL: TAZ promotes cell
proliferation and epithelial-mesenchymal transition and is
inhibited by the hippo pathway. Mol Cell Biol. 28:2426–2436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu H, Subramanian RR and Masters SC:
14-3-3 proteins: Structure, function, and regulation. Annu Rev
Pharmacol Toxicol. 40:617–647. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Ma K, Chen L, Zhu H, Liang S, Liu
M and Xu N: TAZ promotes cell growth and inhibits Celastrol-induced
cell apoptosis. Biosci Rep. 36:pii: e003862016. View Article : Google Scholar
|
|
15
|
Morvaridi S, Dhall D, Greene MI, Pandol SJ
and Wang Q: Role of YAP and TAZ in pancreatic ductal adenocarcinoma
and in stellate cells associated with cancer and chronic
pancreatitis. Sci Rep. 5:167592015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie D, Cui J, Xia T, Jia Z, Wang L, Wei W,
Zhu A, Gao Y, Xie K and Quan M: Hippo transducer TAZ promotes
epithelial mesenchymal transition and supports pancreatic cancer
progression. Oncotarget. 6:35949–35963. 2015.PubMed/NCBI
|
|
17
|
Chen M, Wang M, Xu S, Guo X and Jiang J:
Upregulation of miR-181c contributes to chemoresistance in
pancreatic cancer by inactivating the Hippo signaling pathway.
Oncotarget. 6:44466–44479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higashi T, Hayashi H, Ishimoto T, Takeyama
H, Kaida T, Arima K, Taki K, Sakamoto K, Kuroki H, Okabe H, et al:
miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in
hepatocellular carcinoma cells. Br J Cancer. 113:252–258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tuttle RM, Haugen B and Perrier ND:
Updated American Joint Committee on Cancer/Tumor-Node-Metastasis
Staging System for Differentiated and Anaplastic Thyroid Cancer
(Eighth Edition): What Changed and Why? Thyroid. 27:751–756. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Yue Z, Xiong W, Sun P, You K and
Wang J: TXNIP overexpression suppresses proliferation and induces
apoptosis in SMMC7221 cells through ROS generation and MAPK pathway
activation. Oncol Rep. 37:3369–3376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanger F, Nicklen S and Coulson AR: DNA
sequencing with chain-terminating inhibitors. Proc Natl Acad Sci
USA. 74:pp. 5463–5467. 1977; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan LJ, Ding M, Hou LJ, Cui YT, Li CJ and
Yu DM: Long noncoding RNA TUG1 alleviates extracellular matrix
accumulation via mediating microRNA-377 targeting of PPARγ in
diabetic nephropathy. Biochem Biophys Res Commun. 484:598–604.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Welten SM, Bastiaansen AJ, de Jong RC, de
Vries MR, Peters EA, Boonstra MC, Sheikh SP, La Monica N,
Kandimalla ER, Quax PH and Nossent AY: Inhibition of 14q32
MicroRNAs miR-329, miR-487b, miR-494, and miR-495 increases
neovascularization and blood flow recovery after ischemia. Circ
Res. 115:696–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah VV, Soibam B, Ritter RA, Benham A,
Oomen J and Sater AK: Data on microRNAs and microRNA-targeted mRNAs
in Xenopus ectoderm. Data Brief. 9:699–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang F, Lu J, Peng X, Wang J, Liu X, Chen
X, Jiang Y, Li X and Zhang B: Integrated analysis of microRNA
regulatory network in nasopharyngeal carcinoma with deep
sequencing. J Exp Clin Cancer Res. 35:172016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen WF, Hu YL, Uttarwar L, Passegue E and
Largman C: MicroRNA-126 regulates HOXA9 by binding to the homeobox.
Mol Cell Biol. 28:4609–4619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joladarashi D, Garikipati VNS,
Thandavarayan RA, Verma SK, Mackie AR, Khan M, Gumpert AM, Bhimaraj
A, Youker KA, Uribe C, et al: Enhanced cardiac regenerative ability
of stem cells after ischemia-reperfusion injury: Role of human
CD34+ cells deficient in MicroRNA-377. J Am Coll Cardiol.
66:2214–2226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Q, Zeng L, Chen Y, Lian G, Qian C,
Chen S, Li J and Huang K: Pancreatic cancer epidemiology,
detection, and management. Gastroenterol Res Pract.
2016:89623212016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halder G and Johnson RL: Hippo signaling:
Growth control and beyond. Development. 138:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harvey K and Tapon N: The
Salvador-Warts-Hippo pathway-an emerging tumour-suppressor network.
Nat Rev Cancer. 7:182–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cordenonsi M, Zanconato F, Azzolin L,
Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR,
Poletti A, et al: The Hippo transducer TAZ confers cancer stem
cell-related traits on breast cancer cells. Cell. 147:759–772.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Varelas X: The Hippo pathway effectors TAZ
and YAP in development, homeostasis and disease. Development.
141:1614–1626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie M, Zhang L, He CS, Hou JH, Lin SX, Hu
ZH, Xu F and Zhao HY: Prognostic significance of TAZ expression in
resected non-small cell lung cancer. J Thorac Oncol. 7:799–807.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yue G, Sun X, Gimenez-Capitan A, Shen J,
Yu L, Teixido C, Guan W, Rosell R, Liu B and Wei J: TAZ is highly
expressed in gastric signet ring cell carcinoma. Biomed Res Int.
2014:3930642014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang YP and Tang DX: Expression of
Yes-associated protein in liver cancer and its correlation with
clinicopathological features and prognosis of liver cancer
patients. Int J Clin Exp Med. 8:1080–1086. 2015.PubMed/NCBI
|
|
39
|
Kim JO, Song DW, Kwon EJ, Hong SE, Song
HK, Min CK and Kim DH: miR-185 plays an anti-hypertrophic role in
the heart via multiple targets in the calcium-signaling pathways.
PLoS One. 10:e01225092015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma X, Shen D, Li H, Zhang Y, Lv X, Huang
Q, Gao Y, Li X, Gu L, Xiu S, Bao X, et al: MicroRNA-185 inhibits
cell proliferation and induces cell apoptosis by targeting VEGFA
directly in von Hippel-Lindau-inactivated clear cell renal cell
carcinoma. Urol Oncol. 33:169. e1–11. 2015. View Article : Google Scholar
|
|
41
|
Bao L, Fu X, Si M, Wang Y, Ma R, Ren X and
Lv H: MicroRNA-185 targets SOCS3 to inhibit beta-cell dysfunction
in diabetes. PLoS One. 10:e01160672015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu P, DU F, Yao M, Lv K and Liu Y:
MicroRNA-185 inhibits proliferation by targeting c-Met in human
breast cancer cells. Exp Ther Med. 8:1879–1883. 2014.PubMed/NCBI
|
|
43
|
Wang R, Tian S, Wang HB, Chu DP, Cao JL,
Xia HF and Ma X: MiR-185 is involved in human breast carcinogenesis
by targeting Vegfa. FEBS Lett. 588:4438–4447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miller MK, Bang ML, Witt CC, Labeit D,
Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC and
Labeit S: The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and
DARP as a family of titin filament-based stress response molecules.
J Mol Biol. 333:951–964. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mosavi LK, Cammett TJ, Desrosiers DC and
Peng ZY: The ankyrin repeat as molecular architecture for protein
recognition. Protein Sci. 13:1435–1448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei J, Yu G, Shao G, Sun A, Chen M, Yang W
and Lin Q: CYR61 (CCN1) is a metastatic biomarker of gastric cardia
adenocarcinoma. Oncotarget. 7:31067–31078. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kuonen F, Secondini C and Ruegg C:
Molecular pathways: Emerging pathways mediating growth, invasion,
and metastasis of tumors progressing in an irradiated
microenvironment. Clin Cancer Res. 18:5196–5202. 2012. View Article : Google Scholar : PubMed/NCBI
|